Abstract
In addition to radial glial cells of neurohistogenesis, immature astrocytes with stem-cell-like properties cordon off emerging functional patterns in the developing brain. Astrocytes also can be stem cells during adult neurogenesis, and a proposed potency of injury-associated reactive astrocytes has recently been substantiated. Astrocytic cells might additionally be involved in cancer stem cell-associated gliomagenesis. Thus, there are distinguishing roles for stem-cell-like astrocytes during brain development, in neurogenic niches in the adult, during attempted reactive neurogenesis after brain injury or disease and during brain tumorigenesis.
Introduction
After the close of the neuro-developmental critical period, when most astrocytes have lost their neurogenic and/or cytogenic abilities, injury might induce some otherwise fully differentiated astrocytes to assume a primitive or stem-like profile and take on a neurogenic role [1,2]. Although extensive reactive gliosis is associated with injury and/or disease of the central nervous system (CNS), only a subset of astrocytes undergo this transition and are capable of driving reactive cytogenesis. These unique reactive astrocytes are distinguished by indicators of cellular proliferation (i.e. the incorporation of nucleo-sides such as 3H-thymidine [3] or thymidine analogues such as bromodeoxyuridine [BrdU]) [4] and the expression of certain immature cytoskeletal markers such as nestin and vimentin [5]. Additionally, these select astroglia upregulate the production of certain developmentally regulated extracellular matrix (ECM) molecules (such as tenascin-C [6] and chondroitin-sulfate- and heparin-sulfate-containing proteoglycans [CSPG and HSPG, respectively]) [7,8]. This profile, a proliferative, immature astrocyte residing within the confines of an enriched ECM environment, precisely describes the rare subset of astrocytes that function as neural stem cells. This profile is shared by the three types of astrocyte at the center of this discussion, namely, the aforementioned cytogenic reactive astrocytes, the astrocytic stem cells found within the adult subventricular zone (SVZ), and a third unique subset of astrocytes, the boundary astrocytes, which are found within the early developing neonatal brain and spinal cord. What are the in vivo responsibilities and behaviors of these cells? Besides the astrocytic stem cells of the SVZ, the biology of boundary and reactive astrocytes remains far less well understood. For instance, although reactive glia are often considered to be beneficial within a lesion [9,10], might there also be dangers associated with supporting such a plastic and potent astrocyte? We demonstrate that there are notable similarities in the stem-like behaviors of three key astrocyte subpopulations, those within the adult SVZ, those associated with injury, and a third population associated with cancer. In each case, these shared, stem-like attributes contribute to cell lineage diversity during normal and abnormal tissue genesis (Figure 1). We challenge that astrocytes, displaying the specific collection of elements of cellular immaturity and proliferation confined within an enriched ECM, define what is required for normal, and potentially aberrant, astrocytic brain tissue building.
Figure 1.
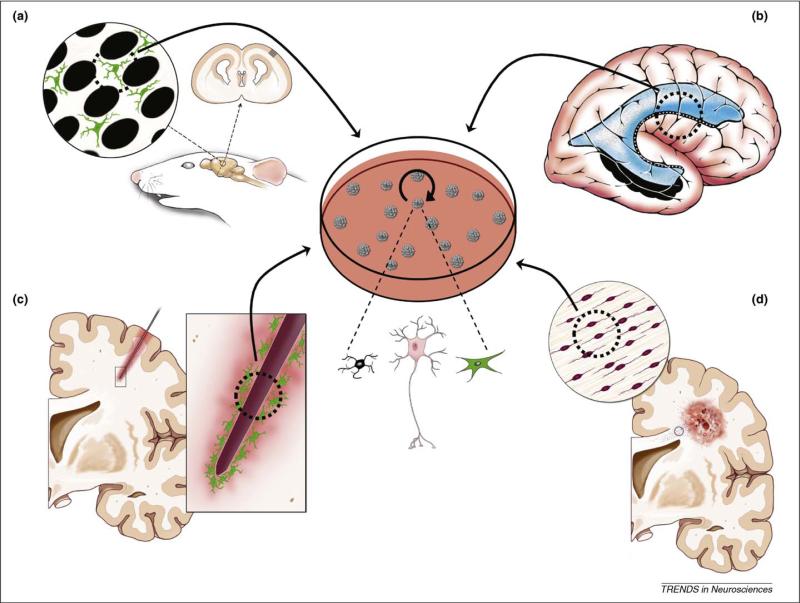
Cartoon depicting the four examples of astrocytic stem cells, in vivo and in vitro, that are the focus of this article. (a) Developmental boundary astrocytes. Boundary astrocytes present in the somatosensory cortical whisker barrel system during early postnatal development. Even though it is predicted that these cells would be both multipotent and clonogenic, and give rise to neurospheres (arrow to the central culture dish), this is, as yet, unproven. (b) Adult neurogenic astrocytes. Multipotent astrocytic stem cells (MASCs) are present in the adult neurogenic niches of the subventricular zone (SVZ) around the lateral ventricle and in the hippocampus (adapted, with permission, from Ref. [65]). These cells have been shown in numerous studies to exhibit stem-cell characteristics including self-renewal, neurosphere generation and the ability to give rise to all three neural lineages: oligodendrocytes (black cell in the center of the figure); neurons (pink cell); and astrocyes (green cell). (c) Reactive astrocytes. Injury-associated astroglial scar astrocytes attempt reactive neurogenesis after, in this case, a penetrating injury in the adult brain. These cells also exhibit multipotency, giving rise to neurospheres and all three neural lineages. (d) Tumorigenic astrocytes. In gliomas, including in this example a cortical glioblastoma, tumor-initiating astrocytic cells might not only give rise to the tumor mass but also contribute to invasion and metastasis after their migration to disparate sites. Tumorigenic astrocytes also have been shown in numerous studies to give rise to multipotent neurospheres, thus exhibiting stem-cell attributes including the contribution to neural cell lineage diversity and self-renewal.
