Abstract
Epstein-Barr virus (EBV), a human gammaherpesvirus, is associated with a series of malignant tumors. These include lymphomas (Burkitt’s lymphoma, Hodgkin’s disease, T/NK-cell lymphoma, post-transplant lymphoproliferative disease, AIDS-associated lymphoma, X-linked lymphoproliferative syndrome), carcinomas (nasopharyngeal carcinoma, gastric carcinoma, carcinomas of major salivary glands, thymic carcinoma, mammary carcinoma) and a sarcoma (leiomyosarcoma). The latent EBV genomes persist in the tumor cells as circular episomes, co-replicating with the cellular DNA once per cell cycle. The expression of latent EBV genes is cell type specific due to the strict epigenetic control of their promoters. DNA methylation, histone modifications and binding of key cellular regulatory proteins contribute to the regulation of alternative promoters for transcripts encoding the nuclear antigens EBNA1 to 6 and affect the activity of promoters for transcripts encoding transmembrane proteins (LMP1, LMP2A, LMP2B). In addition to genes transcribed by RNA polymerase II, there are also two RNA polymerase III transcribed genes in the EBV genome (EBER 1 and 2). The 5′ and internal regulatory sequences of EBER 1 and 2 transcription units are invariably unmethylated. The highly abundant EBER 1 and 2 RNAs are not translated to protein. Based on the cell type specific epigenetic marks associated with latent EBV genomes one can distinguish between viral epigenotypes that differ in transcriptional activity in spite of having an identical (or nearly identical) DNA sequence. Whereas latent EBV genomes are regularly targeted by epigenetic control mechanisms in different cell types, EBV encoded proteins may, in turn, affect the activity of a set of cellular promoters by interacting with the very same epigenetic regulatory machinery. There are EBNA1 binding sites in the human genome. Because high affinity binding of EBNA1 to its recognition sites is known to specify sites of DNA demethylation, we suggest that binding of EBNA1 to its cellular target sites may elicit local demethylation and contribute thereby to the activation of silent cellular promoters. EBNA2 interacts with histone acetyltransferases, and EBNALP (EBNA5) coactivates transcription by displacing histone deacetylase 4 from EBNA2-bound promoter sites. EBNA3C (EBNA6) seems to be associated both with histone acetylases and deacetylases, although in separate complexes. LMP1, a transmembrane protein involved in malignant transformation, can affect both alternative systems of epigenetic memory, DNA methylation and the Polycomb-trithorax group of protein complexes. In epithelial cells LMP1 can up-regulate DNA methyltransferases and, in Hodgkin lymphoma cells, induce the Polycomb group protein Bmi-1. In addition, LMP1 can also modulate cellular gene expression programs by affecting, via the NF-κB pathway, levels of cellular microRNAs miR-146a and miR-155. These interactions may result in epigenetic dysregulation and subsequent cellular dysfunctions that may manifest in or contribute to the development of pathological changes (e.g. initiation and progression of malignant neoplasms, autoimmune phenomena, immunodeficiency). Thus, Epstein-Barr virus, similarly to other viruses and certain bacteria, may induce pathological changes by epigenetic reprogramming of host cells. Elucidation of the epigenetic consequences of EBV-host interactions (within the framework of the emerging new field of patho-epigenetics) may have important implications for therapy and disease prevention, because epigenetic processes are reversible and continuous silencing of EBV genes contributing to patho-epigenetic changes may prevent disease development.
Introduction:
Epstein-Barr virus (EBV), a member of the Gammaherpesvirinae subfamily, is a herpesvirus widespread in human populations1. EBV infects B lymphocytes via the complement receptor CD21, and the B-cell-bound virions can be transferred efficiently to CD21-negative epithelial cells2. Thus, when transmitted orally, EBV enters the epithelial cells of the oropharynx resulting in productive (lytic) infection. On the contrary, the EBV-B cell interaction is usually nonproductive, the latent viral genomes persist as circular episomes and co-replicate with the cellular DNA once per cell cycle.
The association of latent EBV genomes with malignant tumors is well documented. The virus has been discovered in cells derived from endemic Burkitt’s lymphoma samples3. In addition, other lymphomas (X-linked lymphoproliferative syndrome, posttransplant lymphoproliferative disease, AIDS-associated lymphoma, peripheral T/NK-cell lymphoma, Hodgkin’s lymphoma), certain carcinomas (nasopharyngeal carcinoma, gastric carcinoma, carcinomas of the major salivary glands, thymic carcinoma, mammary carcinoma) and a sarcoma (leiomyosarcoma) also regularly carry latent EBV genomes1,4 (Table 1).
Table 1.
Major EBV associated neoplasms (for details see Niller et al, 2007 and 2008).
Lymphomas
|
Carcinomas
|
Sarcoma
|
Sequencing of the 172 kb double stranded linear genome of the prototype EBV strain (B95-8) significantly contributed to the identification of latent, growth-transformation associated proteins of the virus5.
During productive infection all EBV encoded proteins are expressed and concatemeric replication of circular viral DNA templates is initiated at oriLyt (the lytic origin of replication) by the viral DNA polymerase with the help of EBV encoded transactivator and replication proteins6,7,8 (Figure 1)
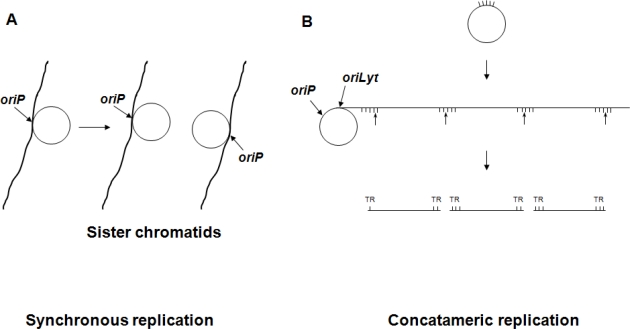
Figure 1.
Alternative origins of EBV DNA replication: In A, the synchronous replication of latent EBV episomes and the cellular DNA (i.e. the chromosomal site where the episome is teethered by EBNA1) is demonstrated. EBNA1 (not shown on the figure) binds to oriP, the latent origin of EBV DNA replication, and it is anchored to a possibly AT rich chromosomal site as well. After replication, duplicated EBV episomes bind to the duplicated sites on adjacent sister chromatids (based on Nanbo et al.10). In B, concatemeric replication is initiated during the lytic cycle at oriLyt. After nuclease cleavage, double stranded linear EBV genomes with variable number of terminal repeats (TRs; their borders are indicated by vertical bars) are generated (based on Sato et al.7).
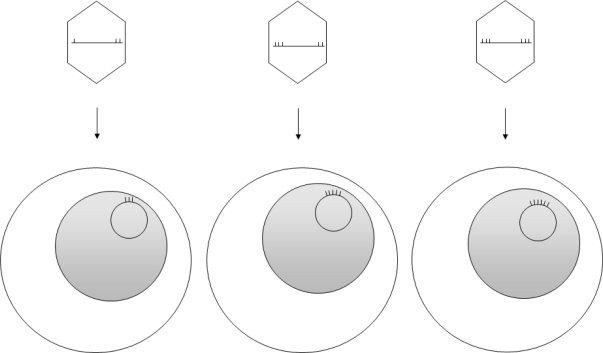
The viral DNA packaged into the virion ends in terminal repetitions (TRs). Soon after in vitro infection of B cells the TRs fuse with each other and circular episomes are generated by amplification of a single copy of the viral DNA. Only a subset of the viral genes are transcribed from the latent EBV episomes that co-replicate with the cellular genome in the emerging B cell clones. It is noteworthy that during latent, non-productive infection, DNA synthesis is initiated at a distinct origin of replication called oriP (that is different from oriLyt), by the cellular DNA replication machinery9,10 (Figure 1). Expression of growth-transformation-associated viral latency genes leads to the establishment of permanently growing (immortal) lymphoblastoid cell lines (LCLs). The major EBV-associated malignancies also carry predominantly circular EBV DNA molecules. EBV infection is apparently important in their development, because analysis of fused TR sequences showed that these tumors are clonal proliferations of cells infected by EBV on a single occasion, presumably in an early stage of tumorigenesis11 (Figure 2).
