Abstract
A homozygous mutation in the gene for β globin, a subunit of adult hemoglobin A (HbA), is the proximate cause of sickle cell disease (SCD). Sickle hemoglobin (HbS) shows peculiar biochemical properties, which lead to polymerizing when deoxygenated. HbS polymerization is associated with a reduction in cell ion and water content (cell dehydration), increased red cell density which further accelerate HbS polymerization. Dense, dehydrated erythrocytes are likely to undergo instant polymerization in conditions of mild hypoxia due to their high HbS concentration, and HbS polymers may be formed under normal oxygen pressure. Pathophysiological studies have shown that the dense, dehydrated red cells may play a central role in acute and chronic clinical manifestations of sickle cell disease, in which intravascular sickling in capillaries and small vessels leads to vaso-occlusion and impaired blood flow in a variety of organs and tissue. The persistent membrane damage associated with HbS polymerization also favors the generation of distorted rigid cells and further contributes to vaso-occlusive crisis (VOCs) and cell destruction in the peripheral circulation. These damaged, dense sickle red cells also show a loss of phospholipid asymmetry with externalization of phosphatidylserine (PS), which is believed to play a significant role in promoting macrophage recognition with removal of erythrocytes (erythrophagocytosis). Vaso-occlusive events in the microcirculation result from a complex scenario involving the interactions between different cell types, including dense, dehydrated sickle cells, reticulocytes, abnormally activated endothelial cells, leukocytes, platelets and plasma factors such as cytokine and oxidized pro-inflammatory lipids. Hydroxycarbamide (hydroxyurea) is currently the only drug approved for chronic administration in adult patients with sickle cell disease to prevent acute painful crises and reduce the incidence of transfusion and acute chest crises. Here, we will focus on consolidated and experimental therapeutic strategies for the treatment of sickle cell disease, including:
agents which reduce or prevent sickle cell dehydration
agents which reduce sickle cell-endothelial adhesive events
nitric oxide (NO) or NO-related compounds
anti-oxidant agents
Correction of the abnormalities ranging from membrane cation transport pathways to red cell-endothelial adhesive events, might constitute new pharmacological targets for treating sickle cell disease.
Introduction:
A homozygous mutation in the gene for β globin, a subunit of adult hemoglobin A (HbA), is the proximate cause of sickle cell disease (SCD). Sickle hemoglobin (HbS) shows peculiar biochemical properties, which lead to polymerizing when deoxygenated. Studies of the kinetics of HbS polymerization following deoxygenation have shown it to be a high order exponential function of haemoglobin concentration, thus highlighting a crucial role for cellular HbS concentration in sickling1,2. HbS polymerization is associated with a reduction in cell ion and water content (cell dehydration), increased red cell density which further accelerate HbS polymerization1–3. Dense, dehydrated erythrocytes are likely to undergo instant polymerization in conditions of mild hypoxia due to their high HbS concentration, and HbS polymers may be formed under normal oxygen pressure.
Pathophysiological studies have shown that the dense, dehydrated red cells may play a central role in acute and chronic clinical manifestations of sickle cell disease, in which intravascular sickling in capillaries and small vessels leads to vaso-occlusion and impaired blood flow in a variety of organs and tissues2,4. The persistent membrane damage associated with HbS polymerization also favors the generation of5 distorted rigid cells and further contributes to vaso-occlusive crisis (VOCs) and cell destruction in the peripheral circulation. These damaged, dense sickle red cells also show a loss of phospholipid asymmetry with externalization of phosphatidylserine (PS), which is believed to play a significant role in promoting macrophage recognition with removal of erythrocytes (erythrophagocytosis), cell apoptosis and activation of coagulation. Although the percentage of dense erythrocytes does not predict the severity of the disease, it has been shown to increase prior to or during the first phase of the painful crisis and to decrease thereafter4,6,7. Vaso-occlusive events in the microcirculation result from a complex scenario involving the interactions between different cell types, including dense, dehydrated sickle cells, reticulocytes, abnormally activated endothelial cells, leukocytes, platelets and plasma factors such as cytokines8,9 and oxidized pro-inflammatory lipids6,10,11.
Hydroxycarbamide (hydroxyurea) is currently the only drug approved for chronic administration in adult patients with sickle cell disease to prevent acute painful crises and reduce the incidence of transfusion and acute chest crises12. Long-term use of hydroxycarbamide has been demonstrated to produce dramatic reductions in mortality and morbidity in patients with sickle cell disease13. Clinical use of hydroxycarbamide in pediatric and adult patients with sickle cell disease is discussed in the next chapter on clinical management (13.2). Decitabine has also been shown to be a promising agent for the modulation on Hb F in sickle cell disease14. We will focus here on therapeutic strategies currently being considered for the treatment of sickle cell disease, which are not based on Hb F modulation. They include:
Use of agents which reduce or prevent sickle cell dehydration
Use of agents which reduce sickle cell-endothelial adhesive events
Use of nitric oxide (NO) or NO-related compounds
Use of antioxidant agents
a). Prevention of sickle red cell dehydration:
One of the distinguishing characteristics of sickle cell disease is the presence of dense erythrocytes, formed as a result of cell dehydration and loss of potassium (K+). These dense red cells generally have a lower HbF content and include both reticulocytes and red cells15. Usually, the dense fraction of erythrocytes has a high percentage of irreversible sickle cells (ISCs), cells that maintain their sickle shape even when fully oxygenated. An inverse correlation has been demonstrated between percentage of ICSs and erythrocyte survival. In vitro and in vivo studies in animal models for sickle cell disease have suggested a crucial role of dehydrated red cells in the pathogenesis of vaso-occlusive events; in fact, the dense, dehydrated red cells might be easily trapped in post capillary venules, promoting micro-vascular obstruction16.
