Abstract
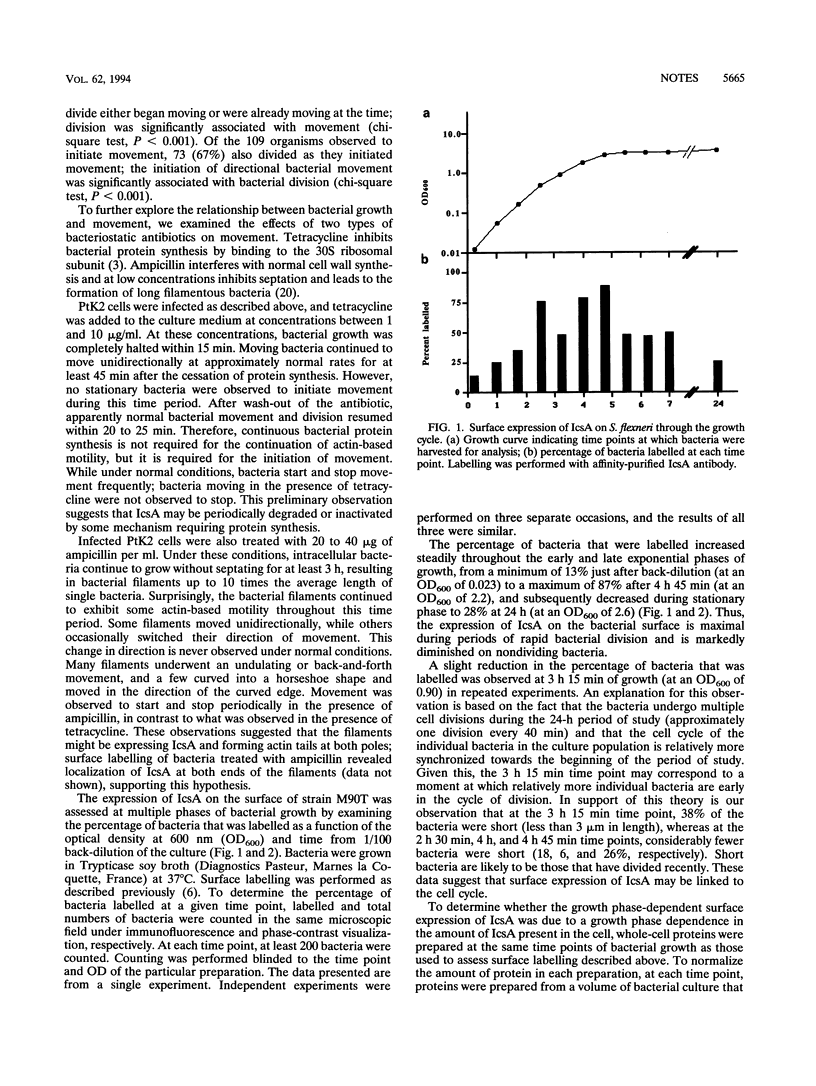
After lysing the phagocytic vacuole, Shigella spp. accumulate filaments of polymerized actin on their surface at one pole, leading to the formation of actin tails that enable them to move through the cytoplasm. We have recently demonstrated that the Shigella protein IcsA is located at the pole that is adjacent to the growing end of the actin tail (M. B. Goldberg, O. Barzu, C. Parsot, and P. J. Sansonetti, J. Bacteriol. 175:2189-2196, 1993). Not every bacterium that is observed within the cytoplasm has an actin tail. The factors that determine when a bacterium will form a tail are unknown. Here we demonstrate that at the moment of initiation of movement, Shigella spp. are frequently in the process of division. Furthermore, the expression of IcsA on the surface of the bacteria occurs in a growth phase-dependent fashion, suggesting that the surface expression of IcsA per se determines the observed association of bacterial division with movement.