Figure 2.
Clonality of EBV-associated neoplasms: The double stranded linear EBV genomes packaged into virions have variable numbers of terminal repeats (TRs, bordered by vertical bars on the figure) at their ends. After infection of cells, the termini fuse forming a circular episome harbouring distinct numbers of TRs. Clones of transformed cells can be characterized by determining the size of the BamHI fragment carrying the TRs (see Raab-Traub and Flynn11).
Host cell phenotype-dependent expression of latent EBV genes: EBV latency types:
The majority of EBV encoded proteins is involved in productive EBV infection and includes transactivators, enzymes necessary for viral DNA amplification and structural components of the virions. There is a sequential order of gene activation in the course of the lytic cycle: immediate early genes (BZLF1 and BRLF1) are expressed first and activate the so called early genes. Late proteins are expressed after viral DNA synthesis12.
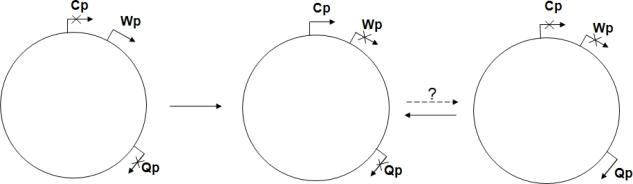
A more restricted pattern of viral gene expression can be observed in latently infected cells. The latent EBV genomes carried by in vitro transformed LCLs and lymphoproliferations developing in recipients of organ or bone marrow transplants express six nuclear proteins (EBNAs). The EBNA1-6 transcripts are initiated at the C promoter (Cp, located to the BamHI C fragment of the viral genome), hence the term „Cp on” latency (also called latency type III)13 (Table 2, Figure 3).
Table 2.
Major latency types of Epstein-Barr virus
| Latency type | Representative cell type | Expressed products |
|---|---|---|
| „Cp off latency” | ||
| Type 0 latency | Resting B cell | EBNA1 (variable ?), LMP2A (?) |
| Type I latency | Burkitt’s lymphoma | EBNA1, EBER1 & 2, BART, microRNAs |
| Type II latency | Hodgkin’s lymphoma | EBNA1, EBER1 & 2, BART, LMP1 & 2, microRNAs |
| Nasopharyngeal and gastric carcinoma | EBNA1, EBER1 & 2, BART, LMP1 (variable expression), LMP2, microRNAs, BARF1 | |
| „Cp on latency” | ||
| Lymphoblastoid cell lines | EBNA1-6, EBER1 & 2, BART, LMP1 & 2, microRNAs |
Figure 3.
Promoter switch in latent EBV episomes: After initial EBV infection of B cells in vitro, Wp (a promoter located to the BamHI W fragment of the EBV genome) is switched on. A giant transcript coding for 6 nuclear antigens (EBNA1-6) is generated but the activity of Wp is transient. The transactivator protein EBNA2 and the EBNA1-bound oriP enhancer may contribute to switching on Cp, a B lymphoblast specific promoter. Burkitt’s lymphoma (BL) cells use a different promoter, Qp, to generate a transcript for EBNA1 only. In vitro cultivated BL cells may switch from Qp to Cp (arrow to the left). This promoter switch results in a phenotypic drift (from memory B cell to activated B cell phenotype). A Cp to Qp switch has been predicted but never observed (broken arrow) (see Niller et al.13).
Cp is active only in B lymphoblasts14. EBNA1 is required for stable maintenance of viral episomes, whereas EBNA2, the major transactivator protein of EBV transactivates both viral promoters (Cp, LMP1p, LMP2Ap, LMP2Bp, see below) and promoters of cellular genes (CD23, CD21). EBNA3-6 modulate the transactivator activity of EBNA2 (see below).
In „Cp on” latency (reviewed by Niller et al.1) one can also detect three transcripts for latent membrane proteins (LMP1, LMP2A, LMP2B), two small RNA molecules (EBER 1 and 2), transcripts derived from the BamHI A fragment of the genome (BARTs, reviewed by Smith15) and a series of microRNAs16,17,18. The transmembrane proteins LMP1 and LMP2 affect various signal transduction pathways. Expression of LMP1 in rodent cells causes malignant transformation. The EBERs and BARTs (and the protein products of BARTs), and the BARF1 protein were also implicated in oncogenesis (reviewed by Niller et al.1). The microRNAs derived from transcripts of the BHRF1 gene and the BART transcripts may target both viral and cellular RNAs affecting thereby the abundance of lytic (BALF5) and latent (LMP1) EBV transcripts as well as certain cellular RNAs (e.g. that of CXCL-11, coding for a chemokine) (for review see Swaminathan19).
In other EBV-carrying cell types Cp is switched off („Cp off” latency). These include memory B cells, Burkitt’s lymphomas (BLs) and BL cell lines that maintain the BL biopsy phenotype (type I BL cell lines), EBV-associated carcinomas (nasopharyngeal carcinoma, gastric carcinoma) and Hodgkin’s lymphomas. In „Cp off latency” typically an alternative promoter (Q promoter or Qp) is used for expression of EBNA1 transcripts, but the transcripts coding for the other five EBNAs are absent. The various cell types carrying silent C promoters may differ from each other regarding the expression of LMPs, BARTs, BARF1 and EBV-encoded microRNAs (latency types 0, I and II, Table 2, Figure 3)1, 15,17.
EBNA1 binds to oriP, the latent origin of EBV replication that acts as a long-range enhancer of Cp and LMP1p. This enhancer activity is essential for EBNA2 expression during immortalization of B cells in vitro20. In the absence of EBNA2 (the major viral transactivator protein in latency type III), the LMP promoters are activated by cellular proteins in nasopharyngeal carcinomas and in Sternberg-Reed cells of Hodgkin’s disease (latency type II), but not in type I BL cell lines or BL biopsies (type I latency) or memory B cells (type 0 latency, characterized by variable Qp activity)1.
It was observed by several laboratories that EBV latency promoter-reporter constructs were active after in vitro transfection into EBV carrying cell lines even if the corresponding latency promoter located on the viral episomes was silent21,22,23,24. This observations suggested that the transfected cells contained the transcription factors necessary for promoter activity and raised the possibility that silencing of latent viral promoters may be associated with their inaccessibility to cellular transcription factors. In the next part we briefly outline the epigenetic modifications and mechanisms potentially regulating promoter accessibility.
