Abstract
Background
This study was designed to determine whether elevated viral load in infants and young children is associated with congenital cytomegalovirus (CMV)-related hearing loss.
Methods
Blood samples were obtained from 135 children with congenital CMV infection. CMV DNA in the peripheral blood was quantitated with a real-time polymerase chain reaction assay. Viral load measurements were analyzed in 3 different age groups (<2 months, 2–12 months, 12–36 months).
Results
In children with symptomatic and asymptomatic infection, CMV DNA levels were not different between children with hearing deficit and those with normal hearing in all 3 age groups. In children with asymptomatic infection, the positive predictive value of a peripheral blood viral load <3500 genomic equivalents per milliliter (ge/mL) at <2 months and 2 to 12 months of age is 8%, and at 12 to 36 months of age is 11.8%. However, the negative predictive value of a viral load <3500 ge/mL is 94.4% at <2 months of age, and 100% at 2 to 36 months of age.
Conclusions
Peripheral blood viral load is not associated with hearing loss in children with congenital CMV infection. However, a viral load of <3500 ge/mL is associated with a lower risk of hearing loss in children born with asymptomatic congenital infection.
Keywords: CMV, viral load, hearing loss
Cytomegalovirus (CMV) is a common cause of congenital infection and a leading cause of sensorineural hearing loss (SNHL) in children worldwide.1,2 Of the 20,000 to 40,000 infants born each year in the United States with congenital CMV infection, about 5% to 10% of children with asymptomatic infection and 40% to 50% of those with symptomatic congenital infection will develop hearing loss.3,4 Although some children with CMV-related SNHL are born with a hearing deficit, the majority will experience delayed-onset loss and continued deterioration of hearing function (progressive hearing loss) during childhood.3,5–8
The pathogenesis of SNHL in children with congenital CMV infection is poorly understood. Limited human temporal bone studies as well as studies in the guinea pig model have demonstrated that viral infection in the inner ear structures is important to the development of SNHL.9–11 Recent studies from our laboratory and others have suggested that higher systemic virus burden in early infancy was associated with CMV-related hearing loss.12–14 To determine whether elevated viral load beyond early infancy is associated with CMV-related SNHL, we examined the value of the peripheral blood (PB) CMV viral load in the prediction of SNHL in a cohort of infants and young children with congenital CMV infection.
MATERIALS AND METHODS
Study Population and Specimens
One hundred ninety-six children with congenital CMV infection born between January 1994 and February 2005 at 3 hospitals in Birmingham, AL, were monitored for hearing loss as part of a natural history study. Congenital CMV infection was identified by the presence of the virus in saliva specimens obtained during the first week of life.15,16 Of the 196 children found to have congenital CMV infection, 61 had insufficient blood samples. The remaining 135 children with available blood samples for testing constituted the study population. The demographic characteristics and hearing outcomes were not different between the study children and children enrolled in the follow-up study with unavailable PB samples (data not shown). Two hundred two PB samples from the 135 study children were available for quantification of CMV DNA and analysis. The results of the PB viral load in samples obtained during the first month of life from 75 of these children were included in a previous report.12 Eighty-five children (73 with normal hearing and 12 with SNHL) had only one blood sample, whereas 50 children (46 with normal hearing and 4 with SNHL) had more than one blood sample available for analysis. For children with hearing loss, only PB samples obtained before or at the time of detection of SNHL, and in children with progressive hearing loss, only samples obtained before documented progression were included in the analysis. The PB samples were processed immediately after collection to obtain DNA preparations from 200 μL of whole blood with commercial spin columns (Qiagen, Valencia, CA) and stored at −20°C.
Infants were classified as having symptomatic infection when they shed CMV during the first week of life and had any of the clinical findings suggestive of congenital infection at birth, including jaundice, petechiae, hepatosplenomegaly, purpura, microcephaly, seizures, lethargy/hypotonia, and/or poor suck.17 One study subject received ganciclovir. This child had progression of hearing loss detected at 6 months of age, received 6 weeks of ganciclovir at the discretion of the clinician, and only PB samples obtained before ganciclovir treatment were included in the analysis.
