Summary
The intensity of the BMP signal is determined by cell surface receptors that phosphorylate Smad1/5/8 at the C-terminus. In addition to this BMP-activated phosphorylation, recent studies have shown that sequential phosphorylations by MAPK and GSK3 kinases can negatively regulate the activity of the pSmad1Cter signal. These phosphorylations in the linker region cause Smad1 to be transported to the centrosomal region, polyubiquitinylated and degraded by the proteasomal machinery. In Xenopus embryos, Wnt signals, which regulate GSK3, induce ectoderm to adopt an epidermal fate and this Wnt effect requires an active BMP-Smad1/5/8 signaling pathway. These findings have profound implications for understanding how dorsal-ventral and anterior-posterior patterning are seamlessly integrated in the early embryonic morphogenetic field.
Introduction
Embryonic patterning in Xenopus is controlled by graded signals along the dorsal-ventral (D-V) and anterior-posterior (A-P) axes. The positional information produced by these signal gradients are seamlessly integrated in each individual embryo. A classic example of the self-regulating nature of this patterning integration is demonstrated by cutting a Xenopus blastula stage embryo in half along its D-V axis: the two halves go on to ultimately form perfectly matching twins. How signals emanating from distant points of a morphogenetic field can communicate to each cell within the field when to differentiate, proliferate or die, is key to understanding how embryos, tissues and organs are patterned. Recent advances have begun to address this issue, with Smad1 identified as a central candidate in this process. Smad1 has been recently shown to be at the crossroads of the BMP/BMP receptor, the FGF/MAPK and Wnt/GSK3 signaling pathways which are integrated through specific phosphorylations in this transcription factor. Here we review how positional information provided by D-V and A-P growth factor gradients in the developing embryo are integrated at the level of Smad1/5/8.
Global positioning in the developing embryo
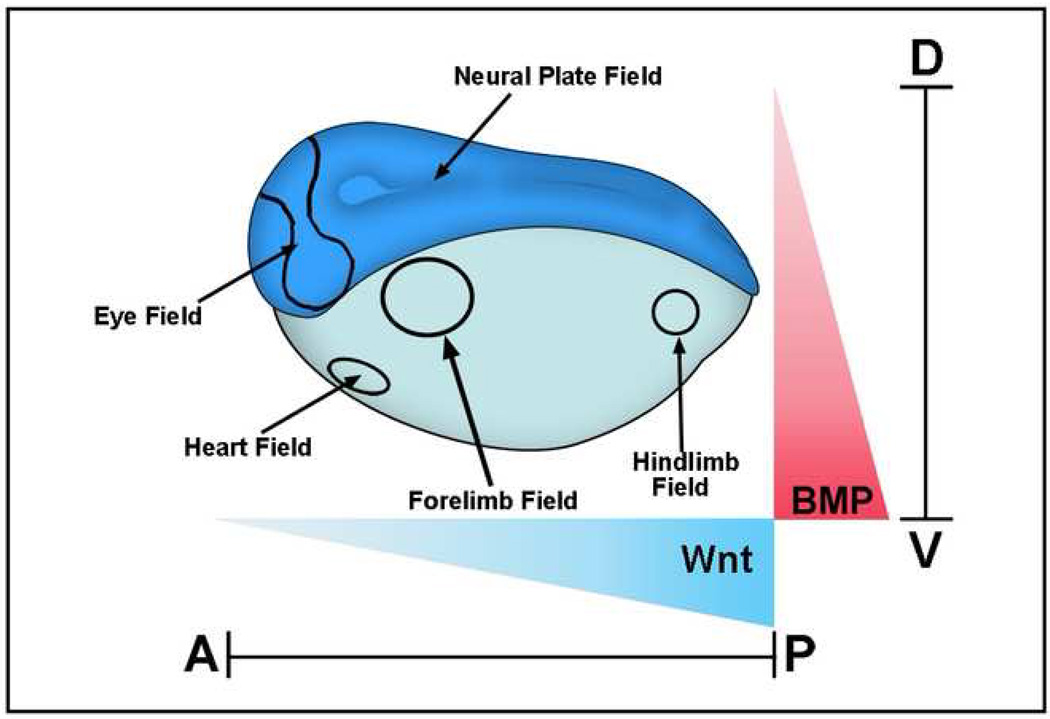
Two important gradients signal in the developing embryo acting together as a global positioning system for the early embryonic morphogenetic field (Figure 1). These morphogen gradients determine the D-V and A-P axes, providing Cartesian coordinates for the body plan that determines where subsequent self-regulating organ morphogenetic fields for parts such as eyes, brain and limbs are placed at later stages of development [1].
