Abstract
BACKGROUND
We investigated time dependence and spatial progression of cardiac function and angiogenesis signaling in a porcine model of chronic myocardial ischemia.
STUDY DESIGN
Yorkshire mini-swine (n = 7/group) were subjected to chronic myocardial ischemia by placing an ameroid constrictor on the left circumflex coronary artery under general anesthesia. Swine were sacrificed after either 4 or 7 weeks of ischemia. Myocardial function, angiographic evidence of angiogenesis, microvessel function, molecular signaling, and levels of apoptosis and oxidative stress were assessed.
RESULTS
Flow reserve was significantly increased at 7 versus 4 weeks. Myocardial function (+dP/dt) improved 1.5-fold by 7 weeks. In the ischemic territory, microvessels at 4 weeks displayed abnormal contraction responses to serotonin, which diminished at 7 weeks. Delta-like ligand 4 protein expression decreased at 7 weeks; expression of vascular endothelial growth factor (VEGF) and phospho-endothelial nitric acid synthase (eNOS) increased. The number of apoptotic cells was decreased at 7 weeks, and antiapoptotic markers heat shock protein (HSP) 27 and HSP 90 were upregulated at 7 weeks. There was an increase in proliferating endothelial cells at 7 weeks as compared with 4 weeks. In the adjacent normal ventricle, microvessels demonstrated smaller contraction responses to endothelin-1 and serotonin at 7 weeks. There was an increase in protein peroxidation in the ischemic territory at 7 weeks.
CONCLUSIONS
Over time, myocardial perfusion, function, and angiogenic signaling improved in the ischemic myocardium and adjacent normal territory compared with what is observed shortly after coronary occlusion.
Cardiovascular disease was responsible for about 35% of all deaths in the United States in 2005.1 Chronic myocardial ischemia, usually atherosclerotic in nature, results from an imbalance between myocardial oxygen supply and demand. Over time, a patient may develop collateral vessels to increase the supply of oxygen to ischemic myocardium in a process known as angiogenesis. However, this process is often deficient in patients with comorbid illnesses such as diabetes mellitus, hypertension, and hyperlipidemia.2
Therapeutic angiogensis is a potential treatment option for patients with coronary artery disease not amenable to percutaneous coronary interventions or coronary artery bypass grafting.3 However, therapeutic angiogenesis has not been successfully transitioned to the clinical phase and there remain many aspects of native and therapeutic collateral formation that need to be defined.4 By further characterizing the process of angiogenesis, we may be able to optimize the timing and targets of collateral stimulating therapies.
In this article, we investigated how ischemic myocardium and normal myocardium adjacent to the injury respond to chronic ischemia. There are classic physiologic studies on the effects and responses of the myocardium to chronic ischemia,5,6 but there are no large animal studies that examine the physiologic, angiographic, microvascular, and molecular changes over time in the normal and ischemic territories during chronic myocardial ischemia. We hypothesized that there would be functional (at the microvascular and left ventricular levels), blood flow, and angiogenic signaling changes over time, as well as between non-ischemic and ischemic myocardial territories.
METHODS
Study design
Adult Yorkshire mini-swine (Parsons Research) were divided into 2 groups that underwent myocardial ischemia for either 4 (n = 9) or 7 (n = 8) weeks. All animals received a normal diet for the entire study. Animals underwent placement of an ameroid constrictor (Research Instruments SW) on the proximal left circumflex coronary artery (LCx). For all surgical procedures, anesthesia was induced with ketamine (10 mg/kg intramuscular) and thiopental 2.5%, and maintained with a gas mixture of oxygen at 2 L/min and 2.0% isoflurane. The animals were intubated and mechanically ventilated at 16 breaths/min via a volume-cycled ventilator (Drager).
During the first procedure (ameroid constrictor placement), gold-labeled microspheres (Biophysics Assay Laboratory) were injected into the left atrium through a left minithoractomy during temporary occlusion of the LCx to determine the exact myocardial territory at risk. Next, the titanium ameroid constrictor (1.75 mm internal diameter) was placed around the proximal LCx. This gradually occludes the artery, causing myocardial ischemia without infarction.7
Four or 7 weeks after ameroid placement, swine were anesthetized and x-ray coronary angiography was completed. The heart was then exposed, and microspheres were injected both at rest and during ventricular pacing (150 beats/min) and followed by euthanasia. The heart was harvested and 2 1-cm thick transverse slices were cut at the midventricular level and sectioned into 8 segments. Samples were divided and flash frozen in liquid nitrogen (molecular studies), placed in 4°C Krebs solution (microvessel reactivity studies), 10% formalin (immunofluorescence studies), or weighed and dried for microsphere perfusion analyses.