Epigenetic modifications and epigenetic regulatory systems:
Modified bases in the DNA of prokaryotes may serve as identification marks that permit discrimination between self and foreign (invading) DNA. Well characterized endodeoxyribonuclease (restriction endonuclease) and DNA methyltransferase (modification methylase) activities, recognizing the same DNA sequence, enable bacterial cells to resist infections by phage or plasmid DNA molecules that are unmethylated or methylated at recognition sites different from those marked on the host DNA (reviewed in Wilson, 1998, Bujnicki, 2001, Murray, 2002). In addition to inhibiting the activity of restriction endonucleases, sequence-specific methylation may modulate (repress or activate) the expression of certain bacterial gene sets as well, influencing thereby bacterial virulence25,26. Modified bases are also involved in postreplicative repair processes in bacteria and may contribute to membrane binding and segregation of the chromosome as well27,28.
The role of base modification mediated by DNA methyltransferases apparently changed in large-genome eukaryotes. Bestor proposed that in these organisms DNA methyltransferases perform a new function, the compartmentalization of the genome29. This would provide an easy access to the unmethylated fraction of the genome by diffusible regulatory factors, while the usually larger, methylated fraction would remain inaccessible for them. One could argue that this novel function of DNA methyltransferases is reflected in the discontinuous, and changing methylation patterns of vertebrate genomes. In contrast to prokaryotes where the methyl-transferases modify essentially all of their recognition sites, in vertebrates there are alternating domains of methylated and unmethylated regions, and the methylation patterns of a certain region may change during development and differentiation (tissue-specific methylation patterns). The unmethylated domains, called CpG islands, are regularly found 5′ to coding sequences of constitutively expressed „housekeeping” genes30,31. It is important to note, however, that most CpG island promoters are unmethylated independently of their activity32,33. Thus, mechanisms other than DNA methylation may also silence CpG island promoters.
In mammals 5-methylcytosine (confined to the sequence 5′-CpG-3′, shortly: CpG) is the predominant modified base found in DNA34,35. Comparison of the amino acid sequence of DNA methyltransferase I (DNMT1), one of the mammalian DNA (cytosine-5)-methyltransferases, to the sequences of bacterial methyltransferases suggested that DNMT1 arose by fusion of a prokaryotic modification methyltransferase gene (coding for the C-terminal catalytic domain) and a second (unknown) gene, coding for a regulatory (N-terminal) domain36,37,38. A key feature of DNMT1 is its preference for hemimethylated DNA as a substrate. Hemimethylated DNA molecules (consisting of one methylated strand and an unmethylated complementary strand) are regularly produced during DNA replication (provided that the parental strands were originally methylated). High affinity of DNMT1 for hemimethylated DNA ensures the clonal propagation of methylation patterns (maintenance methylation). Other mammalian methyltransferases (like the human DNMT3A and DNMT3B) create new methylation patterns by acting on unmethylated DNA substrates (de novo methylation)38. Their N-terminal domains differ from each other and from that of DNMT139.
Methylated CpG dinucleotides may silence promoters either directly, by blocking the binding of transcription factors to their recognition sequences, or indirectly, by attracting methyl-CpG binding proteins. Methyl-CpG binding proteins regularly associate with histone deacetylases and histone methylases. Removal of the acetyl moieties from histone tails and methylation of selected lysine and arginine groups results in a repressive chromatin structure silencing promoter activity40,41,42. These alterations may switch off active promoters and stabilize the silent status of already inactivated genes. Riggs proposed that stable gene silencing by DNA methylation may help progeny cells to „remember” their proper cellular identity, i.e. DNA methylation may provide a memory function43.
Transcriptional repression may depend on CpG methylation density33,44. CpG-poor promoters may retain their activity even if methylated. In somatic cells, a second class of promoters characterized with a moderate or intermediate CpG density (so called „weak CpG island promoters”) are preferentially targeted by de novo methylation33. Typical CpG island promoters (called „strong CpG island promoters” by Weber et al.33) seem to be unmethylated independent of their activity33 although they are regularly inactivated by DNA methylation in neoplasms44. Thus, focal hypermethylation seems to accompany tumorigenesis in parallel or consecutive to overall genomic hypomethylation45,46.
It is interesting to note, that an alternative system of epigenetic memory, the Polycomb-trithorax group of protein complexes, that may operate both independently from and in concert with DNA methylation, ensures a heritable regulation of gene expression via modification of histone tails, too (Bird, 2002, Cernilogar and Orlando, 2005, Laue et al., 2005). In animal cells certain sets of promoters are marked by trimethylation on lysine 27 of histone H3 (H3K27me3), a modification carried out by the enzyme EZH2. EZH2, a component of the Polycomb repressive complex 2 (PRC2) involved in gene silencing in embryonal stem cells was recently shown to attract DNA methyltransferases47 and may contribute, thereby, to permanent gene silencing via DNA hypermethylation in cancer cells48,49.
In contrast to the hypermethylated silent promoters, active promoters are usually unmethylated at CpG dinucleotides and associate with acetylated histones H3 and H4, that form ’acetylation islands’50. Histone H3 di- or trimethylated on lysine 4 (H3K4me2, H3K4me3) is also enriched at active promoters. The presence of these „activating” chromatin modifications may not be sufficient, however, to ensure promoter activity because certain promoters are enriched both in „activating” and „repressive” modifications (’bivalent’ chromatin structure)51. Such ’bivalent’ domains may silence developmental genes in embryonic stem cells. In addition, Weber et al. observed that unmethylated CpG rich promoters may also associate with H3K4me2 in the absence of transcription33.
Epigenetic mechanisms targeting the alternative latency promoters Cp and Qp in lymphoma cells and immortalized lympho-blastoid cell lines:
The role of DNA methylation in regulating Cp and Qp activity has been reviewed earlier (Li and Minarovits, 2003). Briefly, both Cp and Qp can be silenced, when inserted into reporter constructs, by in vitro CpG methylation. Accordingly, Cp is hypermethylated and silent in tumor cells corresponding to „Cp off” latency (including latency type I Burkitt’s lymphoma cells and latency type II Hodgkin’s lymphomas), but unmethylated and active in LCLs and the majority of posttransplant lymphomas („Cp on” latency)24,52, 53, 54. Although in vitro data demonstrated thatCpG methylation could silence Qp as well55, Qp stays unmethylated independently of its activity (i.e. it is unmethylated also in LCLs where it is switched off). Thus, DNA methylation may contribute to the silencing of Cp, but it is not used to silence Qp in vivo.
Episomal EBV genomes are organized into chromatin55. Similarly to cellular promoters41, 56, the activity of latent EBV promoters Cp and Qp also correlated with certain histone modifications marking „open” or „closed” chromatin domains57,58,59,60. In lymphoblastoid cell lines active C promoters were located to AcH3 and AcH4 rich regions60 that are similar to the ’acetylation islands’ characteristic to the active chromatin domains in human T cells50. In contrast, in type I Burkitt’s lymphoma cell lines there was no acetylation island at the inactive Cp. Active Qp, the promoter used in most cases of „Cp off” latency, was selectively enriched in AcH3, AcH4 and H3K4me259,60 in type I Burkitt’s lymphoma cell lines. In lymphoblastoid cell lines, however, the inactive Qp was located to domains poor in acetylated histones.
It was demonstrated by in vivo footprinting that the unmethylated, active Cp was occupied by transcription factors53. In contrast, the absence of interactions with activating transcription factors was characteristic for highly methylated, silent C promoters. It is interesting to note, that epigenetic silencing of Cp may be regarded as a viral escape mechanism, because all of the transcripts encoding the immunodominant viral EBNA proteins are initiated at Cp. Tumor cells actively using Cp can survive, however, in severely immunsuppressed patients (e.g. B cell lymphomas in transplantation recipients and AIDS patients).