Figure 1.
Xenopus gastrula stage embryo showing some of the main morphogenetic fields that form along the D-V (BMP) and A-P (Wnt) axes. These fields are considered self-regulating (as is the early blastula embryo as well) because they can be cut in half and regenerate the whole pattern after transplantation [1]. BMP signals are high in the ventral side and decrease dorsally, while Wnt signals are strong in the posterior and weaken anteriorly. Cells at different positions within these two Cartesian axes read these morphogen gradients and determine the embryonic body plan that specifies the place at which the various organs will be subsequently formed.
The D-V axis is established by BMP (bone morphogenetic protein) signals, which subdivide the ectoderm into central nervous system, neural crest and epidermis. In the mesoderm, from dorsal to ventral, notochord, somite and intermediate (kidney) and lateral (body wall) mesodermal tissues are generated by the BMP gradient. Thus, the D-V system regulates the initial histotypic differentiations of the vertebrate embryo. An elaborate system of extracellular protein-protein interactions regulates this patterning gradient. Chordin, a secreted BMP antagonist, is expressed in the dorsal region of the vertebrate embryo during the gastrula stage, while on the opposite side at the ventral pole BMPs are expressed at high levels [2,3,4]. BMPs activate their receptors (BMPR), which are transmembrane serine/threonine kinases, and these in turn phosphorylate the transcription factor Smad1 at its C-terminal serines. Once phosphorylated, Smad1 translocates into the nucleus and activates or represses BMP-responsive genes [5,6]. BMP antagonists such as Chordin and Noggin inhibit C-terminal phosphorylation of Smad1 and induce the ectoderm to adopt a neural fate [7], while high ventral BMP levels ensure that the ectoderm differentiates into an epidermal cell fate [8]. These two opposing ventral and dorsal signaling centers provide the basis for D-V patterning in embryos such as Xenopus and zebrafish by controlling the intensity of the pSmad1Cter signal a cell will receive.
D-V pattern is self-regulating because the dorsal and ventral signaling centers are under opposite transcriptional control [2,9]. Dorsal genes are transcribed at low Smad1/5/8 activity levels. A decrease in BMP levels triggers transcription of a dorsal BMP (called ADMP) [9]. Ventral center genes are activated by Smad1 and include BMP4/7 and an enzyme of the Tolloid family of zinc metalloproteinases called Xolloid-related that degrades Chordin, liberating BMP and ADMP for signaling [2,3]. At high BMP levels Sizzled, a competitive inhibitor of Tolloids, is transcribed in Xenopus and zebrafish, increasing the stability of the BMP antagonist Chordin (see figure 4 below) [10,11]. In addition, at high BMP levels two BMP-binding proteins called Bambi (BMP and Activin Membrane-Bound Inhibitory Protein) [12] and Crossveinless-2 [13] are produced, further dampening the signal. This Chordin/BMP axis is used universally by bilateral animals for patterning the D-V axis during gastrulation. Drosophila [14] spiders [15], hemichordates [16] and amphioxus [17] all use this conserved ancestral mechanism. Many components of this system have been maintained in evolution, even though an inversion of the D-V axis took place between the invertebrate and chordate body plans [18–20].