All experiments were approved by the Beth Israel Deaconess Medical Center Animal Care and Use Committee. Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 5377-3 1996).
Measurement of global and regional myocardial function
The following indices of global and regional myocardial function were monitored and recorded during the final surgical procedure: mean arterial pressure, developed left ventricular pressure, positive (+dP/dt) and negative (−dP/dt) first derivatives of left ventricular pressure, and segmental shortening in the axes parallel to and perpendicular to the LCx in the area at risk (AAR) for 10 sequential beats using a Sonometrics system, as previously described (Sonometrics Corp).8
X-ray coronary angiography
X-ray coronary angiography was carried out in order to ensure occlusion of the LCx and to assess collateral formation. Recorded images were interpreted by an interventional cardiologist blinded to the treatment groups. Angio-graphic collateral formation was measured according to the Rentrop grading system, which assesses the presence and extension of collateral filling of the coronary epicardial vessels.9
Myocardial perfusion analysis
Myocardial perfusion was determined during each procedure with 15-μm diameter isotope-labeled microspheres (ILMs) (BioPAL) using previously reported methods.10 Briefly, 1.5 × 107 gold-labeled microspheres were injected during temporary LCx occlusion at the time of ameroid placement to identify the area at risk. Lutetium- (rest) and Europium- (pace) labeled ILMs were injected at the final procedure. After euthanasia, 10 transmural left ventricular sections were collected and weighed for ILM assays. The myocardial segments with the lowest counts of gold microspheres were considered the areas at risk. The samples were exposed to neutron beams and microsphere densities per gram of tissue were measured using a gamma counter.
In vitro microvascular studies
After cardiac harvest, coronary arterioles (80 to 180 μm in diameter) from the ischemic territory were dissected and placed in an isolated microvessel chamber, as described previously.11 The microvascular responses to vasoconstricting agents endothelin-1 (ET-1, 10−12 to 10−7mol/L) and serotonin (5-HT, 10−9 to 10−5mol/L) were assessed. All drugs were applied extraluminally. Responses were defined as the percent constriction of the baseline diameter for ET-1 and 5-HT. All reagents were obtained from Sigma-Aldrich.
Molecular studies ?>
Western blotting of myocardial tissue from the ischemic left ventricle (area at risk, AAR) and the adjacent normal ventricle (NV) was performed. Sixty micrograms of total protein was fractionated by 4% to 20% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (Invitrogen) and transferred to polyvinylidene fluoride membranes (Millipore). Each membrane was incubated with the following specific antibodies: anti-vascular endothelial growth factor (VEGF) (Calbiochem), anti-fibroblast growth factor (FGF)-2 (US Biological), anti-NFkB, anti-phospho-endothelial nitric oxide synthase (eNOS) (ser1177), anti-phospho-Akt (ser473), anti-phospho-extracellular signal regulated kinase (ERK) (thr202/tyr 204), anti-heat shock protein (HSP) 27, anti-HSP 90, anti-Bcl-2, anti-Bad, anti-Bax (Cell Signaling), and anti-eNOS antibody (BD Biosciences). Immune complexes were visualized with an enhanced chemiluminescence detection system (Amersham). Bands were quantified by densitometry of autoradiograph films. Ponceau staining was used to ensure equal protein loading.
Myocardial protein oxidative stress
Oxidized proteins were detected using a commercial kit (Oxy-blot, Chemicon International). Tissue homogenates were first incubated with 2,4-dinitrophenylhydrazine, and the carbonyl groups in the protein side chains were derivatized to 2,4-dinitrophenylhydrazine. The dinitrophenylhydrazine-derivatized protein samples were separated by 10% polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were incubated with primary antibody specific to the dinitrophenylhydrazine moiety of the proteins, followed by incubation with a horseradish peroxidase-linked secondary antibody. Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham). Bands were quantified by densitometry of radioautograph films.
Myocardial lipid oxidative stress
Measurement of lipid peroxidation was carried out using a commercial kit to assess levels of free malondialdehyde (MDA), a reactive carbonyl compound produced during decomposition of lipid peroxides (Oxis International), according to the manufacturer’s specifications. Tissue was homogenized in the presence of 5 mM butylated hydroxytoluene to prevent oxidation during homogenization. Homogenates were incubated with N-methyl-2-phenylindole in acetonitrile, probucol, and concentrated hydrochloric acid. MDA forms a conjugate with N-methyl-2-phenylindole, resulting in production of a blue chromogenic signal. Samples were centrifuged and the MDA-containing supernatant was removed. Absorbance was measured at 586 nm and values were analyzed against a standard curve. Concentration of MDA was corrected for protein concentration between samples.
