Abstract
Renin-producing juxtaglomerular cells are connected to each other and to endothelial cells of afferent arterioles by gap junctions containing Connexin 40 (Cx40), abundantly expressed by these two cell types. Here, we generated mice with cell-specific deletion of Cx40 in endothelial and in renin-producing cells, as its global deletion caused local dissociation of renin-producing cells from endothelial cells, renin hypersecretion, and hypertension. In mice lacking endothelial Cx40, the blood pressure, renin-producing cell distribution, and the control of renin secretion were similar to wild-type mice. In contrast, mice deficient for Cx40 in renin-producing cells were hypertensive and these cells were ectopically localized. Although plasma renin activity and kidney renin mRNA levels of these mice were not different from controls, the negative regulation of renin secretion by pressure was inverted to a positive feedback in kidneys lacking Cx40 in renin-producing cells. Thus, our findings show that endothelial Cx40 is not essential for the control of renin expression and/or release. Cx40 in renin-producing cells is required for their correct positioning in the juxtaglomerular area and the control of renin secretion by pressure.
Keywords: afferent arteriole, blood pressure, renal hypertension, renin angiotensin system
Renin-producing cells in the kidney are connected via gap junctions to each other and to adjacent endothelial and mesangial cells. Evidence suggests that Connexin 40 (Cx40) is essential for the formation of these gap junctions, because this connexin subtype is strongly expressed in renin, endothelial, and mesangial cells1–4 and global deletion of Cx40 leads to disappearance of other connexins from renin cells.4 Moreover, global deletion of Cx40 also strongly alters renin cell distribution in the kidney and their responses to physiological manipulations. Renin-producing cells are ectopically located outside the arteriolar vessel wall and renin secretion escapes the physiological negative feedback control by pressure and angiotensin.5–7 As a consequence, Cx40-deficient mice show massive hyperreninemia associated with hypertension. The mechanisms responsible for the disturbed function of the renin–angiotensin system in the absence of Cx40 are unknown.
Interestingly, endothelial as well as renin-producing cells express Cx40, however, it is unknown in which cell type the Cx40 expression is required to enable negative feedback of pressure on renin secretion. Different scenarios can be envisioned: first, both cell types communicate in a crucial manner through gap junctions formed by Cx40; second, Cx40 may be only required in renin-producing cells to synchronize their behavior with respect to renin secretion; and third, Cx40 is only mandatory in endothelial cells, which would suggest that endothelial cells function as pressure sensors, as proposed recently.8 In addition to the cell function, these envisioned communication pathways may also determine the development and location of renin-producing cells. An alteration of the normal spatial relationship between endothelial cells and renin-producing cells in the tubulo-interstitial space possibly interrupts and prevents normal gap junctional coupling between endothelial cells and renin-producing cells. As a result, renin-producing cells are unable to receive and/or interpret those signals that govern renin secretion, which results in defective control of renin secretion as observed in global Cx40 deficiency. Therefore, we were interested in determining the effect of selective deletion of Cx40 either in endothelial cells or in renin-producing cells, on the phenotype and location of renin-producing cells and on the control of renin secretion in comparison with the effects induced by a global Cx40 deficiency. The Tie2 promoter-driven Cre recombinase9 is a suitable tool to generate mice carrying an endothelium-specific gene deletion using the Cre-lox system.10 Similarly, renin promoter-driven Cre recombinase has been successfully used to generate renin cell-specific gene deletion.11 We therefore cross-bred mice carrying a lox P-sites flanked Cx40 gene12 with mice harboring the Cre recombinase under the control of the Tie2 promoter (Tie2-Cre) or the renin promoter (renin-Cre) to generate mice with endothelium or renin cell-specific deletions of Cx40.
RESULTS
Tie2-Cre Cx40fl/fl mice lacked Cx40 immunoreactivity in renal afferent arteriolar endothelial cells, while Cx40 expression was maintained in renin-producing cells and mesangial cells (Figure 1). We confirmed endothelial deletion of Cx40 in the aorta of Tie2-Cre Cx40fl/flmice (Supplementary File S1). Conversely, Cx40 immunoreactivity was absent in renin-producing cells but not in endothelial or in mesangial cells in renin-Cre Cx40fl/fl mice (Figure 2). We therefore assumed that these animals showed the intended cell-specific deletions of Cx40. Cx45 immunoreactivity was not different between the three genotypes under investigation.
Figure 1. Immunostainings of kidney sections of a Connexin 40 (Cx40)fl/fl mouse (control) and Tie2-Cre Cx40fl/fl mouse.