We observed that the active, unmethylated Qp was also contacted by cellular transcription factors and EBNA1, that in addition to oriP has binding sites at Qp as well53. In contrast to Cp, where silencing was correlated with the loss of transcription factor binding, silent Qp was marked by the same protein footprints as its active counterpart. A unique footprint observed only at the silent Q promoters suggested that binding of a cellular repressor might contribute to the repression of Qp activity in „Cp on” latency.
Epigenetic marks at other EBV latency promoters:
In vitro methylation experiments demonstrated that Wp, where transcripts of EBNA1 to 6 are initiated soon after EBV infection of B cells (Figure 3), is a CpG methylation sensitive promoter61. Wp is switched off in LCLs, however, provided that Cp is intact, in parallel with the activation of Cp62. A variable methylation of Wp in LCLs suggests that epigenetic mechanisms other than CpG methylation also contribute to Wp silencing63.
The EBER 1 and 2 transcription units are transcribed by RNA polymerase III. Their activity is also sensitive to in vitro CpG methylation that blocks binding of the nuclear proteins c-myc and ATF to the 5′region of EBER1p64. EBERs are expressed in the major EBV-carrying cell types and the EBER locus is hypomethylated65.
LMP1 expression can be related to the methylation status of the LMP1 promoter. In type I BL lines LMP1p is silent and highly methylated, whereas in LCLs and midline granulomas it is unmethylated and active (reviewed by Li and Minarovits, 2003). In contrast to Cp, LMP1p activity did not correlate with transcription factor occupancy53.
In lymphoid cell lines, expression of LMP2A seems to be regulated by the combinatorial effects of DNA methylation, histone acetylation and the level of histone H3 dimethylated on lysine 4 (H3K4me2, a marker of „open” chromatin)66.
The promoter for the BARTs (also called CSTs including BARF0) RNAs was found to be active and unmethylated in the only nasopharyngeal carcinoma cell line it was looked for67.
Latent Epstein-Barr virus proteins interacting with the cellular epigenetic regulatory machinery:
We demonstrated above that various epigenetic control mechanisms leave cell type specific marks on latent EBV genomes, establishing thereby distinct viral epigenotypes (reviewed by Minarovits,68). In turn, certain EBV encoded proteins may interact with the cellular epigenetic regulatory machinery, affecting thereby the activity of a set of cellular promoters (Table 3).
Table 3.
Latent Epstein-Barr virus products interacting with the cellular epigenetic regulatory machinery
| Latency product | Cellular partner | Putative outcome of the interaction |
|---|---|---|
| EBNA1 | EBNA1 binding site | site-specific demethylation of DNA; activation of adjacent genes; |
| EBNA2 | histone acetyltransferases (p300, CBP, PCAF) | transactivation of genes; |
| EBNA3C (EBNA6) | prothymosin alpha, p300; histone deacetylases (HDAC1 & 2) | modulating EBNA2-mediated transactivation |
| EBNA-LP (EBNA5) | histone deacetylase 4 (HDAC4) | dispacement of HDAC4 from EBNA2-activated promoters; coactivation |
| LMP1 | DNA methyltransferases DNMT1, 3a, 3b; | hypermethylation mediated silencing of promoters; |
| Bmi-1 (a component of PRC1); | silencing of B cell specific and tumor suppressor genes; | |
| marking a set of promoters for de novo methylation by DNMTases; | ||
| up-regulation of transcription (STAT-1, c-MET, HK); | ||
| via the NF-κB pathway: microRNA coding genes | alteration of miR-146a and miR-1 level; modulation of cellular mRNA levels |
Because EBNA1 has binding sites in the human genome69 and it is known that high affinity EBNA1 binding can cause site specific demethylation within oriP, the latent origin of EBV replication70, we suggest that binding of EBNA1 to its cellular target sites may elicit local demethylation and contribute thereby to the activation of silent cellular promoters. Lin et al. demonstrated in model experiments that high affinity of the binding protein and protein binding site occupancy is crucial in targeted demethylation71.
EBNA2, the major viral transactivator protein expressed in „Cp on” latency, may switch on both viral (Cp, LMP1p, LMP2Ap) and cellular promoters (CD21p, CD23p, AML-2p, BATFp, IL-16p) (reviewed by Györy and Minarovits72). EBNA2 activates its target promoters through binding to CBF1 (C promoter binding factor 1), a cellular protein that also interacts with the activated cellular Notch family of proteins73–76. In vivo footprinting confirmed CBF1 binding to the active C promoter and LMP2A promoter but not to their inactive counterparts53,77. In addition to CBF1, EBNA2 also associates with the cellular histone acetyltransferases p300, CBP, and PCAF78. Wang et al.78 speculate that these histone acetyltransferases may enhance the ability of EBNA2 to up-regulate expression of the viral oncogene LMP1 by counteracting the effect of histone deacetylases associated with the silent LMP1 promoter78.
EBNA3C (EBNA6), a nuclear antigen essential for EBV-dependent immortalization of B cells may fine tune (down-modulate) the activity of EBNA2-activated promoters by interacting and competing with prothymosin alpha, a partner of p30079,80. In addition, EBNA3C may also form complexes with histone deacetylases (HDAC1 and 2) in human B cells81. Knight et al81 proposed that EBNA3C may be in separate complexes with histone acetylases and deacetylases affecting the function of distinct viral and cellular promoters.
In contrast to EBNA3C, the partly nuclear, parly cytoplasmic antigen EBNA-LP (EBNA leader protein, EBNA5) strongly up-regulates EBNA2-mediated transcription by relocalizing the histone deacetylase HDAC4 from the nucleus to the cytoplasm82. Based on their observations Portal et al.82 suggested that the coactivator function of EBNA-LP is due to the reduction of the HDAC4 concentration in the nucleus. They also speculated that EBNA-LP coactivates with EBNA2 by displacing HDAC4 (and HDAC4 associated repressors) from EBNA2-bound promoters. Recent data do not support, however, the nucleocytoplasmic model for EBNA-LP mediated LMP1p coactivation, because an EBNA-LP isoform, defective in shuttling to the cytoplasm, could coactivate EBNA2 and associate with HDAC483.
LMP1, a transmembrane protein involved in malignant transformation can affect both alternative systems of epigenetic memory, DNA methylation and the Polycomb-trithorax group of protein complexes. Tsai et al. observed, however, that expression of the EBV-encoded oncoprotein LMP1 (latent membrane protein 1) in epithelial cells up-regulated the expression and activity of cellular DNA methyltransferases 1, 3a and 3b in vitro84. This resulted in hypermethylation of the E-cadherin promoter and down-regulation of E-cadherin gene expression. LMP1 activated DNMT1 via the c-jun NH(2)-terminal kinase/activator protein-1 (JNK-AP-1) signaling pathway85. In addition, formation of a transcriptional repression complex (composed of DNMT1 and histone deacetylase) could be detected on the E-cadherin promoter as a consequence of LMP1 action. How this repressor complex was targeted to the E-cadherin promoter, however, remains to be elucidated. These results observed in epithelial cells are certainly very much relevant to the development of EBV-associated lymphomas as well, because latent EBV infection apparently results in hypermethylation of a set of cellular promoters not only in nasopharyngeal carcinoma86 and EBV-associated gastric carcinoma87,88, but also in typically LMP1 positive Hodgkin lymphomas89,90,91, iatrogenic lymphomas92, and lymphomas developing in AIDS patients93.