Quantification of apoptosis
Apoptotic cells in the myocardium were identified using the ApopTag detection kit according to manufacturer’s specifications (Chemicon Inc). At least 1 cm2 of tissue from the AAR was analyzed from each animal (4 per group). The number of terminal deoxynucleotidyl transferase mediated dUTP nick-end-labeling (TUNEL)-positive cardiomyocytes is expressed as positive cells/cm2.
Immunofluorescence double staining for dividing endothelial cells
Frozen sections (12 μm in thickness) of myocardium from the normal and ischemic territories were formalin fixed for 10 minutes and processed as previously described.12 Antibodies against Ki-67 (Abcam Inc) and platelet endothelial cell adhesion molecule (PECAM)-1 (CD-31, R&D Systems) were simultaneously applied to the sections and incubated overnight at 4°C. Detection was obtained using appropriate secondary antibodies (Jackson ImmunoReaserch). Sections were then mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories). Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc) equipped with a digital camera and 200× magnification (Photodoc). Dividing endothelial cells were counted in a blinded fashion. Structures that were DAPI positive, Ki-67 positive, and within an endothelial cell (CD-31 positive) were considered dividing endothelial cells. Data are presented as number of Ki-67 positive endothelial cells/mm2.
Data analysis
All results are expressed as mean ± standard error of the mean (SEM). Microvessel responses are expressed as percent contraction of the baseline diameter, and were analyzed using 2-way, repeated measures analysis of variance (ANOVA) with a Bonferroni post-hoc test comparing treatment and dose. Western blots were analyzed after digitalization (ScanJet 4c; Hewlett-Packard) using ImageJ 1.41 software (National Institutes of Health). For data analysis, levels of phosphorylated proteins were normalized to total expression levels. Comparisons between 2 groups were analyzed by 2-tailed t-test, and comparisons between 4 groups were analyzed by 1-way ANOVA with a Newman-Keuls multiple comparisons post-hoc test using GraphPad Prism 4 (GraphPad Software Inc). Probability values less than 0.05 were considered significant.
RESULTS
Experimental model
Two animals in the 4-week group and 1 animal in the 7-week group died prematurely. Necropsy did not identify a clear cause of death, but it was most likely related to ventricular arrhythmia. A total of 7 animals were analyzed in each group. The body weights of the animals were not significantly different between the groups at the end of the study (4 weeks, 26.5 ± 1.5 kg; 7 weeks, 31 ± 2.2 kg, p = 0.09).
Coronary angiography
Animals achieved significant myocardial ischemia as the ameroid caused complete occlusion in all cases (Fig. 1A). There was no difference between the 4- and 7-week groups in terms of Rentrop scores. The mean Rentrop score was slightly higher in the 7-week group (4-week, 1.0 ± 0.3; 7-week, 1.3 ± 0.3, p = 0.51).
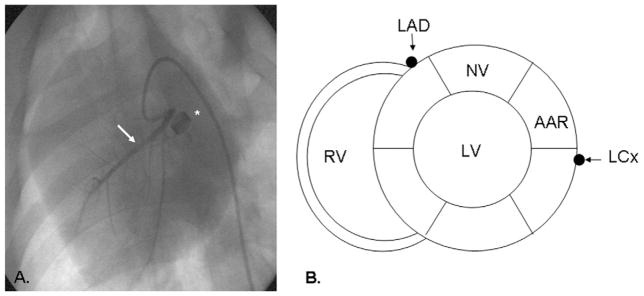
Figure 1.
Coronary angiography and relationship between nonischemic and ischemic myocardium. (A) Coronary angiogram demonstrating occlusion of the left circumflex coronary artery (LCx). Arrow denotes the left anterior descending (LAD) artery, asterisk denotes the ameroid constrictor. (B) Short axis schematic demonstrating the relationship between the nonischemic (NV, normal ventricle) and ischemic (area at risk, AAR) left ventricular (LV) territories. RV, right ventricle.
Left ventricular functional studies
There was no significant difference in the mean arterial pressures between the 2 groups (4 weeks, 48.1 ± 2.9 mmHg; 7 weeks, 55.7 ± 3.2 mmHg, p = 0.12). However, the 7-week group demonstrated significantly increased peak +dP/dt values (4 weeks, 685.7 ± 49 mmHg/sec; 7 weeks, 1015 ± 111 mmHg/sec, p = 0.02). There was no difference in the –dP/dt between the groups (4 weeks, ’989.5 ± 154 mmHg/sec; and 7 weeks, −928 ± 129 mmHg/sec, p = 0.79). There was no difference between the groups in terms of perpendicular segmental shortening in the ischemic territory (4 weeks, 9.6 ± 1.2 mm vs 7 weeks, 6.7 ± 1.0 mm, p = 0.15) (Figure 1B demonstrates relevant anatomy). There was also no difference in parallel segmental shortening (4 weeks, 11.9 ± 2.9 mm vs 7 weeks, 10.4 ± 2.5 mm, p = 0.75).