(a, c) are immunostained for Cx40 (red) and renin (green). b and d are immunostained for Cx40 (red), renin (green), and CD 31 (blue) as an endothelial cell marker. Arrows indicate endothelial staining, arrowheads renin-producing cells, and asterisks intraglomerular mesangial cells. Note the coexistence of Cx40 and CD31 immunoreactivity in the Cx40fl/fl kidney (a, b), whereas Cx40 immunoreactivity is absent in the Tie2-Cre Cx40fl/fl kidney from endothelial cells (c, d). Also, note the similar Cx40 immunoreactivity in the renin-producing cells both in Cx40fl/fl (a, b) and in Tie2-Cre Cx40fl/fl (c, d) kidneys.
Figure 2. Immunostainings of kidney sections of a Connexin 40 (Cx40)fl/fl mouse (control) and renin-Cre Cx40fl/fl mouse.
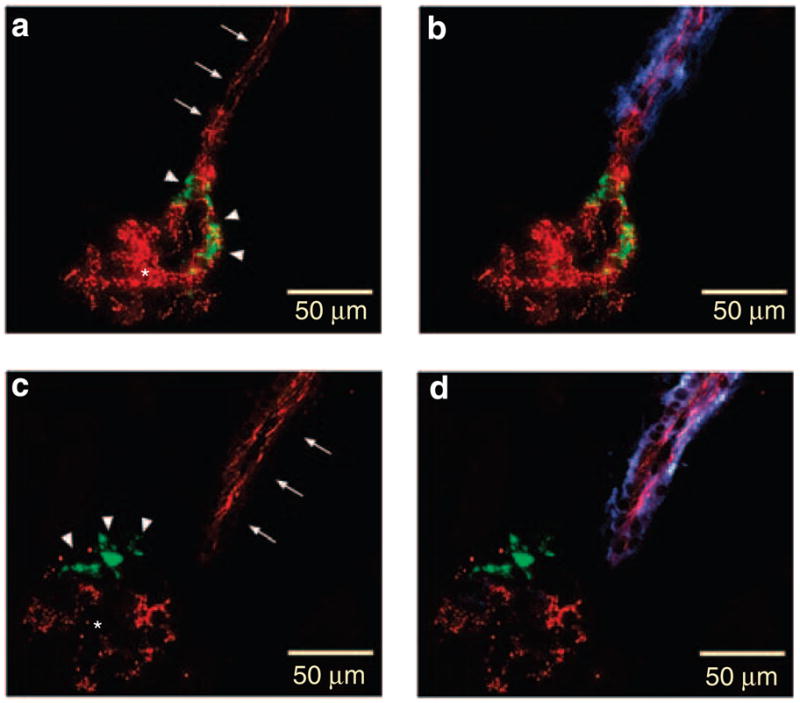
(a, c) are immunostained for Cx40 (red) and renin (green). b and d are immunostained for Cx40 (red), renin (green), and α-smooth muscle actin (blue) as a smooth muscle cell marker in an afferent arteriole. Arrows indicate endothelial staining, arrowheads renin-producing cells and asterisks intraglomerular mesangial cells. Note the coexistence of Cx40 and renin immunoreactivity in the Cx40fl/fl kidney (a, b), whereas Cx40 immunoreactivity is absent in the renin-Cre Cx40fl/fl kidney from renin-producing cells (c, d). Also, note the similar Cx40 immunoreactivity in the endothelium both in Cx40fl/fl (a, b) and in renin-Cre Cx40fl/fl (c, d) kidneys.
In Tie2-Cre Cx40fl/fl mice, mean arterial blood pressure and heart rates were not different from that in Cx40fl/fl mice (Figure 3) and both genotypes showed pressures comparable to previously measured wild-type animals.13 Renin-producing cells in Tie2-Cre Cx40fl/fl were normal in number, form, and location, i.e., they were integral part of the media layer of afferent arterioles at the glomerular vascular pole and indistinguishable from Cx40fl/fl controls (Figure 4a and b). Likewise, plasma renin concentrations and kidney renin mRNA levels were normal in both genotypes Tie2-Cre Cx40fl/fl and Cx40fl/fl (Figures 5 and 6). Salt depletion induced by feeding a low-salt diet in combination with the angiotensin II converting enzyme (ACE) inhibitor, enalapril, (10 mg/kg added to the drinking water) for 7 days led to 10- and 6-fold increases in plasma renin concentration and renin mRNA levels, respectively, in Cx40fl/fl as well as Tie2-Cre Cx40fl/fl mice (Figures 5 and 6). Concomitantly, the number of renin-producing cells in the media layer of afferent arterioles increased in a characteristic retrograde recruitment pattern along afferent arterioles in both Tie2-Cre Cx40fl/fl and Cx40fl/fl mice (Figure 4d and e). Finally, the pressure dependency of renin secretion as assayed in isolated perfused kidney preparations was also not different between Tie2-Cre Cx40fl/fl and Cx40fl/fl mice and showed the typical inverse relationship between pressure and renin secretion (Figure 7).