The study was approved by the University of Alabama at Birmingham Institutional Review Board for Human Use, and informed consent was obtained from the parents or guardians of the children enrolled in the study.
Follow-Up of Children
Study participants were monitored according to a standard protocol and received age-appropriate audiologic evaluations.3 A child was considered to have SNHL when air conduction thresholds at one or more frequencies were greater than 20 dB in one or both ears in conjunction with normal tympanograms, normal otoscopic findings, and/or normal bone conduction thresholds. Progressive hearing loss was defined as sensorineural decrease in hearing of ≥10 dB at any one frequency or auditory brainstem response threshold documented on 2 separate evaluations. Delayed-onset hearing loss was defined as one or more hearing evaluations with a normal threshold documented for each ear before the onset of SNHL.3,6
Real-Time Polymerase Chain Reaction
The investigators who performed the real-time polymerase chain reaction (PCR) were blinded to the results of the audiologic follow-up. CMV viral load was assessed by real-time PCR technique with an ABI 7500 Sequence Detection System (Applied Biosystems, Foster City, CA) utilizing Absolute Low ROX QPCR mix (ABgene, Rockford, IL). CMV primers were selected from the highly conserved AD-1 region of the major envelope glycoprotein B.18–20 Amplification was performed under the following conditions: 1 cycle at 95°C for 15 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. All samples were run with plasmid standards that were constructed from the target sequence. Plasmid standards and samples were run in duplicate and the average values were used to determine the CMV viral load. CMV virus burden in whole blood was expressed as CMV genomic equivalents per milliliter of blood (ge/mL).12 The sensitivity of the assay has been determined to be approximately 200 ge per 1 mL of blood.
Data Analysis
The demographic characteristics, newborn findings, outcome data, and the results of PB real-time PCR were collected on case report forms and entered into SAS V9.1 data sets (SAS Institute, Cary, NC). Viral load measurements were analyzed in 3 different age groups by nonparametric methods: <2 months, 2 to 12 months, and 12 to 36 months, and statistical significance was determined with the Wilcoxon rank sum test. Positive predictive values (PPV) and negative predictive values (NPV) and exact 95% confidence intervals were assessed where appropriate.
RESULTS
The demographic and outcome characteristics of the study children according to their hearing status are shown in Table 1. Twelve percent (16/135) of the children in the study had SNHL. The majority of the study children are African-American and born to single mothers who received their prenatal care at public health clinics. Significantly more children with CMV–related hearing loss were born to mothers younger than 20 years of age (88%) compared with only 47% of the children with normal hearing. More children in the hearing loss group (56%) had symptomatic congenital CMV infection than those with normal hearing (13%, P < 0.001). The mean duration of follow-up for the children with CMV-related hearing loss was 45.0 ± 20.8 months and they underwent a median number of 9 hearing evaluations (range, 1–17), whereas those with normal hearing were monitored for 33.3 ± 22.3 months and had 6 hearing tests (range, 1–12) (Table 1). Progressive hearing loss was observed in 6 of 16 (38%) of the children with hearing deficit and delayed-onset hearing loss was seen in approximately half (7/16) of the children. The median age of onset of hearing loss was 1 month (range, 0–76 months), with SNHL in 2 study children detected at 40 and 76 months of age. Bilateral SNHL was detected in 5 of 16 (31%) of study participants (Table 2). Seventy-five of 76 children from a previous study12 were included in this study and the demographic characteristics were similar between the 75 children from the previous report and the additional 60 children.
TABLE 1.