Myocardial perfusion to the ischemic territory
On gross examination there was no evidence of infarction in any of the animals. The weight of tissues examined for ILM density were not different between groups (4 weeks, 0.77 ± 0.04 g; 7 weeks, 0.83 g, p = 0.24). Transmural blood flow to the ischemic territory at rest was not different between the groups (Fig. 2A). In contrast, the flow reserve, measured as the ability to increase blood flow to the ischemic territory during paced conditions, was significantly increased in the 7-week group. There was a significant increase in blood flow during stress at 4 and 7 weeks (Fig. 2B).
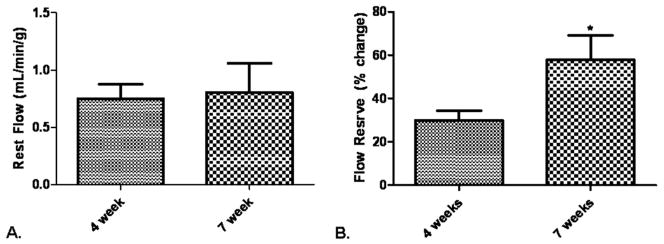
Figure 2.
Blood flow in the ischemic myocardium. (A) At rest, there was no difference in blood flow to the collateral dependent territory, p = 0.85. (B) During paced conditions, the 7-week group had greater flow reserve in the ischemic myocardium, *p = 0.03.
Myocardial microvascular function
Normal ventricle
There was no difference in the baseline diameters of the microvessels in the NV (4 weeks, 156 ± 16.3 μm vs 7 weeks, 125 ± 28.3 μm, p = 0.34). The microvascular contraction response was significantly smaller at 7 weeks in response to both ET-1 and serotonin. The contraction response to ET-1 was significantly lower at 7 weeks. Likewise, the contraction response to serotonin at 7 weeks was significantly lower than at 4 weeks (Figs. 3A and B).
Figure 3.
Microvascular responses in the normal ventricle (NV). (A) The 7-week group demonstrated a decreased contraction response to endothelin-1, *p < 0.001. (B) The 7-week group had a decreased contraction response to serotonin, *p < 0.001. Microvascular responses in the area at risk (AAR). (C) There was no difference between 4 and 7 weeks in the response to endothelin-1 in the ischemic territory, p > 0.05. (D) The animals in the 7-week group had a significantly smaller contraction response to serotonin, *p < 0.001. Line with black nodes, 4 weeks; line with white nodes, 7 weeks.
Area at risk
There was no difference in baseline diameters of the microvessels before application of the drugs in the AAR (4 weeks, 135 ± 17.6 μm vs 7 weeks, 135 ± 9.1 μm, p = 0.98). In the ischemic territory, the microvascular contraction response to serotonin was significantly decreased; there was no change in the response to ET-1. The contraction response to serotonin was significantly lower at 7 weeks. The contractile response to ET-1 was not different between groups (Figs. 3C and 3D).
Levels of lipid oxidative stress
Normal ventricle
There was no difference in the levels of lipid peroxidation at 4 and 7 weeks.
Area at risk
Similarly, there was no difference in levels of lipid peroxidation at 4 and 7 weeks in the ischemic myocardium (Fig. 4A).
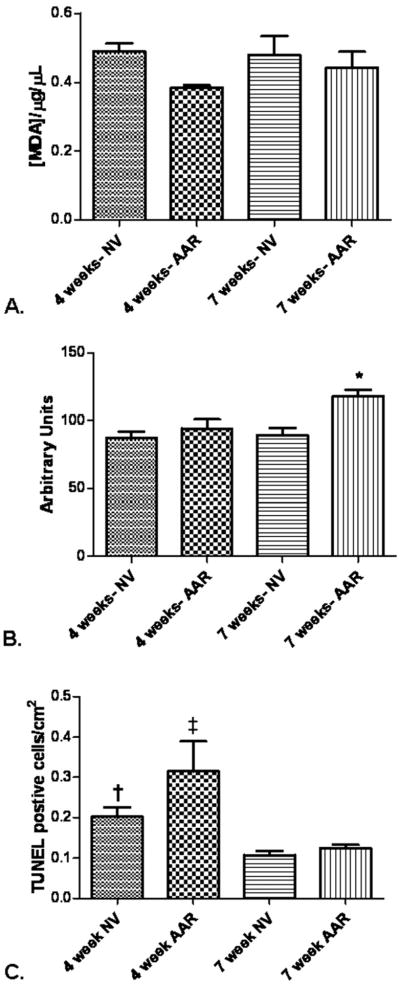
Figure 4.