In this article, we first highlight and discuss the curious connection between stem-like astrocytes and the enriched ECM environments in which they are invariably found (Table 1). A comparison between three distinct astrocyte cell types – those found within neonatal CNS pattern-associated boundaries (Figure 2), the adult SVZ (Figure 3) and the penumbra of a brain lesion (Figure 4) (each capable of driving cytogenesis) – will motivate this initial discussion. Second, informed by the aforementioned discussion, we speculate on gliomagenesis and the possible connection between supporting an active, potentially multipotent, cytogenic astrocyte within the brain and future glioma tumor formation (Figure 5).
Table 1.
Neural stem-like astrocytes
| Cell Type | Definition | Refs |
|---|---|---|
| Boundary astrocyte | A cell that expresses GFAP and developmentally regulated ECM molecules such as various proteoglycans (e.g. CSPG, HSPG) and glycoproteins (e.g. tenascin-C). It is observed throughout the neuraxis at the interface between two functionally different structures during pattern formation. | [28,29,32–34] |
| SVZ neurogenic astrocyte | A cell with morphological and immunophenotypic characteristics of astrocytes. Although a precise surface antigen profile is unknown, these cells are known to express nestin, GFAP and certain ECM molecules. It resides within the SVZ and contributes to olfactory neurogenesis in vivo and gives rise to clonal, self-renewing multipotent neurospheres in vitro. | [12,13,16,17] |
| Reactive astrocyte | An astrocyte that upregulates GFAP, nestin and developmentally regulated ECM proteins in response to injury or disease. Can respond by proliferating and might become neurogenic and/or cytogenic after exposure to particular environmental conditions both in vivo and in vitro. | [2,6,49,69,70] |
| Tumorigenic astrocyte | A transformed astrocytic cell found within human glioma, transgenic mouse models of glioma or in spontaneously transformed rodent SVZ astrocytes. It exhibits multipotency in vitro and contributes to tumorigenesis in vivo. | [54–56,61,71–73] |
Figure 2.
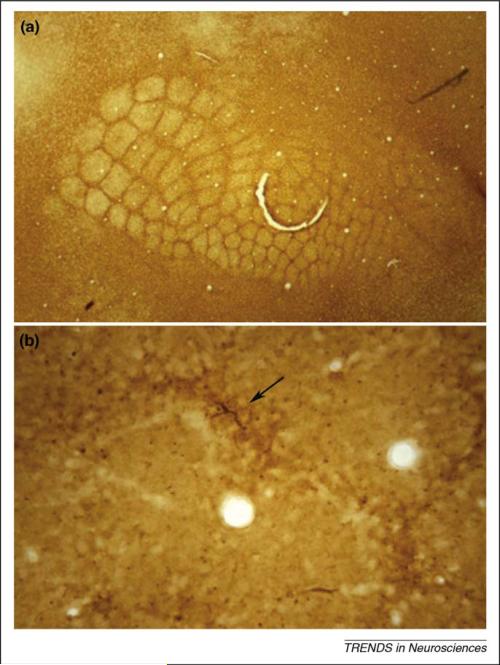
Boundary astrocytes and their expression of developmentally regulated molecules, for example tenascin-C, cordon off developing structures and functional units throughout the neuraxis. In the example presented here, somatosensory cortical barrels are demarcated by boundary astrocytes and ECM molecules. (a) In a flattened tangential section through layer IV of the postnatal day 6 mouse somatosensory cortex, immunoperoxidase staining of the ECM glycoprotein tenascin-C reveals boundaries around all five rows of posteromedial and anterolateral subfield whisker barrels. Each barrel is 200–250 μm in diameter (adapted, with permission, from Ref. [66]). (b) GFAP immunolabeling of the postnatal day 6 barrel field reveals astrocytes and their processes that distribute in boundaries around forming barrel units. A single astrocyte can be seen (see arrow) within a boundary (future inter-barrel septum) between two barrels. Barrel hollows (two transected blood vessels appear in two hollows) exhibit less GFAP staining, except for sparse transected radial glial processes that are more concentrated in the boundaries (appear as immunolabeled punctae) (adapted, with permission, from Ref. [29]).
Figure 3.
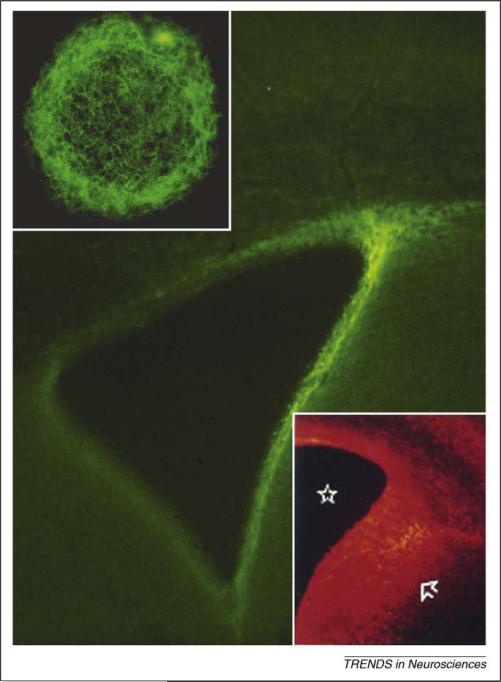
Immunofluorescence for tenascin-C (main figure) and chondroitin sulfate proteoglycans (lower inset) in the neurogenic SVZ surrounding the lateral ventricle as seen in a coronal section through the adult mouse forebrain. In contrast to the late embryonic brain (E17) (lower inset) where chondroitin sulfate proteoglycans and other extracellular matrix (ECM) molecules including tenascin-C are intensely expressed in both the SVZ and ventricular zone (region between star in the lateral ventricle and arrow) and in the overlying cortex, there is little labeling in surrounding structures including the striatum, septum and subcortical white matter. Upper inset shows a single adult human brain neurosphere, derived from an SVZ multipotent astrocytic stem cell (MASC or B-cell) immunostained for tenascin-C. There is a dense tenascin-C matrix surrounding cells of this cultured neurosphere. Figure adapted, with permission, from Refs [46,53,67].
Figure 4.