Thus, prevention of red cell dehydration represents an exciting possible new therapeutic strategy. Studies on membrane permeability in sickle cell disease have shown abnormalities in different specialized membrane-embedded transporters that carry cations, anions and water across the erythrocyte membrane. In the last two decades, studies on the nature and properties of the pathways mediating K+ loss in sickle cell erythrocytes have led to the development of new therapeutic tools to block K+ loss and dehydration.
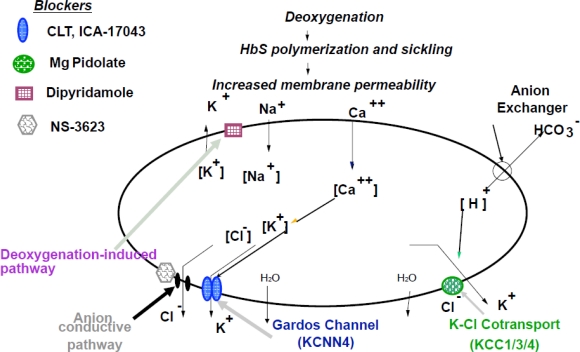
The major pathways for K+ loss during sickle cell dehydration events are the Ca2+-activated K+ channel, known as Gardos channel, operating in parallel with the conductive Cl− pathway and the electroneutral K-Cl cotransport (Figure 1)17–22.
Figure 1.
Schematic diagram of the ion transport pathways involved in sickle cell dehydration and action sites of potential therapeutic blockers: Ca2+activated K+ channel (Gardos channel, KCNN4): Clotrimazole (CLT) and ICA-17043; K-Cl cotransport (KCC1/3/4): Magnesium (Mg) Pidolate; Deoxygenation-induced pathway: Dipyridamole; Anion conductive pathway: NS3623. Deoxygenation induces Hb S polymerization and sickling, with associated increased membrane permeability and abnormal function of different ion transport pathways, resulting in K+, Cl− and water loss and red cell dehydration (modified from De Franceschi L et al. Haematologica 89: 348, 2004).
Ca2+-activated K+ channel (Gardos channel, KCNN4):
Sickle red cells are characterized by increased amounts of calcium, which is functionally and physically sequestered into intracellular vesicles, but maintained in normal concentration in the steady state. The cyclic deoxygenation and HbS polymerization that occurs in sickle red cells has been shown to produce transient increase in free intracellular calcium, which is responsible for large K+ loss with associated Cl− and water loss. This effect is due to activation of a specific Ca-gated K+ channel that was first described by Gardos23. The imidazole antimycotic clotrimazole (CLT) has shown to be a specific inhibitor of the Gardos channel and to prevent sickle cell dehydration in vitro18. In a transgenic mouse model of sickle cell disease, oral administration of CLT was reported to specifically block the Gardos channel, increase the red cell K+ content and reduce red cell dehydration24. The compound was further tested in normal humans (AA) and in sickle cell volunteers (SS), and was shown to be a powerful and effective inhibitor of the erythroid Gardos channel and of sickle red cell dehydration25,26. Further studies led to the development of a novel class of compounds based on the back-bone structure of CLT, which have conserved Gardos channel inhibitory power, but are devoid of the imidazole moiety of CLT, and thus of cytochrome P450 inhibitory effects27. One of these compounds (ICA-17043) has been shown to have 10-fold greater potency than CLT in blocking the Gardos channel in vitro and in vivo to specifically inhibit Gardos channel and prevent K+ loss and red cell dehydration28. Phase I studies in normal human subjects and in sickle cell patients, showed significant blockade of the Gardos channel, in absence of any significant side-effects29. A phase II study showed that ICA-17043 reduced haemolysis and the percentage of dense cells, with a significant amelioration of anaemia in patients with sickle cell disease30. However, a Phase III clinical trial showed no effect of ICA-17043 on the rate of painful events in SCD patients, most likely related with some effects on blood viscosity of red cells displaying an increase survival. No other studies have been planned with this molecule.
Another therapeutic agent, which has been recently shown to modulate the Gardos channel activity, is L-Arginine. Patients with SCD show a state of relative depletion of arginine, which is part of the nitric oxide pathway. L-Arginine supplementation of transgenic sickle cell mice resulted in inhibition of erythrocyte Gardos channel activity and amelioration of red cell dehydration16. A phase II study to test the effect of arginine supplementation have shown no major effects on Gardos channel function and erythrocyte hydration in patients with sickle cell disease31,32.
K-Cl cotransport (KCC1/3/4):
Several forms of K-Cl cotransport have been described in various human and mouse tissues. KCC2 expression seems to be limited to brain cells, while human and mouse erythrocytes seem to possess KCC1, KCC3 and KCC4 isoforms in different and still undetermined ratio. The K-Cl cotransport mediates red cell dehydration in SCD. Studies on K-Cl cotransport function have identified different triggers of activation, such as cell swelling, cell acidification, reduced cell magnesium (Mg2+) content, membrane oxidative damage and urea. Franco et al.22 have also shown that K-Cl cotransport mainly contributes to dehydration of sickle reticulocytes and that deoxygenation of sickle red cells also stimulates K-Cl cotransport in isotonic solutions at pH 7.4 (Figure 1). The relative contribution of the Gardos channel and of the K-Cl cotransport pathway in generating dehydrated, dense sickle red cells is a complex and still unresolved issue.