Demographic Characteristics, Clinical Findings and Follow-up Parameters for the Study Children With Congenital Cytomegalovirus Infection According to their Hearing Status
| Hearing Loss (n = 16) | Normal Hearing (n = 119) | |
|---|---|---|
| Race | ||
| African American | 14 (88%) | 104 (87%) |
| White | 2 (12%) | 15 (13%) |
| Male gender | 9 (56%) | 62 (52%) |
| Maternal marital status | ||
| Single | 15 (94%) | 103 (87%) |
| Married | 1 (6%) | 16 (13%) |
| Maternal prenatal care | ||
| Public health clinics | 15 (94%) | 103 (87%)* |
| Private provider | 1 (6%) | 7 (6%) |
| None | 0 (0%) | 3 (3%) |
| Maternal age | ||
| <20 yr | 14 (88%)† | 56 (47%) |
| ≥20 yr | 2 (12%) | 63 (53%) |
| Symptomatic at birth | 9 (56%) | 15 (13%)‡ |
| Mean duration of follow-up (months ± SD) | 45.0 ± 20.8 | 33.3 ± 22.3 |
| Median number of hearing Evaluations (range) | 9 (1–17)§ | 6 (1–12) |
Data available for 113 subjects.
P = 0.02.
P < 0.001.
P = 0.011.
TABLE 2.
Hearing loss Characteristics of the 16 Children With Cytomegalovirus-Related Hearing Loss
| Delayed onset SNHL* | 7/16 (44%) |
| Age at SNHL detection | |
| <2 mo | 7/16 (44%) |
| 2–12 mo | 5/16 (31%) |
| >12 mo† | 4/16 (25%) |
| Progressive SNHL | 6/16 (38%) |
| Bilateral SNHL | 5/16 (31%) |
Delayed onset SNHL defined as one or more hearing evaluations with a normal threshold documented for each ear before the onset of SNHL.
Two children with SNHL detected after 36 month.
The median PB CMV DNA concentration was higher among children with symptomatic infection (2.93 × 104, 0–5.90 × 106 ge/mL) compared with children who had asymptomatic infection (4.17 × 103, 0–3.40 × 106 ge/mL) in the first 2 months of life, and this difference was statistically significant (P = 0.006). However, there was no difference in PB virus burden between children with asymptomatic and symptomatic congenital infection in samples obtained after the first 2 months of life.
To determine the pattern of change in viral load with time, we analyzed the data from 50 children from whom more than one blood sample was available. The viral load values fluctuated by at least one log in 20 children, increased in 5, decreased in 18, and 7 children had no change in viral load values.
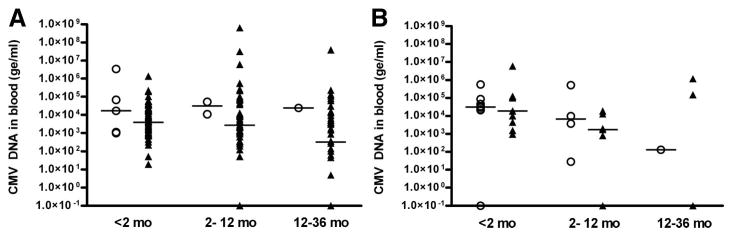
Viral load data were analyzed independently among children with symptomatic infection and those with asymptomatic infection; in each age group, the levels were compared between children with hearing loss and those with normal hearing (Fig. 1). Seven children had undetectable viral concentrations (<200 ge/mL) <2 months of age. Six of these children were born with asymptomatic infection and had normal hearing, whereas one child had symptomatic infection with hearing loss which was detected within the first month of life. When more than 1 PB sample was available from a child in a given age group, only the viral load level in the earliest sample was included in the analysis. Among children less than 2 months of age with asymptomatic infection, median virus burden was not significantly different between the 5 children with hearing loss (1.70 × 104, range: 1.0 × 103–3.40 × 106 ge/mL) and the group of 69 children with normal hearing (3.98 × 103, range: 0–1.36 × 106 ge/mL, P = 0.301). In children less than 2 months of age with symptomatic infection, CMV DNA levels were not different between the group with hearing deficit and those with normal hearing (3.2 × 104, range: 0–5.6 × 105 ge/mL and 1.9 × 104, range: 9.5 × 102–5.9 × 106 ge/mL, respectively, P = 0.847). PB viral load measurements were not significantly different in older infants and children with hearing loss and normal hearing in both symptomatic and asymptomatic infection. In the 2- to 12-month age group, median virus burden in asymptomatic infection was similar for the children with SNHL and those with normal hearing (3.17 × 104, range: 1.08 × 104–5.27 × 104 ge/mL vs. 2.71 × 103, range: 0–6.40 × 108 ge/mL; P = 0.140). Similarly, in symptomatic children between 2–12 months of age, and in both symptomatic and asymptomatic children 12–36 months of age, the amount of viral load was not different between children with hearing loss and those with normal hearing (Figure).