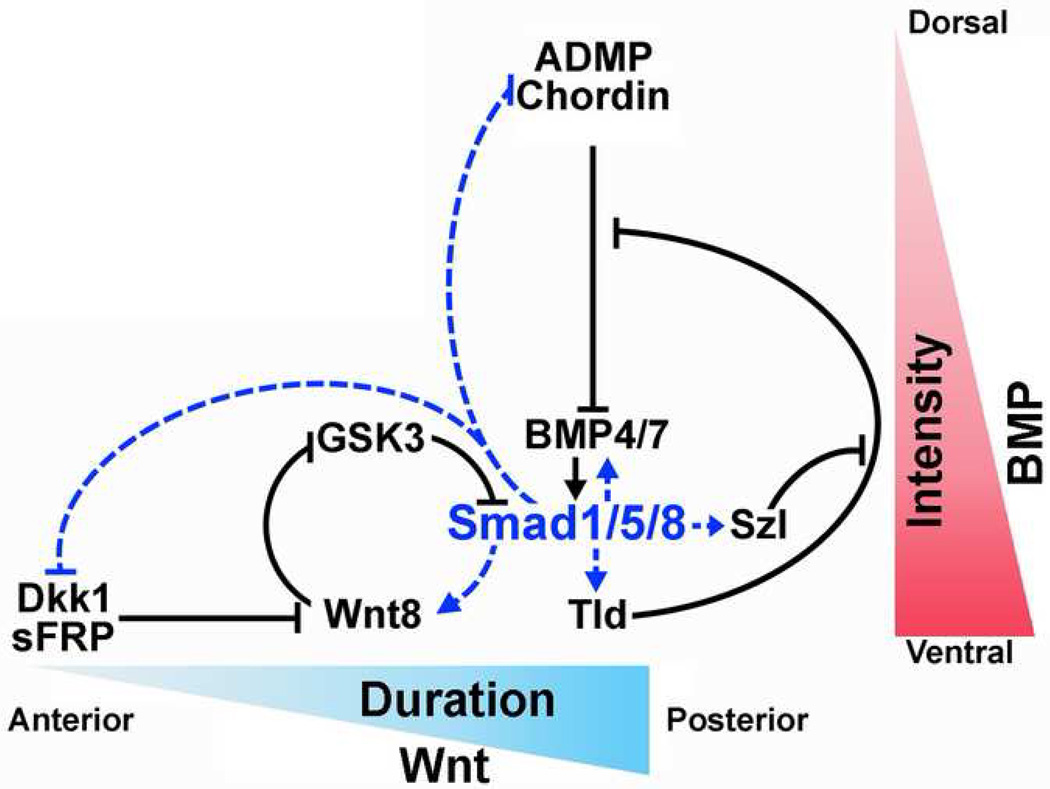
Figure 4.
Model of the integration of the BMP (D-V) and Wnt (A-P) patterning pathways at the level of Smad1/5/8 linker phosphorylations. Blue dotted arrows indicate transcriptional regulation by Smad1/5/8 and black arrows indicate direct protein-protein interactions. All these interactions have been confirmed in Xenopus embryos using loss-of function and overexpression experiments [9, 10, 28]. Not shown here are two BMP inhibitors produced in the ventral side called Crossveinless-2 and Bambi, which serve to dampen the BMP signal [9].
The main A-P morphogenetic gradient is provided by Wnt signals [21,22]. In Xenopus and amphioxus, Wnt signals are highest in the posterior blastopore where xWnt8 is expressed [22,23]. An endogenous gradient of Wnt signals can be visualized in the Xenopus neural plate, with high Wnt signals found in the posterior which decreases anteriorly, and an increasing concentrations of anti-Wnts like Dkk1 (Dickkopf-1) in anterior regions [21,24]. In neuralized Xenopus animal caps, varying doses of Wnt3a induce different A-P markers, while overexpression of Wnt antagonists in Xenopus embryos inhibits posterior markers [22,25]. Studies on planarian regeneration strongly support the view that canonical Wnt signaling specifies posterior cell fate along the A-P axis, since RNAi against β-Catenin causes the ectopic regeneration of head structures instead of tail at posterior amputations [26•,27].