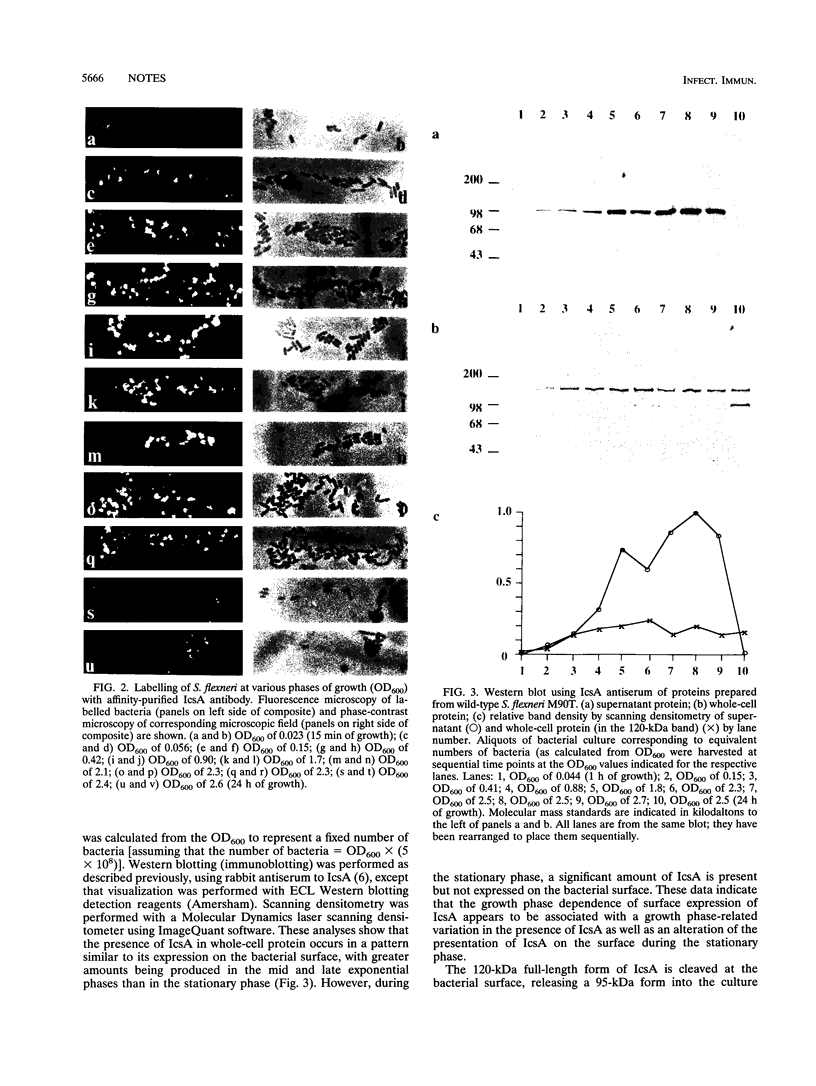
Full text
PDF
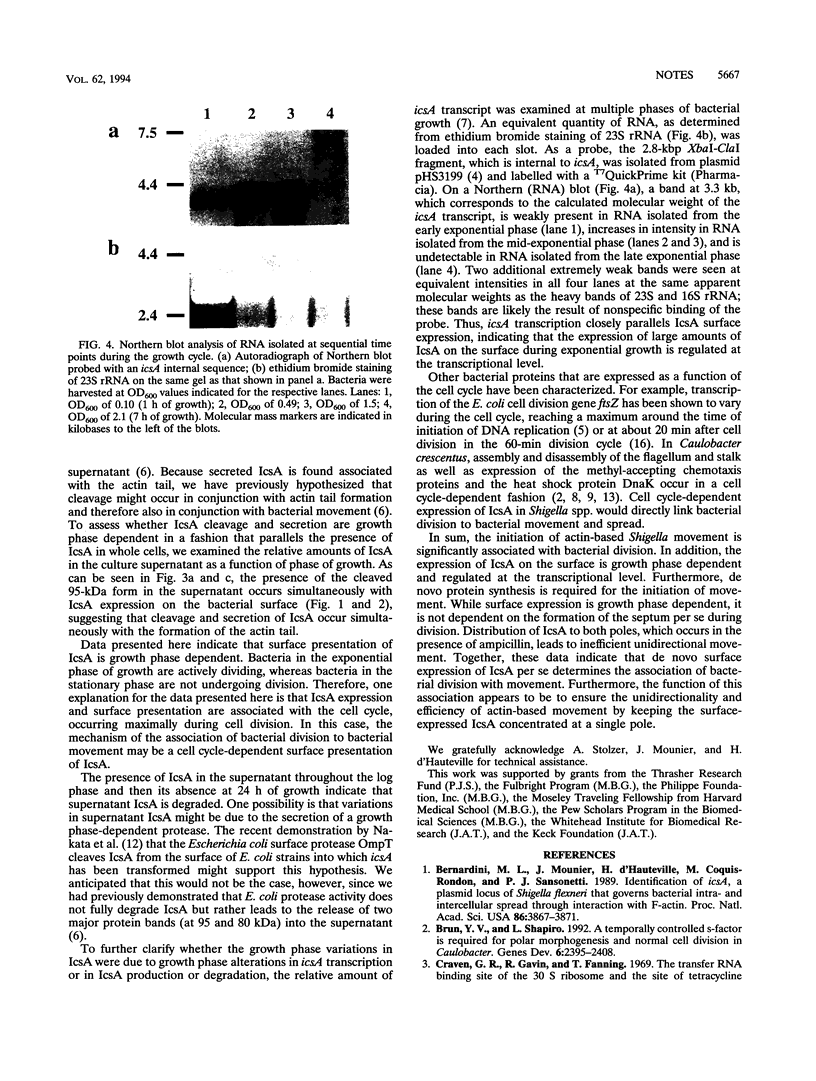

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun Y. V., Shapiro L. A temporally controlled sigma-factor is required for polar morphogenesis and normal cell division in Caulobacter. Genes Dev. 1992 Dec;6(12A):2395–2408. doi: 10.1101/gad.6.12a.2395. [DOI] [PubMed] [Google Scholar]
- Craven G. R., Gavin R., Fanning T. The transfer RNA binding site of the 30 S ribosome and the site of tetracycline inhibition. Cold Spring Harb Symp Quant Biol. 1969;34:129–137. doi: 10.1101/sqb.1969.034.01.019. [DOI] [PubMed] [Google Scholar]
- Garrido T., Sánchez M., Palacios P., Aldea M., Vicente M. Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 1993 Oct;12(10):3957–3965. doi: 10.1002/j.1460-2075.1993.tb06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Calderwood S. B. Transcriptional regulation by iron of a Vibrio cholerae virulence gene and homology of the gene to the Escherichia coli fur system. J Bacteriol. 1990 Dec;172(12):6863–6870. doi: 10.1128/jb.172.12.6863-6870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Bârzu O., Parsot C., Sansonetti P. J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993 Apr;175(8):2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S. L., Gober J. W., Shapiro L. Expression of the Caulobacter heat shock gene dnaK is developmentally controlled during growth at normal temperatures. J Bacteriol. 1990 Jun;172(6):3051–3059. doi: 10.1128/jb.172.6.3051-3059.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S. L., Shapiro L. Differential expression and positioning of chemotaxis methylation proteins in Caulobacter. J Mol Biol. 1984 Sep 25;178(3):551–568. doi: 10.1016/0022-2836(84)90238-9. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Rohde M., Wehland J., Timmis K. N. Intercellular spread of Shigella flexneri through a monolayer mediated by membranous protrusions and associated with reorganization of the cytoskeletal protein vinculin. Infect Immun. 1991 Oct;59(10):3463–3471. doi: 10.1128/iai.59.10.3463-3471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Nakata N., Tobe T., Fukuda I., Suzuki T., Komatsu K., Yoshikawa M., Sasakawa C. The absence of a surface protease, OmpT, determines the intercellular spreading ability of Shigella: the relationship between the ompT and kcpA loci. Mol Microbiol. 1993 Aug;9(3):459–468. doi: 10.1111/j.1365-2958.1993.tb01707.x. [DOI] [PubMed] [Google Scholar]