K-Cl cotransport activity is modulated by red cell Mg content and low Mg2+ levels are associated with abnormal activation of K-Cl cotransport. Some small studies have reported a reduction in red cell Mg2+ content in SCD patients. Thus, oral Mg supplementation with the aim of increasing red cell Mg2+ levels and inhibiting K-Cl cotransport activity may represent a possible therapeutic strategy for ameliorating SCD red cell dehydration16,17. Dietary magnesium supple-mentation in transgenic sickle cell mice has demonstrated that increasing erythrocyte Mg2+ content can ameliorate red cell dehydration. Two uncontrolled trials of oral supplementation with Mg pidolate have been carried out in sickle cell patients, showing a reduction in K-Cl cotransport activity, an increase in red cell K+ and Mg2+ content, an improvement in red cell dehydration and a reduction in the number of painful events17,33. A double-blind, placebo controlled crossover study with Mg pidolate supplementation in children with sickle cell disease did not demonstrate any significant changes in the haematological parameters studied; however, the Mg-pidolate dosage used was markedly lower than that proposed in the previous studies. In a phase I study, the therapeutic association of Mg-pidolate with hydroxyurea have been evaluated in patients with HbSC disease, showing a significant reduction in the activity of the K-Cl cotransport after 3 months of supplementation34.
Recently, it has been reported that infusion of Mg sulfate reduces the length of stay of sickle cell patients hospitalized during vaso-occlusive crises16.
Cl− permeability pathway:
Studies on the conductive Cl− pathway indicate that for red cell dehydration the movement of K+ must be accompanied by that of chloride (or other monovalent anions) to maintain electroneutrality (Figure 1). Elegant sets of studies demonstrate that movement of K+ and dehydration via the Gardos channel can be blocked if the Cl− conductive pathway is inhibited. A specific inhibitor of Cl-conductance has been recently developed (NS3623). NS3623 has been tested in transgenic sickle cell mice and was found to reduce in vivo sickle cell dehydration, with a mild echinocytosis at the highest doses. Unfortunately, NS3623 was not further developed for clinical use because of undesirable side effects observed in human subjects16,19.
b). Anti-adherence therapy in sickle cell disease:
Vaso-occlusive episodes are central events in the pathophysiology of sickle cell disease, causing the clinical manifestations and leading to acute and chronic organ damage. The abnormal adhesive interactions between erythrocyte, reticulocytes, endothelial cells, platelets or soluble mediators may represent a possible new therapeutic target. In addition, SCD patients showed abnormally activated circulating endothelial cells that increase during acute vaso-occlusive crisis suggesting the presence of chronic vascular endothelial damage further worsening during acute events4,35–37. The end-point of anti-adherence therapy is to interfere with the initialization and/or amplification of adhesive events. Although anti-adherence therapy has been mainly studied during acute painful events, its mechanisms of action are only partially known.4,6,38–40
In SCD, the anti-adherence therapeutic strategies (Figure 2) can be divided into:
Molecules interfering with chemical-physical processes during erythrocyte-endothelial adhesion events
Molecules interfering with sickle cell-endothelial adhesive mechanisms
Molecules modulating inflammatory pathways involved in sickle cell-endothelial adhesion
The heme-oxygenase-1 (HO-1) connection
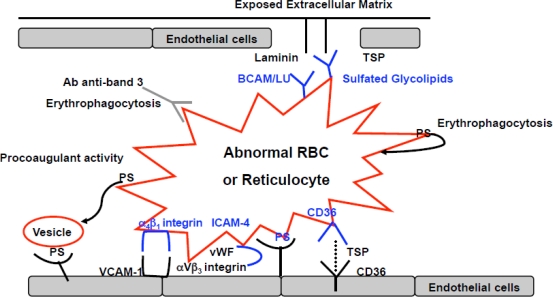
Figure 2.
Schematic diagram of possible therapeutic targets for agents that interfere with adherence of sickle red cells (RBC) or reticulocytes to abnormally activated endothelial cells. PS: phosphatidylserine; TSP: thrombospondine; Ab anti-band 3: natural occurring antibodies (NTAb) anti-band 3; vW: von Willebrand; BCAM/LU: Lutheran blood group protein; ICAM-4: Landstein-Weiner (LW) blood group glycoprotein (modified from De Franceschi L et al. Haematologica 89: 348, 2004).
Molecules interfering with chemical-physical processes during erythrocyte-endothelial adhesion events:
Non-ionic surfactant block copolymer such as RheothRx (Poloxamer 188) lowering viscosity and frictional forces improves microvascular blood flow. RheothRx has been shown to block hydrophobic adhesive interactions (cell-cell, cell-protein or protein-protein interaction) in blood, resulting in reduction of erythrocyte aggregation and red cell adherence to vascular endothelium, with a hypothetical improvement in microvascular flow41. Phase II studies have shown a limited favorable effect in treatment of acute pain crises, when associated with hydroxyurea (HU) in sickle cell children. However, no further clinical development studies are planned for this compound.
Molecules interfering with sickle cell-endothelial adhesive mechanisms:
Recent studies on the sickle cell-endothelium adhesive mechanism have identified different interactions which may have particular therapeutic relevance: a) the integrin α a4bβ1 receptor of fibronectin and the vascular adhesion molecule −1 (VCAM-1), E-selectin and P-selectin; b) the thrombospondin and/or collagen and receptor CD36, present on the surface of endothelial cells, platelets and reticulocyte-rich subpopulations of normal and sickle erythrocytes; c) the sulfate glycolipids, which bind thrombospondin, von Willebrand factor multimer and laminin2,42,43; d) the Lutheran blood group proteins (BCAM/LU), which expression is increased in red cells from SCD patients that bind to α5 subunit of laminin, a component of extracellular subendothelial matrix44,45; e) the ICAM-4 (Landstein-Weiner blood group glycoprotein- LW), which binds αVβ3 integrin receptors on endothelial cells46–49; and f) the exposure of phosphatydyl-serine (PS), detectable in a subpopulation of sickle red cells, which participates in sickle cell adhesion to activated endothelium50–54 (Figure 2). Ex vivo and in vitro experimental studies have shown that thrombospondin- and von Willebrand factor-mediated interaction between sickle red cells and endothelium via α Vβ3 integrin might be blocked by monoclonal antibodies against αVβ3 integrin receptors42,48,55. Recent study with short synthetic peptides interfering with ICAM-4 and α Vβ3 integrin binding have been evaluated in ex vivo system, showing a reduction in sickle erythrocyte adhesion to activated endothelial cells allowing to consider the blocking of this adhesion pathway as possible therapeutic new strategy in treatment of acute sickle cell events48,55.