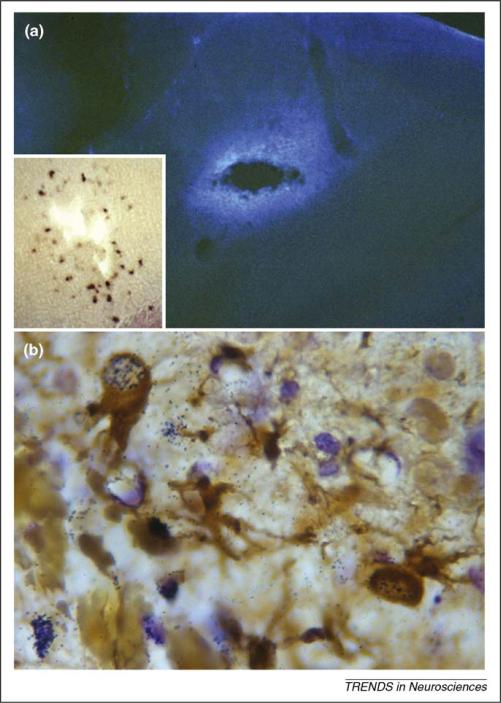
Brain lesions induce certain reactive astrocytes to proliferate and upregulate developmentally regulated ECM proteins including tenascin-C in response to injury. (a) Immunofluorescence for the tenascin-C glycoprotein after a penetrating injury to the adult mouse brain (3 day survival) results in upregulation of this ECM protein just around the injury cavity. Inset in (a) shows in situ hybridization for tenascin-C mRNA in the lesioned cortex 3 days after a stab injury just around the injury cavity (adapted, with permission, from Ref. [6]). (b) GFAP immunoperoxidase and tritiated thymidine autoradiography in the cerebral cortex of a postnatal week 3 mouse after a penetrating stab lesion (lower left side of the figure). In addition to birthday-identified vascular elements, GFAP+ reactive astrocytes (brown) near the injury site are also found to proliferate in response to the stab wound (black autoradiographic signal over their nuclei) which, along with their expression of tenascin-C, parts (a) and (b) are suggestive of a possible attempt at reactive neurogenesis. Figure adapted, with permission, from Ref. [68].
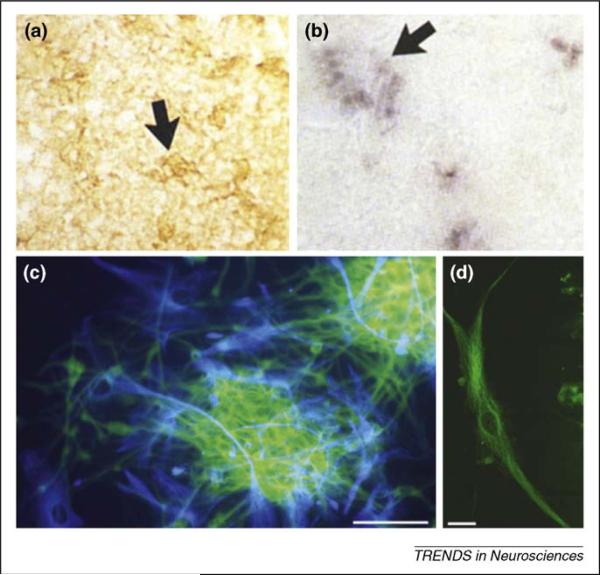
Figure 5.
Human gliomas, both in situ and in vitro, express reactive and neurogenic astrocytic cell markers. (a) and (b) Tenascin-C protein immunoperoxidase (a) and mRNA following in situ hybridization (b) show expression of the ECM protein in fixed tissue specimens from a human anaplastic astrocytoma. The dense areas of immunoperoxidase labeling in part (a) presumably correspond to clusters of tenascin-C riboprobe-positve cells from the same tumor specimen shown in part (b) (see arrows). (c) Two attached neurospheres derived from a human glioblastoma specimen indicate the diversity of cell types generated within and growing out from the attached spheres (β-III tubulin shown as green and GFAP shown as blue). (d) A single β-III tubulin+ cell from one of these spheres exhibits a morphology suggestive of migratory behavior as seen in invasive cells observed from these specimens (D.J. Silver, unpublished; see migratory cells as depicted crossing the corpus callosum in Figure 1d). These glioblastoma-derived neurospheres also express tenascin-C, in addition to other markers associated with normal astrocytic stem cells. Figure adapted, with permission, from Ref. [54]. Scale bar in part (c) = 100 μm, (d) = 10 μm.
The relationship between boundary and neurogenic astrocytes
The subgranular zone of the hippocampal dentate gyrus and the SVZ lining the lateral walls of the lateral ventricles represent the only two regions within the adult mammalian brain that support ongoing neurogenesis throughout life (Figure 1b). Within these rare germinal niches, astrocytes functioning as neural stem cells [11,12] begin the cascade of events that continually renew the granule and periglomerular interneurons of the olfactory bulb [13] and granule neurons of the adult hippocampus [14,15]. In vivo, the neurogenic process within the SVZ begins with a glial fibrillary acidic protein (GFAP)-expressing astrocytic stem cell, also known as a ‘B-cell’ [13]. These unique neurogenic astrocytes, or what we refer to as multipotent astrocytic stem cells (MASCs) [12,16–18], are the direct descendants of embryonic radial glia [19,20] and, unlike mature cortical astrocytes, maintain a thin process, including a cilium, tethering them to the lateral ventricular wall and ventricular cavity [21]. The rare and quiescent B-cells have been reported to give rise to a population of highly proliferative, however less potent, progenitor cells, referred to as transit amplifiers or ‘C-cells’. Finally, from this putative intermediate progenitor pool comes a population of young, immature neurons known as ‘A-cells’, which migrate forward over a tremendous distance through the rostral migratory stream (RMS) before a select few mature and integrate into the olfactory bulb neural circuitry as new interneurons [13,22,23]. Interestingly, a recent publication by Danilov and colleagues [24] has challenged the canonical B → C → A cell genesis cascade, suggesting that the multipotent B-cell astrocyte might actually give rise to its neuroblast progeny directly, without the C-cell intermediate. Coincidently, these results are in accord with our own in vitro model of SVZ neurogenesis, wherein cultured astrocytic stem cells are seen to robustly and directly generate a population of young neurons [25]. Whether the B-cell astrocyte is directly neurogenic or requires a less potent intermediary, its activity is limited to the specific regions within the adult brain where the expression of the ECM is greatest. Under normal circumstances, the ECM molecules CSPG, HSPG and tenascin-C are all intensely expressed strictly within the SVZ in the adult brain (Figure 3). Thus, the coincidence of enriched matrix and persistent cell genesis, although still largely unquantified, must assuredly be of fundamental importance [26,27].
