Abstract
Our understanding of the molecular and pathophysiological mechanisms underlying the disease process in patients with thalassaemia intermedia (TI) has substantially increased over the past decade. TI encompasses a wide clinical spectrum of beta-thalassaemia phenotypes. Some TI patients are asymptomatic until adult life, whereas others are symptomatic from as young as 2 years of age. A number of clinical complications commonly associated with TI are rarely seen in thalassaemia major, including extramedullary hematopoiesis, leg ulcers, gallstones, thrombosis and pulmonary hypertension. There are a number of options currently available for managing patients with TI, including transfusion therapy, iron chelation therapy, modulation of foetal haemoglobin production and haematopoietic stem cell transplantation. However, at present, there are no clear guidelines for an orchestrated optimal treatment plan.
Introduction:
Thalassaemia was defined as a clinical entity in 1925 by Dr. Thomas B. Cooley at the annual meeting of the American Pediatric Society where he presented five young children with severe anaemia, splenomegaly, and peculiar bone abnormalities1. Almost all patients with β-thalassaemia syndromes were later classified as having thalassaemia minor or thalassaemia major (TM). However, a small number of patients did not fit into this categorical distribution. Sturgeon was the first to describe this group in the American literature. He suggested the term thalassaemia intermedia (TI) to describe patients who had clinical manifestations that are too severe to be termed minor and too mild to be termed major2. Ever since, our knowledge of the molecular basis and pathophysiology of TI has progressed significantly in the last decade, including an increased understanding of the genetic mutations that lead to the TI phenotypes3,4. The clinical presentation and sequelae of TI span a wide spectrum of severity. The natural history of the disease allows the emergence of several complications that are relatively unique to TI compared to TM5. As such, the optimal course of management for TI patients has been hard to identify and several controversies remain regarding the best treatment plan6. This review summarizes molecular and clinical characteristics of TI. Moreover, currently practiced treatment options and insights onto future perspectives are discussed.
Molecular Understanding
The clinical manifestations of thalassaemia result from defects in one of two types of haemoglobin (Hb) polypeptide chains (alpha or beta). For Hb to function properly, the number of alpha-chains must precisely match the number of beta-chains; thalassaemia is caused by an imbalance in globin chain synthesis. The beta-thalassaemias, including TI, arise from defective gene function leading to the partial suppression of beta-globin protein production. The extent of suppression varies from patient to patient and dictates the clinical severity of disease. Most TI patients are homozygotes or compound heterozygotes for beta-thalassaemia, meaning that both beta-globin loci are affected3. Less commonly, only a single beta-globin locus is affected, the other being completely normal.1 The mild clinical characteristics of TI compared with TM major result primarily from three different mechanisms:3,7
Inheritance of a mild or silent beta-chain mutation. Rather than a complete absence of beta-chain synthesis, the level of synthesis is subnormal. This leads to a smaller imbalance between the number of alpha-and beta-chains compared with an absence of beta-chains.
Co-inheritance of determinants associated with increased gamma-chain production. The increased number of gamma-chains helps to neutralize the large proportion of unbound alpha-chains.
Co-inheritance of alpha-thalassaemia. This helps to suppress the synthesis of alpha-chains, causing less of an alpha/beta-chain imbalance.
The phenotype of TI may result from the increased production of alpha-globin chains by a triplicated or quadruplicated alpha genotype associated with beta-heterozygosity.8–10 Table 1 shows β-globin mutations in TI and TM that have a direct effect on modifying the amount of excess alpha-chains, such as inheritance of abnormal alpha- or gamma-chain genes. Tertiary modifiers are polymorphisms occurring at loci involved in bone, iron and bilirubin metabolism which can affect clinical expression, although these are thought to be of relatively little importance. Recent studies of the JAK2 cytoplasmic tyrosine kinase, which has a vital role in signal transduction from several haemopoietic growth factor receptors, revealed a V617F mutation that was implicated in a variety of diseases mainly related to myelo-proliferative disorders including polycythaemia vera, essential thrombocythaemia, and idiopathic myelofibrosis. TI patients, however, do not show increased expression of this mutation.11
Table 1.
Prevalent beta-globin mutations in thalassaemia intermedia and thalassaemia major in patients of Mediterranean origin.7
| Mutation | Thalassemia Intermedia n(%) | Thalassemia major n(%) |
|---|---|---|
| cd 39 C->T | 72 (24) | 136 (53.5) |
| IVSI-110 G->T | 52 (17) | 58 (23) |
| IVSI-6 T->C | 94 (31.5) | 16 (6.3) |
| IVSI-1 G->A | 8 (2.7) | 19 (7.4) |
| IVSII-1 G->A | 14 (4.7) | 10 (3.9) |
| IVSII-745 C->G | 11 (3.7) | 9 (3.5) |
| −101 C->T | 10(3.3) | - |
| cd6 - A | 10(3.3) | 3 (1.2) |
| −87 C->G | 8 (2.7) | - |
| δβSiciliana | 13 (4.3) | - |
| Lepore Boston | 2 (0.7) | - |
| IVSI-5 G->A | - | 1 (0.4) |
| IVSI-5 G->C | 1 (0.3) | - |
| IVSII-844 G->C | 1 (0.3) | - |
| IVSI-2 T->A | 1 (0.3) | - |
| cd 44 - C | - | 1 (0.4) |
| cd 8 - AA | 1 (0.3) | 1 (0.4) |
| Total | 298 (100) | 254 (100) |
Environmental factors including social conditions, nutrition and the availability of medical care have also been implicated as key players in the phenotypic variability of TI.12
A number of studies have attempted to classify patients with TI according to the severity of their condition, although these studies have had only limited success.13,14 A recent study described the development of a phenotype scoring system that successfully sub-classified TI patients into three separate groups: mild, moderate or severe 15. The severity of TI was graded according to a number of clinical features, such as age at presentation, severity of anaemia, extent of growth retardation and bone marrow hyperplasia, blood transfusion requirements and need for splenectomy. This classification could prove useful for relating genotype to phenotype and for developing separate treatment guidelines for different disease severities. However, further studies would be required to confirm the reliability and utility of this approach.