Although the oncoprotein LMP1 can up-regulate the PcG protein Bmi-194, there are no data regarding the contribution of PcG (or trithorax group) proteins to the regulation of latent EBV promoters. Up-regulation of Bmi-1 is mediated by NF-κB signaling both in EBV-positive and EBV-negative Hodgkin’s lymphoma cells. Dutton et al.94 suggested that by activating NF-κB, and thereby up-regulating Bmi-1, LMP1 may contribute to the loss of B-cell identity in EBV carrying Hodkins’s lymphomas via down-regulating a series of B-cell markers (CD21/MS4A1, BLK, LY9). In EBV-negative disease other activators of NF-κB may substitute for LMP1. We speculate that Bmi-1, as a component of polycomb repressor complex 1 (PRC1), may act by marking a set of promoters for de novo methylation by DNA methyltransferases. In addition, Bmi-1, using the same mechanism, may down-regulate a series of tumor suppressor genes (including IGSF4 and ATM), too. Bmi-1 also mediates up-regulation of certain genes that are transcriptional targets of LMP1 (STAT1 and c-MET and HK). These genes code for signaling molecules and a hexokinase maintaining high glycolytic activity and seem to play an important role in lymphomagenesis.
It is worthy to mention that LMP1 may affect the levels of certain cellular microRNAs (and modulate thereby cellular gene expression programs) by activating the NF-κB pathway. LMP1 induced expression of miR-146a, a microRNA that may affect the interferon response (Cameron et al., 2008), and elevated the level of miR-155, a microRNA implicated in the development of B cell lymphomas 95.
Stem cell chromatin patterns in B-cell lymphomas:
Burkitt lymphomas (BLs) and diffuse large B-cell lymphomas (DLBCLs) seem to share a set of de novo methylated genes (e.g. HOXB13, CALCA, NEFL, PROK2) that are repressed by the polycomb repressive complex 2 (PRC2) in embryonic stem cells, in spite of the differences of BLs and DLBCLs regarding morphology, genetic background, and transcriptional pattern96. The aberrant DNA methylation observed in lymphomas was absent in adult stem and progenitor cells. Based on these data Martin-Subero et al.96 suggested that BLs and DLBCLs may originate from cells with stem cell features or acquire such features during lymphomagenesis by epigenetic remodeling.
Tumor specific and EBV-associated gene expression and CpG methylation changes in lymphomas:
The epigenetic alterations of the host genomes in EBV-associated neoplasms (lymphomas and carcinomas) were reviewed recently97.
It is obvious that certain common tumor suppressor and tumor-associated genes were found to be silenced by DNA methylation in more than one lymphoma types in independent studies. One could also discern, however, methylation profiles that seem to be unique for individual lymphoma types. The apparently specific contribution of EBV to the alteration of gene expression in HD biopsies98 remains to be correlated with the corresponding epigenetic changes. Elucidation of the temporal sequence of genetic events (e.g. the Ig/c-myc translocation in BL), epigenetic alterations (e.g. stem cell-like epigenetic marks of BL cells) and EBV infection (an early event during the genesis of BL) will be indispensable in case of each EBV positive lymphoma type for a better understanding of lymphoma development and progression. In addition, the patho-epigenetic changes induced by EBV, similarly to those elicited by a growing number of other viruses and certain bacteria may offer new opportunitis for therapeutic intervention.
Table 4.
Cellular genes silenced by promoter hypermethylation in EBV-associated lymphomas and derived cell lines. For detailed discussion see Niller et al.97
| Gene | Lymphoma | ||
|---|---|---|---|
| BL | HD | Iatrogenic/AIDS-associated | |
| ABF1 | + | − | |
| BCL2-family (proapoptotic members) | + | ||
| BCMA | + | ||
| BOB.1/OBF.1 | + | ||
| CHK2 kinase | + | ||
| Cyclin D2 | + | ||
| CD19 | + | ||
| CD20 | + | ||
| CD79B | + | ||
| CD59/HRF20 | + | ||
| DAPK | + | ± | + |
| DLC1 | + | ± | |
| FHIT | + | ||
| GADD25G | + | ||
| GSTP1 | + | ||
| LCK | + | ||
| MAPK10/JNK3 | + | ± | |
| O6-methylguanine-DNA MTase | + | ||
| p16/CDK4A | + | ± | − |
| p15/CDK4B | + | ± | − |
| p57/KIP2 | + | ||
| p73 | + | ||
| PCDH10 | + | + | |
| PLK2 | + | ||
| PU.1 | + | ||
| PTPN13/FAP1 | + | ||
| RASSF1A | − | + | − |
| SYK | + | ||
| WNT5A | + | ||
BL: Burkitt’s lymphoma; HD: Hodgkin’s disease; iatrogenic lymphomas include post-transplant lymphoproliferative disorders (PTLDs) and methotrexate-related lymphomas.
Symbols: frequent inactivation by promoter methylation, +; infrequent inactivation by promoter methylation, −/+; typically unmethylated promoter, −.
Footnotes
This article is available from: http://www.mjhid.org/article/view/5080
References
- 1.Niller HH, Wolf H, Minarovits J. Epstein-Barr Virus. In: Minarovits J, Gonczol E, Valyi-Nagy T, editors. Latency Strategies of Herpesviruses. Springer; 2007. pp. 154–191. [Google Scholar]
- 2.Shannon-Lowe CD, Neuhierl B, Baldwin G, Rickinson AB, Delecluse H. Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc Natl Acad Sci USA. 2006;103:7065–7070. doi: 10.1073/pnas.0510512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Minarovits J. Host cell-dependent expression of latent Epstein-Barr virus genomes: Regulation by DNA methylation. Advances in Cancer Res. 2003;89:133–156. doi: 10.1016/s0065-230x(03)01004-2. [DOI] [PubMed] [Google Scholar]
- 5.Dillner J, Kallin B. The Epstein-Barr virus proteins. Adv Cancer Res. 1988;50:95–158. doi: 10.1016/s0065-230x(08)60436-4. [DOI] [PubMed] [Google Scholar]
- 6.Hammerschmidt W, Sugden B. Identification and characterization of orilyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988;55:427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- 7.Sato H, Takimoto T, Tanaka S, Tanaka J, Raab-Traub N. Concatemeric replication of Epstein-Barr virus: structure of termini in virus producer and newly transformed cell lines. J Virol. 1990;64:5295–5300. doi: 10.1128/jvi.64.11.5295-5300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fixman ED, Hayward GS, Hayward D. Transacting requirements for replication of Epstein-Barr virus ori-Lyt. Virology. 1992;66:5030–5029. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates JL, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr virus genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci USA. 1984;81:3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 2007;26:4252–4262. doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus is a marker of clonal cell proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- 12.Miller G. Epstein-Barr virus: Biology, pathogenesis and medical aspects. In: Fields BN, Knipe DM, editors. Virology. Raven Press; New York: 1990. pp. 1921–1958. [Google Scholar]