The binding between thrombospondin, von Willebrand factor and laminin, which mediates sickle cell-endothelial adherence, might be blocked by anionic polysaccharides such as high molecular weight dextran sulfate or chondroitin sulfate2,42, 43.
An additional therapeutic approach to block sickle cell adhesion to endothelial cells is heparin that might interfer with sickle cell adhesion to endothelial cells through P-selectin56–59 or binding to TSP that can mediate the interactions between sickle erythrocytes and the vascular endothelial surface. A double blind randomized trial with tinzaparin in SCD patients during acute VOCs has documented a reduction of severity and duration of the VOCs51,60.
Molecules modulating inflammatory pathways involved in sickle cell-endothelial adhesion:
Chronic inflammatory state has been described in SCD patients characterized by increase plasma levels of acute phase proteins, of soluble cytokines such as IL1β, IL6, TNF- α and endothelin-1 (ET-1) that are further elevated during acute VOCs. These factors participate to leukocyte chemotasis, modulate vascular tone and contribute in sickle cell related tissue damage. Thus, anti-inflammatory therapy has been propose to interfere with inflammatory storm and abnormal vascular activation61. Sulfasalazine is an anti-inflammatory molecule and can inhibit the transcription of nuclear factor NF-kB and interferring with endothelial cell activation62–65. Transgenic sickle cell mice treated with sulfasalazine show a reduction in activated circulating endothelial cells, and in VCAM-1, ICAM and E-selectin vascular wall endothelial expression. In a pilot study, the administration of sulfasalazine to sickle cell patients results in reduction in the abnormal endothelial activation4. Another possible strategy aimed at reducing the adhesion of sickle red cells to vascular endothelium is the inhibition of interactions between leukocytes already adherent to endothelium and sickle red cells during vaso-occlusive events66. Based on the in vitro evidence that immunoglobulin (Ig) significantly reduces the binding of sickle red cells to neutrophils in transgenic sickle cell mice, the infusion of Ig in vivo was shown to inhibit the interaction between sickle red cells and leukocytes in the cremasteric venules, suggesting that Ig may act either by inhibitiing the interactions between sickle red cells and leukocytes and/or by reducing the number of adherent leukocytes66. In humans, three out of four sickle cell patients treated with infusion of Ig showed some beneficial effect, whereas in the fourth case the treatment accelerated a vaso-occlusive crisis66,67. Since Ig infusion might be related to severe side effects such as renal toxicity and thrombosis, it should be used with caution in sickle cell patients.
Recent studies have shown the important role of ET-1 in acute sickle cell related VOCs in a mouse model for SCD68,69. The block of ET-1 actions was obtained directly by the ET-1 receptors antagonist, Bosentan, evaluating its effects on SCD mouse kidney as target organ and indirectly by the inhibition of phosphodiesterase-4 with Rolipram in a model of early pulmonary hypertension68,69. Bosentan is actually under evaluation in a phase III clinical trial in SCD patients with pulmonary hypertension70.
The possibility of delivering oxygen directly to sickled red cells entrapped in partially obstructed vessels has also been explored. Perflubron-based fluorocarbon emulsion (PFE) decreases the peripheral vascular resistance ex vivo in the mesocaecal vasculature of rats, due more to its ability to dissolve oxygen than to its ability to modify the vascular tone71.
Recently, Hebbel et al have reported the beneficial effects of histone deacetylase inhibitors on vascular pathology in mouse model for sickle cell disease72. The Authors observed multiple therapeutic effects of these compounds as: (i) inducers of HbF; (ii) iron chelators; (iii) modulators of vascular damage and abnormal activation sickle cell related (i.e.: reduction of VCAM-1 expression).
The heme-oxygenase-1 (HO-1) connection:
In different sickle cell mouse models under steady state, Belcher et al have shown up-regulation of cytoprotective gene as heme oxygenase-1 (HO-1) and a reduction of the sickle cell related organ damage when pathological mice were treated with heme oxygenase-1 products63,73. In SCD patients in steady state, the gene expression profiling of circulating leukocytes has shown increased HO-1 and biliverdin reductase as well as in kidney and in circulating endothelial cells74,75, suggesting an induction of cell protective systems in response to chronic inflammatory stress characterizing SCD. However, the still open question is whether these cytoprotective systems are rapidly and further induced driving a continuous cellular protection during acute sickle cell vaso-occlusive crisis.
c). Nitric Oxide (NO) based therapies in sickle cell disease:
Nitric oxide (NO) is a potent vasodilator and inhibitor of vascular remodeling and also affects the multi-step cascade of events involved in leukocyte, platelet and endothelial activation. NO is generated from L-Arginine by endothelial cells via constitutive (eNOS) and inflammatory inducible nitric oxide synthases (iNOS). SCD is characterized by relative reduction in NO bioavaibility that contributes to endothelial abnormal activation and SCD organ damage. In addition chronic hemolysis leading to increase the plasma levels of hemoglobin that is an efficient NO buffer, contributes in reducing NO levels in SCD.