When Wnt antagonists such as Dkk are overexpressed in Xenopus embryos the result is a strongly dorsalized phenotype, with a neural plate expanded at the expense of epidermal tissue. This phenotype is very similar to that of embryos injected with BMP antagonists such as Chordin [28••]. This puzzling observation suggested that Wnt antagonists might regulate BMP signaling in novel ways. Integration of multiple signaling pathways at the level of Smad1 phosphorylations proved productive, with its serine-rich linker region shown to be highly regulated by two different signaling pathways (Figure 2).
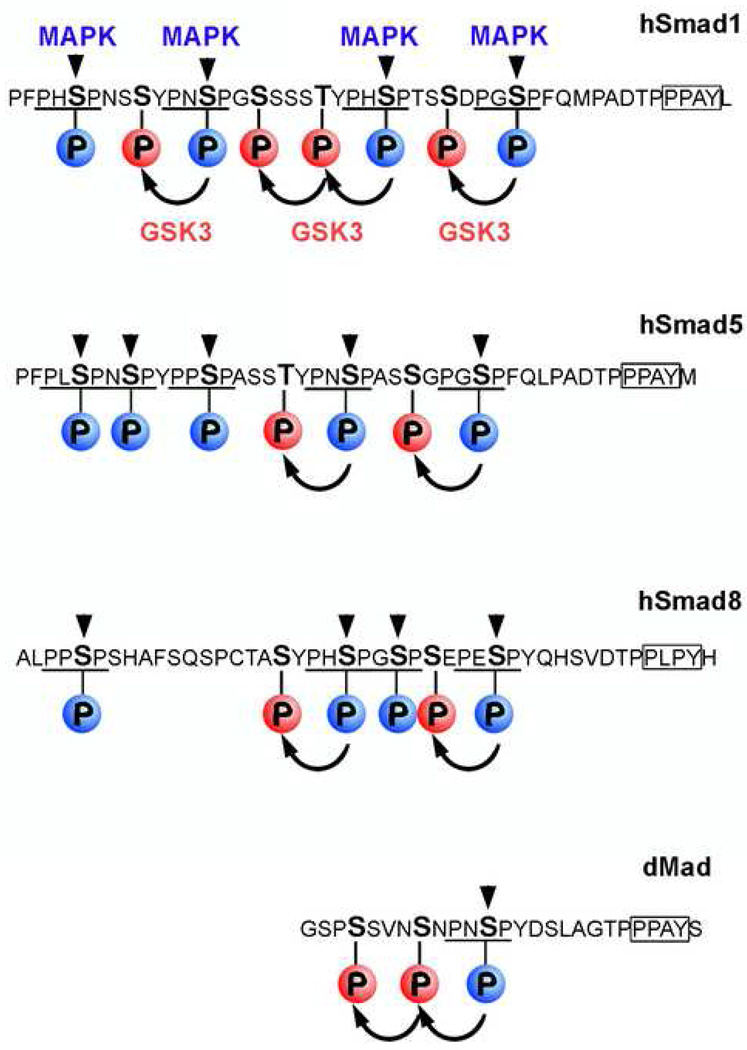
Figure 2.
Sequence analysis of BMP-dependent Smad1/5/8 linker regions showing that the MAPK and GSK3 phosphorylation sites are conserved. These MAPK/GSK3 phosphorylation sites are also present in Mad the Drosophila. MAPK sites (PXSP) are indicated in blue and GSK3 sites in red. The Smurf E3 ubiquitin ligase binding site (PPAY) located 9 amino acids after the last MAPK site is boxed.