- O'Neill E. A., Bender R. A. Cell-cycle-dependent polar morphogenesis in Caulobacter crescentus: roles of phospholipid, DNA, and protein syntheses. J Bacteriol. 1989 Sep;171(9):4814–4820. doi: 10.1128/jb.171.9.4814-4820.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévost M. C., Lesourd M., Arpin M., Vernel F., Mounier J., Hellio R., Sansonetti P. J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992 Oct;60(10):4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Newland J. W., Tall B. D., Formal S. B., Hale T. L. Intracellular spread of Shigella flexneri associated with the kcpA locus and a 140-kilodalton protein. Infect Immun. 1989 Feb;57(2):477–486. doi: 10.1128/iai.57.2.477-486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin A., Joseleau-Petit D., D'Ari R. Transcription of the ftsZ gene and cell division in Escherichia coli. J Bacteriol. 1990 Mar;172(3):1392–1399. doi: 10.1128/jb.172.3.1392-1399.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Mounier J., Prévost M. C., Mège R. M. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 1994 Mar 11;76(5):829–839. doi: 10.1016/0092-8674(94)90358-1. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Hellio R., Sansonetti P. J. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992 Mar;60(3):1031–1040. doi: 10.1128/iai.60.3.1031-1040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Prevost M. C., Hellio R., Sansonetti P. J. Stress fiber-based movement of Shigella flexneri within cells. Infect Immun. 1991 May;59(5):1723–1732. doi: 10.1128/iai.59.5.1723-1732.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Hauteville H., Sansonetti P. J. Phosphorylation of IcsA by cAMP-dependent protein kinase and its effect on intracellular spread of Shigella flexneri. Mol Microbiol. 1992 Apr;6(7):833–841. doi: 10.1111/j.1365-2958.1992.tb01534.x. [DOI] [PubMed] [Google Scholar]