Figure 3. Blood pressure in awake Connexin 40 (Cx40)fl/fl, Tie2-Cre Cx40fl/fl, and renin-Cre Cx40fl/fl mice as measured by telemetry.
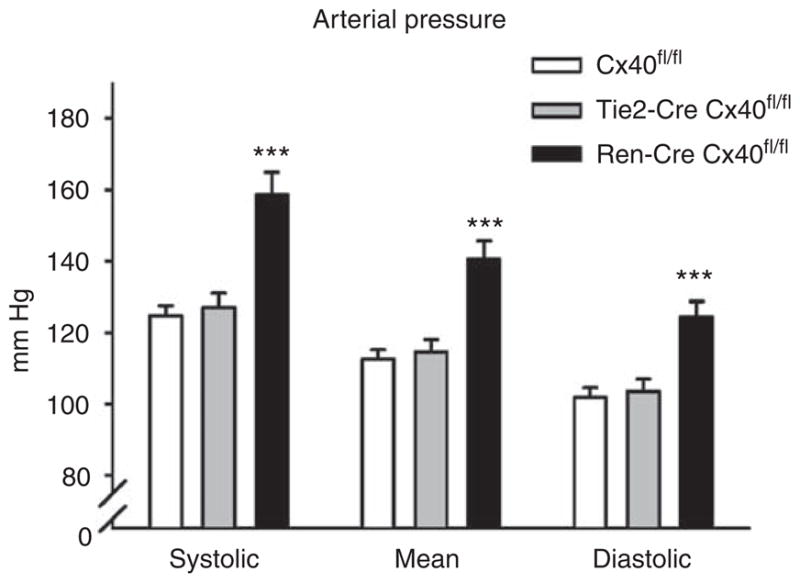
Systolic, mean, and diastolic pressures were increased in renin-Cre Cx40fl/fl mice. Heart rates were not different between genotypes (Cx40fl/fl, 582±24; Tie2-Cre Cx40fl/fl, 563±11; renin-Cre Cx40fl/fl, 622±14 beats/min). Data are means ± s.e.m. of at least nine animals of each genotype. Asterisk indicates P<0.001 vs Cx40fl/fl and Tie2-Cre Cx40fl/fl.
Figure 4. Immunostainings of kidney sections of a connexin 40 (Cx40)fl/fl mouse (control), Tie2-Cre Cx40fl/fl and renin-Cre Cx40fl/fl mouse.
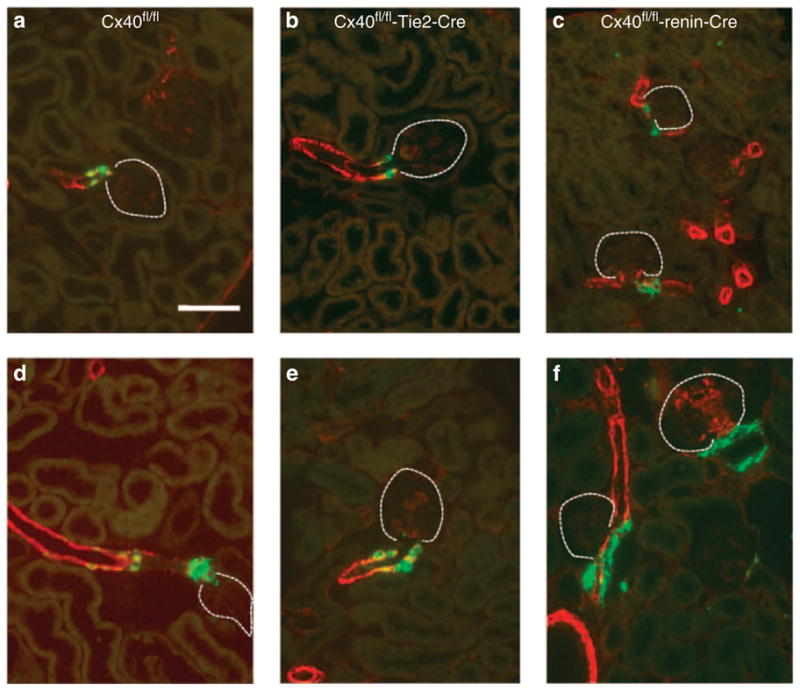
Immunohistochemistry of renin (green) and α-smooth muscle actin (red) in kidney sections of Connexin 40 (Cx40)fl/fl (a, d), Tie2-Cre Cx40fl/fl (b, e) and renin-Cre Cx40fl/fl (c, f) mice under normal salt diet (a–c) or treated with low-salt diet (0.02%) and enalapril (10 mg/kg) (d–f). Coexpression of renin and α-smooth muscle actin is indicated by yellow color. Dotted lines mark glomeruli. Bar = 50 μm. In Cx40fl/fl and Tie2-Cre Cx40fl/fl kidneys, renin-expressing cells are located typically in the walls of afferent arterioles. In renin-Cre Cx40fl/fl kidneys, renin-expressing cells are located mainly outside the vessel walls.