MAPK and Smad1 inhibition
The first evidence that multiple signaling pathways can be integrated at the level of Smad1 phosphorylation was discovered in human cultured cell lines [29]. It was demonstrated that Smad1 is a target of mitogen-activated protein kinases (MAPK), activated by epidermal growth factor receptor (EGFR). Phosphorylation by MAPKs was shown to occur at canonical Erk (Extracellular signal-regulated kinase) PXS[PO3]P sites in the linker region of Smad1 (Figure 2) which were inhibitory to the BMP-activated pSmad1Cter signal. These linker phosphorylations are present in all BMP-activated Smads (1/5/8). Intriguingly, a single PXSP site is also found in Mad the Drosophila Smad1 homologue (Figure 2). Phosphorylation in the linker region serves to terminate the pSmad1Cter signal by promoting the polyubiquitinylation of the protein [28••,31••]. The Smurf1 E3-ubiquitin ligase binds to a PPXY sequence [30] located 9 amino acids downstream of the MAPK phosphorylation sites in the linker domain (Figure 2). After MAPK phosphorylation, Smad1 is exported from the nucleus and degraded in cytoplasmic proteasomes [28••,31••].
This discovery proved important as it had long been known that active signals such as fibroblast growth factors (FGFs) and insulin-like growth factors (IGFs) [32,33] were potent neural inducers in Xenopus ectoderm. Embryologists were puzzled by this, for the same neural phenotype could be attained by inhibiting BMP signals with antagonists such as Chordin [34]. Further analysis of these phosphorylation sites demonstrated that IGF and FGF activate MAPK/Erk, which in turn induced neural tissue by phosphorylating Smad1 in the linker region [34–36]. Overexpression of phosphorylation-resistant mutants of Smad1 in Xenopus embryos increased epidermal development with a concomitant decrease in neural tissue, whereas wild-type Smad1 injected embryos displayed only minimal neural plate specification changes [34,36]. These studies demonstrated that Smad1 is hyperactive in the absence of MAPK phosphorylation and that in vivo MAPK plays a key role in the regulation of Smad1 activity.
Knock-in mice containing Smad1 forms that are insensitive to phosphorylation by MAPK in the linker region display phenotypes in the gastrointestinal epithelium and the reproductive tract [37]. Recently it has been confirmed that FGFs inhibit BMP signaling by phosphorylating the linker region of Smad1: in mouse embryonic fibroblasts (MEFs) from these phosphorylation-resistant Smad1 mice, FGF loses its ability to inhibit a BMP signaling reporter gene [31••]. This result shows that FGF requires the MAPK phosphorylation sites within the linker region of Smad1 to inhibit BMP signaling.
In Drosophila the Mad signal can also be modulated by a MAPK-related kinase called nemo-like kinase (Nlk) [38•]. Nlk an enzyme known to be involved in the Wingless/Wnt pathway, phosphorylates Mad at a conserved serine in its amino-terminal domain. This phosphorylation inhibits BMP signaling by preventing nuclear accumulation of pMadCter, thus inhibiting the activation of BMP-responsive genes [38•].
Wnt signals through Smad1
A novel branch of the canonical Wnt pathway was revealed by the discovery that Smad1 receives a second inhibitory phosphorylation in its linker region by the glycogen synthase kinase (GSK3) serine/threonine kinase [28••,31••]. GSK3 normally phosphorylates “primed” or pre-phosphorylated proteins [39], as in the case of β-Catenin [40]. The canonical Wnt pathway signals by inhibiting GSK3, which results in the stabilization of β-Catenin protein, which then translocates into the nucleus where it functions as a transcriptional co-activator [41]. In the case of Smad1/5/8, MAPK provides the priming phosphorylation for GSK3 (Figures 2 and 3). Once this double phosphorylation occurs, Smad1 is transported to the pericentrosomal region where it is degraded by the proteasomal machinery, thus terminating the BMP/pSmad1Cter signal (Figure 3) [28••]. The centrosome, in addition to its functions during mitotic division, is now known to also serve as the proteolytic centre of the cell [42]. GSK3 activity is inhibited by Wnt signals by becoming localized to LRP6-signalosome membrane vesicles, which contain LRP6, Frizzled, Dishevelled, Axin and GSK3 [43••]. In the presence of Wnt, the stability of Smad1 is increased, causing the duration of the BMP/pSmad1Cter signal to be prolonged [28••]. In this way, Wnt can signal through Smad1.