Although the clinical phenotypes of thalassaemia minor, intermedia and major differ, there are some similarities. There is an increasing awareness of the need for accurate diagnosis in order to achieve optimal patient management and to avoid over or under treatment3,16. The accurate identification of TI versus thalassaemia minor and TM can be difficult if based on clinical presentation alone, although certain differentiating parameters have been established. In general, TI is characterized by Hb levels maintained around 7–10 g/dL without the need for regular blood transfusions, by more severe red blood cell (RBC) abnormalities than thalassaemia minor, by a varying degree of spleen enlargement, and by skeletal changes such as expansion of the facial bones and obliteration of the maxillary sinuses, which causes protrusion of the upper jaw.5,6,16 Criteria for differentiating TM from TI at presentation are summarised in Table 2.
Table 2.
Criteria to differentiate thalassaemia major from intermedia at presentation.
| Parameter | Thalassaemia major more likely | Thalassaemia intermedia more likely |
|---|---|---|
| Clinical | ||
| Presentation (years) | <2 | >2 |
| Liver/spleen enlargement | Severe | Moderate to severe |
| Haematological | ||
| Hb level (g/dL) | 6–7 | 7–10 |
| HbF (%) | >50 | 10–50 (may be up to 100%) |
| HbA2 (%) | <3.5 | >3.5 |
| Genetic | ||
| Parents | Both carriers of high HbA2 β-thalassaemia | One or both atypical carriers:
|
| Molecular | ||
| Type of mutation | Severe | Mild/silent |
| Co-inheritance of α-thalassaemia | No | Yes |
| Hereditary persistence of HbF | No | Yes |
| δβ-thalassaemia | No | Yes |
| Gγ Xmn1 polymorphism | No | Yes |
Hb = haemoglobin; HbF = foetal haemoglobin
Pathophysiology
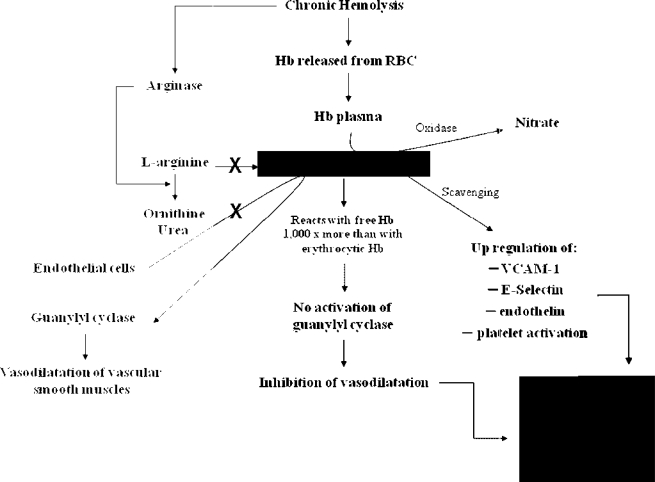
Three main factors are responsible for the clinical manifestations of TI: ineffective erythropoiesis, chronic haemolytic anaemia and iron overload. The severity of clinical sequelae primarily depends on the underlying molecular defects. Alpha-chains are highly unstable and precipitate within erythroid precursors in the bone marrow, causing membrane damage and cell death – this is ineffective erythropoiesis.17 Hypertrophy of erythroid marrow in medullary and extramedullary sites, a consequence of severe ineffective erythropoiesis, results in characteristic deformities of the skull and face and may also cause cortical thinning and pathological fractures of long bones.16,18 The degree of ineffective erythropoiesis is the primary determinant of the development of anaemia, while peripheral haemolysis of mature RBCs and an overall reduction in Hb synthesis are secondary. However, haemolysis per se has been linked to the development of a hypercoagulable state and pulmonary hypertension (PHT) in this patient population.19,20 Damaged circulating RBCs expose negatively charged phosphatidyl-serine residues through the ‘Flip-Flop’ phenomenon.21 These along with activation of platelets, endothelial cells and monocytes along with dysfunction of the coagulation system have been associated with thromboembolic phenomenon, especially in splenectomised TI patients21. Moreover, haemolysis carries a role in the dysregulation of nitric oxide (NO) homeostasis which is correlated with PHT and probably thrombotic phenomena (Figure 1).22
Figure 1.
Nitric oxide dependent pathway leading to pulmonary hypertension and, possibly, thrombotic phenomena in thalassaemia intermedia patients (Hb = haemoglobin, RBC = red blood cell, PHT = pulmonary hypertension, VCAM = vascular cell adhesion molecule).
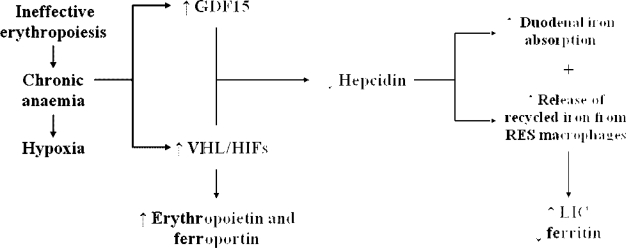
In contrast to patients with TM, in whom iron loading occurs mainly as a result of transfusion therapy, patients with TI accumulate iron primarily due to increased intestinal iron absorption.23 The chronic anaemia and ineffective erythropoiesis that are characteristic of TI are associated with reduced expression of hepcidin, a hepatic peptide that plays a central role in iron homeostasis.23 Recent studies indicate that growth and differentiation factor 15 (GDF15), secreted by erythroid precursors, is significantly increased in patients with TM, inhibiting hepcidin production.24 In addition, other studies indicate that hypoxia-inducible transcription factors (HIFs) control iron homeostasis by coordinating the downregulation of hepcidin and the upregulation of erythropoietin and ferroportin. Under normal conditions, HIFs play a useful role by mobilizing iron and supporting erythrocyte production in response to anaemia/hypoxia.25–27 However, the same mechanism may contribute to the harmful accumulation of iron in response to the chronic anaemia of TI. The combination of ineffective erythropoiesis (leading to increased GDF15) and chronic anaemia/hypoxia (altering the expression of HIF) results in hepcidin suppression, increased iron absorption and increased release of recycled iron from the reticuloendothelial (RE) system. This results in depletion of macrophage iron, and relatively low levels of serum ferritin. The situation in TI is similar to that seen in patients with hereditary hemochromatosis syndromes, which is characterized by impaired hepcidin production (Figure 2).
