Abstract
Rationale
This phase I study was conducted to determine the maximum tolerated dose (MTD) of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, with 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) in patients with advanced colorectal cancer (CRC). Bevacizumab was later included as standard of care at the MTD.
Patients and Methods
Patients received FOLFOX4 with escalating doses of erlotinib: dose level (DL) 1, 50 mg; DL 2, 100 mg; and DL 3, 150 mg once daily continuously. Bevacizumab 5 mg/kg days 1 and 15 was added at the MTD upon Food and Drug Administration approval. Correlative studies included pharmacokinetics, pharmacodynamics was assessed in paired skin biopsies, and fluorodeoxyglucose positron emission tomography scans.
Results
Fifteen patients received 60 cycles (120 FOLFOX treatments). Two dose-limiting toxicities (DLTs) were seen at DL 3: intolerable grade 2 rash (Common Terminology Criteria for Adverse Events version 2) lasting > 1 week, and grade 4 neutropenia. Dose level 2 was expanded to 6 more patients, this time adding bevacizumab, and 1 DLT of grade 3 mucositis occurred. As expected, the primary toxicities were cytopenias, diarrhea, rash, and fatigue. There were 2 occurrences of pneumatosis. One patient experienced an unrelated grade 4 myocardial infarction before starting chemotherapy. No pharmacokinetic drug interactions were observed. The Response Evaluation Criteria in Solid Tumors response rate was 11 of 14 (78%), median progression-free survival was 9.5 months, and median overall survival was 30 months. Three patients are currently alive > 3 years, with 1 having no evidence of disease.
Conclusion
The MTD of erlotinib with FOLFOX4 with or without bevacizumab is 100 mg daily. The regimen appeared to increase toxicity but showed activity in patients with CRC.
Keywords: Epidermal growth factor receptor, Pharmacokinetics, Tyrosine kinase
Introduction
Colorectal cancer (CRC) is the second-leading cause of cancer death in both sexes in the United States, accounting for over 51,370 deaths in 2010.1 Although 6 new drugs have been approved for CRC in the past decade, including both cytotoxic agents and targeted therapies, the 5-year survival remains in the single digits.2 Moreover, though only a subset of patients benefit from each drug or drug combination, the determinants of benefit and the exact effects of anticancer agents on tumor tissues remain major unknowns. New agents and strategies are desperately needed.
The epidermal growth factor receptor (EGFR) is a validated target in multiple cancer types, including CRC.3 Two strategies to block the proproliferative, prometastatic, and proangiogenic signaling of EGFR include monoclonal antibodies (MoAbs) and small molecules. Monoclonal antibodies bind to the extracellular domain of EGFR in the inactive configuration and compete for ligand binding, whereas small molecules reversibly compete with adenosine triphospate for the intracellular tyrosine kinase (TK) domain of EGFR and block activation on that basis. No small-molecule inhibitors are Federal Drug Administration (FDA)–approved for CRC. There are 2 EGFR-targeting MoAbs approved for chemotherapy- resistant CRC: cetuximab4 and panitumumab.5 Recent studies have shown that patients harboring a KRAS mutation do not benefit from MoAbs,6 and that combining these MoAbs with triple-agent combination chemotherapy including the vascular endothelial growth factor (VEGF)–targeting drug bevacizumab actually worsens patient outcomes.7,8
Erlotinib is a small-molecule TK inhibitor against EGFR approved for chemotherapy-resistant lung cancer9 and previously untreated pancreatic cancer in combination with gemcitabine.10 In lung cancer, it has been shown that specific activating mutations in EGFR confer sensitivity to small-molecule inhibitors such as gefitinib, 11,12 but these mutations in CRC are exceedingly rare.12 The response rates (RRs) of monotherapy trials for both gefitinib13,14 and erlotinib15 have been negligible in CRC. Phase II combination studies in patients with CRC, however, have shown fairly impressive activity of erlotinib combined with capecitabine/oxaliplatin16,17 and 5-fluorouracil (5-FU)/leucovorin (LV)/oxaliplatin (FOLFOX)/ bevacizumab,18 and gefitinib with FOLFOX19–22 and capecitabine/ oxaliplatin.23 Interestingly, some combination regimen trials have reported excessive toxicity, including our own 5-FU/LV/irinotecan (FOLFIRI)/erlotinib study,24 FOLFIRI/gefitinib,25,26 mXELOX (modified capecitabine/oxaliplatin) or mFOLFOX/bevacizumab/ erlotinib,27 and bolus irinotecan/5-FU/LV (IFL)/gefitinib.28 Very few of these studies have incorporated either pharmacokinetic or pharmacodynamic objectives. In addition, none of these regimens has been advanced to definitive phase III testing.