Recent studies have focused on inhaled NO for the treatment of tissue damage in various ischaemic syndromes, including cardiovascular disease, pulmonary hypertension, and acute lung distress syndromes. The possible therapeutic role of inhaled NO has been studied in different animal models of lung injury induced by ischaemia/reperfusion. Inhaled NO prevents leukocyte migration and reduces the permeability of the peripheral microvasculature. In association with surfactant, inhaled NO alleviates alveolar edema and reduces bronchoalveolar leukocyte and neutrophil infiltration in animal models of ischaemic lung injury. A placebo-controlled randomized clinical trial of inhaled NO in SCD has recently reported beneficial results in the treatment of acute vaso-occlusive crisis, although the mechanism of action remains unknown. Plasma NO metabolites are decreased in SCD patients during vaso-occlusive crisis associated with severe pain and also in acute chest syndrome32. A decrease in exhaled NO has been reported in sickle cell patients, suggesting a role for NO in the pathogenesis of the pulmonary complications76. In a transgenic mouse model of sickle cell disease, it has been shown that inhaled NO provides protection during ischaemia/reperfusion lung injury, in which endothelial NO production is reduced77,78.
In addition NO-donor as polynitroxy-albumine and nitroxic- albumine have been shown to be effective in reducing inflammatory state in a SCD mouse strain and to reduce the hypoxia induce lung damage in another mouse model of acute VOCs79,80.
Another possible therapeutic strategy for increased NO production in sickle cell disease is supplementation of L-Arginine. Morris et al showed that L-Arginine supplementation alone induces an unexpected decrease in NO metabolite production11,81. In a subsequent pilot study, an increase in NO metabolites was observed when L-Arginine was co-administrated with HU, suggesting that the combination treatment may have a synergistic effect on NO production2,31. A phase II trial on L-Arginine supplementation in SCD has shown no effects on NO levels and on erythrocyte features.
d). Antioxidant agents in sickle cell disease:
SCD is characterized by a pro-oxidant environment due to high production of reactive oxygen species (ROS) related to increased levels of free pathological iron and heme groups associated with a reduction in antioxidant systems such as GSH82–85. Studies in vitro on SCD red cells have shown that iron chelation by deferipone (L1) reduce the sickle red cell membrane susceptibility to iron mediated oxidative damage82,86. In vivo study on SCD patients supplemented with L-glutamate to increase GSH and glutamate levels have shown some improvement of chronic pain83.
Conclusions:
In conclusion, the emerging picture for treatment of sickle cell disease is that abnormalities ranging from membrane cation transport pathways to red cell membrane proteins structure and function, or red cell-endothelial adhesive events, might constitute new pharmacological targets for treating sickle cell disease. Prospective therapy for SCD need to combine molecules with different pharmacological targets in order to increase their therapeutic efficacy and to reduce their side effects (e.g., volume-controlling drugs and either hydroxyurea or anti-adhesive molecules).
Footnotes
This article is available from: http://www.mjhid.org/article/view/5239
References
- 1.Eaton WA, Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 3.Ballas SK, Smith ED. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood. 1992;79:2154–2163. [PubMed] [Google Scholar]
- 4.Solovey AA, Solovey AN, Harkness J, Hebbel RP. Modulation of endothelial cell activation in sickle cell disease: a pilot study. Blood. 2001;97:1937–1941. doi: 10.1182/blood.v97.7.1937. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe C, Klitz W, D’Harlingue K, Cheng S, et al. Confirmation of an association between the TNF(-308) promoter polymorphism and stroke risk in children with sickle cell anemia. Stroke. 2007;38:2241–2246. doi: 10.1161/STROKEAHA.107.483115. [DOI] [PubMed] [Google Scholar]
- 6.Hebbel RP. Adhesive interactions of sickle erythrocytes with endothelium. J Clin Invest. 1997;100:S83–86. [PubMed] [Google Scholar]
- 7.Kuypers FA, Yuan J, Lewis RA, Snyder LM, et al. Membrane phospholipid asymmetry in human thalassemia. Blood. 1998;91:3044–3051. [PubMed] [Google Scholar]
- 8.Archer DR, Stiles JK, Newman GW, Quarshie A, et al. C-reactive protein and interleukin-6 are decreased in transgenic sickle cell mice fed a high protein diet. J Nutr. 2008;138:1148–1152. doi: 10.1093/jn/138.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum C, Peace D, Rich E, Van Besien K. Granulocyte colony-stimulating factor-based stem cell mobilization in patients with sickle cell disease. Biol Blood Marrow Transplant. 2008;14:719–723. doi: 10.1016/j.bbmt.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Ou J, Ou Z, Jones DW, Holzhauer S, et al. L-4F, an apolipoprotein A-1 mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation. 2003;107:2337–2341. doi: 10.1161/01.CIR.0000070589.61860.A9. [DOI] [PubMed] [Google Scholar]
- 11.Belcher JD, Marker PH, Geiger P, Girotti AW, et al. Low-density lipoprotein susceptibility to oxidation and cytotoxicity to endothelium in sickle cell anemia. J Lab Clin Med. 1999;133:605–612. doi: 10.1016/s0022-2143(99)90191-9. [DOI] [PubMed] [Google Scholar]