FIGURE 1.
Results of tests measuring levels of CMV DNA in PB at 3 different age ranges from children enrolled in the study with congenital CMV infection with asymptomatic (A) and symptomatic (B) infection at birth that had hearing loss (○) and normal hearing (▲). The results are expressed as genomic equivalents per mL of blood (ge/mL). The horizontal bars represent median values. In children with asymptomatic and symptomatic infection, median CMV DNA levels were not different between children with SNHL and those with normal hearing in all 3 age groups, analyzed by the Wilcoxon rank sum test. Note: the median VL of symptomatic children with normal hearing in the 12 to 36 month age group is 0 ge/mL.
To determine the usefulness of PB viral load measurements in predicting SNHL in children with congenital CMV infection, we calculated the PPV and NPV of PB viral load ≤3500 and >3500 ge/mL in children with asymptomatic and those with symptomatic infection (Tables, Supplemental Digital Content 1, http://links.lww.com/A1114 and Supplemental Digital Content 2, http://links.lww.com/A1115). Among children with asymptomatic infection, only 2 of 36 children with a PB viral load measurement lower than 3500 ge/mL at <2 months of age had hearing deficit, resulting in a NPV of 94.4% (Table, Supplemental Digital Content 1, http://links.lww.com/A1114). However, the PPV for a viral load measurement >3500 ge/mL at <2 months of age was only 7.9%. None of the 26 children at 2 to 12 months of age and 0 of 25 children 12 to 36 months of age with PB virus burden ≤3500 ge/mL had SNHL resulting in a NPV of 100% (Table, Supplemental Digital Content 2, http://links.lww.com/A1115). However, PPVs for SNHL were poor in both of these age groups for a PB virus burden >3500 ge/mL.
In children with symptomatic infection, the PPV and NPV were poor in all age groups (Table, Supplemental Digital Content 2, http://links.lww.com/A1115). At <2 months of age, the PPV of a PB virus load >3500 ge/mL for SNHL is 50% and the NPV of a viral load ≤3500 ge/mL is 66.7%. Five of 6 children with a PB virus burden of ≤3500 ge/mL at 2 to 12 months of age had normal hearing resulting in a NPV of 83.3%. The PPV in this age range for a viral load >3500 ge/mL was 60%. In children aged 12 to 36 months with symptomatic infection, neither of the 2 children with PB viral load >3500 ge/mL had SNHL resulting in a PPV of 0%. The NPV in symptomatic children 12 to 36 months of age with normal hearing with a PB virus load of ≤3500 ge/mL was 75.0.0% (Table, Supplemental Digital Content 2, http://links.lww.com/A1115).