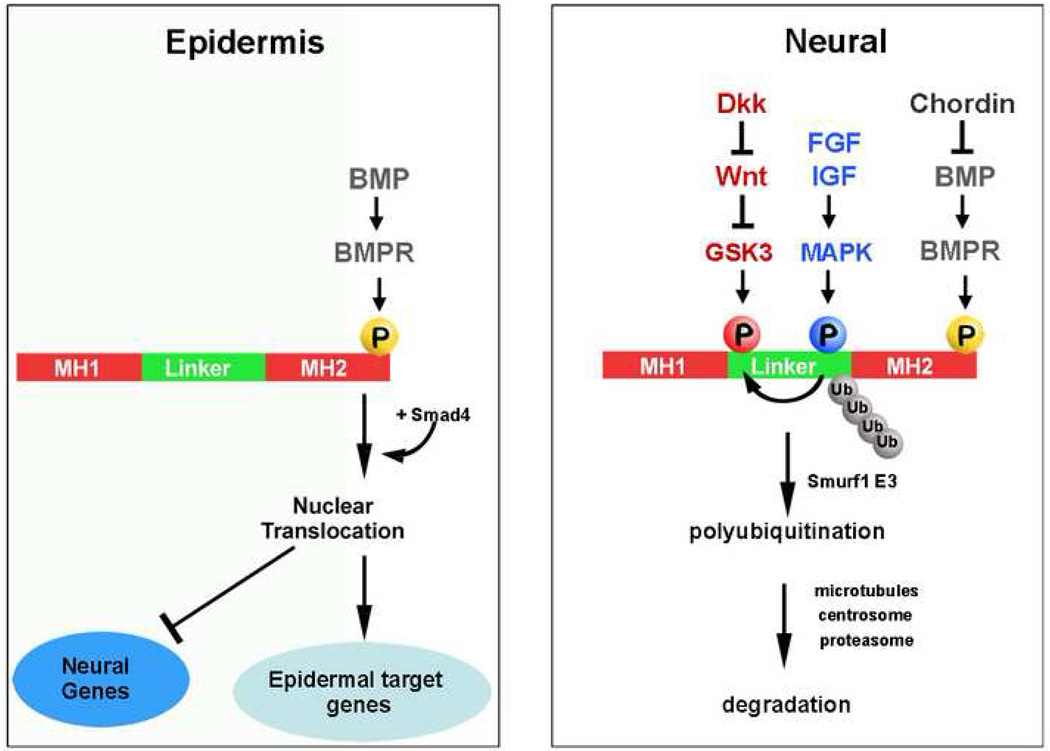
Figure 3.
Phosphorylations of the transcription factor Smad1 determine the type of cell differentiation; BMP provides the intensity of the pSmad1Cter and Wnt prolongs its duration. The double phosphorylation of Smad1 by MAPK and GSK3 is required for its ubiquitination and termination of the pSmad1Cter signal through degradation of the activated transcription factor in the proteasome [28••]. MH1 and MH2 refer to the conserved Mad homology domains present in Smad transcription factors.
Integrating the D-V and A-P morphogenetic gradients
In the context of the gastrula embryo the BMP pathway determines the intensity of the BMP signal, high in the ventral and low in the dorsal region (Figure 1 and 4). In the A-P axis, Wnts prolong the duration of the BMP/Smad signal near the posterior blastopore and this duration shortens anteriorly, due to the Wnt activity gradient (Figure 4) [22]. This model implies that both the D-V (BMP) and A-P (Wnt) axes can be integrated at the level of Smad1 linker phosphorylation. Epistatic experiments confirmed that certain effects of canonical Wnt signaling are mediated through Smad1. In dissociated Xenopus ectodermal cells [8,36] the overexpression of xWnt8 in Xenopus embryos induced epidermis differentiation, and this branch of the canonical Wnt pathway was blocked by co-injection of a dominant-negative Smad5 [28••] that blocks all BMP/Smad1/5/8 signaling [44]. Therefore, epidermal induction by Wnt requires an active BMP/Smad signal. These effects of Wnt signaling on epidermal cell differentiation do not require Tcf3 (T-cell factor 3) but, intriguingly, require β-Catenin [28••].