The purpose of this study was to determine the maximum tolerated dose (MTD) of erlotinib in combination with FOLFOX4 (with bevacizumab added at the MTD confirmation portion of the study in keeping with standard of care) in patients with firstor second-line advanced CRC. Secondary objectives included pharmacokinetic analysis of erlotinib when given alone and in combination with FOLFOX4, fluorodeoxyglucose positron emission tomography (FDG-PET) scans to predict biologic effects and patient outcomes, and to explore the biologic effects of erlotinib using serial skin biopsies and plasma EGFR.
Patients and Methods
Eligibility
Following Institutional Review Board approval, patients with histologically confirmed advanced colorectal adenocarcinoma with or without previous treatment were enrolled in this study. Eligibility criteria also included the following: age ≥ 18 years (no upper limit); Eastern Cooperative Oncology Group performance status of 0 or 1; life expectancy > 3 months; measurable disease (defined as > 1 cm on spiral computed tomography [CT] scan); adequate organ function including bone marrow (leukocytes > 3000/μL, absolute neutrophil count ≥ 1500/μL, platelets > 100,000/μL), liver (total bilirubin < 2.0 mg/dL, aspartate aminotransferase/ alanine aminotransferase < 2.5 × upper limit of normal (ULN) for patients without liver metastases, < 5 × ULN for patients with liver metastases), coagulation (partial thromboplastin time < 40 seconds, prothrombin time (PT) < 2 seconds more than ULN), and kidneys (serum creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 60 mL/ min/1.73 m2 for patients with creatinine above ULN). Exclusion criteria included previous oxaliplatin for metastatic disease (and > 120 days since completion of adjuvant therapy); thromboembolic events within 6 months; grade > 1 neuropathy; major surgery within 28 days, or nonhealed surgical wounds; corneal abnormalities; pregnancy; known brain metastases; uncontrolled medical illnesses; inability to take oral medications; and HIV positivity. Written informed consent was obtained as per federal and institutional guidelines before treatment.
Dosage and Drug Administration
Cohorts of 3 patients received FOLFOX4 (oxaliplatin 85 mg/m2 day 1, LV 200 mg/m2 days 1-2, 5-FU bolus 400 mg/m2 days 1-2, then 5-FU infusion 600 mg/m2 over 22 hours days 1-2) on day 1 and 15 of each 28-day cycle. Erlotinib (dose level [DL] 1, 50 mg; DL 2, 100 mg; and DL 3, 150 mg) was administered orally daily as a 7-day “lead-in” monotherapy to permit single-agent pharmacokinetic analysis and pharmacodynamic assessment, followed by combination treatment with FOLFOX4 and erlotinib given once daily without break. Tolerability at a particular dose level was defined as the occurrence of dose-limiting toxicity (DLT) in ≤ 33% of the patients during the first 6-week cycle (≤ 2 subjects in a 6-subject cohort). Bevacizumab 5 mg/kg intravenously on days 1 and 15 was added upon FDA approval as part standard care during the expansion of DL 2.
Toxicity Assessment
Toxicity was assessed weekly during the first cycle, then every 2 weeks thereafter using the National Cancer Institute Common Toxicity Criteria version 2. Responses were determined clinically and radiologically every 2 cycles (2 months) of therapy using Response Evaluation Criteria in Solid Tumors (RECIST). Dose-limiting toxicity was defined as treatment-related grade 4 neutropenia > 5 days, grade 3 or 4 neutropenia with fever (> 38.5°C), grade 3 or 4 nonhematologic toxicity (excluding nausea, vomiting, and diarrhea unless appropriate supportive care was in use); and grade 2 symptomatic toxicity persisting for longer than 7 days despite appropriate supportive care (in order to ensure that the combination would be tolerable for long-term treatment). Dose-limiting toxicity was determined by toxicity during the first cycle (28 days) only. Dose reductions of erlotinib, 5-FU, and oxaliplatin (approximately 25%) were implemented for grade ≥ 3 nonhematologic and hematologic toxicities. Oxaliplatin was also dose reduced for grade ≥ 2 thrombocytopenia, and erlotinib could be held and/or dose reduced for intolerable grade 2 rash or diarrhea despite optimal supportive care. No dose reductions were performed for LV or bevacizumab.