Levels of lipid and protein oxidative stress and apoptosis. (A) There was no difference in levels of lipid peroxidation between the groups, p = 0.15. (B) There was no difference in the level of protein peroxidation in the normal ventricle (NV) between 4 and 7 weeks, p = 0.60, but in the 7-week area at risk (AAR) there was a significant increase in protein peroxidation, *p = 0.01. (C) The number of TUNEL-positive cells was increased in the 4-week NV and 4-week AAR, †p < 0.05 when 4-week NV is compared with 4-week AAR, ‡p = 0.005 when 4-week AAR is compared with all other groups.
Levels of protein oxidative stress
Normal ventricle
In the normally perfused left ventricular territory, there was no change in the level of protein peroxidation in the normal ventricle between the 2 groups.
Area at risk
In the chronically ischemic LCx territory, the level of protein peroxidation significantly increased at 7 weeks (Fig. 4B).
Levels of apoptosis
The number of TUNEL-positive cells was highest in the AAR of the 4-week animals, which was significantly higher than the 4-week NV and 7-week AAR (Fig. 4C).
We then examined the levels of pro- and antiapoptotic protein expression in the normal and ischemic territories. The antiapoptotic protein Bcl-2 trended toward the lowest expression in the 4-week AAR (p = 0.07). There was no difference in the expression of the proapoptotic protein Bad between the groups (p = 0.64).
Endothelial proliferation
The number of proliferating endothelial cells in the ischemic and adjacent normal tissue was determined by immunofluorescence costaining with proliferation marker Ki-67 and endothelial marker platelet endothelial cell adhesion molecule (PECAM)-1. There was a significant increase in the number of dividing endothelial cells in the 7-week groups (Figs. 5A to 5C).
Figure 5.
Quantification of dividing endothelial cells by immunofluorescence double staining. (A) Double staining for endothelium CD-31 (1) and Ki-67 (2) with 4′,6-diamidino-2-phenylindole (DAPI) (3) allow for the visualization of dividing endothelial cells, which appear pink in the combined image (4). In this representative section for the 4-week group, there are few dividing endothelial cells. (B) A representative myocardial section from the 7-week group demonstrates an increased number of dividing endothelial cells. All images are taken at 200× magnification. The line represents 10 μm. (C) Quantification of Ki-67-positive endothelial cells demonstrated significant increases in the 7-week normal ventricle (NV) and area at risk (AAR), *p < 0.001 as compared with 4-week NV and AAR. There were no differences between 4-week NV and AAR and 7-week NV and AAR.
Protein expression levels
Normal ventricle
At 4 weeks, expression of VEGF was significantly higher (3.6-fold) than it was in the 7-week group (Fig. 6A). There was a significant, 4-fold increase in phospho-endothelial nitric oxide synthase (eNOS) (ser1177) expression at 4 weeks compared with 7 weeks (Fig. 6B). The level of delta-like ligand 4 was increased 1.2-fold in the 7-week group, though this did not reach significance. Expression of phospho-extracellular signal regulated kinase ERK(thr202/tyr204)was in creased 2.5-foldinthe7-weekgroup versus in the 4-week group (Fig. 5C). Likewise, apoptosis inducing factor (AIF) increased 1.8-fold, HSP 27 increased 2.6-fold, and HSP 90 increased 1.9-fold in the 7-week group versus the 4-week group (Figs. 6D to 6F). There was no difference in the expression levels of fibroblast growth factor (FGF)-2 (p = 0.18), total Akt (p = 0.12), or phospho-Akt (thr308) (p = 0.26) between the 4-week and 7-week groups.
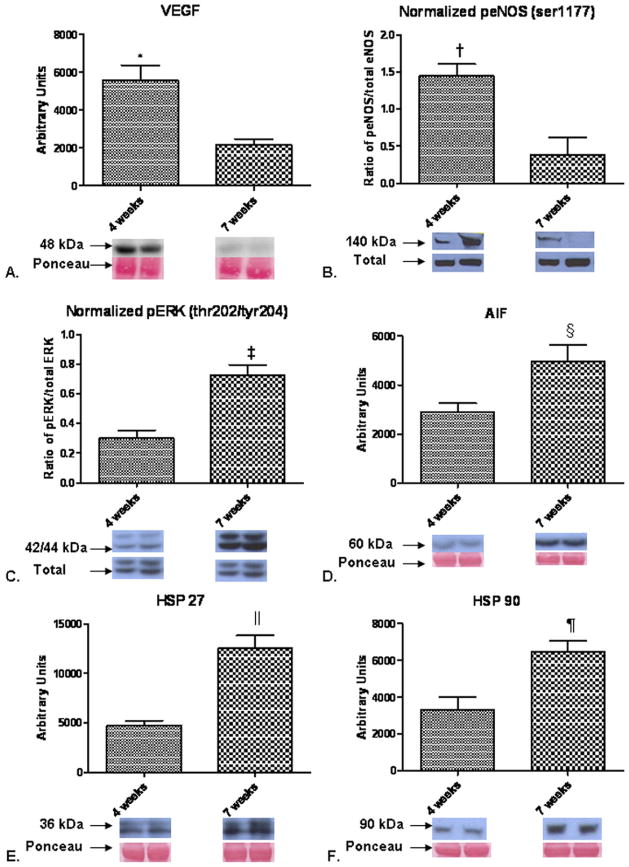
Figure 6.