Figure 5. Plasma renin concentrations in Connexin 40 (Cx40)fl/fl, Tie2-Cre Cx40fl/fl, and renin-Cre Cx40fl/fl mice under normal salt diet or after low-salt diet (0.02%) combined with enalapril (10 mg/kg added to the drinking water) for 7 days.

Data are means ± s.e.m., six animals in each group. Asterisks indicate P<0.05 vs normal salt diet.
Figure 6. Renin mRNA abundance in kidneys of Connexin 40 (Cx40)fl/fl, Tie2-Cre Cx40fl/fl, and renin-Cre Cx40fl/fl mice during normal salt diet or after low-salt diet (0.02%) combined with enalapril (10 mg/kg).

Data are means ± s.e.m., six animals in each group. Asterisks indicate P<0.05 vs normal salt diet.
Figure 7. Pressure-dependent renin secretion in isolated perfused kidneys from Connexin 40 (Cx40)fl/fl, Tie2-Cre Cx40fl/fl, and renin-Cre Cx40fl/fl mice.

Renin secretion increased with enhanced pressure in renin-Cre Cx40fl/fl mice, whereas in Tie2-Cre Cx40fl/fl as well as Cx40fl/fl controls, the normal inverse relationship was found. Data are means ± s.e.m. of four kidneys of each genotype.
In contrast, renin-Cre Cx40fl/fl mice had an elevated blood pressure (Figure 3), but heart rates, plasma renin concentrations, and kidney renin mRNA levels were not different from the respective values measured in Cx40fl/fl mice (Figures 5 and 6). However, localization, but not the number of renin-producing cells, was different between renin-Cre Cx40fl/fl and Cx40fl/fl mice. In renin-Cre Cx40fl/fl animals, renin-expressing cells were mainly localized outside the media layer of afferent arterioles and seem to have a different fibroblast-like phenotype (Figures 4c and 8a). The atypical localization and presence of renin-producing cells in the interstitium in renin-Cre Cx40fl/fl mice became more apparent after treatment of the animals with low-salt diet in combination with the ACE inhibitor, enalapril. In the course of this treatment, the number of renin-expressing cells markedly increased outside the vessel walls in the juxtaglomerular interstitium and occasionally into the glomerular stalk (Figure 4f and 8b). However, plasma renin concentration and renal renin mRNA concentrations increased in renin-Cre Cx40fl/fl mice to similar levels as observed in Cx40fl/fl mice (Figures 5 and 6). The pressure dependency of renin secretion determined in the isolated perfused kidney was substantially deteriorated in renin-Cre Cx40fl/fl mice. Instead of the typical inverse relationship between renin secretion and renal perfusion pressure as seen in Cx40fl/fl mice, renin secretion was even positively related to perfusion pressure in renin-Cre Cx40fl/fl kidneys (Figure 7).
Figure 8. Immunostainings of kidney sections of a renin-Cre Connexin 40 (Cx40)fl/fl mouse.