DISCUSSION
Previous studies examining the relationship between virus burden and the risk for hearing loss in children with congenital CMV infection demonstrated that higher viral load during early infancy was associated with an increased risk of SNHL.12–14 In the majority of children with CMV-related hearing loss, the impairment occurs beyond early infancy and in more than half of those, the hearing deficit continues to progress. In the present study, we explored whether PB viral load during infancy and early childhood can be used to predict hearing loss. As the frequency and natural history of SNHL in children with asymptomatic congenital CMV infection is different from that in symptomatic children,3 data in the 2 groups of children were analyzed independently. This analysis showed no association between viral load and hearing loss in all 3 age groups examined. Furthermore, our results showed that PB viral burden has a poor positive predictive value for CMV-related hearing loss. On the other hand, asymptomatic children with a PB viral load level of ≤3500 ge/mL appeared to be at lower risk for SNHL as only 2 of 36 children in the <2 months age group, 0 of 26 children in the 2 to 12 months age group, and 0 of 25 children in the ≥12 months age group with PB viral load ≤3500 ge/mL had SNHL. Together, these findings indicate that in individual children with congenital CMV infection, an elevated viral load measurement may not be useful in identifying a child at risk for CMV-related hearing loss. However, this data suggests that a low viral load in children with asymptomatic infection is associated with a lower risk for hearing deficit.
We could not confirm the association between systemic virus burden during early infancy and SNHL in children with asymptomatic congenital CMV infection that was observed in our previous study.12 The real-time PCR assay protocol used to determine the viral load in both studies was identical. Although most of the study infants in the <2 months age group were included in the previous report, the addition of fifteen asymptomatic children with normal hearing and one child with SNHL has discounted our previous findings. The association between viral load in early infancy and hearing loss was not observed in this study. There was considerable overlap in the amount of viral load between the groups with and without hearing loss and this overlap could explain the lack of an association between PB viral load and hearing loss in early infancy. Similar findings were reported in an earlier study that examined the relationship between CMV viremia and hearing loss in 50 infants with symptomatic congenital CMV infection with CNS involvement participating in phase II and phase III ganciclovir treatment trials.21 The findings of that study showed that although baseline viremia correlated with SNHL, an increase in viral load was not predictive of hearing loss.
The data from the current study are not consistent with studies by other investigators that have reported an association between higher PB viral load and SNHL. Lanari et al13 examined a cohort of 37 infants with congenital CMV with clinical follow-up of more than 12 months. They reported that mean blood viral loads were higher in newborns that developed sequelae than those who did not. However, their study only included 1 child with hearing loss resulting from asymptomatic infection. A group of investigators from London examined the association between dried blood spot viral load and hearing loss in a group of 34 children with confirmed congenital CMV infection that did not receive ganciclovir therapy. They reported that CMV DNA viral load in the newborn period was significantly correlated with SNHL. However, the study population contained mainly children born with symptomatic infection at birth (22/34), and only 9 of 34 (26%) of the study cohort had normal hearing.14 Our current study includes the largest cohort of children with asymptomatic congenital CMV infection that has been examined for the association between virus burden and SNHL. In addition, the number of hearing impaired children (16/135, 12%) in our study is similar to the reported rates of CMV-related SNHL and thus, is more reflective of the overall group of children with congenital CMV infection. This argues against a selection bias which may have influenced the results of the other reports in the literature.
Although the natural history of CMV-related hearing loss has been well documented in large cohort studies, the pathogenesis of CMV-related hearing loss is poorly understood. The data from a limited number of human temporal bone studies and the guinea pig model of congenital CMV infection suggest that CMV can infect both the epithelium and neural tissue of the inner ear and that damage can occur as a result of direct viral mediated injury to the neural tissue or secondary to host derived inflammatory responses.9–11,22,23 Systemic virus burden has been shown to correlate with CMV disease in immunocompromised hosts including allograft recipients and HIV-infected individuals.24–28 These findings, together with recent reports suggesting an association between high systemic virus burden and SNHL has lead to the hope that PB viral load measurements could be useful to predict CMV-related hearing loss as well as a surrogate marker to assess the effectiveness of antiviral therapy in preventing or reducing the incidence and severity of CMV-related SNHL.12–14 However, the lack of an association between virus burden and hearing loss in children with congenital CMV infection in the present study suggests that it is premature to use systemic virus burden as a reliable surrogate marker of the amount of virus replication in the inner ear. Therefore, the results of our study argue against the use of PB viral load to predict SNHL in individual children with congenital CMV infection and to monitor the effectiveness of antiviral therapy.