Drug Assay and Pharmacokinetic Analysis
Erlotinib
Pharmacokinetic studies were performed when erlotinib was administered alone during the “lead in” and in combination during the first cycle. Blood samples were obtained pretreatment and 1, 2, 4, 6, 10, and 24 hours posttreatment on day −7 and cycle 1 day 1. Pretreatment samples were also obtained on cycle 1 days 3, 8, 15, and 22. Blood samples were collected in heparinized tubes and were processed by centrifugation at 1000 g at 4°C for 10 minutes. Plasma was stored at −70°C until analysis. Erlotinib and its metabolite OSI-420 concentrations in plasma were measured using a validated liquid chromatography-tandem mass spectrometry (LC-MS-MS) analytical method over the range of 10-10000 ng/mL and 1-1000 ng/mL, respectively.29 This method does not distinguish between the isomers OSI-420 and OSI-413. Therefore, the isomer concentrations will be reported as OSI-420.30
The pharmacokinetics of erlotinib were assessed by compartmental analysis utilizing ADAPT II.31 The pharmacokinetics of OSI-420 were not assessed by compartmental analysis because of previous reported findings by population pharmacokinetic analysis in which the metabolite is consistently approximately 10% of erlotinib exposure.32 The erlotinib data were fit to a 1-compartment linear model using weighted least-squares regression. For several patients, 1 or more blood samples were not obtained or obtained improperly and were not usable for analysis. Any sample that was documented to not be a pretreatment sample (ie, within 3 hours before the next dose or after a dose) was not used in subsequent analysis. After excluding concentrations from these samples, the total number of concentration-time observations per individual was relatively small compared with the number of parameters estimated. Therefore, an iterative 2-stage approach was implemented to estimate pharmacokinetic parameters for all patients. First, a 1-compartment model was fitted to individual erlotinib plasma concentration-versus-time data from 4 patients and 7 pharmacokinetic sampling periods with complete concentration data (7 observations over a 24-hour sampling period) using weighted least-squares estimation. Parameters estimated in this manner were used to establish Bayesian priors for the structural model parameters that included: volume of the central compartment (Vc), absorption rate constant (Ka), and elimination rate constant (Ke). An iterative 2-stage approach was used, in each iteration updating the Bayesian priors, until the mean estimates of all parameters differed by < 5% from the previous mean estimate, which was our arbitrarily predefined stopping point.33 In the final model fit, data from both pharmacokinetic sampling periods for 14 patients, including those with incomplete observations (2-6 observations; time of last plasma concentration 10-24 hours), were analyzed using a Bayesian algorithm to estimate individual structural parameters. Calculated secondary pharmacokinetic parameters included half-life (t1/2) and apparent systemic clearance (Cls/F). Area under the curve (AUC) was calculated as dose divided by Cls/F. Cmax and Tmax values were obtained from the observed values for both erlotinib and OSI-420. Cmin at steady state (Css,min) was calculated for each patient by taking the average of the Cmin after 1 week of erlotinib dosing when steady state was achieved in each patient. The accumulation factor was calculated from the equation 1/(1-e−Ke ×τ), where Ke was from the single-dose (“lead-in”) period and τ was the dosing interval of 24 hours. The accumulation factor was used to correct single-dose (“lead-in”) exposure parameters for comparison with multiple-dose (combination) data.
5-Fluorouracil
5-Fluorouracil pharmacokinetic studies were performed on cycle 1 day 1 and cycle 1 day 15. Serial sampling of venous blood was obtained pretreatment and at 4 and 21.5 hours into the infusion. Blood samples were collected in heparinized tubes and were processed by centrifugation within 30 minutes at 1000 g at 4°C for 10 minutes. Plasma was stored at −20°C until analysis using a modification of a high-performance liquid chromatography method using an ultraviolet spectrophotometer.34 5-Fluorouracil was quantitated over the range of 50-10000 ng/mL. Css was calculated as the mean of the plasma concentrations. Systemic clearance (CLs) was calculated by dividing the infusion rate by Css.