In the developing neonatal brain, neurogenesis is not quite so limited, yet it is still defined by a germinal ECM. Over 20 years ago, our laboratory and others described transient patterns of enriched ECM expression within the early postnatal rodent brain. These matrix patterns seemed to outline functionally different emerging brain structures and, as such, engendered substantial controversy over their particular role during brain development (for review, see Ref. [28]). During the course of these investigations, a subpopulation of astrocytes was brought to light that were consistently found intimately associated with these patterns. Situated within the ECM boundary itself, these unique cells became known as boundary-associated astrocytes or, simply, ‘boundary astrocytes’ (Figures 1a and 2). Although the original finding characterized these astrocytes within the developing somatosensory cortical barrel field [29,30] (Figures 1a and 2b), subsequent investigations ultimately revealed boundary astrocytes associated with patterning neostriatal striosomes and subcortical brainstem nuclei [31], emerging olfactory glomeruli [32], the laminating optic tectum [33] and the roof plate of the embryonic spinal cord [34], suggesting that these interesting cells might be present extensively across the entire neuraxis. At the time, we were unsure of the importance of these cells. With their characteristic hypertrophic GFAP-expressing cytoskeleton and accompanied expression of the neurite growth inhibitory molecules CSPG and tenascin-C, the boundary astrocyte intimated a strong resemblance to the so-called ‘reactive’ astrocyte of the adult brain. Interestingly, as we elaborate later, only now, in light of our current understanding of cytogenic astrocytes, have we begun to appreciate that the behavioral and molecular hallmarks of neural stem cells might be present in the boundary astrocyte population.
At first glance, boundary astrocytes might seem to be a bit out of place in the context of a stem cell story. They are certainly not, as yet, members of the neural stem cell canon. After all, although these unique cells express many of the same immunophenotypic markers commonly associated with neural stem and progenitor cells, they have not yet been isolated ex vivo and tested for their ability to repeatedly self-renew and generate clonal multipotent progeny. We believe that boundary astrocytes are potential stem cells: cells that typically do not function as stem cells but are capable of doing so under certain conditions [35]. We've based this hypothesis on the following pieces of correlative evidence. First, similar to the B-cell of the adult mammalian SVZ [19,20], boundary astrocytes are directly descended from radial glia – the first genuine stem cells of the developing CNS [36–38]. Evidence for this descent is clear based on our early morphological analyses of mouse whisker barrel boundary astrocytes. Very early on, observable only during the first weeks of postnatal development, these unique cells echo one of the hallmarks of radial glial morphology – they secure a direct connection to the pial surface by way of a lengthy radial process. The direct descent from radial glia is important because it hints at the possibility that the key set of intrinsic genetic and epigenetic factors required for stemness has been transferred more-or-less completely to boundary astrocytes [39]. Just as the direct descendant of the B-cell (i.e. the C-cell) is more primitive than its neuroblast progeny [13], might the boundary astrocyte also maintain a more primitive, stem-cell-like quality than other more mature astrocytes? At this point, this aspect of our argument is more of a thought experiment than anything else. Although clearly not definitive, we are intrigued by this genealogical parallel and what it might mean regarding stemness in the population of boundary astrocytes.
It would be presumptive to state that simply having a lineage relationship with radial glia predicates stemness. After all, ventricular ciliated ependymal cells are lineage associated with radial glia [40], and both our own laboratory [12] and others [13,16] have convincingly ruled out the possibility of an ependymal neural stem cell within the brain. Interestingly, Frisen and collaborators [41] have recently presented compelling evidence that ependymal cells, in contrast to the situation within the brain, might mediate a reactive cytogenesis after spinal cord injury. Nonetheless, focusing specifically on the brain, additional evidence presented by Laywell and colleagues [12] bolsters our argument that these unique cortical boundary astrocytes might have the capability of displaying neural stem-like character. Laywell and colleagues challenged astrocytes (isolated from various regions within the late embryonic, early postnatal and adult mammalian CNS) with the neurosphere assay – an in vitro bioassay used to test these tissues for the presence of neural stem-like cells [42]. Remarkably, the authors found that until the close of the second postnatal week, the cerebral cortex harbors a population of astrocytic cells, which displays the neural stem-cell attribute of neurosphere formation [12]. Notably, during this same time period, the expression of the germinal ECM undergoes a dramatic shift in expression pattern and intensity. Initially expressed broadly at high levels (Figure 3, lower inset), the matrix is progressively spatially restricted until it exclusively defines the adult SVZ (Figure 3). Put another way, cortical boundary astrocytes retract their radial glial-like connections to the pia and undergo other aspects of terminal differentiation in precisely the same interval of time that coincides with the end of the critical window for cortical neurosphere formation and the withdrawal of the germinal ECM into the adult germinal center [43]. Is the boundary astrocyte the neurosphere-forming cell of the neonatal cortex? As we stated before, the boundary astrocyte has never been directly isolated and assessed for neural stemness; however, taken together, these two lines of inquiry strongly suggest that, given the proper conditions, boundary astrocytes might represent a unique cortical and/or CNS potential stem cell.