Recently, work in zebrafish embryos has shown that histotypic differentiation along the A-P axis is controlled by distinct temporal intervals of BMP signaling, with an early specification of anterior ventrolateral cells followed by a later specification of more posterior ventrolateral cell fates [45•]. These findings may provide an interesting temporal dimension to the proposed mechanism of integrating the D-V and A-P axes by Smad1 linker phosphorylation.
The integration of BMP and Wnt at the level of Smad1 may also have implications for human disease. Although BMPs are the main regulators of bone morphogenesis, most human mutations that affect the pathogenesis of osteoblast differentiation diseases such as osteoporosis and osteopetrosis were found to affect the Wnt co-receptor LRP5, or the secreted LRP5/6 antagonists Dkk1 and Sclerostin [46,47]. Since Wnt signaling increases the duration of BMP/Smad1/5/8 signals (Figure 3), in future it will be interesting to investigate whether these bone formation syndromes are mediated by Smad1/5/8 activity regulated by the Wnt pathway.
Conclusions and prospects
Figure 4 summarizes how embryonic pattern may be achieved by a self-regulating mechanism integrating both D-V and A-P gradients. In this model, BMP would regulate the intensity of the signal, while Wnt would regulate the duration of the Smad1/5/8 signal. Gene promoters may be affected differently by these two qualities -intensity and duration- of the same transcription factor. One prediction of the model is that A-P genes will be more sensitive to the duration of the Smad1 signal. In future it will be important to identify promoters that are particularly sensitive to the duration of the Smad1 signal. In the hard-wired mechanism reviewed here, the BMP and Wnt signals, acting through Smad1/5/8, would regulate coordinately hundreds of downstream target genes. Perhaps this strategy provides a more robust way of obtaining a perfect patterned embryo time after time. In this view, the eventual vertebrate body plan would be encrypted in the phosphorylation sites of a transcription factor.
The realization that Smad1/5/8 is a key player in integrating embryonic patterning signals clarifies old questions and generates many new ones. One interesting question that arises is whether Wingless in Drosophila can stabilize Mad, placing this D-V patterning transcription factor in a central role for A-P patterning. If such a node of signal integration functioned in fruit flies, this could have deep implications for the evolution and development of the body plans of all bilateral animals [20].
Acknowledgements
We thank members of our laboratory for critical readings of this manuscript. Our work was supported by the NIH (HD21502-21) and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edward Eivers, Email: eeivers@mednet.ucla.edu.
Luis C. Fuentealba, Email: lfuentea@ucla.edu.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.De Robertis EM, Morita E, Cho KY. Gradient fields and homeobox genes. Development. 1991;112:669–678. doi: 10.1242/dev.112.3.669. [DOI] [PubMed] [Google Scholar]
- 2.De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Bio. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Def Res. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- 4.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 6.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 7.Harland R. Neural induction. Curr Opin Genet Dev. 2000;10:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PA, Hemmati-Brinvalou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 9.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- 12.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signaling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 13.Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akiyama-Oda Y, Oda H. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development. 2006;133:2347–2357. doi: 10.1242/dev.02400. [DOI] [PubMed] [Google Scholar]
- 16.Lowe CJ, Terasaki M, Wu M, Freeman RM, Runft L, Kwan K, Haigo S, Aronowicz J, Lander E, Gruber C, et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 2006;4:1603–1619. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Satou Y, Holland ND, Shin-I T, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- 18.Arendt D, Nubler-Jung K. Innversion of dorsoventral axis? Nature. 1994;371:26. doi: 10.1038/371026a0. [DOI] [PubMed] [Google Scholar]
- 19.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;38:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 20.De Robertis EM. Evo-Devo: variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signaling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 22.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;6:425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 23.Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 25.McGrew LL, Hoppler S, Moon RT. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech Dev. 1997;69:105–114. doi: 10.1016/s0925-4773(97)00160-3. [DOI] [PubMed] [Google Scholar]
- 26. Gurley KA, Rink JC, Sanchez-Alvarado A. β-Catenin defines head versus tail identity during planarian regeneration and homeostatis. Science. 2008;319:323–327. doi: 10.1126/science.1150029. Presents experiments showing that beta-catenin functions as a molecular switch to specify the anteroposterior identity during regeneration and homeostasis in planarians.