The current study does have limitations. The study children were followed for varying durations and PB samples were not available at all of the follow-up visits. Among the 50 children with more than one blood sample, there was no clear pattern to the change in viral load in individual children and the virus burden levels varied greatly over time. The absence of a predictable pattern in longitudinal virus burden measurements suggests that the lack of an association between viral load and hearing loss in our study is not likely due to the arbitrary categorization of the study children into 3 age groups. However, future studies with a more consistent patient follow-up are needed to carefully assess the dynamics of PB DNAemia in children with congenital infection. An additional limitation of the present study is that the hearing loss group was followed longer (47.6 ± 22.8 months) than those with normal hearing (33.3 ± 22.7 months). To reduce the bias from the differential follow-up, only samples obtained in the first 36 months of follow-up were included in the analysis. Because of the small numbers of children with delayed-onset and/or progressive SNHL, we were unable to determine if PB CMV DNA levels are associated with hearing loss. Studies with larger numbers of children with these types of SNHL are needed. Finally, the quantitative PCR assays to measure CMV viral loads have not been standardized across the various laboratories and the assay used in the present study was developed in our laboratory. Therefore, the results shown in this study may not be directly extrapolated to other populations in which different PCR techniques with different primers, probes, and detection systems are used.
Supplementary Material
Acknowledgments
Supported in part by the National Institutes of Health, the National Institute of Child Health and Human Development grant (P01 HD 10699), the National Institute of Allergy and Infectious Diseases (P01 AI43681, T32 AI052069), The National Institute on Deafness and Other Communication Disorders (R01 DC02139) and the General Clinical Research Center (M01 R00032).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pidj.com).
References
- 1.Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control: summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1990;13:315–329. doi: 10.1093/clinids/13.2.315. [DOI] [PubMed] [Google Scholar]
- 2.Stagno S. Cytomegalovirus. In: Remington JS, Klein JO, Wilson CB, et al., editors. Infectious Diseases of the Fetus and Newborn Infant. 6. Philadelphia, PA: W.B. Saunders Company; 2006. pp. 389–424. [Google Scholar]
- 3.Dahle AJ, Fowler KB, Wright JD, et al. Longitudinal investigations of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11:283–290. [PubMed] [Google Scholar]
- 4.Harris S, Ahlfors K, Ivarsson SA, et al. Congenital cytomegalovirus infection and sensorineural hearing loss. Ear Hear. 1984;5:352–355. doi: 10.1097/00003446-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Fowler KB, Dahle AJ, Boppana SB, et al. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135:60–64. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 6.Fowler KB, McCollister FP, Dahle AJ, et al. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–630. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 7.Williamson WD, Demmler GJ, Percy AK, et al. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90:862–866. [PubMed] [Google Scholar]
- 8.Morton CC, Nance WE. Newborn hearing screening–a silent revolution. N Engl J Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 9.Rarey KE, Davis LE. Temporal bone histopathology 14 years after cytomegalic inclusion disease: a case study. Laryngoscope. 1993;103:904–909. doi: 10.1288/00005537-199308000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Strauss M. Human cytomegalovirus labyrinthitis. Am J Otolaryngol. 1990;11:292–298. doi: 10.1016/0196-0709(90)90057-3. [DOI] [PubMed] [Google Scholar]
