Abstract
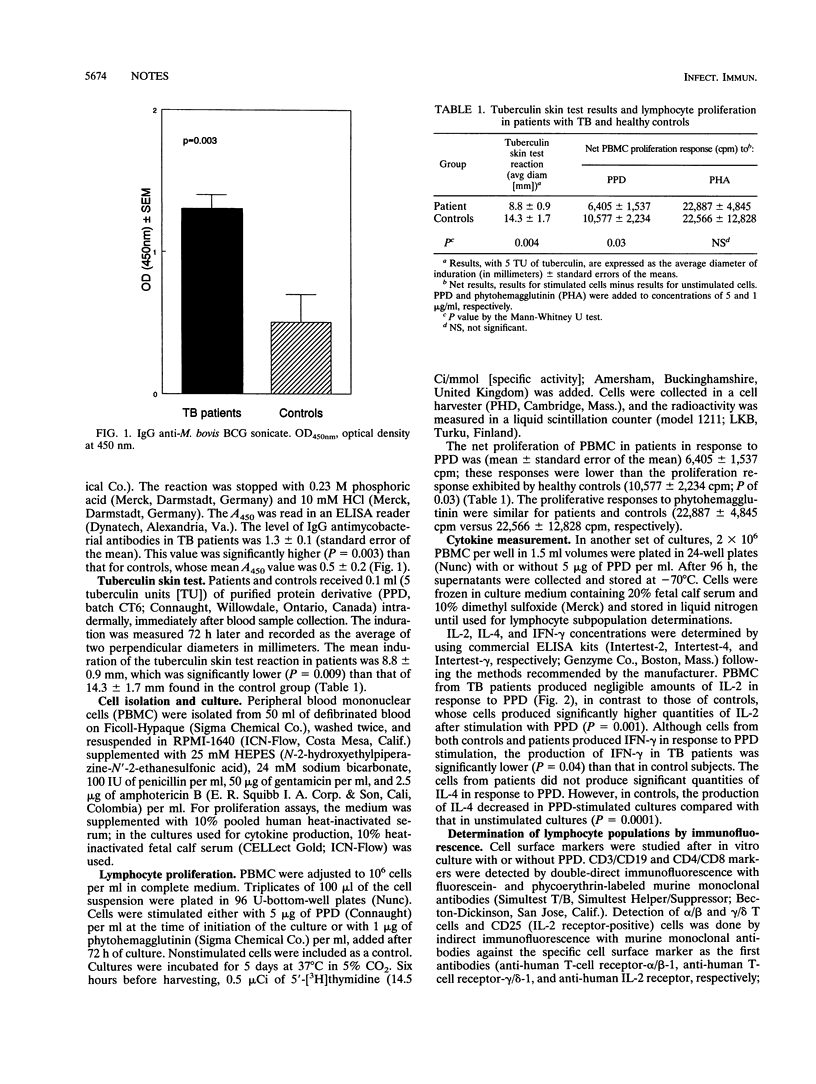
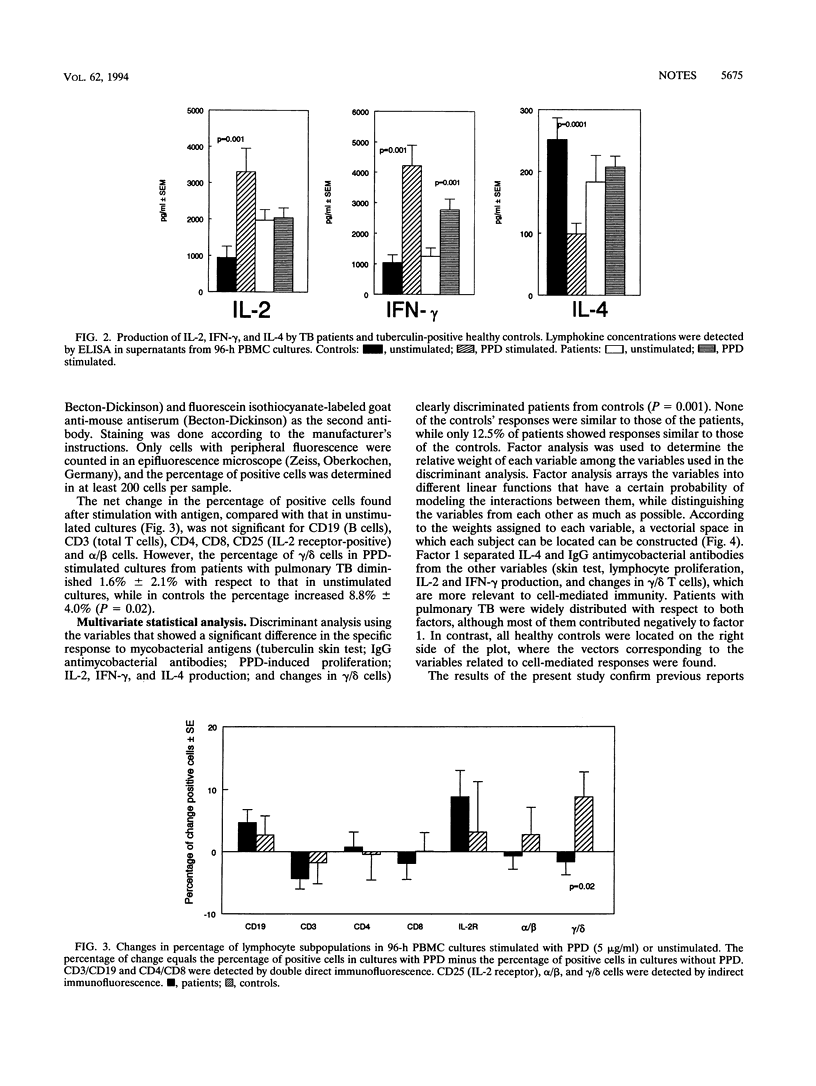
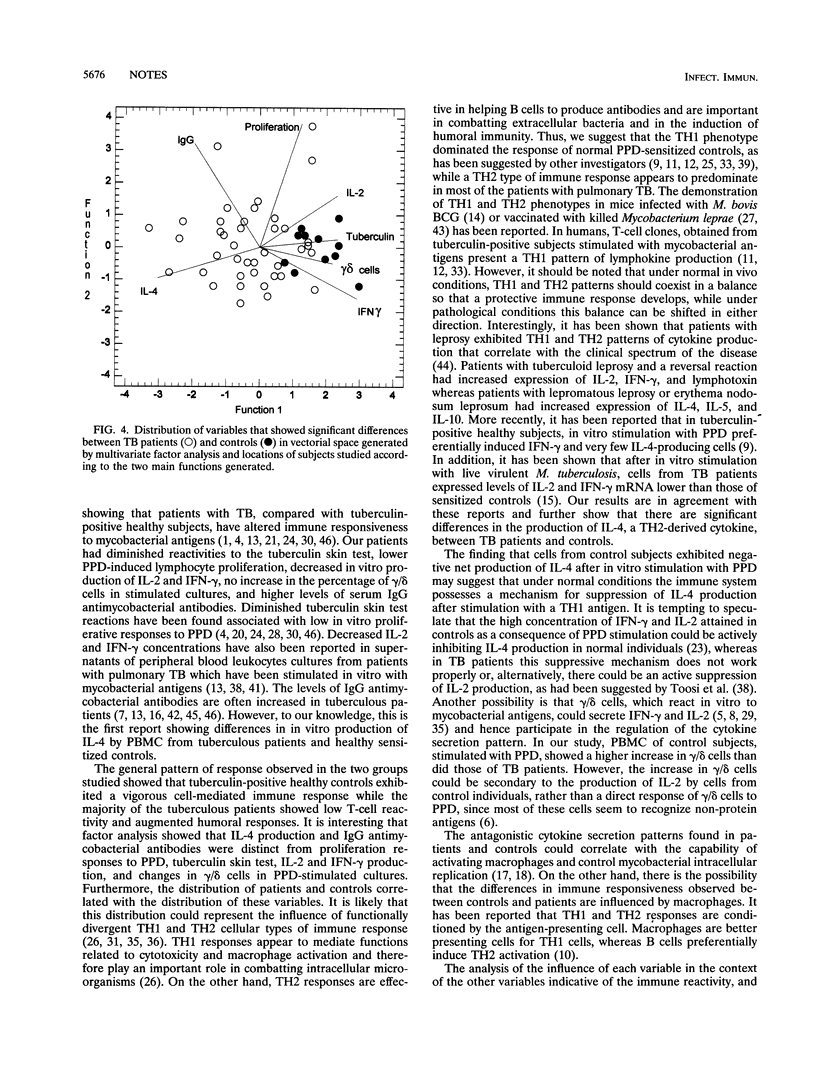
The aim of the present study was to determine the profile of immune responsiveness that differentiates patients with tuberculosis (TB) from healthy tuberculin-positive controls. Forty-five patients with pulmonary TB and 16 healthy tuberculin-positive controls, all human immunodeficiency virus negative, were studied. Patients had decreased reactivity to tuberculin, diminished proliferative response to purified protein derivative (PPD), lower concentrations of interleukin-2 (IL-2) and gamma interferon in PPD-stimulated cultures, no increase in the percentage of gamma/delta cells in PPD-stimulated cultures, and higher immunoglobulin G antimycobacterial antibodies compared with control subjects. Furthermore, controls exhibited decreased production of IL-4 by PPD-stimulated cells. Multivariate discriminant and factor analyses demonstrated divergent patterns of immune reactivity against mycobacterial antigens. The association of IL-4 and immunoglobulin G antibody levels in patients, in contrast to the high reactivity to tuberculin, increased proliferation to PPD, and higher levels of IL-2 and gamma interferon observed in healthy controls suggested that most TB patients exhibit a TH2 pattern of immune responsiveness while tuberculin-positive healthy individuals have a TH1 pattern.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Tawil N. G., Thewaini A. J. Study of the immunological status of patients with pulmonary tuberculosis. Scand J Immunol. 1978;8(4):333–338. doi: 10.1111/j.1365-3083.1978.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristizábal L., García L. F. Formation of total and stable E-rosettes by lymphocytes from patients with pulmonary tuberculosis stimulated by Con A or PPD. J Clin Lab Immunol. 1985 Jan;16(1):31–36. [PubMed] [Google Scholar]
- Beck J. S., Potts R. C., Kardjito T., Grange J. M. T4 lymphopenia in patients with active pulmonary tuberculosis. Clin Exp Immunol. 1985 Apr;60(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R., Malaviya A. N., Narayanan S., Rajgopalan P., Kumar R., Bharadwaj O. P. Spectrum of immune response abnormalities in different clinical forms of tuberculosis. Am Rev Respir Dis. 1977 Feb;115(2):207–212. doi: 10.1164/arrd.1977.115.2.207. [DOI] [PubMed] [Google Scholar]