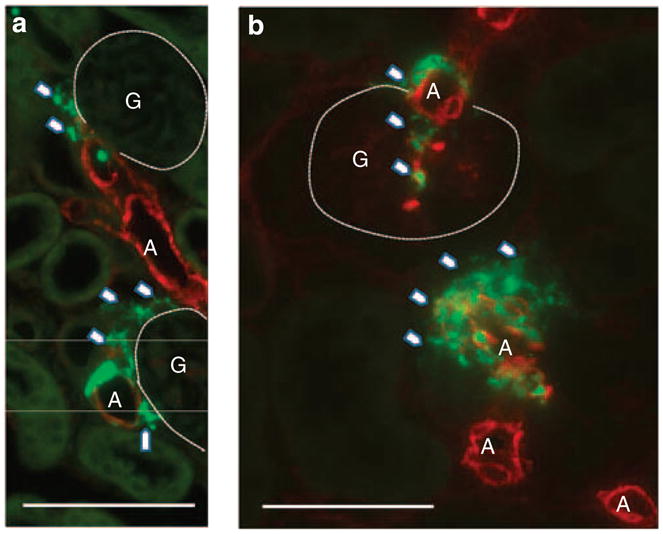
Higher power magnifications of renin-expressing cells (renin in green) and smooth muscle cells (α-smooth muscle actin in red) in kidneys of renin-Cre Connexin 40 (Cx40)fl/fl mice under normal salt diet (a) or treated with low salt and enalapril (b). Arrowheads highlight atypically located renin-expressing cells. Note the increase in the number of renin-expressing cells during treatment with low-salt diet, which occasionally extend into the glomerular stalk, and the development of plaque-like patterns. Bar = 50 μm. A, arteriole; G, glomerulus.
DISCUSSION
Our data show that mice deficient for Cx40 in renin-producing cells, but not mice lacking Cx40 in endothelial cells, show an elevated blood pressure, alterations of the intrarenal distribution of renin-producing cells, and a disturbed pressure regulation of renin secretion that resemble those seen in mice with a general deletion of Cx40.5,7 In Tie2-Cre Cx40fl/fl mice, endothelial cells cannot form Cx40 gap junctions with adjacent cells. Therefore, our data suggest that these abnormalities seen in mice with a global deficiency of Cx40 primarily result from a defective Cx40 signaling within renin-producing cells, rather than from an interruption of Cx40-related gap junctional signaling within preglomerular endothelial or between endothelial cells and renin-producing cells. However, cell-specific deletion of Cx40 in renin-producing cells does not completely mimic the phenotype of global Cx40 deficiency. In mice with global Cx40 deficiency, plasma renin concentrations and renin mRNA levels are already increased under normal salt diet. While salt depletion induced by low-salt diet in combination with an ACE inhibitor produces only a minor additional effect on the plasma renin concentrations, the renin mRNA levels, and the number of renin-expressing cells in global Cx40-deficient mice, salt depletion is very effective in enhancing these parameters of renin release in cell-specific Cx40-deficient mice. In fact, both genotypes, mice devoid of Cx40 in endothelial as well as renin-producing cells, are not different from the respective controls or wild-type animals in this regard. It therefore appears that mice with global Cx40 deletion are already in a basal state of renin expression, which is also evident in mice with only a renin-cell specific deletion of Cx40 (and wild-type animals) under severe salt depletion.
In contrast to deficiency of Cx40 in renin-producing cells, its lack in endothelial cells was not associated with defects in renin production or release and these animals were also normotensive. This suggests that either signals from endothelial cells delivered through gap junctions are not critical at all for appropriate renin secretion or that endothelial Cx40 is a dispensable component of a gap junctional signaling pathway from endothelial to renin-producing cells. In favor of this, latter assumption argues the fact that preglomerular endothelial cells express, in addition to Cx40, also abundant amounts of Cx37,4 and Cx37 may substitute for the lack of Cx40. However, recently it has been shown that expression of Cx37 is strongly dependent on Cx40 and the lack of Cx40 results in a strong decrease of Cx37 expression in the endothelial cell membrane of arterioles.13 Moreover, general deletion of Cx37 has no major impact on the renin system.14 Therefore, we suggest from the present data that gap junctional signaling between endothelial cells or from endothelial to renin-producing cells is not required for intact feedback inhibition of pressure on renin release or for the correct positioning of renin-producing cells.
In contrast, selective lack of Cx40 in renin-producing cells impairs the negative feedback control of pressure on renin secretion in the perfused isolated kidney. This shows the critical importance of Cx40 in renin-producing cells, which may function either to interconnect these cells to synchronize their behavior or alternatively supply hemichannels as suggested recently.15 Another possibility could be that it is primarily the ectopic position of Cx40 deficient renin-producing cells that renders their secretory behavior insensitive to the renal perfusion pressure.
Although renin cell-specific deletion mimicks the behavior of Cx40 null kidneys with respect to pressure feedback in vitro, important differences are observed in the intact animal. Surprisingly, renin-Cre Cx40fl/fl mice are hypertensive in spite of normal renin activity, which raises an important question, namely why is the basal activity of the renin system markedly different between mice with global Cx40 and mice with renin cell-specific Cx40 deletions?