Figure 2.
Pathophysiology of iron overload in patients with thalassaemia intermedia. GDF15 = growth differentiation factor 15; HIF = hypoxia-inducible transcription factor; RES = reticuloendothelial system; VHL = von Hippel-Lindau; LIC = liver iron concentration.
By contrast, in transfused TM patients iron is preferentially distributed to the RE system, stimulating ferritin synthesis and its release to the circulation and leading to high serum ferritin levels28. Blood transfusions modify hepcidin production by altering all three factors responsible for the clinical manifestations of TI described above namely: (i) by correcting anaemia, transfusion therapy will suppress ineffective erythropoiesis and the associated increase in GDF15; (ii) by correcting anaemia, blood transfusions will prevent the hypoxia responsible for increased HIF production and hypoxia-associated suppression of hepcidin; and (iii) increased body iron and transferrin saturation will stimulate hepcidin production. The result will be a relative increase in hepcidin production in polytransfused TM patients countering the hepcidin-inhibitory effects of anaemia and ineffective erythropoiesis. These considerations may explain the differences in iron homeostasis characterizing TM compared with untransfused TI patients. Thus, although the rate of iron loading may be slower than that in patients with TM, TI patients will inevitably suffer from iron overload which in turn can cause a number of serious complications including cardiac failure and endocrine abnormalities such as diabetes mellitus and hypogonadism.
Clinical Sequelae
As a consequence of the aforementioned pathophysiology, several complications have been identified in patients with TI. Some of these complications have been termed unique to TI as compared to TM, especially in the splenectomised, transfusion naïve patient (Table 3). Cholelithiasis Gallstones are much more common in TI than in TM because of ineffective erythropoiesis and peripheral haemolysis. Recently, unrelated genetic factors such as uridine 5′-diphospho-alpha-D-glucose (UDPG) deficiency (Gilbert’s syndrome) have been reported to increase gallstone formation in patients with thalassaemia29. For this reason, the gallbladder should be inspected during splenectomy and a cholecystectomy performed if necessary, particularly if the patient is experiencing symptomatic gallstones. This should be undertaken to prevent cholecystitis, which can have serious consequences in the splenectomised patient.
Table 3.
Prevalence of complications in thalassaemia intermedia vs. major.5
| Complication (% of patients affected) | Thalassaemia intermedia | Thalassaemia major | ||
|---|---|---|---|---|
| Lebanon | Italy | Lebanon | Italy | |
| (n = 37) | (n = 63) | (n = 40) | (n = 60) | |
| Splenectomy | 90 | 67 | 95 | 83 |
| Cholecystectomy | 85 | 68 | 15 | 7 |
| Gallstones | 55 | 63 | 10 | 23 |
| Extramedullary haemopoiesis | 20 | 24 | 0 | 0 |
| Leg ulcers | 20 | 33 | 0 | 0 |
| Thrombotic events | 28 | 22 | 0 | 0 |
| Cardiopathy* | 3 | 5 | 10 | 25 |
| Pulmonary hypertension† | 50 | 17 | 10 | 11 |
| Abnormal liver enzymes | 20 | 22 | 55 | 68 |
| HCV infection | 7 | 33 | 7 | 98 |
| Hypogonadism | 5 | 3 | 80 | 93 |
| Diabetes mellitus | 3 | 2 | 12.5 | 10 |
| Hypothyroidism | 3 | 2 | 15 | 11 |
Fractional shortening < 35%.
Defined as pulmonary artery systolic pressure > 30 mmHg; a well-enveloped tricuspid regurgitant jet velocity could be detected in only 20 patients, so frequency was assessed in these patients only.