Positron Emission Tomography Scans
Fluorodeoxyglucose PET scans were performed at baseline and after 2 cycles (8 weeks) of combination therapy in a standardized fashion at the Johns Hopkins Hospital. A PET/CT scanner was used for localization of activity, and scans were read blindly by a single interpreter. Patients fasted 6 hours before the scan, and 10-20 mCi of 18F-FDG was injected intravenously. Segmented attenuation correction and iterative reconstruction was performed using Discovery LS or RX software (GE Healthcare, Waukesha, WI). Standardized uptake values (SUVs) of tumor sites were corrected for lean body mass. Results are reported using 1999 European Organization for Research and Treatment of Cancer recommendations.35
Measurement of Plasma EGFR
Plasma samples from study patients treated with erlotinib were collected at baseline (before erlotinib treatment on day −7), and on days 1 and 15 of the first cycle of combination therapy. Plasma was separated by centrifugation and stored at −80°C until the time of analysis. Commercial immunoenzymatic-based assays (enzyme-linked immunosorbent assay) were used for the quantification of the circulating levels of EGFR (Oncogene Research Products, San Diego, CA) according to the instructions provided by the manufacturers.
Serial Skin Biopsies
Skin biopsies were performed at baseline (before treatment) and after 5 days of treatment with single-agent erlotinib during the lead-in period. Samples from 3 mm punch biopsies were placed into formalin and paraffin-embedded. Immunohistochemical analysis was performed for total EGFR, phospho-EGFR, p27, mitogen-activated protein kinase (MAPK), phospho-MAPK, and p27.
Statistical Methods
The primary endpoints were to determine the MTD and toxicity of this combination, and to explore antitumor activity. For secondary endpoints, progression-free survival (PFS) and overall survival (OS) times were calculated using the Kaplan-Meier method. Plasma levels of EGFR were normalized to the pretreatment value in each patient using the following equation: ([pretreatment]−[posttreatment])/( pretreatment) × 100. For pharmacodynamic assessments, comparisons between means and proportions were done using Student t test and χ2 method, respectively, and 2-sided analysis of variance (ANOVA) tests were used to compare values at each time point. For pharmacokinetic analysis, parameters were summarized using descriptive statistics. Differences between the pharmacokinetic parameters between study periods were evaluated statistically by use of a Wilcoxon matched-pairs signed-rank test.
Results
Fifteen patients (7 men and 8 women; median age, 55 years; range, 39-76 years) with advanced CRC received 60 cycles (120 FOLFOX treatments; Table 1). Most patients had colon cancer as their primary site (9 colon/6 rectal), had undergone previous surgery for CRC (n = 13), and were previously untreated. Four patients had received previous chemotherapy for advanced disease, generally irinotecan-based as previous oxaliplatin was an exclusion criterion.
Table 1.
Patient Characteristics
| Characteristic, n | Value (n = 15) |
|---|---|
| Median Age, Years (Range) | 55 (39–76) |
| Sex | |
| Male | 7 |
| Female | 8 |
| Race | |
| Caucasian | 12 |
| African American | 3 |
| ECOG Performance Status | |
| 0 | 8 |
| 1 | 7 |
| Primary Site | |
| Colon | 9 |
| Rectum | 6 |
| Previous Treatment | |
| Chemotherapy | 4 |
| Surgery | 13 |
Abbreviation: ECOG = Eastern Cooperative Oncology Group
Safety
The study enrolled 3 patients each at DL 1, DL 2, and DL 3 of erlotinib and FOLFOX4. There were 2 DLTs at DL 3: intolerable grade 2 maculopapular rash lasting > 1 week despite holding erlotinib, and grade 4 neutropenia lasting > 5 days. Thus, DL 2 was expanded with 6 more patients, this time adding bevacizumab as it had become standard of care. One patient had an unrelated grade 4 myocardial infarction before the administration of any chemotherapy, and is not including in the efficacy or toxicity analysis. There was 1 DLT at dose level 2 with bevacizumab (grade 3 mucositis; Table 2). There were no DLTs attributable to bevacizumab, and overall the toxicities at DL 2 appeared similar (albeit with limited patients) with and without bevacizumab. Thus, DL 2 using erlotinib 100 mg daily was declared the MTD.
Table 2.