Hypertension, in spite of normal circulating renin levels and kidney renin mRNA levels, was similarly detected in mice in which the coding sequence, but not the promoter of Cx40, is replaced by Cx45 (Cx40KI45 mice). Thus, native Cx40-expressing cells such as renin-producing cells, endothelial cells, and mesangial cells express Cx45 instead of Cx40 in these animals.16,17 Because gap junctions formed by Cx45 show a lower conductivity than Cx4018 and such lower coupling results in a moderate renin phenotype,18 one may challenge the efficacy of the Cre-lox recombination in renin-Cre Cx40fl/fl mice, with remaining coupling being present. However, we were unable to detect Cx40 in renin cells (Figure 2) and the renin-Cre recombinase carrying mice that were used have been shown to be very effective in other models as well,19 which argues in favor of a complete cell-specific deletion of Cx40. As remaining coupling is therefore unlikely, the discrepancy between hypertension and renin activity suggests, at a first glance, that hypersecretion of renin is not the primary cause for the hypertension. Blood pressure measurements revealed that the reduction in systolic blood pressure during ACE inhibition was greater in renin-Cre Cx40fl/fl mice than in Cx40fl/fl controls, suggesting a renin-dependent component of hypertension (Table 1). However, even after ACE inhibition, blood pressure remained at higher levels in renin-Cre Cx40fl/fl mice than in Cx40fl/fl controls, suggesting also a renin-independent component of hypertension. Similarly, in mice with global Cx40 deficiency, hyperreninemia is unlikely to be the exclusive cause of hypertension because inhibitors of the renin angiotensin system also do not abrogate the blood pressure difference between wild-type and Cx40-deficient mice.5,20 It should be emphasized, however, that circulating renin levels and kidney renin mRNA levels are relatively increased in renin-Cre Cx40fl/fl and Cx40KI45 mice and even more pronounced in mice lacking Cx40 globally. Physiologically, elevated pressure depresses renin synthesis and renin secretion in the sense of a true negative feedback.21 However, because the negative feedback control of renin secretion by pressure is absent in Cx40-deficient mice, in renin-Cre Cx40fl/fl, and also in Cx40KI45 mice (not shown), inappropriate high renin-secretion rates most likely contribute, importantly, to the development and maintenance of elevated blood pressure. In addition, an impaired tubuloglomerular feedback inhibition in Cx40 null mice6,22 may facilitate renin secretion in the intact animal.
Table 1.
Influence of combined low salt and enalapril treatment on systolic blood pressure in Cx40fl/fl and in renin-Cre Cx40fl/fl mice
| Systolic blood pressure (mm Hg) |
|||
|---|---|---|---|
| Normal salt | Low salt+enalapril | Δ | |
| Cx40fl/fl | 120±4 | 92±4 | −28±3 |
| Renin-CreCx40fl/fl | 150±6 | 102±4 | −48±4 |
| P-value | <0.05 | <0.05 | <0.05 |
| n | 7 | 8 | |
Abbreviation: Cx40, Connexin 40.
Δ Indicates changes in systolic blood pressure after treatment with low-salt diet and enalapril.
In conclusion, the present data show that feedback control exerted by pressure on renin secretion and the positioning of renin-expressing cells requires Cx40-dependent signaling (most likely through gap junctions) in renin-producing cells, but not within endothelial cells, or between these two cell types. Although animals that are deficient for Cx40 in renin-producing cells are hypertensive, plasma renin activity is not different from controls and thus in light of their pressure only relatively enhanced. However, possible renin cell-independent mechanisms contribute to excess renin and hypertension in Cx40 null animals, but they remain unclear at the moment. As the renin promoter is active also in extrarenal tissues during development of the organism, we cannot yet distinguish in the present study between renal and extrarenal Cx40-related mechanisms that contribute to high blood pressure in animals with impaired Cx40 function.
MATERIALS AND METHODS
Mice
Tie2-Cre mice9 were obtained from M Yanagisawa (University of Texas, Dallas, TX, USA). Mice with a floxed Cx40 gene and renin-Cre mice were used as described previously.11,12 Mice were mated to generate mice being homozygous for the floxed Cx40 gene (Cx40fl/fl) carrying either Tie2-Cre, renin-Cre, or no Cre recombinase, which served as controls. Mice were genotyped by PCR from tail-tip biopsies using primers as described.9,11,12
Measurement of arterial pressure by radiotelemetry
Mice were anesthetized with isoflurane (1–1.5%) and surgically implanted with microminiaturized radiotelemeters (TA11PA-C10, Data Sciences International, St Paul, MN, USA) and the catheter was introduced into the left carotid artery. After stabilization and recovery (24 h), arterial pressure was recorded each day at the same time for 4 days as described previously.16 Heart rate was determined offline from the pressure curve.