- 12.Charache S, Terrin ML, Moore RD, Dover GJ, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg MH, Barton F, Castro O, Pegelow CH, et al. Effect of Hydroxyurea on Mortality and Morbidity in Adult Sickle Cell Anemia: Risks and Benefits Up to 9 Years of Treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 14.Saunthararajah Y, Molokie R, Saraf S, Sidhwani S, et al. Clinical effectiveness of decitabine in severe sickle cell disease. Br J Haematol. 2008;141:126–129. doi: 10.1111/j.1365-2141.2008.07027.x. [DOI] [PubMed] [Google Scholar]
- 15.Fabry ME, Nagel RL. Heterogeneity of red cells in the sickler: a characteristic with practical clinical and pathophysiological implications. Blood Cells. 1982;8:9–15. [PubMed] [Google Scholar]
- 16.De Franceschi L, Corroche R. Established and experimental treatments for sickle cell disease. Haematologica. 2004;89:348–356. [PubMed] [Google Scholar]
- 17.De Franceschi L, Bachir D, Galacteros F, Tchernia G, et al. Oral magnesium supplements reduce erythrocyte dehydration in patients with sickle cell disease. J Clin Invest. 1997;100:1847–1852. doi: 10.1172/JCI119713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brugnara C, de Franceschi L, Alper SL. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J Clin Invest. 1993;92:520–526. doi: 10.1172/JCI116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugnara C, De Franceschi L, Bennekou P, Alper SL, Christophersen P. Novel therapies for prevention of erythrocyte dehydration in sickle cell anemia. Drug News Perspect. 2001;14:208–220. doi: 10.1358/dnp.2001.14.4.858404. [DOI] [PubMed] [Google Scholar]
- 20.Lew VL, Ortiz OE, Bookchin RM. Stochastic nature and red cell population distribution of the sickling-induced Ca2+ permeability. J Clin Invest. 1997;99:2727–2735. doi: 10.1172/JCI119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGoron AJ, Joiner CH, Palascak MB, Claussen WJ, Franco RS. Dehydration of mature and immature sickle red blood cells during fast oxygenation/deoxygenation cycles: role of KCl cotransport and extracellular calcium. Blood. 2000;95:2164–2168. [PubMed] [Google Scholar]
- 22.Franco RS, Thompson H, Palascak M, Joiner CH. The formation of transferrin receptor-positive sickle reticulocytes with intermediate density is not determined by fetal hemoglobin content. Blood. 1997;90:3195–3203. [PubMed] [Google Scholar]
- 23.Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- 24.De Franceschi L, Saadane N, Trudel M, Alper SL, et al. Treatment with oral clotrimazole blocks Ca(2+)-activated K+ transport and reverses erythrocyte dehydration in transgenic SAD mice. A model for therapy of sickle cell disease. J Clin Invest. 1994;93:1670–1676. doi: 10.1172/JCI117149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brugnara C, Armsby CC, Sakamoto M, Rifai N, et al. Oral administration of clotrimazole and blockade of human erythrocyte Ca(++)-activated K+ channel: the imidazole ring is not required for inhibitory activity. Journal of Pharmacology & Experimental Therapeutics. 1995;273:266–272. [PubMed] [Google Scholar]
- 26.Brugnara C, Gee B, Armsby CC, Kurth S, et al. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. Journal of Clinical Investigation. 1996;97:1227–1234. doi: 10.1172/JCI118537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNaughton-Smith GA, Burns JF, Stocker JW, Rigdon GC, et al. Novel inhibitors of the Gardos channel for the treatment of sickle cell disease. J Med Chem. 2008;51:976–982. doi: 10.1021/jm070663s. [DOI] [PubMed] [Google Scholar]
- 28.Stocker JW, De Franceschi L, McNaughton-Smith GA, Corrocher R, et al. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- 29.Ataga KI, DeCastro LM, Swerdlow P, Saunthararajay Y, Smith W. Efficacy and safety of the Gardos channel inhibitor, ICA-17043, in patients with sickle cell anemia. Blood. 2004;104:33a. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- 30.Ataga KI, Smith WR, De Castro LM, Swerdlow P, et al. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111:3991–3997. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- 31.Morris CR, Kato GJ, Poljakovic M, Wang X, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez BL, Kreshak AA, Morris CR, Davis-Moon L, et al. L-arginine levels are diminished in adult acute vaso-occlusive sickle cell crisis in the emergency department. Br J Haematol. 2003;120:532–534. doi: 10.1046/j.1365-2141.2003.04109.x. [DOI] [PubMed] [Google Scholar]
- 33.De Franceschi L, Bachir D, Galacteros F, Tchernia G, et al. Oral magnesium pidolate: effects of long-term administration in patients with sickle cell disease. Br. J Haematol. 2000;108:248–289. doi: 10.1046/j.1365-2141.2000.01861.x. [DOI] [PubMed] [Google Scholar]
- 34.Hankins JS, Wynn LW, Brugnara C, Hillery CA, et al. Phase I study of magnesium pidolate in combination with hydroxycarbamide for children with sickle cell anaemia. Br J Haematol. 2008;140:80–85. doi: 10.1111/j.1365-2141.2007.06884.x. [DOI] [PubMed] [Google Scholar]
- 35.Solovey A, Gui L, Ramakrishnan S, Steinberg MH, Hebbel RP. Sickle cell anemia as a possible state of enhanced anti-apoptotic tone: survival effect of vascular endothelial growth factor on circulating and unanchored endothelial cells. Blood. 1999;93:3824–3830. [PubMed] [Google Scholar]