Above and beyond their radial glial ancestry, the idea that cortical boundary astrocytes might be potential stem cells is motivated by the second key feature at the center of this perspective: the highly unique makeup of the ECM environment in which neural stem and/or progenitor cells are found. There is a growing body of literature that suggests a connection between the stemness of a cell and the ECM environment in which it is maintained (for review, see Ref. [26,44]). For example, consider the adult mammalian SVZ and RMS. The cells of the SVZ and RMS are confined within a uniquely matrix-rich environment – an environment in which the ECM molecules HSPG and CSPG and glycoproteins such as tenascin-C [27,45,46] are strongly expressed. Although the precise part played by the environment in SVZ neurogenesis remains unknown, we contend that these surroundings serve to physically sequester the cells of the niche, preserving them in a state of perpetual immaturity. Similarly, boundary astrocytes of the early postnatal cortex themselves reside in an enriched matrix environment, which is strikingly similar to that of the adult germinal SVZ. Consider, for example, early postnatal murine somatosensory cortical boundary astrocytes; these particular boundary astrocytes reside in the spaces, or septae, that will eventually demarcate the walls of the adult murine whisker barrel field (Figures 1a and 2b). In the neonatal brain, these highly patterned spaces are defined by their intense expression of certain lectin-binding glycoconjugates and confine the boundary astrocytes like the neurogenic cells of the SVZ and RMS are confined. If the neural stem cell character of the SVZ B-cell is dependent, at least in part, on this unique primordial ECM environment, might such an environment support this same character in the boundary astrocyte? We concede that environment is, as yet, an unproven metric for stemness, especially in these days of the induced pluripotent stem cell [47,48] where the intrinsic programming of a cell has cast a long shadow over less well-quantified extrinsic influences.
Reactive neurogenic astrocytes in injury or disease
What becomes of the neonatal boundary astrocyte after the downregulation of the ECM boundaries? Might there be a carry-over of these interesting cortical astrocytes in the adult brain? One of the main hallmarks of a lesion to the adult CNS is the glial scar (for review, see Ref. [49]). Interestingly, glial scarring is associated with an enriched ECM – an environment that is highly reminiscent to the SVZ and to neonatal pattern-associated boundaries. Similar to boundary astrocytes, astrocytes within and surrounding the lesion, referred to as reactive astrocytes, are identified by exaggerated or hypertrophic GFAP-expressing processes. Additionally, reactive glia begin to express many of the same markers commonly associated with the neurogenic stem and progenitor cells of the SVZ and, to a lesser extent, the boundary astrocytes of the neonatal cortex. Furthermore, a few (only those immediately adjacent to the lesion itself) begin to secrete the matrix proteins that define the lesion (Figure 4a, inset). At this point, it is crucial to note that this discussion pertains exclusively to a unique subpopulation of reactive astrocytes. Only those reactive astrocytes that directly circumscribe the lesion and adopt the aforementioned profile (i.e. cellular immaturity, proliferation and ECM production) are germane (Figures 1c and 4). Previously, it has been postulated that the re-expression of these primitive markers suggests these select reactive glia are potential stem cells [1]. Furthermore, we propose that the boundary astrocytes and the specific reactive astrocytes of interest are similar, lineally related cell types. Both cell types present an immature astroglial phenotype within a common matrix environment, suggesting that both the intrinsic and extrinsic factors required for stemness are accounted for. The Götz laboratory [2] has recently proven this postulate correct in an elegant article describing the neural stem-like attributes of reactive glia both in vitro and in vivo.
The notion of astroglial scar formation and the reappearance of boundary molecules intimates the possibility that brain injury or disease might re-induce normally highly controlled developmental programs for neurogenesis. Penetrating brain injuries result in astroglial scars that exhibit an enhanced expression of ECM proteins including tenascin-C and various proteoglycans [6,7,50] (Figure 4a, and inset). It is now accepted that within the glial scar there are cycling cells that exhibit an enhanced expression of astrocytic GFAP [2]. These recent results synergize with earlier studies showing reactive astrocytes exhibiting an enhanced expressed of the neural stem cell marker nestin [5]. There is now no question that after brain injury or disease, the release of growth factors, cytokines and other factors from at-risk or dying cells, in addition to vascular-and immune-related elements, might support a reactive cyto- or neurogenesis.
In the report by Buffo and colleagues [2], the authors combined a powerful new conditional glutamate aspartate transporter (GLAST) transgenic mouse and astrocyte-targeted lentiviral vectors. In doing so, they were able to discern cortical reactive astrocyte de-differentiation (‘upregulating developmental features’) in the injured adult mouse cortex. The authors showed that penetrating cortical lesions induced proliferation of the local, resident astrocyte population that contributed to reactive astrogliosis and that:
‘...the adult cerebral cortex mature astrocytes lack expression of GFAP, nestin, vimentin, and tenascin-C and do not proliferate. However, these proteins are contained in some reactive glial cells, some of which proliferate...and in more immature glia such as radial glia and postnatal glial progenitors, which also proliferate...Because our fate mapping analysis now reveals that reactive astroglia derive from mature astrocytes, these data suggest that astrocytes exposed to injury may indeed resume properties of glia present at earlier developmental stages...’ [2].
Whether these cells are in fact lineage associated with radial glia, boundary astrocytes or B-cell astrocytes remains to be determined. However, their common developmental behaviors, including an ability to maintain proliferation and execute neurogenic programs, along with distinct developmental molecular expression patterns, suggests that they hold promise for successful CNS regeneration and exuberant growth, for example neoplasia (elaborated on later). We propose that the boundary astrocyte, the B-cell astrocyte and the reactive astrocyte have similar if not identical phenotypes in a common matrix environment.
Abnormal astrocytic neurogenesis after oncogenic transformation
Why should a cell that is responsible for protecting and facilitating neural function, for example by generating all of the cells of the brain during development or contributing to adult neurogenesis, change its job and contribute to tissue overgrowth? Stem cells do have an innate purpose of generating tissue, and there is no question that altered oncogene expression, loss of tumor suppressor genes, epigenetics and environmental carcinogens can result in such potent cells changing their nature and generating too much tissue. As discussed by Weissman and others [51], it is also possible that cancer-initiating cells take on stem-cell characteristics rather than starting out as stem cells gone awry but, as discussed earlier, certain astrocytic cells clearly exhibit a variety of structural and behavioral phenotypic changes during development and injury that create the potential to become hyperplastic [52].