- 27.Petersen CP, Reddien PW. Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science. 2008;319:327–330. doi: 10.1126/science.1149943. [DOI] [PubMed] [Google Scholar]
- 28. Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. This study reports that the duration of the pSmad1Cter signal is prolonged by canonical Wnt signals. Epistatic experiments provide evidence that dorsoventral and anteroposterior patterning gradients are integrated at the level of Smad1 phosphorylations.
- 29.Kretzschmar M, Doody J, Massagué J. Opposing BMP and EGF signaling pathways converge on the TGF-β family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- 31. Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massagué J. Balancing BMP signaling through integrated outputs into the Smad linker. Mol Cell. 2007;25:441–454. doi: 10.1016/j.molcel.2007.01.006. This work demonstrates that once Smad1 is phosphorylated in the linker region by MAPK, it then is recognized by Smurf1 (an E3 ubiquitin ligase) and polyubiquitinated resulting in either degradation or cytoplasmic retention..
- 32.Stern CD. Neural induction: 10 years on since the 'default model'. Curr Opin Cell Biol. 2006;6:692–697. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Pera EM, Wessely O, Li SY, De Robertis EM. Neural and head induction by insulin-like growth factor signals. Dev Cell. 2001;1:655–665. doi: 10.1016/s1534-5807(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 34.Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sater AK, El-Hodiri HM, Goswami M, Alexander TB, Al-Sheikh O, Etkin LD, Akif Uzman J. Evidence for antagonism of BMP-4 signals by MAP kinase during Xenopus axis determination and neural specification. Differentiation. 2003;71:434–444. doi: 10.1046/j.1432-0436.2003.7107006.x. [DOI] [PubMed] [Google Scholar]
- 36.Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aubin J, Davy A, Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–1494. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng YA, Rahnama M, Wang S, Sosu-Sedzorme W, Verheyen EM. Drosophila Nemo antagonizes BMP signaling by phosphorylation of Mad and inhibition of its nuclear accumulation. Development. 2007;134:2061–2071. doi: 10.1242/dev.02853. This study reports that Nemo-like kinase, a MAPK, can inhibit MAD signaling in the Drosophila wing by phosphorylating Mad in the MH1 domain.
- 39.Cohen P, Frame S. The renaissance of GSK3. Nat rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual kinase. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 41.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 42.Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6:194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- 43. Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes disheveled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. This important study reports that Wnt signals cause GSK3 and axin to become localized to LRP6-signalosome membrane vesicles, causing β-catenin stabilization.
- 44.Beck CW, Whitman M, Slack JM. The role of BMP signaling in the outgrowth of the Xenopus tail bud. Dev Biol. 2001;238:303–314. doi: 10.1006/dbio.2001.0407. [DOI] [PubMed] [Google Scholar]
- 45. Tucker JA, Mintzer KA, Mullins MC. The BMP Signaling Gradient Patterns Dorsoventral Tissues in a Temporally Progressive Manner along the Anteroposterior Axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. Comprehensive study proposing that A-P patterning is modulated throughout gastrulation by BMP signaling, with an early specification of anterior ventrolateral cells followed by specification of more posterior ventrolateral cell fates at later time intervals.
- 46.Koay MA, Brown MA. Genetic disorders of the LRP5-Wnt signaling pathway affecting the skeleton. Trends Mol Med. 2005;11:129–137. doi: 10.1016/j.molmed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Semenov MV, He X. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem. 2006;281:38276–38284. doi: 10.1074/jbc.M609509200. [DOI] [PubMed] [Google Scholar]