Abstract
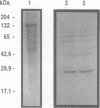
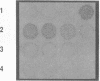
Group B streptococci (GBS) are important pathogens in neonatal sepsis, pneumonia, and meningitis. The ability of GBS to invade the collagen-rich amniotic membrane of the placenta has been shown in vitro. In the presence of GBS, the collagen fibrils of the amnion appear disordered, suggesting a role for GBS in premature rupture of membranes. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Sephadex G-200 column chromatography, and gelatin zymograms were used in this study to characterize cell-associated collagenolytic activities of GBS. The synthetic peptide 2-furanacryloyl-Leu-Gly-Pro-Ala (FALGPA), which mimics the primary structure of collagen, was degraded by GBS USF704, a clinical isolate from the placenta of a septic newborn. Cells of GBS USF704 (9 x 10(7) CFU/ml) hydrolyzed 902 nmol of FALGPA over a 24-h period. As reported for zinc metalloenzymes such as collagenase, the hydrolysis of FALGPA by GBS was inhibited by addition of EDTA or 1,10-phenanthroline. Boiling of the cells resulted in loss of activity, while higher activity was observed with crude GBS cell lysates (hydrolysis of 970 nmol of FALGPA in 1.5 h). Antiserum raised against collagenase from Clostridium histolyticum was found to cross-react with cell-associated proteins produced by GBS and to inhibit GBS FALGPA hydrolysis. Twenty-five additional GBS clinical isolates were screened and found to have various levels of FALGPA hydrolytic activity. These observations suggest a cell-associated collagenolytic activity by GBS which may be involved in premature rupture of membranes and neonatal disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres D. C., Lim C. K. Modification of the pendant ring of podophyllotoxin. Cancer Chemother Pharmacol. 1982;7(2-3):99–101. doi: 10.1007/BF00254529. [DOI] [PubMed] [Google Scholar]
- Dao M. L. An improved method of antigen detection on nitrocellulose: in situ staining of alkaline phosphatase conjugated antibody. J Immunol Methods. 1985 Oct 10;82(2):225–231. doi: 10.1016/0022-1759(85)90354-0. [DOI] [PubMed] [Google Scholar]
- Galask R. P., Varner M. W., Petzold C. R., Wilbur S. L. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984 Apr 1;148(7):915–928. doi: 10.1016/0002-9378(84)90534-9. [DOI] [PubMed] [Google Scholar]
- Han S., Blumenfeld O. O., Seifter S. Specific identification of collagens and their fragments by clostridial and anti-collagenase antibody. Anal Biochem. 1992 Mar;201(2):336–342. doi: 10.1016/0003-2697(92)90348-b. [DOI] [PubMed] [Google Scholar]
- Helmig R., Halaburt J. T., Uldbjert N., Thomsen A. C., Stenderup A. Increased cell adherence of group B streptococci from preterm infants with neonatal sepsis. Obstet Gynecol. 1990 Nov;76(5 Pt 1):825–827. doi: 10.1097/00006250-199011000-00020. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993 Dec;57(4):823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. E., Kanarek K. S., Lim D. V. Group B streptococcal colonization patterns in mothers and their infants. J Clin Microbiol. 1984 Sep;20(3):438–440. doi: 10.1128/jcm.20.3.438-440.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen K. K., Makinen P. L. Purification and properties of an extracellular collagenolytic protease produced by the human oral bacterium Bacillus cereus (strain Soc 67). J Biol Chem. 1987 Sep 15;262(26):12488–12495. [PubMed] [Google Scholar]
- Matorras R., Garcia Perea A., Omeñaca F., Usandizaga J. A., Nieto A., Herruzo R. Group B streptococcus and premature rupture of membranes and preterm delivery. Gynecol Obstet Invest. 1989;27(1):14–18. doi: 10.1159/000293607. [DOI] [PubMed] [Google Scholar]
- McGregor J. A., French J. I., Lawellin D., Franco-Buff A., Smith C., Todd J. K. Bacterial protease-induced reduction of chorioamniotic membrane strength and elasticity. Obstet Gynecol. 1987 Feb;69(2):167–174. [PubMed] [Google Scholar]
- Pass M. A., Gray B. M., Khare S., Dillon H. C., Jr Prospective studies of group B streptococcal infections in infants. J Pediatr. 1979 Sep;95(3):437–443. doi: 10.1016/s0022-3476(79)80531-4. [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Shibano Y., Morihara K., Fukushima J., Inami S., Keil B., Gilles A. M., Kawamoto S., Okuda K. Structural gene and complete amino acid sequence of Vibrio alginolyticus collagenase. Biochem J. 1992 Feb 1;281(Pt 3):703–708. doi: 10.1042/bj2810703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura G. S., Kuypers J. M., Smith S., Raff H., Rubens C. E. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun. 1994 Jun;62(6):2450–2458. doi: 10.1128/iai.62.6.2450-2458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Steinbrink D. R. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal Biochem. 1981 May 15;113(2):356–365. doi: 10.1016/0003-2697(81)90089-0. [DOI] [PubMed] [Google Scholar]