Determination of renin mRNA by real-time PCR
Total RNA was isolated from the frozen kidneys as described by Chomczynski M and Sacchi.23 The complementary DNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Superscript, Invitrogen, Karlsruhe, Germany). For quantification of renin mRNA expression, real time reverse transcriptase-PCR was performed using a Light Cycler Instrument (Roche Diagnostics, Mannheim, Germany) and the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany), and β-actin as a control. The primers were chosen to span over the two exon–intron borders, to avoid amplification of genomic DNA. To verify the accuracy of the amplicon, a melting curve analysis was performed after amplification, and PCR products were analyzed on an ethidium bromide-stained agarose gel. For amplification of mouse renin and β-actin complementary DNAs, the following primers were used: renin: 5′-ATGAAGGGGGTGTCTGTGGGG-3′ (sense), 5′-ATGCGGGGAGGGTGGGCACCT-3′ (antisense); β-actin: 5′-CGGGATCCCCGCCCTAGGCACCAGGGTG-3′ (sense), 5′-GGAATTAGGCTGGGGTGTTGAAGGTCTCAAA-3′ (antisense).
Determination of plasma renin concentration
For determination of plasma renin concentration, the blood samples taken from the tail vein were centrifuged and the plasma was incubated for 1.5 h at 37°C, with plasma from bilaterally nephrectomized male rats as renin substrate.5 The generated angiotension-I (ng/ml/h) was determined using radioimmunoassay (Byk & DiaSorin Diagnostics, Dietzenbach, Germany).
Isolated perfused mouse kidney
The isolated perfused mouse kidney model has been described in detail previously.5 Briefly, the animals were anesthetized with an intraperitoneal injection of 12 mg/kg xylazine (Rompun, Bayer, Leverkusen, Germany) and 80 mg/kg ketamine-HCl (Curamed, Karlsruhe, Germany), the abdominal aorta was cannulated, the right kidney was excised, placed in a thermostated moistening chamber, and perfused at constant pressure (90 mmHg). Using an electronic feedback control, perfusion pressure could be changed and held constant in a pressure range between 40 and 140 mmHg. Finally, the renal vein was cannulated and the venous effluent was collected for determination of renin activity and venous blood flow. The basic perfusion medium consisted of a modified Krebs–Henseleit solution supplemented with 6 g/100 ml bovine serum albumin and with freshly washed human red blood cells (a 10% hematocrit). For the determination of renin secretion rates, three samples of the venous effluent were taken in intervals of 2 min during each experimental period. Renin activity in the venous effluent was determined by radioimmunoassay (Byk & DiaSorin Diagnostics) as described previously.5 Renin secretion rates were calculated as the product of the renin activity and the venous flow rate (ml/min*g kidney weight).
Immunohistochemistry for renin and α-smooth muscle actin
The expression of renin and α-smooth muscle actin was localized by immunohistochemistry. In brief, kidneys were perfusion-fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Immunolabeling was performed on 5-μm paraffin sections. After blocking with 10% horse serum, 1% bovine serum albumin in phosphate-buffered saline (PBS), sections were incubated with either anti-renin (Davids Immunotechnologie, Regensburg, Germany) or anti-smooth muscle actin (Beckman Coulter, Immunotech, Marseille, France) antibodies overnight at 4°C, followed by incubation with a fluorescent secondary antibody (Dianova, Hamburg, Germany).
Immunohistochemistry for Cx40
Antibodies used for immunostaining of Cx40 and α-smooth muscle actin were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). Antibodies against CD31 were obtained from R&D Systems (Minneapolis, MN, USA). Kidneys were frozen unfixed in TissueTek OCT embedding medium (Sakura Finetek, Heppenheim, Germany) and sectioned at 5 μm with a cryostat. Without further storing, sections were fixed in methanol at −20°C for 20 min; washed three times in PBS; and blocked in a buffer containing PBS, 1% bovine serum albumin, and 10% horse serum for 30 min. Primary antibodies were diluted in the same blocking solution using anti-Cx40 (1:100), anti-renin (1:200), anti-CD31 (1:100), and anti-α-smooth muscle actin (1:10) in respective combinations, incubating sections at 4°C overnight. On the next day, sections were washed three times in PBS containing 1% bovine serum albumin and incubated with combinations of cyanine 2, TRITC, or cyanine 5 secondary antibodies (Dianova) for 90 min at room temperature, diluted 1:400, 1:300, and 1:400, respectively. After washing in PBS, sections were mounted with Dakocytomation glycergel mounting medium (Dako, Glostrup, Denmark) and viewed with an Axiovert microscope (Zeiss, Jena, Germany).