- 13.Niller HH, Salamon D, Banati F, Schwarzmann F, Wolf H, Minarovits J. The LCR of EBV makes Burkitt’s lymphoma endemic. Trends Microbiol. 2004;12:495–499. doi: 10.1016/j.tim.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Contreras-Brodin BA, Anvret M, Imreh S, Altiok E, Klein G, Masucci MG. B cell phenotype-dependent expression of the Epstein-Barr virus nuclear antigens EBNA-2 to EBNA-6: studies with somatic cell hybrids. J Gen Virol. 1991;72:3025–33. doi: 10.1099/0022-1317-72-12-3025. [DOI] [PubMed] [Google Scholar]
- 15.Smith P. Epstein-Barr virus complementary strand transcripts (CSTs/BARTs) and cancer. Semin Cancer Biol. 2001;11:469–476. doi: 10.1006/scbi.2001.0414. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer S, Zavolan M, Grässer FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C. Identification of virus-encoded microRNAs. Science. 2004;314:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 17.Cai X, Schafer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, Cullen BR. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog. 2006;2:e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaminathan S. Noncoding RNAs produced by oncogenic human herpesviruses. J Cell Physiol. 2008;216:321–326. doi: 10.1002/jcp.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altmann M, Pich D, Ruiss R, Wang J, Sugden B. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV’s transforming genes. Proc Natl Acad Sci USA. 2006;103:14188–14193. doi: 10.1073/pnas.0605985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woisetschlaeger M, Jack L, Strominger JL, Speck S. Mutually exclusive usage of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc Natl Acad Sci USA. 1989;86:6498–6502. doi: 10.1073/pnas.86.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walls D, Perricaudet M. Novel downstream elements upregulate transcription initiated from an Epstein-Barr virus latent promoter. EMBO J. 1991;10:143–151. doi: 10.1002/j.1460-2075.1991.tb07930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minarovits J, Hu LF, Minarovits-Kormuta S, Klein G, Ernberg I. Sequence-specifc methylation inhibits the activity of the Epstein-Barr virus LMP1 and BCR2 enhancer-promoter regions. Virology. 1994;200:661–667. doi: 10.1006/viro.1994.1229. [DOI] [PubMed] [Google Scholar]
- 24.Robertson KD, Hayward D, Ling PD, Samid D, Ambinder R. Transcriptional activation of the Epstein-Barr virus latency C promoter after 5-azacytidine treatment: evidence that demethylation at a single CpG site is crucial. Mol Cell Biol. 1995;1995;15:6150–6159. doi: 10.1128/mcb.15.11.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heithoff DM, Sinsheimer RL, Low DA, Mahan MJ. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284:967–970. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 26.Balbontin R, Rowley G, Pucciarelli MG, Lopez-Garrido J, Wormstone Y, Lucchini S, Garcia-Del Portillo F, Hinton JC, Casadesus J. DNA adenine methylation regulates virulence gene expression in Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188:8160–8168. doi: 10.1128/JB.00847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pukkila PJ. Telling right from wrong: a role for DNA methylation. Trends in Genetics. 1987;3:1–2. [Google Scholar]
- 28.Ogden GB, Pratt MJ, Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988;54:127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- 29.Bestor TH. DNA methylation: evolution of a bacterial immune function into a regulator of gene expression and genome structure in higher eukaryotes. Phil Trans R Soc Lond B. 1990;326:179–187. doi: 10.1098/rstb.1990.0002. [DOI] [PubMed] [Google Scholar]
- 30.Bird AP. CpG islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 31.Bird AP. CpG islands as gene markers in the vertebrate nucleus. Trends in Genetics. 1987;3:342–347. [Google Scholar]
- 32.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 33.Weber M, Hellmann I, Stadler MB, Ramos L, Pääbo S, Rebhan M, Schübeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation int he human genome. Nature Genetics. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 34.Razin A, Riggs AD. DNA methylation and gene function. Science. 1980;210:604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- 35.Paulsen M, Tierling S, Walter J. DNA methylation in the mammalian genome. In: Trost J, editor. Epigenetics. Caister Academic Press; Norfolk, UK: 2008. pp. 1–21. [Google Scholar]
- 36.Bestor T, Laudano A, Mattaliano R, Vernon I. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. J Mol Biol. 1988;203:971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- 37.Yen RWC, Vertino PM, Nelkin BD, Yu IJ, Eldeiry W, Cumarraswamy A, Lennon GG, Trask BJ, Celano P, Baylin SB. Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res. 1992;20:2287–2291. doi: 10.1093/nar/20.9.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Emburgh BO, Robertson KD. DNA methyltransferases and methyl-CpG binding proteins as multifunctional regulators of chromatin structure and development. In: Trost J, editor. Epigenetics. Caister Academic Press; Norfolk, UK: 2008. pp. 23–61. [Google Scholar]
- 39.Xie S, Wang Z, Okano M, Nogami M, Li Y, He WW, Okumura K, Li E. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236:87–95. doi: 10.1016/s0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- 40.Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 41.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:2889–2897. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 42.Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T. The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem. 2003;278:4035–4040. doi: 10.1074/jbc.M210256200. [DOI] [PubMed] [Google Scholar]
- 43.Riggs AD. DNA methylation and late replication probably aid cell memory, and type I DNA reeling could aid chromosome folding and enhancer function. Phil Trans R Soc Lond B. 1990;326:285–297. doi: 10.1098/rstb.1990.0012. [DOI] [PubMed] [Google Scholar]
- 44.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–74. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 45.Ehrlich M. DNA hypomethylation and cancer. In: Ehrlich M, editor. DNA Alterations in Cancer. Eaton publishing; Natick, MA: 2000. pp. 273–291. [Google Scholar]
- 46.Zilberman D. The human promoter methylome. Nature Genetics. 2007;39:442–443. doi: 10.1038/ng0407-442. [DOI] [PubMed] [Google Scholar]
- 47.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelor C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuchs F. The Polycomb group protein EZH2 controls DNA methylation. Nature. 2006;439:871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 48.Schlesinger Y, Straussman R, Keshet I, Farkash S, Hecht M, Zimmerman J, Eden E, Yakhini Z, Ben-Shushan E, Reubinoff B, Bergman Y, Simon I, Cedar H. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nature Genetics. 2007;39:232–236. doi: 10.1038/ng1950. [DOI] [PubMed] [Google Scholar]