Adverse Events (Worst Incidence, Grade > 1) Related to Study Medications
| Adverse Event | Dose Level 1 FOLFOX/Erlotinib 50 mg (n = 3) | Dose Level 2A (FOLFOX/Erlotinib 100 mg (n = 3); and 2B (With Bevacizumab (n = 5)a | Dose Level 3 FOLFOX/Erlotinib 150 mg (n = 3) |
|---|---|---|---|
| General | |||
| Dehydration | – | Grade 3:1 | Grade 2:1; Grade 3:1 |
| Fatigue | Grade 2:1 | Grade 2:3 | – |
| Fever | – | Grade 2:1 | Grade 2:1 |
| Dermatologic | |||
| Alopecia | – | Grade 2:1 | – |
| Dry skin | – | – | – |
| Hand-foot skin reaction | – | Grade 2:1 | Grade 2:1 |
| Maculopapular rash | Grade 2:1 | Grade 2:3 | Grade 2:3b,c |
| Gastrointestinal | |||
| Abdominal pain | Grade 2:1 | Grade 2:2 | Grade 2:1 |
| Anorexia | – | Grade 2:1; Grade 3:1 | – |
| Diarrhea | Grade 2:1; Grade 3:1 | Grade 2:4; Grade 3:2 | Grade 3:3 |
| Mucositis | – | Grade 2:1; Grade 3:1c | – |
| Pneumatosis | Grade 3:1 | – | Grade 3:1 |
| Nausea/vomiting | – | Grade 2:2 | – |
| Laboratory, Chemical | |||
| Alkaline phosphatase elevated | – | – | Grade 2:1; Grade 3:1 |
| ALT elevation | – | Grade 2:1 | Grade 2:1 |
| AST elevation | – | Grade 2:1 | Grade 2:1 |
| Elevated bilirubin | – | Grade 2:1 | Grade 3:1 |
| Laboratory, Hematologic | |||
| Neutropenia | Grade 2:1; Grade 3:1 | Grade 2:2; Grade 3:2; Grade 4:1 | Grade 2:1; Grade 4:1c |
| Hemoglobin | – | Grade 2:1 | Grade 1:2; Grade 3:2 |
| Thrombocytopenia | – | Grade 2:1; Grade 3:1 | – |
| Other | |||
| Thrombosis/embolism | – | Grade 3:1 | – |
One patient who did not receive FOLFOX/erlotinib/bevacizumab not included.
One patient’s rash did not improve to tolerable within 2 weeks, and thus was graded as a DLT.
These adverse events indicate DLT.
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; DLT = dose-limiting toxicity; FOLFOX = infusional 5-fluorouracil/leucovorin/oxaliplatin
The most frequent toxicities were rash (100% grade 1–2), diarrhea (100% grade 1–3; 43% grade 3), neuropathy (100% grade 1–2), fatigue (92% grade 1–2), and mucositis (71% grade 1–3, 7% grade 3). Elevated liver function tests were also common, but nearly all participants (93%) had liver metastases. Dose delays of FOLFOX4 were required in 57% of patients at some point in their treatment course, generally because of gastrointestinal toxicities and cytopenias, with reductions in 43%. Dose delays for erlotinib occurred in 36%, generally because of rash, but dose reduction was required in only 1 patient (7%). Interestingly, there were 2 cases of pneumatosis (presenting with mild abdominal pain but otherwise asymptomatic) detected on CT scan after cycle 1, for which patients were taken off study immediately. Both episodes resolved with supportive care only and did not recur in subsequent lines of chemotherapy. Aside from the aforementioned myocardial infarction, reasons for discontinuation of protocol treatment were progressive disease (PD), (5) neuropathy (2), pneumatosis (2), geographic/logistical (2), surgery (1), allergic reaction (1), and noncompliance (1).
Efficacy
Response was evaluated after every 2 cycles (8 weeks) using CT scanning and RECIST. The combination was highly active in this patient population, with a RR of 78% (Figure 1). At the 100 mg and 150 mg dose levels, every patient had a partial response (PR) except 1, who experienced stable disease (minor response) and went on to have curative resection and remains disease free at 3.5 years after enrollment. One other patient underwent curative-intent surgery but recurred 1 year later. The median PFS was 9.5 months, and OS was 30 months. There was no correlation between rash and response, although every patient had at least a grade 1 rash. As expected, there was a strong correlation between median PFS and OS (Spearman correlation coefficient, 0.81; P < .001).