- Born W. K., O'Brien R. L., Modlin R. L. Antigen specificity of gamma delta T lymphocytes. FASEB J. 1991 Sep;5(12):2699–2705. doi: 10.1096/fasebj.5.12.1717333. [DOI] [PubMed] [Google Scholar]
- Constant P., Davodeau F., Peyrat M. A., Poquet Y., Puzo G., Bonneville M., Fournié J. J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994 Apr 8;264(5156):267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Oxtoby M. J., Pinto E., Moreno E. The immune spectrum in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1981 May;123(5):556–559. doi: 10.1164/arrd.1981.123.5.556. [DOI] [PubMed] [Google Scholar]
- De Libero G., Casorati G., Giachino C., Carbonara C., Migone N., Matzinger P., Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral gamma/delta T cells. J Exp Med. 1991 Jun 1;173(6):1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. E., Schein M. F., Cauthen G. M., Geiter L. J., Selin M. J., Good R. C., O'Brien R. J. Evaluation of the clinical usefulness of mycobacterial skin test antigens in adults with pulmonary mycobacterioses. Am Rev Respir Dis. 1992 May;145(5):1160–1166. doi: 10.1164/ajrccm/145.5.1160. [DOI] [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Johnson B. J., McMurray D. N. Cytokine gene expression by cultures of human lymphocytes with autologous Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994 Apr;62(4):1444–1450. doi: 10.1128/iai.62.4.1444-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Yamamoto K., Shibata M., Kurematsu S., Mitsuhashi O., Kuze A. IgG antibody level to mycobacterial glycoprotein in pulmonary tuberculosis by ELISA. Eur J Respir Dis. 1987 Jul;71(1):37–41. [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity against intracellular bacteria: biological effector functions and antigen specificity of T lymphocytes. Curr Top Microbiol Immunol. 1988;138:141–176. [PubMed] [Google Scholar]