Extramedullary haematopoiesis
Extra-medullary haematopoiesis (EMH) is a compensatory mechanism where bone marrow activity increases in an attempt to overcome the chronic anaemia of TI, leading to the formation of erythropoietic tissue masses that primarily affect the spleen, liver and lymph nodes. These masses can be detected by magnetic resonance imaging (MRI). They may cause neurological problems such as spinal cord compression and paraplegia, and intrathoracic masses.30,31 Extramedullary haemato-poiesis can be managed by radiotherapy (since haematopoietic tissue is highly radiosensitive)32, transfusion therapy or hydroxyurea.30,33,34
Leg ulcers
Leg ulcers are more common in older than in younger patients with TI. It is unclear why ulcers develop in some patients who are maintained at relatively low Hb levels and have the same amount of foetal Hb (HbF) as others in whom ulcers do not develop. The skin at the extremities of elderly TI patients can be thin due to reduced tissue oxygenation, and this makes the subcutaneous tissue fragile and increases the risk of lesions from minimal trauma. Once an ulcer has started to develop it is very painful and difficult to cure, although regular blood transfusions may provide some relief in persistent cases. Simple measures may be beneficial, such as keeping the patient’s legs and feet raised above the level of the heart for 1–2 hours during the day or sleeping with the end of the bed raised. Zinc supplementation35 and pentoxifylline, which alters the rheological properties of the RBCs,36 can help accelerate the healing of ulcers. Hydroxyurea also has some benefit, either alone or in combination with erythropoietin.37 In addition, the use of an oxygen chamber can provide moderate relief since tissue hypoxia may be an underlying cause of the ulceration.38
Thromboembolic events
Taher et al.39 analyzed data from 8860 thalassaemia patients (6670 TM and 2190 TI). In this study thromboembolic events (TEE) occurred 4.38 times more frequently in TI than TM patients, with more venous events occurring in TI and more arterial events occurring in TM. In another series of TI patients, 24 patients (29%) developed either deep vein thrombosis (DVT), pulmonary embolism, or portal vein thrombosis during a 10-year follow up.40 In a recent survey involving nine Italian pediatric thalassaemia centres, TEE was observed in 4% of 683 patients with TM and in 9.6% of 52 patients with TI.41 Studies collectively revealed that the incidence of TEE is higher in splenectomised patients.39,40 The incidence of overt stroke in TI patients is low;8 however, a study done to assess the rate of asymptomatic brain damage in patients with benign haemoglobinopathies reported that 37.5% of patients with TI showed silent brain ischemia.42
Pulmonary hypertension and congestive heart failure
Pulmonary hypertension (PHT) is prevalent in patients with TI (59.1%),20 and is thought to be the primary cause of congestive heart failure in this patient population. A retrospective analysis showed that splenectomised females with significant anaemia, thrombocytosis and elevated ferritin levels, were at greatest risk for developing PHT.43 Preliminary results have demonstrated that PHT is reversible by blood transfusion and treatment with aspirin and warfarin. Several echocardiographic studies have confirmed that cardiac ejection fraction is rarely affected in TI.44 Nevertheless, patients with TI often have an increased cardiac output, and left ventricular wall dimensions proportional to the dilutional volume overload secondary to chronic anaemia.45
As anaemia and iron overload are uncommon in well-transfused and chelated TM patients, they are likely to be at the heart of the pathophysiology of PHT. A recent study demonstrated a significant correlation between iron overloading in the liver and pulmonary artery systolic pressure independent of left ventricular filling pressures.46 Regular transfusion and iron chelation therapy is therefore indicated in TI patients who are well-stratified according to the early detection of PHT indices. Sildenafil has also been successfully used to treat PHT,47 although data from large patient numbers are lacking in TI.
Pregnancy and related complications
Women with TI may have spontaneous successful pregnancies although complications during pregnancy may occur48. The chronic anaemia of TI can cause an increase in spontaneous abortions, pre-term labour and intrauterine growth retardation, while endocrine complications due to haemo-siderosis are common49. The course and outcome of 19 pregnancies was assessed in 16 women with thalassaemia, including four with TI50. All pregnancies were uneventful and elective Caesarean section was performed in each case. The mean birth weight of the babies was 3000 g and all were normal except for one case of omphalocele. The largest study to date assessed 44 TI women who had 83 pregnancies, all spontaneous, 30 from Lebanon and 53 from Italy.51 These pregnancies resulted in 20.5% abortions, 77.1% live-births and 2 intrauterine foetal deaths at 26 and 36 weeks’ gestation. The mean gestational age at delivery was 36.5 weeks and mean birth weight was 2551 g. In pregnancies progressing > 20 weeks’ gestation, pre-term delivery and intrauterine growth restriction (IUGR) were noted in 31.8% and 24.2% of cases, respectively. In those complicated by IUGR, Caesarean delivery (CS) rate was 87.5%. Two women (Italy) developed severe alloimmune haemolytic anaemia. One progressed to cardiac failure at 35 weeks’ gestation and had CS. The other underwent CS for IUGR and non-reassuring foetal heart monitoring and was scheduled for a splenectomy postpartum. Worsening alloimmune anaemia also developed in 2 women from Lebanon who required splenectomy within eight weeks postpartum. Transfusion was required in 35/44 women during pregnancy (79.5%), with 27.3% requiring transfusion during pregnancy for the first time. The lowest mean Hb level was 6.7±2.0 vs. 8.3±1.2 g/dL in Lebanon and Italy respectively. The average ferritin level before pregnancy was 885.2 ± 658.9 vs.1232.8 ± 902.9 μg/L after pregnancy. In total, CS was performed in 48 pregnancies (72.7%), the indications being elective (41.7%), repeat (31.2%) and obstetrical (27.1%). Pregnancy outcome was similar between Lebanon and Italy with the exception of a significantly higher rate of live births in Italy.51
Folic acid deficiency is common in TI and occurs due to poor absorption, low dietary intake, or, most significantly, an increased demand for folic acid from active bone marrow. This is a particular concern in pregnancy since deficiency can cause neural tube defects, such as spina bifida, in the growing foetus. During pregnancy, women with TI should therefore be given oral folic acid supplementation (around 1 mg/day), and should be carefully monitored in order to assess the need for transfusion therapy and to avoid haemodynamic compromises. The major fear of initiating transfusions during pregnancy is the development of alloantibodies. These can aggravate anaemia and progress into severe haemolytic anaemia refractory to transfusions and thus increase the complication rate. CS may be required in TI patients due to the associated cephalopelvic disproportion secondary to skeletal deformity and short stature, especially in non-transfused women. Splenectomy is usually performed in TI women for decreased levels of haemoglobin, hyperactivity of the spleen, leukopenia and symptomatic thrombocytopenia.5,6
Management
There are a number of options currently available for managing patients with TI, including splenectomy, transfusion therapy, iron chelation therapy, modulation of HbF production and haematopoietic stem cell transplantation.