Protein expression in the normal ventricle. (A) Vascular endothelial growth factor (VEGF) expression was increased in the 4-week group, *p = 0.003. (B) Expression of phospho-endothelial nitric oxide synthase (eNOS) was increased in the 4-week group. Presented as the ratio of phospho-eNOS to total eNOS expression, †p = 0.02. (C) The level of phospho-extracellular signal regulated kinase (ERK) was increased at 7 weeks, ‡p = 0.001. (D) The level of apoptosis inducing factor (AIF) was increased at 7 weeks, §p = 0.02. (E) Expression of heat shock protein (HSP) 27 was increased in the 7-week group, ||p = 0.001. (F) HSP 90 expression was increased in the 7-week group, ¶p = 0.01.
Area at risk
Most of the proteins in the angiogenic signaling pathway were increased in the ischemic myocardium at 7 weeks when compared with 4 weeks. These included a 2.6-fold increase in VEGF, a 4.9-fold increase in phospho-eNOS (ser1177), and a 2.5-fold increase in phospho-ERK (thr202/tyr204) (Figs. 7A to 7C). Expression of delta-like ligand-4, a marker of early angiogenesis, was increased 2.2-fold in the 4-week group versus the 7-week group (Fig. 6D). HSP 27 continued to be upregulated 1.9-fold by 7 weeks, as did HSP 90, which was 2.3-fold greater in the 7-week group (Figs. 7E and 7F). The expression of AIF was 1.5-fold greater in the 7-week group. There was no difference in expression levels of FGF-2 (p = 0.27), total Akt (p = 0.70), or phospho-Akt (thr308) (p = 0.77) between 4 week and 7 weeks.
Figure 7.
Protein expression in the area at risk. (A) Expression of vascular endothelial growth factor (VEGF) was significantly higher in the 7-week group, *p = 0.02. (B) Phospho-endothelial nitric oxide synthase (eNOS) expression was increased at 7 weeks, †p = 0.01. (C) Levels of phospho-extracellular signal regulated kinase (ERK) were increased in the 7-week group, ‡p = 0.05. (D) Expression of delta-like ligand (Dll) 4 was decreased at 7 weeks, §p = 0.03. (E) Heat shock protein (HSP) 27 expression was increased at 7 weeks, ‡p = 0.05. (F) Levels of HSP 90 were increased at 7 weeks, ||p = 0.004.
DISCUSSION
This study elucidated temporal and spatial changes that occur at the microvascular and molecular levels in a swine model of chronic myocardial ischemia. Microvascular function improved and dividing endothelial cells increased after 7 weeks of myocardial ischemia. These findings are in agreement with previous research in this area.13 On the other hand, our study resulted in a number of unexpected findings, including increased expression of VEGF and phospho-eNOS in the normal territory at 4 weeks, an increase in protein peroxidation in the ischemic territory (AAR) at 7 weeks, and increased AIF in the face of fewer apoptotic cells in the 7-week AAR. Overall, we believe these findings will lead to a better understanding of the effects of chronic ischemic insults to the heart over time.
Chronic ischemia was associated with an improvement in global left ventricular function over time in this study. The improved function may be, in part, a result of less microvascular dysfunction, as evidenced by decreased contraction responses at 7 weeks in the NV and AAR to ET-1 and serotonin. Serotonin has variable effects on endothelium, but often has a vasoconstrictive effect during ischemia via interaction with 5-HT1B receptors. This receptor functions through phospholipase C signaling and leads to increased local thromboxane A2.14 ET-1, a vasoactive protein expressed during myocardial ischemia, causes vasoconstriction through endothelin A receptors, which work by activation of protein kinase C-α (PKC-α).15 Upregulation of PKC can inhibit eNOS phosphorylation.16 So, increased levels of phospho-eNOS in the ischemic territory at 7 weeks may be secondary to decreased ET-1. Additionally, serotonin and ET-1 have been shown to act synergistically to result in greater contraction responses in a nitric oxide dependent manner.17 In light of this, the improved left ventricular function may also be a result of decreased levels of vasoconstrictors and an increase in vasodilators, leading to augmented blood flow and more abundant collateral formation in the ischemic myocardium.13 The flow data in this study are in agreement with data from swine studies of ameroid occlusion done in Dr Bloor’s laboratory.18,19 In work by Roth and colleagues,18 they demonstrated no difference in rest flow to the ischemic territory, but increased flow reserve, the ability to increase blood flow to the ischemic territory during stress, at 7 weeks of ischemia as compared with 3 weeks.