- 36.Ortiz A. Circulating endothelial cells in sickle cell anemia. N Engl J Med. 1998:338, 1162. author reply 1162–1163. [PubMed] [Google Scholar]
- 37.Parise LV, Telen MJ. Erythrocyte adhesion in sickle cell disease. Curr Hematol Rep. 2003;2:102–108. [PubMed] [Google Scholar]
- 38.Hebbel RP, Vercellotti G, Nath KA. A Systems Biology Consideration of the Vasculopathy of Sickle Cell Anemia: The Need for Multi-Modality Chemo-Prophylaxsis. Cardiovasc Hematol Disord Drug Targets. 2009. [DOI] [PMC free article] [PubMed]
- 39.Hebbel RP. The systems biology-based argument for taking a bold step in chemoprophylaxis of sickle vasculopathy. Am J Hematol. 2009;84:543–545. doi: 10.1002/ajh.21474. [DOI] [PubMed] [Google Scholar]
- 40.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orringer EP, Casella JF, Ataga KI, Koshy M, et al. Purified poloxamer 188 for treatment of acute vaso-occlusive crisis of sickle cell disease: A randomized controlled trial. Jama. 2001;286:2099–2106. doi: 10.1001/jama.286.17.2099. [DOI] [PubMed] [Google Scholar]
- 42.Kaul DK, Tsai HM, Liu XD, Nakada MT, et al. Monoclonal antibodies to alphaVbeta3 (7E3 and LM609) inhibit sickle red blood cell-endothelium interactions induced by platelet-activating factor. Blood. 2000;95:368–374. [PubMed] [Google Scholar]
- 43.Barabino GA, Liu XD, Ewenstein BM, Kaul DK. Anionic polysaccharides inhibit adhesion of sickle erythrocytes to the vascular endothelium and result in improved hemodynamic behavior. Blood. 1999;93:1422–1429. [PubMed] [Google Scholar]
- 44.Hines PC, Zen Q, Burney SN, Shea DA, et al. Novel epinephrine and cyclic AMP-mediated activation of BCAM/Lu-dependent sickle (SS) RBC adhesion. Blood. 2003;101:3281–3287. doi: 10.1182/blood-2001-12-0289. [DOI] [PubMed] [Google Scholar]
- 45.Murphy MM, Zayed MA, Evans A, Parker CE, et al. Role of Rap1 in promoting sickle red blood cell adhesion to laminin via BCAM/LU. Blood. 2005;105:3322–3329. doi: 10.1182/blood-2004-07-2881. [DOI] [PubMed] [Google Scholar]
- 46.Zennadi R, Hines PC, De Castro LM, Cartron JP, et al. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood. 2004;104:3774–3781. doi: 10.1182/blood-2004-01-0042. [DOI] [PubMed] [Google Scholar]
- 47.Zennadi R, De Castro L, Eyler C, Xu K, et al. Role and regulation of sickle red cell interactions with other cells: ICAM-4 and other adhesion receptors. Transfus Clin Biol. 2008;15:23–28. doi: 10.1016/j.tracli.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Kaul DK, Liu XD, Zhang X, Mankelow T. Peptides based on alphaV-binding domains of erythrocyte ICAM-4 inhibit sickle red cell-endothelial interactions and vaso-occlusion in the microcirculation. Am J Physiol Cell Physiol. 2006;291:C922–930. doi: 10.1152/ajpcell.00639.2005. [DOI] [PubMed] [Google Scholar]
- 49.Mankelow TJ, Spring FA, Parsons SF, Brady RL, et al. Identification of critical amino-acid residues on the erythroid intercellular adhesion molecule-4 (ICAM-4) mediating adhesion to alpha V integrins. Blood. 2004;103:1503–1508. doi: 10.1182/blood-2003-08-2792. [DOI] [PubMed] [Google Scholar]
- 50.Sabina RL, Wandersee NJ, Hillery CA. Ca(2+)-CaM activation of AMP deaminase contributes to adenine nucleotide dysregulation and phosphatidylserine externalization in human sickle erythrocytes. Br J Haematol. 2008 doi: 10.1111/j.1365-2141.2008.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gayen Betal S, Setty BN. Phosphatidylserine-positive erythrocytes bind to immobilized and soluble thrombospondin-1 via its heparin-binding domain. Transl Res. 2008;152:165–177. doi: 10.1016/j.trsl.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hebbel RP. Adhesion of sickle red cells to endothelium: myths and future directions. Transfus Clin Biol. 2008;15:14–18. doi: 10.1016/j.tracli.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Kuypers FA, Styles LA. The role of secretory phospholipase A2 in acute chest syndrome. Cell Mol Biol (Noisy-le-grand) 2004;50:87–94. [PubMed] [Google Scholar]
- 54.Kuypers FA, de Jong K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cell Mol Biol (Noisy-le-grand) 2004;50:147–158. [PubMed] [Google Scholar]
- 55.Finnegan EM, Barabino GA, Liu XD, Chang HY, et al. Small-molecule cyclic alpha V beta 3 antagonists inhibit sickle red cell adhesion to vascular endothelium and vasoocclusion. Am J Physiol Heart Circ Physiol. 2007;293:H1038–1045. doi: 10.1152/ajpheart.01054.2006. [DOI] [PubMed] [Google Scholar]
- 56.Kato GJ, Martyr S, Blackwelder WC, Nichols JS, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohan JS, Lip GY, Wright J, Bareford D, Blann AD. Plasma levels of tissue factor and soluble E-selectin in sickle cell disease: relationship to genotype and to inflammation. Blood Coagul Fibrinolysis. 2005;16:209–214. doi: 10.1097/01.mbc.0000164431.98169.8f. [DOI] [PubMed] [Google Scholar]
- 58.Wood K, Russell J, Hebbel RP, Granger DN. Differential expression of E- and P-selectin in the microvasculature of sickle cell transgenic mice. Microcirculation. 2004;11:377–385. doi: 10.1080/10739680490437559. [DOI] [PubMed] [Google Scholar]
- 59.Blum A, Yeganeh S, Peleg A, Vigder F, et al. Endothelial function in patients with sickle cell anemia during and after sickle cell crises. J Thromb Thrombolysis. 2005;19:83–86. doi: 10.1007/s11239-005-1377-7. [DOI] [PubMed] [Google Scholar]
- 60.Qari MH, Aljaouni SK, Alardawi MS, Fatani H, et al. Reduction of painful vaso-occlusive crisis of sickle cell anaemia by tinzaparin in a double-blind randomized trial. Thromb Haemost. 2007;98:392–396. [PubMed] [Google Scholar]