Clonal neurospheres derived from the human brain exhibit a profound heterogeneity of stem and progenitor cells when derived from the neurogenic SVZ and hippocampus [53]. Along with their ardent responsiveness to changes in growth conditions, this could likewise lead to distinctive examples of cell lineage diversity of their progeny after oncogenic transformation, for example, the varieties of cell phenotypes seen in the gliomas, especially glioblastoma (Figures 1d and 5c,d). Glioma stem-like cells were originally discovered in our studies that exploited the same neurosphere culture approaches used for studying normal neural stem cells [54], and these findings were corroborated and expanded upon in numerous studies that followed showing the presence of stem cell-like, tumor-initiating cells in human glioblastoma multiforme (GBM) [55–57]. In our study, as seen in studies of normal adult human brain stem and/or progenitor cells, cells expressed transcripts and proteins associated with stemness including tenascin-C, notch and nestin (a transcriptome indicative of common programs for growth and differentiation between normal SVZ B-cells, reactive astrocytes and astrocytes found within gliomas) (Figure 5a,b). To this end, it is interesting that an anti-tenascin-C approach has been developed for treating human gliomas [58].
In a general sense, the underlying function of any stem cell, be it a potential stem cell [35] (e.g. a boundary or reactive astrocyte) or an actual stem cell (e.g. a B-cell) is to generate tissue; however, concomitant with this function is an inherent vulnerability. Active cell division opens a cycling cell to the possibility of accumulating mutations and aneuploid divisions – the consensus inroads to oncogenic transformation. With reactive glia, we're presented with a primitive, stem-like cell; this is a cell fully capable of driving cytogenesis [50] but outside of the self-governing confines of the adult neurogenic niches [23]. In this final section, we explore the possible consequences of reactive astrocytic cytogenesis specifically related to brain cancers and the cancer stem cell hypothesis.
In 1858, the ‘father of cellular pathology’, Rudolf Virchow, began work on the embryonal-rest theory of cancer. Based only upon his own detailed histological analyses, Virchow appreciated similarities between the cell types present in certain adult tumors and those of the developing embryo. These likenesses lead to the conclusion that cancers were the inevitable byproducts of displaced embryonic tissues. In 1875, his student Julius Cohnheim extended and clarified this hypothesis. Cohnheim postulated that adult tissues harbor a residue of dormant embryonic cells or ‘embryonal rests’. These resting, otherwise silent cells, when made active, hold the potential to develop into cancers. These brilliantly intuitive ideas, first articulated a century and a half ago, speak directly to modern discussions of the cancer stem-cell hypothesis.
There is debate within the cancer field concerning the existence and nature of so-called cancer stem cells (for review, see Refs [51,59,60]). There are those who, similar to Virchow and Cohnheim, argue that cancer is the product of an initially stem-like cell gone awry. Although the current literature no longer refers to these cells as ‘embryonal rests’, they are nonetheless resident, genetically aberrant, tissue-specific stem cells. Alternatively, investigators have also entertained the idea of a once-mature, newly dedifferentiated cell that has, in the process of aberrant dedifferentiation, acquired stem-cell characteristics. However, considering this perspective on stem-like reactive glia, a third option emerges: rather than the novel acquisition of stem cell characteristics, in reactive glia we might be dealing more accurately with the reacquisition of dormant stem cell properties. Furthermore, because of this trait, we contend that aberrant reactive glia might serve as a novel biological substrate for brain cancer initiation and provide a yet unexplored therapeutic target for brain cancer research.
Bachoo and colleagues [61] have inadvertently provided strong evidence for a reactive astrocytic tumor-initiating cell. Working to elucidate the molecular mechanisms underscoring the most common and most devastating adult brain cancer (grade IV astrocytoma, also known as GBM), these investigators chanced upon a fundamental mechanism used by mature astrocytes to preserve terminal differentiation. The authors reasoned, based upon the genetic profile of an ‘average’ GBM, that three commonly altered factors (the p16INK4a and p19ARF tumor suppressors and the epidermal growth factor [EGF] receptor) might be responsible for oncogenic cellular transformation and resultant gliomagenesis. Remarkably, beginning initially in mature astrocytes with a dual knockout, the authors found that the combined loss of p16INK4a and p19ARF resulted in dedifferentiation back to a more primitive, putatively stem-like state. When induced by the forced overexpression of the EGF receptor, INK4a/ARF–/– transgenic knockout mice developed a GBM-like tumor. Ironically, Bachoo and investigators perhaps wrongfully concluded that the driving force behind the tumor formation was the synergy of the three genetic mutations rather than the aberrant astrocyte cell of origin. Because the Götz laboratory [2] would not confirm reactive astrocytic cytogenesis for another six years, we certainly cannot fault these authors such a conclusion; however, in light of current findings, we would like to offer a reinterpretation of these data. We challenge that Bachoo and colleagues essentially generated an aberrant reactive astrocyte. They clearly established that p16 and p19 signaling has a vital role in preserving terminal differentiation in mature glia. If these loci are compromised, the affected astroglia dedifferentiate back to a more primitive stem-like state. In the context of a lesion, the ECM surroundings presumably concentrate growth factors around these deregulated reactive astrocytes. In conjunction, secondary EGF receptor expression activation exacerbates the proliferative potential of these cells, which, in this case, becomes highly problematic. Ultimately, these cells are induced into a highly mitotic state, proliferating in an uncontrolled manner that ultimately manifests as glioma.
Our reinterpretation of the conclusions drawn by Bachoo and colleagues, namely that certain reactive astrocytes might serve as a substrate for oncogenic transformation, inflammatory as it might be, is not without precedent in the literature. The first line of support coincidently comes from an independent follow-up study to the Bachoo report. Using the same p16–/p19–/EGFRHigh genetic lesion model introduced in the Bachoo study, Ligon and colleagues demonstrated that ablating the basic helix–loop–helix transcriptional repressor protein Olig2 in these already genetically aberrant mice successfully abrogated the onset of gliomagenesis [62]. Such a robust result is powerfully striking in and of itself; however, additional work championed by Chen and colleagues the following year brings these results in line with our aforementioned reinterpretation. Chen and colleagues [4] presented a careful examination of the part played by Olig2 in reactive astrogliosis after cortical injury. Strikingly, when these investigators ablated Olig2 specifically from the GFAP+ reactive astrocyte compartment of the lesion, they noted a significant decrease in the proliferation of these lesion-associated reactive glia. Moreover, the authors illustrate that the once proliferative reactive astrocytes affected by the Olig2 ablation are the same nestin+, vimentin+ potentially stem-like reactive glia at the center of the argument presented here. Thus, the same genetic lesion that completely abolishes glioma tumor-formation in the p16–/p19–/EGFRHigh animal confers a similar suppression of proliferation specifically to reactive astrocytes. Fraser and colleagues [63] provided a second line of support for our re-analysis of the Bachoo findings. The authors presented a careful analysis of another genetic lesion common to many cancers including glioblastoma: loss of the tumor suppressor gene PTEN (phosphatase and tensin homolog). Remarkably, PTEN ablation specifically from within the GFAP-expressing astrocyte compartment generated primitive (i.e. nestin+, vimentin+), putatively stem-like reactive astrocytes with a significantly enhanced proliferative potential, along with hypertrophy of cells and enlarged brains. As noted by the authors, altering genes such as PTEN can ‘...render astroglial cells susceptible to neoplastic transformation or malignant progression...’ Thus, reactive astrocytes have proven uniquely sensitive to many of the constellation of specific genetic aberrations commonly associated with glioma tumorigenesis, bolstering our reinterpreted hypothesis and presenting them as likely candidates for oncogenic transformation.