Splenectomy
As per expert opinion, the current indications for splenectomy in TI include growth retardation or poor health, leukopenia, thrombocytopenia, increased transfusion demand, or symptomatic splenomegaly.6 Clinical observations, however, have suggested that splenectomy in TI can contribute to an increased susceptibility to TEE and PHT39,40,43. This calls for a review of splenectomy as a procedure of choice, especially with its potential role in increasing TI-related complications and the inherent risk of infection associated with the procedure even for individuals without haematological disorders.52
Transfusion and iron chelation therapy
In patients with TM, a remarkable improvement in life expectancy and prevention of morbidity has been achieved in recent decades 53. This is mainly attributed to improved methods of blood transfusion, better understanding of iron toxicity, and evolution in iron chelation therapy.53 On the other hand, TI has been regarded as a clinical entity with limited complicationsso as the general approach was to avoid early blood transfusions and the associated need for chelation therapy. However, increasing evidence is delineating the benefit of transfusion therapy in decreasing the incidence of complications as PHT, TEE, and EMH, to name a few.30,39,54 Thus although the common practice was to initiate transfusion when complications ensue, it may be worthwhile start transfusion therapy earlier as a preventive approach which will also help alleviate the increased risk of alloimmunisation with delayed initiation of transfusion.55 Although earlier introduction of blood transfusions will increase the rate of iron accumulation, effective methods of iron chelation are now available 56,57, and the benefits of transfusion therapy would greatly outweigh the cost and inconvenience of iron chelation therapy.
The initiation of iron chelation therapy in patients with TI depends not only on the amount of excess iron, but also on the rate of iron accumulation, the duration of exposure to excess iron and various other factors in individual patients. A direct assessment of LIC is recommended, either by biopsy or by a non-invasive method such as R2 MRI. Where LIC measurement is not possible, threshold serum ferritin values of 400–500 ng/mL (which are lower than those generally accepted in patients with TM) could be considered as an indicator for initiation of iron chelation therapy. Chelation therapy should generally be initiated if LIC exceeds 7 mg/g dry weight of liver tissue, however lower levels of LIC for initiation of chelation therapy must be considered particularly now with the availability of oral iron chelators.6
Modulation of foetal haemoglobin production
Increasing the synthesis of HbF can help alleviate anaemia and therefore improve the clinical status of patients with TI 58. Production of HbF is reactivated during recovery from marrow suppression after treatment with cytotoxic drugs, therefore it is postulated that these agents may alter the pattern of erythropoiesis and increase the expression of gamma-chain genes. Several cytotoxic agents with this effect have been identified, including cytosine arabinoside and hydroxyurea.59–61 Recently published results from Iran, evaluating six years of hydroxyurea therapy in transfusion dependent patients with TI, are encouraging. A significant decrease in the need for blood transfusions was observed in many patients; the need was completely obviated in some patients.62 Erythropoietin has also been shown to increase HbF levels in some patients with TI58. Preliminary trials with intravenous and oral butyric acid derivatives have shown increases in foetal and total Hb levels in patients with TI,63–66 and the acceptable safety profile of these agents makes them promising therapeutic targets. It is unclear how butyrates stimulate gamma-globin production or why some patients respond to treatment while others do not.
However, the overall trial results with HbF-stimulating agents are somewhat disappointing. Studies using combined treatments have shown greater promise than the individual agents alone67. Further clinical evaluation is required to clarify the value of this approach, especially in view of the reduced oxygen delivery capacity of HbF, as this might favour the implementation of a target Hb level higher than 10 g/dL in response to increased need (e.g. PHT, coronary heart disease and chronic obstructive pulmonary disease (COPD)) and an increased ratio of HbF/HbA.
Haematopoietic stem cell transplantation
Haematopoietic stem cell transplantation (HSCT), where the marrow of an affected patient is replaced from the stem cells of an uaffected donor, is an established treatment for beta-thalassaemia. Although successful HSCT can offer a cure, it can be unsuccessful (e.g. if the thalassaemia returns), may lead to complications (e.g. graft-versus-host disease, growth impairment, neurological complications), and can even result in death;68–71 the risk for a failed transplantation depends primarily on the health and age of the patient. The decision as to which patients are eligible for transplantation is complex and is related to both the quality of life and expected survival-time of the transplanted patient, when compared with supportive care only. This is particularly relevant in patients with TI, especially in those who are only mildly affected. Due to the risks involved, transplantation is considered appropriate only for patients with a human leukocyte antigen (HLA)-matched donor, which comprises only 30–40% of all beta-thalassaemia patients, at most.72 As HLA type is genetically determined, there is a 25% chance that any two siblings will be a match.
Conclusion
Our understanding of the molecular and pathophysiological mechanisms underlying the disease process in patients with TI has substantially increased over the past decade. Today, a large body of evidence documents the incidence and consequences of the different clinical complications associated with the disease. Although there are still currently no clear guidelines for the optimal management plan of this disease entity, the emerging body of evidence looks promising.
Declaration Of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study did not receive external funding.