- 61.Kaul DK, Liu XD, Choong S, Belcher JD, et al. Anti-inflammatory therapy ameliorates leukocyte adhesion and microvascular flow abnormalities in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2004;287:H293–301. doi: 10.1152/ajpheart.01150.2003. [DOI] [PubMed] [Google Scholar]
- 62.Bao B, Prasad AS, Beck FW, Snell D, et al. Zinc supplementation decreases oxidative stress, incidence of infection, and generation of inflammatory cytokines in sickle cell disease patients. Transl Res. 2008;152:67–80. doi: 10.1016/j.trsl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Belcher JD, Mahaseth H, Welch TE, Vilback AE, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005;288:H2715–2725. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 64.Arruda MA, Rossi AG, de Freitas MS, Barja-Fidalgo C, Graca-Souza AV. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-kappaB. J Immunol. 2004;173:2023–2030. doi: 10.4049/jimmunol.173.3.2023. [DOI] [PubMed] [Google Scholar]
- 65.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, et al. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- 66.Turhan A, Jenab P, Bruhns P, Ravetch JV, et al. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103:2397–2400. doi: 10.1182/blood-2003-07-2209. [DOI] [PubMed] [Google Scholar]
- 67.Chang J, Shi PA, Chiang EY, Frenette PS. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111:915–923. doi: 10.1182/blood-2007-04-084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabaa N, de Franceschi L, Bonnin P, Castier Y, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest. 2008;118:1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Franceschi L, Platt OS, Malpeli G, Janin A, et al. Protective effects of phosphodiesterase-4 (PDE-4) inhibition in the early phase of pulmonary arterial hypertension in transgenic sickle cell mice. FASEB J. 2008;22:1849–1860. doi: 10.1096/fj.07-098921. [DOI] [PubMed] [Google Scholar]
- 70.Benza RL. Pulmonary hypertension associated with sickle cell disease: pathophysiology and rationale for treatment. Lung. 2008;186:247–254. doi: 10.1007/s00408-008-9092-8. [DOI] [PubMed] [Google Scholar]
- 71.Kaul DK, Liu X, Nagel RL. Ameliorating effects of fluorocarbon emulsion on sickle red blood cell-induced obstruction in an ex vivo vasculature. Blood. 2001;98:3128–3131. doi: 10.1182/blood.v98.10.3128. [DOI] [PubMed] [Google Scholar]
- 72.Hebbel RP, Vercellotti GM, Pace B, Solovey A, Kollander R, Abanou C, Nguyen J, Belcher JD, Abdulla F, Osifuye S, Eaton JW, Kelm R, Slungaard A. Blood. 2009. [DOI] [PMC free article] [PubMed]
- 73.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, et al. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nath KA, Grande JP, Haggard JJ, Croatt AJ, et al. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jison ML, Munson PJ, Barb JJ, Suffredini AF, et al. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Girgis RE, Qureshi MA, Abrams J, Swerdlow P. Decreased exhaled nitric oxide in sickle cell disease: relationship with chronic lung involvement. Am J Hematol. 2003;72:177–184. doi: 10.1002/ajh.10284. [DOI] [PubMed] [Google Scholar]
- 77.Weiner DL, Hibberd PL, Betit P, Cooper AB, et al. Preliminary assessment of inhaled nitric oxide for acute vaso-occlusive crisis in pediatric patients with sickle cell disease. Jama. 2003;289:1136–1142. doi: 10.1001/jama.289.9.1136. [DOI] [PubMed] [Google Scholar]
- 78.de Franceschi L, Baron A, Scarpa A, Adrie C, et al. Inhaled nitric oxide protects transgenic SAD mice from sickle cell disease-specific lung injury induced by hypoxia/reoxygenation. Blood. 2003;102:1087–1096. doi: 10.1182/blood-2002-07-2135. [DOI] [PubMed] [Google Scholar]
- 79.de Franceschi L, Malpeli G, Scarpa A, Janin A, et al. Protective effects of S-nitrosoalbumin on lung injury induced by hypoxia-reoxygenation in mouse model of sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:L457–465. doi: 10.1152/ajplung.00462.2005. [DOI] [PubMed] [Google Scholar]
- 80.Mahaseth H, Vercellotti GM, Welch TE, Bowlin PR, et al. Polynitroxyl albumin inhibits inflammation and vasoocclusion in transgenic sickle mice. J Lab Clin Med. 2005;145:204–211. doi: 10.1016/j.lab.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 82.Shalev O, Hebbel RP. Extremely high avidity association of Fe(III) with the sickle red cell membrane. Blood. 1996;88:349–352. [PubMed] [Google Scholar]
- 83.Morris CR, Suh JH, Hagar W, Larkin S, et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood. 2008;111:402–410. doi: 10.1182/blood-2007-04-081703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aslan M, Canatan D. Modulation of redox pathways in neutrophils from sickle cell disease patients. Exp Hematol. 2008;36:1535–1544. doi: 10.1016/j.exphem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Reid M, Badaloo A, Forrester T, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in adults with sickle cell disease. Am J Physiol Endocrinol Metab. 2006;291:E73–79. doi: 10.1152/ajpendo.00287.2005. [DOI] [PubMed] [Google Scholar]
- 86.Shalev O, Repka T, Goldfarb A, Grinberg L, et al. Deferiprone (L1) chelates pathologic iron deposits from membranes of intact thalassemic and sickle red blood cells both in vitro and in vivo. Blood. 1995;86:2008–2013. [PubMed] [Google Scholar]