We've chosen to present the aforementioned study and our reinterpretation in the interests of scholarly provocation. It suggests the possibility that a reactive astrocyte (a cell that has been induced back to a more immature state) could acquire the constellation of mutations necessary for oncogenic transformation. Furthermore, it hints at the possibility that such an aberrant reactive astrocyte could be bolstered by extrinsic factors in its environment. However, it is also somewhat artificial in that the glial reactivity and the acquisition of the cancer-initiating phenotype is genetically engineered rather than chanced upon spontaneously. There is an interesting clinical observation linking prior traumatic brain injury to the onset of certain brain cancers later in life. Presumably, a few of the reactive glia associated with such an injury could provide the biological substrate for transformation. Hasegawa and colleagues [64] have made an instructive attempt to bring clarity and weight to such a clinical correlate of aberrant reactive astrocytic tumor initiation. The authors reasoned, just as we've done here with SVZ neurogenesis, that reactive astrocytic cytogenesis should be the product of an interplay between intrinsic and extrinsic factors. As such, the output of a tumorigenic reactive astrocyte might be bolstered if presented with the germinal-like environment of a glial scar and dampened when presented with the unsupportive environment of a healthy CNS. The authors transplanted an astrocytic rodent glioma cell line (C6 glioma) into a control and into previously contused rodent spinal cords. Invariably, within only 6 weeks of engraftment, the previously injured animals presented with hemorrhagic tumors whereas the uninjured controls did not. The reactive injury-associated environment thus provides a molecular niche that seems to be conducive for tumor growth, again containing cellular and molecular elements in common with the normal tissue-generating environment of CNS developmental (boundaries) and adult (SVZ and astroglial scar) neurogenesis.
Conclusion
In conclusion, we've presented a synthesis of the current neural stem and cancer stem-cell literature while attempting to make the case for two interrelated hypothetical ideas. First, stemness is the result of an inextricable link between seed and soil – between cell and environment. We've argued that tissue generation emerges from a combination of cell-intrinsic developmental programming and the primordial environment fostered by an enriched ECM. We've illustrated that this rare combination is present within neonatal CNS patterning boundaries, the adult SVZ and the penumbra of an injury to the CNS – only those specific regions to which we attribute the presence of neural stem or stem-like cells and the process of neurogenesis. Second, we've presented our interpretation of a possible consequence of reintroducing a tissue-generating cell back into the adult brain. More specifically, we've presented a possible ontogeny for glioblastoma, beginning from a sub-population of stem-like boundary astrocytes that might subsequently re-emerge as transformed stem-like reactive glia within the adult brain. Ultimately, the re-emergence of these immature, aberrant stem-like astrocytes might regrettably offer a substrate for neoplastic transformation. Thus, because these three cell types – the neonatal boundary astrocyte, the post-natal reactive astrocyte and the astrocytic tumor initiator – might actually be three separate manifestations of essentially the same cell, they might represent future targets for therapeutics aimed at encouraging appropriate reactive neuro-genesis after injury or disease and limiting the extent of neoplastic transformation at the root of gliomagenesis.
Acknowledgements
We thank our collaborators and colleagues who helped contribute insights to this article. We thank David Peace for his invaluable assistance in graphics generation. We thank the McKnight Brain Research Foundation (www.mbi.ufl.edu) for support, and the National Institute of Neurological Disorders and Stroke (www.ninds.nih.gov) and The National Institute of Diabetes and Digestive and Kidney Diseases (www.niddk.nih.gov) for grant support of our research.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Steindler DA, et al. Boundary molecules during brain development, injury, and persistent neurogenesis – in vivo and in vitro studies. Prog. Brain Res. 1998;117:179–196. doi: 10.1016/s0079-6123(08)64016-9. [DOI] [PubMed] [Google Scholar]
- 2.Buffo A, et al. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takamiya Y, et al. Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats. Brain Res. 1988;466:201–210. doi: 10.1016/0165-3806(88)90045-4. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, et al. The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J. Neurosci. 2008;28:10983–10989. doi: 10.1523/JNEUROSCI.3545-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin RC, et al. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol. Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- 6.Laywell ED, et al. Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2634–2638. doi: 10.1073/pnas.89.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeon RJ, et al. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Properzi F, et al. Heparan sulphate proteoglycans in glia and in the normal and injured CNS: expression of sulphotransferases and changes in sulphation. Eur. J. Neurosci. 2008;27:593–604. doi: 10.1111/j.1460-9568.2008.06042.x. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann JE, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myer DJ, et al. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 11.Imura T, et al. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J. Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laywell ED, et al. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 14.Seri B, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiasson BJ, et al. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J. Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia AD, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 18.Zheng T, et al. Neurogenic astrocytes transplanted into the adult mouse lateral ventricle contribute to olfactory neurogenesis, and reveal a novel intrinsic subependymal neuron. Neuroscience. 2006;142:175–185. doi: 10.1016/j.neuroscience.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 19.Merkle FT, et al. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 20.Merkle FT, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doetsch F, et al. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishi K. Golgi studies on the development of granule cells of the rat olfactory bulb with reference to migration in the subependymal layer. J. Comp. Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- 23.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danilov AI, et al. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia. 2009;57:136–152. doi: 10.1002/glia.20741. [DOI] [PubMed] [Google Scholar]