- Kleinhenz M. E., Ellner J. J. Antigen responsiveness during tuberculosis: regulatory interactions of T cell subpopulations and adherent cells. J Lab Clin Med. 1987 Jul;110(1):31–40. [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B. Is macrophage death on the field of battle essential to victory, or a tactical weakness in immunity against tuberculosis? Clin Exp Immunol. 1990 Jun;80(3):301–303. doi: 10.1111/j.1365-2249.1990.tb03285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- McMurray D. N., Echeverri A. Cell-mediated immunity in anergic patients with pulmonary tuberculosis. Am Rev Respir Dis. 1978 Nov;118(5):827–834. doi: 10.1164/arrd.1978.118.5.827. [DOI] [PubMed] [Google Scholar]
- Moreno C., Rees A. J. Striking the right balance; the role of cytokines in mycobacterial disease. Clin Exp Immunol. 1993 Oct;94(1):1–3. doi: 10.1111/j.1365-2249.1993.tb05967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R. Cytokines: is there biological meaning? Curr Opin Immunol. 1991 Jun;3(3):311–314. doi: 10.1016/0952-7915(91)90029-z. [DOI] [PubMed] [Google Scholar]
- Mutis T., Kraakman E. M., Cornelisse Y. E., Haanen J. B., Spits H., De Vries R. R., Ottenhoff T. H. Analysis of cytokine production by Mycobacterium-reactive T cells. Failure to explain Mycobacterium leprae-specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients. J Immunol. 1993 May 15;150(10):4641–4651. [PubMed] [Google Scholar]
- Nash D. R., Douglass J. E. Anergy in active pulmonary tuberculosis. A comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest. 1980 Jan;77(1):32–37. doi: 10.1378/chest.77.1.32. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Onwubalili J. K., Scott G. M. Immune status in tuberculosis and response to treatment. Tubercle. 1988 Jun;69(2):81–94. doi: 10.1016/0041-3879(88)90070-0. [DOI] [PubMed] [Google Scholar]
- Restrepo L. M., Barrera L. F., Garcia L. F. Natural killer cell activity in patients with pulmonary tuberculosis and in healthy controls. Tubercle. 1990 Jun;71(2):95–102. doi: 10.1016/0041-3879(90)90003-q. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Carswell J. W., Stanford J. L. Preliminary evidence for the trapping of antigen-specific lymphocytes in the lymphoid tissue of 'anergic' tuberculosis patients. Clin Exp Immunol. 1976 Oct;26(1):129–132. [PMC free article] [PubMed] [Google Scholar]