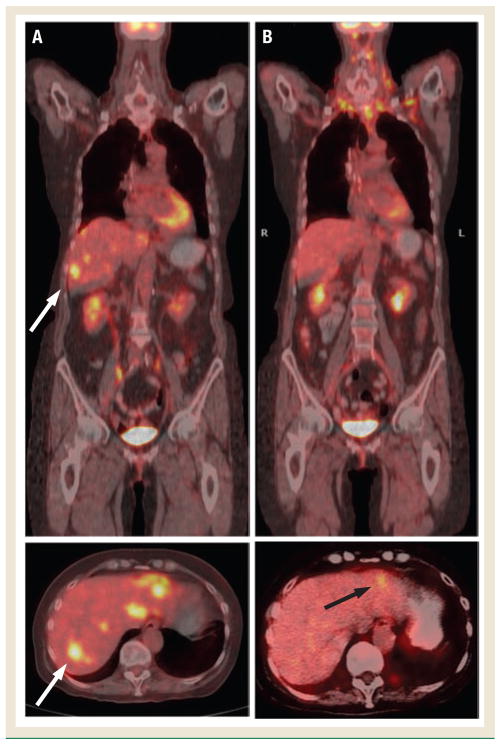
Figure 1.
Positron Emission Tomography/Computed Tomography–Fused Images at Baseline (A) and Following Two Cycles of FOLFOX/Erlotinib Without Bevacizumab (B)
White arrows show metastatic deposit in the right lobe of the liver at baseline. After 2 cycles, the patient had a 64% (standard deviation, 7%) decrease in SUV max-lean, and residual activity was seen in the left lobe only (black arrow). The patient also had partial response per Response Evaluation Criteria in Solid Tumors
Positron Emission Tomography
Fourteen patients underwent PET/CT scans at baseline and 112 target lesions were identified in a blinded fashion. Thirteen patients underwent PET/CT after 2 cycles of chemotherapy. The mean activity of injected 18F-FDG was 16.7 mCi (standard deviation [SD], 4.63), with a mean uptake of 61.8 minutes (SD, 7.67). Between the baseline and the on-treatment scan, the maximum SUV corrected for lean body mass (SUV lean-max) decreased from a mean of 6.93 (deviation, 3.32) down to 2.55 (deviation, 1.36), P < .01. The partial metabolic response (PMR) rate was 78%, with all patients experiencing a decrease in SUV lean-max from 26% to 80%. Three patients had new lesions detected on PET despite a decrease of > 25% on SUV lean-max of the baseline lesions; of these, 1 patient had PD on RECIST, 1 had SD, and 1 had a PR and remains free of disease > 3 years later, indicating that this was a false-positive finding.
Pharmacokinetics
Erlotinib exposure was significantly higher when administered in combination with FOLFOX (Table 3). These differences were due to comparing single-dose (“lead-in”) versus multiple-dose (combination) pharmacokinetics as erlotinib accumulates because of a 36-hour half-life.32 When correcting the single-dose data with the accumulation factor, there was no significant difference in exposure parameters between the “lead-in” and multiple-dose erlotinib exposure parameters (Cmax, P = .56; AUC, P = .49). 5-Fluorouracil plasma concentrations were consistent with previously published values and systemic clearance was not markedly affected by co-administration of erlotinib (P = .97).
Table 3.
Erlotinib and 5-Fluorouracil Pharmacokinetic Parametersa
| Pharmacokinetic Parameter | DL 1 | DL 2b | DL 3 | |||
|---|---|---|---|---|---|---|
| Single Agent | Combination | Single Agent | Combination | Single Agent | Combination | |
| Erlotinib | ||||||
| Cmax (ng/mL) | 610.8 ± 100.8 (3) | 1152.2 ± 426.3 (3) | 985.4 ± 575.5 (8) | 1993.0 ± 980.5 (7) | 1958.4 ± 1158.5 (3) | 4446.5 ± 1066.6 (3) |
| Tmax (hour) | 1.08 (1.00–2.00, 3) | 4.00 (2.00–4.08, 3) | 2.00 (1.03–4.02, 8) | 2.00 (1.00–10.00, 7) | 6.00 (1.00–24.08, 3) | 4.02 (1.03–4.17, 3) |
| AUC (ng/hour/mL) | 14172, 16276 (2) | 10848, 61493 (2) | 40231 ± 49115 (7) | 52684 ± 35964 (7) | 39894, 62060 (2) | 154957 ± 75031 (3) |
| OSI-420 | ||||||
| Cmax (ng/mL) | 60.8 ± 16.1 (3) | 86.1 ± 26.4 (3) | 67.8 ± 49.1 (8) | 158.3 ± 105.9 (7) | 193.2 ± 92.6 (3) | 748.0 ± 269.5 (3) |
| Tmax (hour) | 2.00 (1.08–2.00, 3) | 4.00 (2.00–6.00, 3) | 3.00 (1.03–25.03, 8) | 2.00 (1.00–10.00, 7) | 9.95 (2.07–24.08, 3) | 6.00 (4.17–8.12, 3) |
| OSI-420:Erlotinib Cmin ratio | 0.085 ± 0.002 (3) | 0.081 ± 0.005 (3) | 0.074 ± 0.028 (8) | 0.069 ± 0.021 (8) | 0.185 ± 0.076 (3) | 0.202 ± 0.085 (3) |
| 5-Fluorouracil Cl (liter/hour) | 207.9 ± 36.0 (3) | 182.2 ± 20.9 (3) | 261.4 ± 141.4 (7) | 243.7 ± 93.6 (8) | 200.3, 217.4 (2) | 221.5, 313.2 (2) |
Values are reported as the arithmetic mean ± standard deviation (n). The Tmax is reported as the median (range). When n ≤ 2, individual values are reported.