The increase in protein peroxidation in the AAR at 7 weeks may be a sequela of long-term ischemia. Chronic myocardial ischemia has been associated with increases in reactive oxygen species, which are responsible for increasing levels of oxidative stress. Increased oxidative stress has been linked to left ventricular remodeling and the development of heart failure in the setting of chronic ischemia, and limiting the formation of reactive oxygen species is thought to be beneficial.20 Increased expression of AIF may be involved in controlling the level of oxidative stress. Although AIF is involved with inducing apoptosis, it is also known to be an antioxidant.21 In this case, we demonstrated increased AIF expression, but a decreased number of apoptotic cells in the 7-week group. This suggests that the role of AIF in this case is not related to apoptosis. So AIF may be functioning to decrease oxidant stress, which may help limit ventricular remodeling. Markers of cellular stress, HSP 27 and HSP 90, were increased at 7 weeks in both the NV and AAR. Both of these proteins are associated with cell survival and antiapoptotic actions.22
Evaluation of proteins involved with angiogenesis allowed an examination of how signaling may evolve over time. Early in the ischemic process, angiogenic proteins (VEGF and phospho-eNOS) were more highly expressed in the NV. This may reflect an attempt to vasodilate adjacent blood vessels to increase regional blood flow. Both proteins are well known vasodilators, and increasing local blood flow may help mitigate ischemic damage. Production of these factors may also be acting in a paracrine fashion to not only increase angiogenesis in the ischemic territory, but also to act in homing endothelial progenitor cells to the site of injury.23 By 7 weeks, both VEGF and phospho-eNOS were more abundant in the ischemic territory. Upregulation of these angiogenic signals in the AAR at 7 weeks is likely responsible for the increased number of dividing endothelial cells, an indicator of ongoing angiogenesis.
Delta-like ligand 4 is part of the notch signaling pathway and is involved in vessel sprouting, an early step in the formation of new blood vessels.24 Its expression was higher at 4 weeks than at 7 weeks, indicating that blood vessel modifications are underway by 4 weeks after ischemia. These data may be of value in therapeutic angiogenesis, as treatments can be tailored to exploit the specific changes occurring during myocardial ischemia. Currently there are groups exploring the use of matrices applied to the epicardium during cardiac surgery to enhance angiogenesis in ischemic myocardium.25 In this model, adding timed release of specific angiogenic and cell survival factors may enhance angiogenesis. For example, local stimulation of delta-like ligand 4 expression early in an ischemic process may lead to increased vessel sprouting and, therefore, greater vessel density.
There were limitations to this study. In most situations, the porcine coronary circulation closely mimics the physiology and pathophysiology of the human coronary circulation, but this may not necessarily be the case in this experiment. These experiments were performed under general anesthesia with isoflurane. This inhaled anesthetic agent is a known coronary vasodilator and may have influenced the flow measurements. However, we believe that any vasodilatory side effects would be relatively equal among and between the groups. Additionally, we measured flow in transmural myocardial segments. It is known that the distribution of collaterals is not equal, and most tend to develop in the endo- and mid-myocardium.19 This also may have altered the flow measurements, but differences should be equal among and between the groups. Finally, we measured only 2 time points, 4 and 7 weeks, and functional and molecular markers are likely further changing over time.
In this study, we were able to expand on previous physiologic studies with microvascular and molecular data. We demonstrated improved left ventricular function, decreased microvascular contraction responses and levels of apoptosis, increased oxidative stress, and differential expression of angiogenic proteins.
Acknowledgments
Funding for this project was provided to FWS by NHLBI (RO1HL46716, RO1HL69024, and RO1HL85647), NIH T32-HL076130 (RMO), NIH 5T32-HL0074 (MPR) and the Irving Bard Memorial Fellowship (MPR, LMC, RMO).
Abbreviations and Acronyms
- AAR
area at risk (ischemic myocardial territory)
- AIF
apoptosis inducing factor
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal regulated kinase
- ET-1
endothelin-1
- HSP
heat shock protein
- ILM
isotope-labeled microspheres
- LCx
left circumflex coronary artery
- MDA
malondialdehyde
- NV
normal ventricle (nonischemic myocardial territory)
- VEGF
vascular endothelial growth factor
Footnotes
Presented at the American College of Surgeons 95th Annual Clinical Congress, Surgical Forum, Chicago, IL, October 2009.