Footnotes
This article is available from: http://www.mjhid.org/article/view/4709
References
- 1.Weatherall DJ. Thalassaemia: the long road from bedside to genome. Nat Rev Genet. 2004;5:625–631. doi: 10.1038/nrg1406. [DOI] [PubMed] [Google Scholar]
- 2.Sturgeon P, Itano HA, Bergren WR. Genetic and biochemical studies of intermediate types of Cooley’s anaemia. Br J Haematol. 1955l;1:264–277. doi: 10.1111/j.1365-2141.1955.tb05509.x. [DOI] [PubMed] [Google Scholar]
- 3.Galanello R, Cao A. Relationship between genotype and phenotype. Thalassemia intermedia. Ann NY Acad Sci. 1998;850:325–333. doi: 10.1111/j.1749-6632.1998.tb10489.x. [DOI] [PubMed] [Google Scholar]
- 4.Weatherall D. The molecular basis for phenotypic variability of the common thalassaemias. Mol Med Today. 1995;1:15–20. doi: 10.1016/1357-4310(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 5.Taher A, Ismaeel H, Cappellini MD. Thalassaemia Intermedia: Revisited. Blood Cells Mol Dis. 2006;37:12–20. doi: 10.1016/j.bcmd.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Cappellini MD, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Guidelines for the Clinical Management of Thalassaemia. 2nd Revised Edition. Thalassaemia International Federation; 2008. [PubMed] [Google Scholar]
- 7.Camaschella C, Mazza U, Roetto, Gottardi E, Parziale A, Travi M, Fattore S, Bacchiega D, Fiorelli G, Cappellini MD, A, et al. Genetic interactions in thalassemia intermedia: analysis of beta-mutations, alpha-genotype, gamma-promoters, and beta-LCR hypersensitive sites 2 and 4 in Italian patients. Am J Hematol. 1995;48:82–87. doi: 10.1002/ajh.2830480203. [DOI] [PubMed] [Google Scholar]
- 8.Camaschella C, Kattamis AC, Petroni D, Roetto A, Sivera P, Sbaiz L, Cohen A, Ohene-Frempong K, Trifillis P, Surrey S, Fortina P. Different hematological phenotypes caused by the interaction of triplicated alpha-globin genes and heterozygous beta-thalassemia. Am J Hematol. 1997;55:83–88. doi: 10.1002/(sici)1096-8652(199706)55:2<83::aid-ajh6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Sampietro M, Cazzola M, Cappellini MD, Fiorelli G. The triplicated alpha-gene locus and heterozygous beta thalassaemia: a case of thalassaemia intermedia. Br J Haematol. 1983;55:709–710. doi: 10.1111/j.1365-2141.1983.tb02854.x. [DOI] [PubMed] [Google Scholar]
- 10.Beris P, Solenthaler M, Deutsch S, Darbellay R, Tobler A, Bochaton-pialat ML, Gabbiani G. Severe inclusion body beta-thalassaemia with haemolysis in a patient double heterozygous for beta(0)-thalassaemia and quadruplicated apla-globin gene arrangement of the anti-4.2 type. Br J Haematol. 1999;105:1074–80. doi: 10.1046/j.1365-2141.1999.01451.x. [DOI] [PubMed] [Google Scholar]
- 11.Taher A, Shammaa D, Bazarbachi A, Itani D, Zaatari G, Greige L, Otrock ZK, Mahfouz RA. Absence of JAK2 V617F mutation in thalassemia intermedia patients. Mol Biol Rep. 2009;36:1555–1557. doi: 10.1007/s11033-008-9350-0. [DOI] [PubMed] [Google Scholar]
- 12.Weatherall DJ. Thalassemia intermedia: cellular and molecular aspects. J Hematol. 2001;86:186–188. [Google Scholar]
- 13.Ho PJ, Hall GW, Luo LY, Weatherall DJ, Thein SL. Beta-thalassaemia intermedia: is it possible consistently to predict phenotype from genotype? Br J Haematol. 1998;100:70–78. doi: 10.1046/j.1365-2141.1998.00519.x. [DOI] [PubMed] [Google Scholar]
- 14.Rund D, Oron-Karni P, Filon D, Goldfarb A, Rachmilewitz E, Oppenheim A. Genetic analysis of beta-thalassemia intermedia in Israel: diversity of mechanisms and unpredictability of phenotype. Am J Hematol. 1997;54:16–22. doi: 10.1002/(sici)1096-8652(199701)54:1<16::aid-ajh3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Phadke SR, Agarwal S. Phenotype score to grade the severity of thalassemia intermedia. Indian J Pediatr. 2003;70:477–481. doi: 10.1007/BF02723137. [DOI] [PubMed] [Google Scholar]
- 16.Camaschella C, Cappellini MD. Thalassemia intermedia. Haematologica. 1995;80:58–68. [PubMed] [Google Scholar]
- 17.Olivieri NF. The beta-thalassemias. N Engl J Med. 1999;341:99–109. doi: 10.1056/NEJM199907083410207. [DOI] [PubMed] [Google Scholar]
- 18.Cappellini MD, Cerino M, Marelli S, et al. Thalassemia intermedia: clinical aspects and management. Haematologica. 2001;86:194–196. [Google Scholar]
- 19.Taher A, Abou-Mourad Y, Abchee A, Zallouaa P, Shamseddine A. Pulmonary thromboembolism in beta-thalassemia intermedia: are we aware of this complication? Hemoglobin. 2002;26:107–112. doi: 10.1081/hem-120005447. [DOI] [PubMed] [Google Scholar]
- 20.Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J, Loukopoulos D. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97:3411–3416. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 21.Taher AT, Otrock ZK, Uthman I, Cappellini MD. Thalassemia and hypercoagulability. Blood Rev. 2008;22:283–292. doi: 10.1016/j.blre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Ataga KI, Cappellini MD, Rachmilewitz EA. Beta-thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. Br J Haematol. 2007;139:3–13. doi: 10.1111/j.1365-2141.2007.06740.x. [DOI] [PubMed] [Google Scholar]