- 49.Ohm JE, McGarvey KM, Yu X, Cheng L, Schuebel KE, Cope L, Mohammad H, Chen W, Daniel VC, Yu W, Berman DM, Jenuwein T, Pruitt K, Sharkis SJ, Watkins DN, Herman JG, Baylin S. A stem cell-like chromatin may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nature Genetics. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roh TY, Cuddapah S, Zhao K. Active chromatin domains are defined by acetylation islands defined by genome-wide mapping. Genes Dev. 2005;19:542–555. doi: 10.1101/gad.1272505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 52.Robertson KD, Manns A, Swinnen LJ, Zong JC, Gulley ML, Ambinder RF. CpG methylation of the major Epstein-Barr virus latency promoter in Burkitt’s lymphoma and Hodgkin’s disease. Blood. 1996;88:3129–3136. [PubMed] [Google Scholar]
- 53.Salamon D, Takacs M, Ujvari D, Uhlig J, Wolf H, Minarovits J, Niller HH. Protein-DNA-binding and CpG methylation at nucleotide resolution of latency-associated promoters Qp, Cp and LMP1p of Epstein-Barr virus. J Virol. 2001;75:2584–2596. doi: 10.1128/JVI.75.6.2584-2596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao Q, Robertson KD, Manns A, Hildesheim A, Ambinder RF. The Epstein-Barr virus major latent promoter Qp is constitutively active, hypomethylated and methylation sensitive. J Virol. 1998;72:7075–7083. doi: 10.1128/jvi.72.9.7075-7083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dyson P, Farrell PJ. Chromatin structure of Epstein-Barr virus. J Gen Virol. 1985;66:1931–1940. doi: 10.1099/0022-1317-66-9-1931. [DOI] [PubMed] [Google Scholar]
- 56.Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem Cell Biol. 2005;83:344–353. doi: 10.1139/o05-041. [DOI] [PubMed] [Google Scholar]
- 57.Alazard N, Gruffat H, Hiriart E, Sergeant A, Manet E. Differential hyperacetylation of histones H3 and H4 upon promoter-specific recruitment of EBNA2 in Epstein-Barr virus chromatin. J Virol. 2003;77:8166–8172. doi: 10.1128/JVI.77.14.8166-8172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chau CM, Lieberman PM. Dynamic chromatin boundaries delineate a latency control region of Epstein-Barr virus. J Virol. 2004;78:12308–12319. doi: 10.1128/JVI.78.22.12308-12319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Day L, Chau CM, Nebozhyn M, Rennekamp AJ, Showe M, Lieberman PM. Chromatin profiling of Epstein-Barr virus latency control region. J Virol. 2007;81:6389–6401. doi: 10.1128/JVI.02172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fejer G, Koroknai A, Banati F, Györy I, Salamon D, Wolf H, Niller HH, Minarovits J. Latency type-specific distribution of epigenetic marks at the alternative promoters Cp and Qp of Epstein-Barr virus. J Gen Virol. 2008;89:1364–1370. doi: 10.1099/vir.0.83594-0. [DOI] [PubMed] [Google Scholar]
- 61.Jansson A, Masucci M, Rymo L. Methylation at discrete sites within the enhancer region regulatest he activity of the Epstein-Barr virus BamHI W promoter in Burkitt lymphoma cell lines. J Virol. 1992;66:62–69. doi: 10.1128/jvi.66.1.62-69.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woisetschlaeger M, Jin XW, Yandava CN, Furmanski LA, Strominger JL, Speck SH. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc Natl Acad Sci U S A. 1991;88:3942–6. doi: 10.1073/pnas.88.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elliott J, Goodhew EB, Krug LT, Shaknowsky N, Yoo L, Speck SH. Variable methylation of the Epstein-Barr virus Wp EBNA gene promoter in B-lymphoblastoid cell lines. J Virol. 2004;78:14062–14065. doi: 10.1128/JVI.78.24.14062-14065.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banati F, Koroknai A, Salamon D, Takacs M, Minarovits-Kormuta S, Wolf H, Nimmer HH, Minarovits J. CpG-methylation silences the activity of the RNA polymerase III transcribed EBER-1 promoter of Epstein-Barr virus. FEBS Letters. 2008;582:705–709. doi: 10.1016/j.febslet.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 65.Minarovits J, Hu LF, Marcsek Z, Minarovits-Kormuta S, Klein G, Ernberg I. RNA polymerase III-transcribed EBER 1 and 2 transcription units are expressed and hypomethylated in the major Epstein-Barr virus-carrying cell types. J Gen Virol. 1992;73:1687–1692. doi: 10.1099/0022-1317-73-7-1687. [DOI] [PubMed] [Google Scholar]
- 66.Gerle B, Koroknai A, Fejer G, Bakos A, Banati F, Szenthe K, Wolf H, Niller HH, Minarovits J, Salamon D. Acetylated histone H3 and H4 mark the upregulated LMP2A promoter of Epstein-Barr virus in lymphoid cells. J Virol. 2007;81:13242–13247. doi: 10.1128/JVI.01396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Jesus O, Smith PR, Spender LC, Elgueta KC, Niller HH, Huang D, Farrell PJ. Updated Epstein-Barr virus (EBV) DNA sequence and analysis of a promoter for the BART (CST, BARF0) RNAs of EBV. J Gen Virol. 2003;84:1443–1450. doi: 10.1099/vir.0.19054-0. [DOI] [PubMed] [Google Scholar]
- 68.Minarovits J. Epigenotypes of latent herpesvirus genomes. Current Topics Microbiol Immunol. 2006;310:61–80. doi: 10.1007/3-540-31181-5_5. [DOI] [PubMed] [Google Scholar]
- 69.Dresang LR, Vereide DT, Sugden B. Identifying sites bound by Epstein-Barr virus nuclear antigen 1 (EBNA1) int he human genome: defining a position-weighted matrix to predict sites bound by EBNA1 in viral genomes. J Virol. 2009;83:2930–2940. doi: 10.1128/JVI.01974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsieh CL. Evidence that protein binding specifies sites of DNA demethylation. Mol Cell Biol. 1999;19:46–56. doi: 10.1128/mcb.19.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin IG, Tomzynski TJ, Ou Q, Hsieh CL. Modulation of DNA binding protein affinity directly affects target site demethylation. Mol Cell Biol. 2000;20:2343–2349. doi: 10.1128/mcb.20.7.2343-2349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Györy I, Minarovits J. Epigenetic regulation of lymphoid specific gene sets. Biochem Cell Biol. 2005 Jun;83(3):286–95. doi: 10.1139/o05-020. [DOI] [PubMed] [Google Scholar]
- 73.Ling PD, Hsieh JD, Ruf IK, Rawlins DE, Hayward SD. EBNA-2 upregulation of Epstein-Barr virus promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J Virol. 1994;68:5375–5383. doi: 10.1128/jvi.68.9.5375-5383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsieh JJD, Hayward SD. Masking the CBF1/RBPJκ transcriptional repression domain by Epstein-Barr virus EBNA2. Science. 1995;268:560–563. doi: 10.1126/science.7725102. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh JJD, Nofziger D, Weinmaster G, Hayward D. Epstein-Barr virus immortalization: Notch2 interacts with CBF1 and blocks differentiation. J Virol. 1997;71:1938–1945. doi: 10.1128/jvi.71.3.1938-1945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Höfelmayr H, Strobl LJ, Marschall G, Bornkamm GW, Zimmer-Strobl U. Activated Notch 1 can transiently substitute for EBNA2 in the maintenance of proliferation of LMP1-expressing immortalized B cells. J Virol. 2001;75:2033–2040. doi: 10.1128/JVI.75.5.2033-2040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salamon D, Takacs M, Schwarzmann F, Wolf H, Minarovits J, Niller HH. High-resolution methylation analysis and in vivo protein-DNA binding at the promoter of the viral oncogene LMP2A in B cell lines carrying latent Epstein-Barr virus genomes. Virus Genes. 2003;27:57–66. doi: 10.1023/a:1025124519068. [DOI] [PubMed] [Google Scholar]