Author Contributions
Study conception and design: Robich, Sellke
Acquisition of data: Robich, Osipov, Chu, Feng, Burgess, Oyamada, Laham
Analysis and interpretation of data: Robich, Osipov, Chu, Burgess, Clements, Laham
Drafting of manuscript: Robich
Critical revision: Robich, Clements, Sellke
Disclosure Information: Dr Sellke is a consultant and received honoraria from Novo Nordisk and Cubist Pharmaceutical; he has research support from Ikaria Inc and Capstone Therapeutics; and he was an expert witness and received a support from the law firms representing Pfizer in Celebrex/Bextra litigation. All other authors have nothing to disclose.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Boodhwani M, Sellke FW. Therapeutic angiogenesis in diabetes and hypercholesterolemia: influence of oxidative stress. Anti-oxid Redox Signal. 2009;11:1945–1959. doi: 10.1089/ars.2009.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg LS. Vasculogenesis, angiogenesis, and arteriogenesis: Mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Bonow RO, Chronos NA, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73–86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 5.O’Konski MS, White FC, Longhurst J, et al. Ameroid constriction of the proximal left circumflex coronary artery in swine. A model of limited coronary collateral circulation. Am J Cardio-vasc Pathol. 1987;1:69–77. [PubMed] [Google Scholar]
- 6.White FC, Carroll SM, Magnet A, Bloor CM. Coronary collateral development in swine after coronary artery occlusion. Circ Res. 1992;71:1490–1500. doi: 10.1161/01.res.71.6.1490. [DOI] [PubMed] [Google Scholar]
- 7.Millard RW. Induction of functional coronary collaterals in the swine heart. Basic Res Cardiol. 1981;76:468–473. doi: 10.1007/BF01908345. [DOI] [PubMed] [Google Scholar]
- 8.Sodha NR, Clements RT, Feng J, et al. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986;74:469–476. doi: 10.1161/01.cir.74.3.469. [DOI] [PubMed] [Google Scholar]
- 10.Boodhwani M, Voisine P, Ruel M, et al. Comparison of vascular endothelial growth factor and fibroblast growth factor-2 in a swine model of endothelial dysfunction. Eur J Cardiothorac Surg. 2008;33:645–650. doi: 10.1016/j.ejcts.2007.12.016. discussion 651–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tofukuji M, Metais C, Li J, et al. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation. 1998;98:II242–246. discussion II247–248. [PubMed] [Google Scholar]
- 12.Boodhwani M, Sodha NR, Mieno S, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I-31–37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 14.Robich MP, Araujo EG, Feng J, et al. Altered coronary microvascular serotonin receptor expression after coronary artery bypass grafting with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2010;139:1033–1040. doi: 10.1016/j.jtcvs.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Liu Y, Khabbaz KR, et al. Endothelin-1-induced contractile responses of human coronary arterioles via endothelin-A receptors and PKC-alpha signaling pathways. Surgery. 2010;147:798–804. doi: 10.1016/j.surg.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Signorello MG, Segantin A, Leoncini G. The arachidonic acid effect on platelet nitric oxide level. Biochem Biophys Acta. 2009;1791:1084–1092. doi: 10.1016/j.bbalip.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Villalon AL, Amezquita YM, Monge L, et al. Endothelin-1 potentiation of coronary artery contraction after ischemia-reperfusion. Vascul Pharmacol. 2008;48:109–114. doi: 10.1016/j.vph.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Roth DM, Maruoka Y, Rogers J, et al. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol. 1987;253:H1279–1288. doi: 10.1152/ajpheart.1987.253.5.H1279. [DOI] [PubMed] [Google Scholar]
- 19.White FC, Roth DM, Bloor CM. Coronary collateral reserve during exercise induced ischemia in swine. Basic Res Cardiol. 1989;84:42–54. doi: 10.1007/BF01907002. [DOI] [PubMed] [Google Scholar]
- 20.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 21.van Empel VP, Bertrand AT, van der Nagel R, et al. Downregulation of apoptosis-inducing factor in harlequin mutant mice sensitizes the myocardium to oxidative stress-related cell death and pressure overload-induced decompensation. Circ Res. 2005;96:e92–e101. doi: 10.1161/01.RES.0000172081.30327.28. [DOI] [PubMed] [Google Scholar]
- 22.Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 23.Walsh K, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest. 2007;117:3176–3179. doi: 10.1172/JCI34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limbourg A, Ploom M, Elligsen D, et al. Notch ligand delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100:363–371. doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Ilzarbe M, Agbulut O, Pelacho B, et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]