- 25.Scheffler B, et al. Phenotypic and functional characterization of adult brain neuropoiesis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 27.Kerever A, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 28.Steindler DA. Glial boundaries in the developing nervous system. Annu. Rev. Neurosci. 1993;16:445–470. doi: 10.1146/annurev.ne.16.030193.002305. [DOI] [PubMed] [Google Scholar]
- 29.Cooper NG, Steindler DA. Monoclonal antibody to glial fibrillary acidic protein reveals a parcellation of individual barrels in the early postnatal mouse somatosensory cortex. Brain Res. 1986;380:341–348. doi: 10.1016/0006-8993(86)90232-5. [DOI] [PubMed] [Google Scholar]
- 30.Cooper NG, Steindler DA. Lectins demarcate the barrel subfield in the somatosensory cortex of the early postnatal mouse. J. Comp. Neurol. 1986;249:157–169. doi: 10.1002/cne.902490204. [DOI] [PubMed] [Google Scholar]
- 31.Steindler DA, et al. Glycoconjugate boundaries during early postnatal development of the neostriatal mosaic. J. Comp. Neurol. 1988;267:357–369. doi: 10.1002/cne.902670306. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez Mde L, et al. Role of astroglial extracellular matrix in the formation of rat olfactory bulb glomeruli. Exp. Neurol. 1993;123:91–105. doi: 10.1006/exnr.1993.1143. [DOI] [PubMed] [Google Scholar]
- 33.Miskevich F. Laminar redistribution of a glial subtype in the chick optic tectum. Brain Res. Dev. Brain Res. 1999;115:103–109. doi: 10.1016/s0165-3806(99)00048-6. [DOI] [PubMed] [Google Scholar]
- 34.Snow DM, et al. Molecular and cellular characterization of the glial roof plate of the spinal cord and optic tectum: a possible role for a proteoglycan in the development of an axon barrier. Dev. Biol. 1990;138:359–376. doi: 10.1016/0012-1606(90)90203-u. [DOI] [PubMed] [Google Scholar]
- 35.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 36.Hartfuss E, et al. Characterization of CNS precursor subtypes and radial glia. Dev. Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 37.Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J. Comp. Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- 38.Noctor SC, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 39.Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J. Neurosci. 1997;17:8300–8312. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spassky N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meletis K, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- 43.Cooper NG, Steindler DA. Critical period-dependent alterations of the transient body image in the rodent cerebral cortex. Brain Res. 1989;489:167–176. doi: 10.1016/0006-8993(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 44.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercier F, et al. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J. Comp. Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 46.Thomas LB, et al. Young neurons from the adult subependymal zone proliferate and migrate along an astrocyte, extracellular matrix-rich pathway. Glia. 1996;17:1–14. doi: 10.1002/(SICI)1098-1136(199605)17:1<1::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 49.Silver J, Miller JH. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 50.Brodkey JA, et al. Focal brain injury and upregulation of a developmentally regulated extracellular matrix protein. J. Neurosurg. 1995;82:106–112. doi: 10.3171/jns.1995.82.1.0106. [DOI] [PubMed] [Google Scholar]
- 51.Passegue E, et al. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl. Acad. Sci. U. S. A. 2003;100(Suppl. 1):11842–11849. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng T, et al. Transplantation of an indigenous neural stem cell population leading to hyperplasia and atypical integration. Cloning Stem Cells. 2002;4:3–8. doi: 10.1089/153623002753631995. [DOI] [PubMed] [Google Scholar]
- 53.Suslov ON, et al. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ignatova TN, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 55.Galli R, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 56.Hemmati HD, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 58.Reardon DA, et al. Antitenascin-C monoclonal antibody radioimmunotherapy for malignant glioma patients. Expert Rev. Anticancer Ther. 2007;7:675–687. doi: 10.1586/14737140.7.5.675. [DOI] [PubMed] [Google Scholar]
- 59.Adams JM, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008;68:4018–4021. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
- 60.Vescovi AL, et al. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 61.Bachoo RM, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 62.Ligon KL, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fraser MM, et al. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 64.Hasegawa K, Grumet M. Trauma-induced tumorigenesis of cells implanted into the rat spinal cord. J. Neurosurg. 2003;98:1065–1071. doi: 10.3171/jns.2003.98.5.1065. [DOI] [PubMed] [Google Scholar]
- 65.Steindler DA, Pincus DW. Stem cells and neuropoiesis in the adult human brain. Lancet. 2002;359:1047–1054. doi: 10.1016/S0140-6736(02)08096-0. [DOI] [PubMed] [Google Scholar]
- 66.Steindler DA, et al. Boundaries defined by adhesion molecules during development of the cerebral cortex: the J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Dev. Biol. 1989;131:243–260. doi: 10.1016/s0012-1606(89)80056-9. [DOI] [PubMed] [Google Scholar]
- 67.Gates MA, et al. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J. Comp. Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 68.Laywell ED, Steindler DA. Boundaries and wounds, glia and glycoconjugates. Cellular and molecular analyses of developmental partitions and adult brain lesions. Ann. N. Y. Acad. Sci. 1991;633:122–141. doi: 10.1111/j.1749-6632.1991.tb15603.x. [DOI] [PubMed] [Google Scholar]
- 69.Reier PJ, Houle JD. The glial scar: its bearing on axonal elongation and transplantation approaches to CNS repair. Adv. Neurol. 1988;47:87–138. [PubMed] [Google Scholar]
- 70.Steindler DA, Laywell ED. Astrocytes as stem cells: nomenclature, phenotype, and translation. Glia. 2003;43:62–69. doi: 10.1002/glia.10242. [DOI] [PubMed] [Google Scholar]
- 71.Uhrbom L, et al. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62:5551–5558. [PubMed] [Google Scholar]
- 72.Quiñones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp. Neurol. 2007;205:313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Siebzehnrubl FA, et al. Spontaneous in vitro transformation of adult neural precursors into stem-like cancer cells. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00189.x. DOI: 10.1111/j.1750-3639.2008.00189 ( www.interscience.wiley.com) [DOI] [PMC free article] [PubMed]