- 78.Wang L, Grossman SR, Kieff E. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter. Proc Natl Acad Sci USA. 2000;97:430–435. doi: 10.1073/pnas.97.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cotter MA, II, Robertson ES. Modulation of histone acetyltransferase activity through interaction of Epstein-Barr Nuclear Antigen 3C with prothymosin alpha. Mol Cell Biol. 2000;20:5722–5735. doi: 10.1128/mcb.20.15.5722-5735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Subramanian C, Hasan S, Rowe M, Hottinger M, Orre R, Robertson ES. Epstein-Barr virus nuclear antigen 3C and prothymosin alpha interact with the p300 transcriptional coactivator at the CH1 and CH3/HAT domains and cooperate in regulation of transcription and histone acetylation. J Virol. 2002;76:4699–4708. doi: 10.1128/JVI.76.10.4699-4708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knight JS, Lan K, Subramanian C, Robertson ES. Epstein-Barr virus nuclear antigen 3C recruits histone deacetylase activity and associates with the corepressors mSin3A and NCoR in human B-cell lines. J Virol. 2003;77:4261–72. doi: 10.1128/JVI.77.7.4261-4272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Portal D, Rosendorff A, Kieff E. Epstein-Barr nuclear antigen leader protein coactivates transcriotion through interaction with histone deacetylase 4. Proc Natl Acad Sci USA. 2006;103:19278–19283. doi: 10.1073/pnas.0609320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ling PD, Tan J, Peng R. Nuclear-cytoplasmic shuttling is not required for the Epstein-Barr virus EBNA-LP transcriptional coactivation function. J Virol. 2009;83:7109–29. doi: 10.1128/JVI.00654-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsai CN, Tsai CL, The KP, Chang HY, Chang YS. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the down-regulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc Natl Acad Sci USA. 2002;99:10084–10089. doi: 10.1073/pnas.152059399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai CL, Li HP, Lu YL, Hsueh C, Liang Y, Chen CL, Tsao SW, The KP, Yu JS, Chang YS. Activation of DNA methyltransferase 1 by EBV LMP1 involves c-Jun NH2-terminal kinase signaling. Cancer Res. 2006;66:11668–11676. doi: 10.1158/0008-5472.CAN-06-2194. [DOI] [PubMed] [Google Scholar]
- 86.Kwong J, Lo KW, To KF, Teo PM, Johnson PJ, Huang DP. Promoter hypermethylation of multiple genes in nasopharyngeal carcinoma. Clin Cancer Res. 2002;8:131–137. [PubMed] [Google Scholar]
- 87.Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-Barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang MS, Uozaki H, Chong JM, Ushiku T, Sakuma K, Ishikawa S, Hino R, Barua RR, Iwasaki Y, Arai K, Fujii H, Nagai H, Fukayama M. CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr Virus. Clin Cancer Res. 2006;12:2995–3002. doi: 10.1158/1078-0432.CCR-05-1601. [DOI] [PubMed] [Google Scholar]
- 89.Murray PG, Qiu GH, Fu L, Waites ER, Srivastava G, Heys D, Agathanggelou A, Latif F, Grundy RG, Mann JR, Straczynsky J, Crocker J, Parkes SE, Ambinder RF, Young LS, Tao Q. Frequent inactivation of the RASSF1A tumor suppressor gene in Hodgkin’s lymphoma. Oncogene. 2004;23:1326–1331. doi: 10.1038/sj.onc.1207313. [DOI] [PubMed] [Google Scholar]
- 90.Doerr JR, Malone CS, Fike FM, Gordon MS, Soghomonian SV, Thomas RK, Tao Q, Murray PG, Diehl V, Teitell MA, Wall R. Patterned CpG methylation of silenced B cell gene promoters in classical Hodgkin lymphoma-derived and primary effusion lymphoma cell lines. J Mol Biol. 2005;350:631–40. doi: 10.1016/j.jmb.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 91.Ushmorov A, Leithäuser F, Sakk O, Weinhaüsel A, Popov SW, Möller P, Wirth T. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood. 2006;107:2493–500. doi: 10.1182/blood-2005-09-3765. [DOI] [PubMed] [Google Scholar]
- 92.Au WY, Ma ES, Choy C, Chung LP, Fung TK, Liang R, Kwong YL. Therapy-related lymphomas in patients with autoimmune diseases after treatment with disease-mofifying anti-rheumatic drugs. Am J Hematol. 2006;81:5–11. doi: 10.1002/ajh.20508. [DOI] [PubMed] [Google Scholar]
- 93.Rossi D, Gaidano G, Gloghini A, Deambrogi C, Franceschetti S, Berra E, Cerri M, Vendramin C, Conconi A, Viglio A, Muti G, Oreste P, Morra E, Paulli M, Capello D, Carbone A. Frequent aberrant promoter hypermethylation of O6-methylguanine-DNA methyltransferase and death-associated protein kinase genes in immunodeficiency-related lymphomas. Br J Haematol. 2003;123:475–478. doi: 10.1046/j.1365-2141.2003.04644.x. [DOI] [PubMed] [Google Scholar]
- 94.Dutton A, Woodman CB, Chukwuma MB, Wei W, Vockerodt M, Baumforth KR, Flavell JR, Taylor AM, Young LS, Murray PG. Bmi-1 is induced by the Epstein-Barr virus viral oncogene LMP-1 and regulates the expression of target genes in Hodgkin lymphoma cells. Blood. 2007;109:2597–2603. doi: 10.1182/blood-2006-05-020545. [DOI] [PubMed] [Google Scholar]
- 95.Lu F, Weidmer A, Liu CG, Volinia S, Croce CM, Lieberman PM. Epstein-Barr virus-induced miR-155 attenuates NF-κB signaling and stabilizes latent virus persistence. J Virol. 2008;82:10436–10443. doi: 10.1128/JVI.00752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin-Subero J, Kreuz M, Bibikova M, Bentink S, Ammerpohl O, Wickham-Garcia E, Rosolowski M, Richter J, Lopez-Serra L, Ballestar E, Berger H, Agirre X, Bernd HW, Calvanese V, Cogliatti SB, Drexler HG, Fan JB, Fraga MF, Hansmann ML, Hummel M, Klapper W, Korn B, Küppers R, MacLeod RAF, Möller P, Ott G, Pott C, Prosper F, Rosenwald A, Schwaenen C, Schübeler D, Seifert M, Stürzenhofecker B, Weber M, Wessendorf S, Loeffler M, Trümper L, Stein H, Spang R, Esteller M, Barker D, Hasenclever D, Siebert R. New insights into the biology and origin of mature agressive B-cell lymphomas by combined epigenomic, genomic, and transcriptional profiling. Blood. 2009;113:2488–2497. doi: 10.1182/blood-2008-04-152900. [DOI] [PubMed] [Google Scholar]
- 97.Niller HH, Wolf H, Minarovits J. Epigenetic dysregulation of the host cell genome in Epstein-Barr virus-associated neoplasia. Seminars in Cancer Biology. 2009;19:158–164. doi: 10.1016/j.semcancer.2009.02.012. [DOI] [PubMed] [Google Scholar]; Noyer-Weidner M, Jentsch S, Pawlek B, Günthert U, Trautner TA. Restriction and modification in Bacillus subtilis: DNA methylation potential of the related bacteriophages Z, SPR, SP beta phi 37 and rho 11. J Virol. 1983;46:446–453. doi: 10.1128/jvi.46.2.446-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garcia JF, Camacho FI, Morente M, Fraga M, Montalban C, Alvaro T, Bellas C, Castano A, Diez A, Flores T, Martin C, Martinez MA, Mazorra F, Menárguez J, Mestre MJ, Mollejo M, Saez AI, Sanchez L, Piris MA, Spanish Hodgkin Lymphoma Study Group Hodgkin and Sternberg-Reed cells harbor alterations int he major tumor suppressor pathways and cell-cycle checkpoints: analysis using tissue microarrays. Blood. 2003;101:681–689. doi: 10.1182/blood-2002-04-1128. [DOI] [PubMed] [Google Scholar]