Statistical analysis
Values are given as means ± s.e.m. Differences between groups were analyzed using analysis of variance and Bonferroni adjustment for multiple comparisons. P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The expert technical assistance provided by Ann M’Bangui is gratefully acknowledged. The authors’ work was financially supported by grants of the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 699 to CW, FS, AK; WA 2137/2-1 to CW and AK; and WI 2071/2-1 to CdW).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
File S1. Cx40 expression (red) in the aorta of Cx40fl/fl (A), TIE2-Cre Cx40fl/fl (B), and Renin-Cre Cx40fl/fl (C) mice.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
References
- 1.Hwan Seul K, Beyer EC. Heterogeneous localization of connexin 40 in the renal vasculature. Microvasc Res. 2000;59:140–148. doi: 10.1006/mvre.1999.2216. [DOI] [PubMed] [Google Scholar]
- 2.Haefliger JA, Demotz S, Braissant O, et al. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int. 2001;60:190–201. doi: 10.1046/j.1523-1755.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int. 2005;68:1171–1185. doi: 10.1111/j.1523-1755.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz L, Janssen-Bienhold U, Kurtz A, et al. Connexin expression in renin-producing cells. J Am Soc Nephrol. 2009;20:506–512. doi: 10.1681/ASN.2008030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner C, de Wit C, Kurtz L, et al. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz L, Schweda F, de Wit C, et al. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 7.Krattinger N, Capponi A, Mazzolai L, et al. Loss of connexin 40 alters renin production causing hypertension. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 8.Gomez RA, Sequeira Lopez ML. Who and where is the renal baroreceptor?: the connexin hypothesis. Kidney Int. 2009;75:460–462. doi: 10.1038/ki.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kisanuki YY, Hammer RE, Miyazaki J, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 10.Sabrane K, Kruse MN, Fabritz L, et al. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequeira López ML, Pentz ES, Nomasa T, et al. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell. 2004;6:719–728. doi: 10.1016/s1534-5807(04)00134-0. [DOI] [PubMed] [Google Scholar]
- 12.Chadjichristos CE, Scheckenbach KEL, van Veen TAB, et al. Endothelial-specific deletion of Cx40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation. 2010;121:123–131. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 13.de Wit C. Different pathways with distinct properties conduct dilations in the microcirculation in vivo. Cardiovasc Res. 2010;85:604–613. doi: 10.1093/cvr/cvp340. [DOI] [PubMed] [Google Scholar]
- 14.Wagner C, Kurtz L, Schweda F, et al. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflugers Arch. 2009;459:151–158. doi: 10.1007/s00424-009-0707-6. [DOI] [PubMed] [Google Scholar]
- 15.Toma I, Bansal E, Meer EJ, et al. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1769–R1776. doi: 10.1152/ajpregu.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfle SE, Schmidt VJ, Hoepfl B, et al. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ Res. 2007;101:1292–1299. doi: 10.1161/CIRCRESAHA.107.163279. [DOI] [PubMed] [Google Scholar]
- 17.Schweda F, Kurtz L, de Wit C, et al. Substitution of connexin40 with connexin45 prevents hyperreninemia and attenuates hypertension. Kidney Int. 2009;75:482–489. doi: 10.1038/ki.2008.637. [DOI] [PubMed] [Google Scholar]
- 18.Saez JC, Berthoud VM, Branes MC, et al. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 19.Neubauer B, Machura K, Chen M, et al. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol. 2009;296:F1006–F1012. doi: 10.1152/ajprenal.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Wit C, Roos F, Bolz SS, et al. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics. 2003;13:169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura A, Johns EJ. Renal nerves, renin, and angiotensinogen gene expression in spontaneously hypertensive rats. Hypertension. 1995;25:581–586. doi: 10.1161/01.hyp.25.4.581. [DOI] [PubMed] [Google Scholar]
- 22.Just A, Kurtz L, de Wit C, et al. Connexin 40 mediates the tubuloglomerular feedback contribution to renal blood flow autoregulation. J Am Soc Nephrol. 2009;20:1577–1585. doi: 10.1681/ASN.2008090943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