- 23.Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, Nemeth E. Liver iron concentrations and urinary hepcidin in b-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 24.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, Eling TE, Childs R, Ganz T, Leitman SF, Fucharoen S, Miller JL. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 25.Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40:132–138. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111:924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 27.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pakbaz Z, Fischer R, Fung E, Nielsen P, Harmatz P, Vichinsky E. Serum ferritin underestimates liver iron concentration in transfusion independent thalassemia patients as compared to regularly transfused thalassemia and sickle cell patients. Pediatr Blood Cancer. 2007;49:329–332. doi: 10.1002/pbc.21275. [DOI] [PubMed] [Google Scholar]
- 29.Borgna-Pignatti C, Rigon F, Merlo L, Chakrok R, Micciolo R, Perseu L, Galanello R. Thalassemia minor, the Gilbert mutation, and the risk of gallstones. Haematologica. 2003;88:1106–1109. [PubMed] [Google Scholar]
- 30.Chehal A, Aoun E, Koussa S, Skoury H, Koussa S, Taher A. Hypertransfusion: a successful method of treatment in thalassemia intermedia patients with spinal cord compression secondary to extramedullary hematopoiesis. Spine. 2003;28:E245–249. doi: 10.1097/01.BRS.0000067282.47308.4D. [DOI] [PubMed] [Google Scholar]
- 31.Castelli R, Graziadei G, Karimi M, Cappellini MD. Intrathoracic masses due to extramedullary hematopoiesis. Am J Med Sci. 2004;328:299–303. doi: 10.1097/00000441-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Smith PR, Manjoney DL, Teitcher JB, Choi KN, Braverman AS. Massive hemothorax due to intrathoracic extramedullary hematopoiesis in a patient with thalassemia intermedia. Chest. 1988;94:658–660. doi: 10.1378/chest.94.3.658. [DOI] [PubMed] [Google Scholar]
- 33.Saxon BR, Rees D, Olivieri NF. Regression of extramedullary haemopoiesis and augmentation of fetal haemoglobin concentration during hydroxyurea therapy in beta thalassaemia. Br J Haematol. 1998;101:416–419. doi: 10.1046/j.1365-2141.1998.00719.x. [DOI] [PubMed] [Google Scholar]
- 34.Cario H, Wegener M, Debatin KM. Treatment with hydroxyurea in thalassemia intermedia with paravertebral pseudotumors of extramedullary hematopoiesis. Ann Hematol. 2002;81:478–482. doi: 10.1007/s00277-002-0501-4. [DOI] [PubMed] [Google Scholar]
- 35.Gupta VL, Choubey BS. RBC survival, zinc deficiency, and efficacy of zinc therapy in sickle cell disease. Birth Defects Orig Artic Ser. 1987;23:477–483. [PubMed] [Google Scholar]
- 36.Dettelbach HR, Aviado DM. Clinical pharmacology of pentoxifylline with special reference to its hemorrheologic effect for the treatment of intermittent claudication. J Clin Pharmacol. 1985;25:8–26. doi: 10.1002/j.1552-4604.1985.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 37.al Momen AK. Recombinant human erythropoietin induced rapid healing of a chronic leg ulcer in a patient with sickle cell disease. Acta Haematol. 1991;86:46–48. doi: 10.1159/000204799. [DOI] [PubMed] [Google Scholar]
- 38.Gimmon Z, Wexler MR, Rachmilewitz EA. Juvenile leg ulceration in beta-thalassemia major and intermedia. Plast Reconstr Surg. 1982;69:320–325. doi: 10.1097/00006534-198202000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Taher A, Isma’eel H, Mehio G, Bignamini D, Kattamis A, Rachmilewitz EA, Cappellini MD. Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost. 2006;96:488–491. [PubMed] [Google Scholar]
- 40.Cappellini MD, Robbiolo L, Bottasso BM, Coppola R, Fiorelli AP, Mannucci AP. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111:467–473. doi: 10.1046/j.1365-2141.2000.02376.x. [DOI] [PubMed] [Google Scholar]
- 41.Borgna Pignatti C, Carnelli V, Caruso V, Dore F, De Mattia D, Di Palma A, Di Gregorio F, Romeo MA, Longhi R, Mangiagli A, Melevendi C, Pizzarelli G, Musumeci S. Thromboembolic events in beta thalassemia major: an Italian multicenter study. Acta Haematol. 1998;99:76–79. doi: 10.1159/000040814. [DOI] [PubMed] [Google Scholar]
- 42.Manfre L, Giarratano E, Maggio A, Banco A, Vaccaro G, Lagalla R. MR imaging of the brain: findings in asymptomatic patients with thalassemia intermedia and sickle cell-thalassemia disease. AJR Am J Roentgenol. 1999;173:1477–1480. doi: 10.2214/ajr.173.6.10584785. [DOI] [PubMed] [Google Scholar]
- 43.Atichartakarn V, Likittanasombat K, Chuncharunee S, Chandanamattha P, Worapongpaiboon S, Angchaisuksiri P, Aryurachai K. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol. 2003;78:139–145. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 44.Aessopos A, Farmakis D, Deftereos S, Tsironi M, Tassiopoulos S, Moyssakis I, Karagiorga M. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–1530. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 45.Gharzuddine WS, Kazma HK, Nuwayhid IA, Bitar FF, Koussa SF, Moukarbel GV, Taher AT. Doppler characterization of left ventricular diastolic function in beta-thalassaemia major. Evidence for an early stage of impaired relaxation. Eur J Echocardiogr. 2002;3:47–51. doi: 10.1053/euje.2001.0114. [DOI] [PubMed] [Google Scholar]
- 46.Isma’eel H, Chafic AH, Rassi FE, Inati A, Koussa S, Daher R, Gharzuddin W, Alam S, Taher A. Relation between iron-overload indices, cardiac echo-Doppler, and biochemical markers in thalassemia intermedia. Am J Cardiol. 2008;102:363–367. doi: 10.1016/j.amjcard.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 47.Derchi G, Forni GL, Formisano F, Cappellini MD, Galanello R, D’Ascola G, Bina P, Magnano C, Lamagna M. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90:452–458. [PubMed] [Google Scholar]
- 48.Savona-Ventura C, Bonello F. Beta-thalassemia syndromes and pregnancy. Obstet Gynecol Surv. 1994;49:129–137. doi: 10.1097/00006254-199402000-00025. [DOI] [PubMed] [Google Scholar]