- 11.Woolf NK. Guinea pig model of congenital CMV-induced hearing loss: a review. Transplant Proc. 1991;23:32–34. discussion 34. [PubMed] [Google Scholar]
- 12.Boppana SB, Fowler KB, Pass RF, et al. Congenital Cytomegalovirus Infection: association between Virus Burden in Infancy and Hearing Loss. J Pediatr. 2005;146:817–823. doi: 10.1016/j.jpeds.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 13.Lanari M, Lazzarotto T, Venturi V, et al. Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. Pediatrics. 2006;117:e76–e83. doi: 10.1542/peds.2005-0629. [DOI] [PubMed] [Google Scholar]
- 14.Walter S, Atkinson C, Sharland M, et al. Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Arch Dis Child Fetal Neonatal Ed. 2008;93:F280–F285. doi: 10.1136/adc.2007.119230. [DOI] [PubMed] [Google Scholar]
- 15.Balcarek KB, Warren W, Smith RJ, et al. Neonatal screening for congenital cytomegalovirus infection by detection of virus in saliva. J Infect Dis. 1993;30:1433–1436. doi: 10.1093/infdis/167.6.1433. [DOI] [PubMed] [Google Scholar]
- 16.Warren WP, Balcarek KB, Smith R, et al. Comparison of Rapid Methods of Detection of Cytomegalovirus in Saliva with Virus Isolation in Tissue Culture. J Clin Microbiol. 1992;30:786–789. doi: 10.1128/jcm.30.4.786-789.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93–99. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Britt WJ, Mach M. Human cytomegalovirus glycoproteins. Intervirology. 1996;39:401–412. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- 19.Chou S. Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology. 1992;188:388–390. doi: 10.1016/0042-6822(92)90771-g. [DOI] [PubMed] [Google Scholar]
- 20.Fox JC, Griffiths PD, Emery VC. Quantification of human cytomegalovirus DNA using the polymerase chain reaction. J Gen Virol. 1992;73(pt 9):2405–2408. doi: 10.1099/0022-1317-73-9-2405. [DOI] [PubMed] [Google Scholar]
- 21.Bradford RD, Cloud G, Lakeman AD, et al. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J Infect Dis. 2005;191:227–233. doi: 10.1086/426456. [DOI] [PubMed] [Google Scholar]
- 22.Harris JP, Fan JT, Keithley EM. Immunologic responses in experimental cytomegalovirus labyrinthitis. Am J Otolaryngol. 1990;11:304–308. doi: 10.1016/0196-0709(90)90059-5. [DOI] [PubMed] [Google Scholar]
- 23.Woolf NK, Koehrn FJ, Harris JP, et al. Congenital cytomegalovirus labyrinthitis and sensorineural hearing loss in guinea pigs. J Infect Dis. 1989;160:929–937. doi: 10.1093/infdis/160.6.929. [DOI] [PubMed] [Google Scholar]
- 24.Deayton JR, Sabin CA, Prof, Johnson MA, et al. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. 2004;363:2116–2121. doi: 10.1016/S0140-6736(04)16500-8. [DOI] [PubMed] [Google Scholar]
- 25.Emery VC, Sabin CA, Cope AV, et al. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 26.Erice A, Tierney C, Hirsch M, et al. Cytomegalovirus (CMV) and human immunodeficiency virus (HIV) burden, CMV end-organ disease, and survival in subjects with advanced HIV infection (AIDS Clinical Trials Group Protocol 360) Clin Infect Dis. 2003;37:567–578. doi: 10.1086/375843. [DOI] [PubMed] [Google Scholar]
- 27.Gor D, Sabin C, Prentice HG, et al. Longitudinal fluctuations in cytomegalovirus load in bone marrow transplant patients: relationship between peak virus load, donor/recipient serostatus, acute GVHD and CMV disease. Bone Marrow Transplant. 1998;21:597–605. doi: 10.1038/sj.bmt.1701139. [DOI] [PubMed] [Google Scholar]
- 28.Spector SA, Hsia K, Crager M, et al. Cytomegalovirus (CMV) DNA load is an independent predictor of CMV disease and survival in advanced AIDS. J Virol. 1999;73:7027–7030. doi: 10.1128/jvi.73.8.7027-7030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.