- Scott P., Kaufmann S. H. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991 Oct;12(10):346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Fujiwara H., Teraoka O. Nonspecific recruitment of lymphocytes in purified protein derivative-induced lymphocyte proliferative response of patients with tuberculosis. Infect Immun. 1982 Aug;37(2):702–709. doi: 10.1128/iai.37.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Klion A., Henriksen-DeStefano D., Zemtsov A., Davidson D. M., Davidson M., Friedman-Kien A. E., Le J. Defective gamma-interferon production in peripheral blood leukocytes of patients with acute tuberculosis. J Clin Immunol. 1986 Mar;6(2):146–151. doi: 10.1007/BF00918747. [DOI] [PubMed] [Google Scholar]
- Viljanen M. K., Eskola J., Tala E. Enzyme-linked immunosorbent assay (ELISA) for antibodies to purified protein derivative of tuberculin (PPD). IgM-, IgA- and IgG- anti-PPD antibodies in active pulmonary tuberculosis. Eur J Respir Dis. 1982 May;63(3):257–262. [PubMed] [Google Scholar]
- Walker K. B., Butler R., Colston M. J. Role of Th-1 lymphocytes in the development of protective immunity against Mycobacterium leprae. Analysis of lymphocyte function by polymerase chain reaction detection of cytokine messenger RNA. J Immunol. 1992 Mar 15;148(6):1885–1889. [PubMed] [Google Scholar]
- Yamamura M., Wang X. H., Ohmen J. D., Uyemura K., Rea T. H., Bloom B. R., Modlin R. L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992 Aug 15;149(4):1470–1475. [PubMed] [Google Scholar]
- Zeiss C. R., Radin R. C., Williams J. E., Levitz D., Phair J. P. Detection of immunoglobulin G antibody to purified protein derivative in patients with tuberculosis by radioimmunoassay and enzyme-linked immunosorbent assay. J Clin Microbiol. 1982 Jan;15(1):93–96. doi: 10.1128/jcm.15.1.93-96.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz S. J., Ostrow J. H., Van Arsdel P. P., Jr Humoral and cellular immunity in the anergic tuberculosis patient. A prospective study. J Allergy Clin Immunol. 1974 Jan;53(1):20–26. doi: 10.1016/0091-6749(74)90095-5. [DOI] [PubMed] [Google Scholar]
- elGhazali G. E., Paulie S., Andersson G., Hansson Y., Holmquist G., Sun J. B., Olsson T., Ekre H. P., Troye-Blomberg M. Number of interleukin-4- and interferon-gamma-secreting human T cells reactive with tetanus toxoid and the mycobacterial antigen PPD or phytohemagglutinin: distinct response profiles depending on the type of antigen used for activation. Eur J Immunol. 1993 Nov;23(11):2740–2745. doi: 10.1002/eji.1830231103. [DOI] [PubMed] [Google Scholar]