- 49.Skordis N, Christou S, Koliou M, Pavlides N, Angastiniotis M. Fertility in female patients with thalassemia. J Pediatr Endocrinol Metab. 1998;11:35–943. [PubMed] [Google Scholar]
- 50.Karagiorga-Lagana M. Fertility in thalassemia: the Greek experience. J Pediatr Endocrinol Metab. 1998;11:945–951. [PubMed] [Google Scholar]
- 51.Nassar A, Naja M, Cesaretti C, Eprassi B, Cappellini MD, Taher A. Pregnancy outcome in patients with beta-thalassemia intermedia at two tertiary care centers, in Beirut and Milan. Haematologica. 2008;93:1586–1587. doi: 10.3324/haematol.13152. [DOI] [PubMed] [Google Scholar]
- 52.Cadili A, de Gara C. Complications of splenectomy. Am J Med. 2008;121:371–375. doi: 10.1016/j.amjmed.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, Romeo MA, Forni GL, Gamberini MR, Ghilardi R, Piga A, Cnaan A. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–1193. [PubMed] [Google Scholar]
- 54.Aessopos A, Kati M, Meletis J. Thalassemia intermedia today: should patients regularly receive transfusions? Transfusion. 2007;47:792–800. doi: 10.1111/j.1537-2995.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 55.Eder AF, Chambers LA. Noninfectious complications of blood transfusion. Arch Pathol Lab Med. 2007;13:708–718. doi: 10.5858/2007-131-708-NCOBT. [DOI] [PubMed] [Google Scholar]
- 56.Cossu P, Toccafondi C, Vardeu F, Sanna G, Frau F, Lobrano R, Cornacchia G, Nucaro A, Bertolino F, Loi A, De Virgiliis S, Cao A. Iron overload and desferrioxamine chelation therapy in beta-thalassemia intermedia. Eur J Pediatr. 1981;137:267–271. doi: 10.1007/BF00443255. [DOI] [PubMed] [Google Scholar]
- 57.Pootrakul P, Sirankapracha P, Sankote J, Kachintorn U, Maungsub W, Sriphen K, Thakernpol K, Atisuk K, Fucharoen S, Chantraluksri U, Shalev O, Hoffbrand AV. Clinical trial of deferiprone iron chelation therapy in beta-thalassaemia/haemoglobin E patients in Thailand. Br J Haematol. 2003;122:305–310. doi: 10.1046/j.1365-2141.2003.04412.x. [DOI] [PubMed] [Google Scholar]
- 58.Olivieri NF. Reactivation of fetal hemoglobin in patients with beta-thalassemia. Semin Hematol. 1996;33:24–42. [PubMed] [Google Scholar]
- 59.Arruda VR, Lima CS, Saad ST, Costa FF. Successful use of hydroxyurea in beta-thalassemia major. N Engl J Med. 1997;336:964. doi: 10.1056/NEJM199703273361318. [DOI] [PubMed] [Google Scholar]
- 60.Bradai M, Abad MT, Pissard S, Lamraoui F, Skopinski L, de Montalembert M. Hydroxyurea can eliminate transfusion requirements in children with severe beta-thalassemia. Blood. 2003;102:1529–1530. doi: 10.1182/blood-2003-01-0117. [DOI] [PubMed] [Google Scholar]
- 61.Dixit A, Chatterjee TC, Mishra P, Choudhry DR, Mahapatra M, Tyagi S, Kabra M, Saxena R, Choudhry VP. Hydroxyurea in thalassemia intermedia-a promising therapy. Ann Hematol. 2005;84:441–446. doi: 10.1007/s00277-005-1026-4. [DOI] [PubMed] [Google Scholar]
- 62.Karimi M, Darzi H, Yavarian M. Hematologic and clinical responses of thalassemia intermedia patients to hydroxyurea during 6 years of therapy in Iran. J Pediatr Hematol Oncol. 2005;27:380–385. doi: 10.1097/01.mph.0000174386.13109.28. [DOI] [PubMed] [Google Scholar]
- 63.Perrine SP, Ginder GD, Faller DV, Dover GH, Ikuta T, Witkowska HE, Cai SP, Vichinsky EP, Olivieri NF. A short-term trial of butyrate to stimulate fetalglobin- gene expression in the beta-globin disorders. N Engl J Med. 1993;328:81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- 64.Cappellini MD, Graziadei G, Ciceri L, Comino A, Bianchi P, Porcella A, Fiorelli G. Oral isobutyramide therapy in patients with thalassemia intermedia: results of a phase II open study. Blood Cells Mol Dis. 2000;26:105–111. doi: 10.1006/bcmd.2000.0283. [DOI] [PubMed] [Google Scholar]
- 65.Sher GD, Ginder GD, Little J, Yang S, Dover GJ, Olivieri NF. Extended therapy with intravenous arginine butyrate in patients with beta-hemoglobinopathies. N Engl J Med. 1995;332:1606–1610. doi: 10.1056/NEJM199506153322404. [DOI] [PubMed] [Google Scholar]
- 66.Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:43–49. [PubMed] [Google Scholar]
- 67.Olivieri NF, Rees DC, Ginder GD, Thein SL, Brittenham GM, Waye JS, Weatherall DJ. Treatment of thalassaemia major with phenylbutyrate and hydroxyurea. Lancet. 1997;350:491–492. doi: 10.1016/s0140-6736(05)63080-2. [DOI] [PubMed] [Google Scholar]
- 68.Piga A, Longo F, Voi V, Facello S, Miniero R, Dresow B. Late effects of bone marrow transplantation for thalassemia. Ann N Y Acad Sci. 1998;850:294–299. doi: 10.1111/j.1749-6632.1998.tb10486.x. [DOI] [PubMed] [Google Scholar]
- 69.Apperley JF. Bone marrow transplant for the haemoglobinopathies: past, present and future. Baillieres Clin Haematol. 1993;6:299–325. doi: 10.1016/s0950-3536(05)80074-5. [DOI] [PubMed] [Google Scholar]
- 70.Uckan D, Cetin M, Yigitkanli I, Tezcan I, Tuncer M, Karasimav D, Oguz KK, Topçu M. Life-threatening neurological complications after bone marrow transplantation in children. Bone Marrow Transplant. 2005;35:71–76. doi: 10.1038/sj.bmt.1704749. [DOI] [PubMed] [Google Scholar]
- 71.Khojasteh NH, Zakernia M, Ramzi M, Haghshenas M. Bone marrow transplantation for hematological disorders-Shiraz experience. Indian J Pediatr. 2002;69:31–32. doi: 10.1007/BF02723773. [DOI] [PubMed] [Google Scholar]
- 72.Rund D, Rachmilewitz E. Advances in the pathophysiology and treatment of thalassemia. Crit Rev Oncol Hematol. 1995;20:237–254. doi: 10.1016/1040-8428(95)00162-Z. [DOI] [PubMed] [Google Scholar]