DL2a (FOLFOX/erlotinib 100 mg) and 2b (with bevacizumab).
Abbreviations: AUC = area under the curve; DL = dose level; FOLFOX = infusional 5-fluorouracil/leucovorin/oxaliplatin
Plasma and Skin Pharmacodynamics
Circulating levels of EGFR in the plasma were measured on days at baseline (day –7), and after 1 week of monotherapy (day 1) and 2 weeks on combination therapy (day 15). Overall, there was an increase in the amount of soluble EGFR from a mean of 33047 ng/ mL (standard error, 2154) baseline (day –7 of lead-in); to 37433 ng/mL (standard error, 3376) day 1; to 40059 ng/mL (standard error, 3140) on day 15, but these differences were not statistically significant (ANOVA; P = .25). Only 5 patients (36%) had sustained upregulation (3) or downregulation (2) of serum EGFR over the 2 measurements. There were no statistically significant correlations between circulating EGFR levels at any time point with RECIST response, PET response, PFS, or OS.
Similarly, there were no clear trends with regard to immunohistochemical staining of multiple EGFR pathway members, and the proliferation marker Ki-67 and apoptotic marker p27, in the serial skin biopsies performed at baseline and day 7. EGFR pathway markers assessed included total and phosphorylated (activated) EGFR and MAPK. Study patients showed both increases and decreases in the assessed markers without any correlation between toxicity, RECIST response, PET response, PFS, or OS. The protocol did not include archival tumor collection, so tumor KRAS and BRAF testing was not performed.
Discussion
This study demonstrates a tolerable dose of erlotinib at the 100 mg daily dose level in combination with standard FOLFOX4 in patients with advanced CRC. Although the number of patients is very limited, the addition of bevacizumab in the final dose confirmation cohort did not appear to alter the tolerability of this combination. Toxicities were greater than seen with standard FOLFOX4,36 or FOLFOX4/bevacizumab,37 in the advanced CRC population. The regimen was highly active, with a RECIST and PET response (PMR) response rate of 78%, and PFS and OS of 9.5 and 30 months, respectively. This is comparable to other larger studies of combination cytotoxics and oral EGFR inhibitors in advanced CRC such as the phase II trial of FOLFOX4/gefitinib (IFOX, n = 45), in which the RR was 72%, PFS was 9.3 months, and OS was 20.5 months19; and phase II study of capecitabine, oxaliplatin, and erlotinib (CAPOX/erlotinib; n = 32), in which the RR was 25%, PFS was 5.4 months, and OS was 14.7 months in a second-line population.16
It was expected that erlotinib would add the toxicity of rash and worsen diarrhea, which is an overlapping toxicity with 5-FU. In the phase I study, 25% and 86% of patients treated with erlotinib at 100 mg/day and 150 mg/day experienced diarrhea, but grade 3 diarrhea occurred only at the 200-mg dose level.38 In the large phase III NO16966 trial in patients with CRC, FOLFOX4 was associated with grade 3 diarrhea in 11%, and thus the 43% rate of grade 3 diarrhea seen in this combination study was higher than expected. Because severe diarrhea often occurred after 1 month of combination therapy outside the DLT window, it was not dose limiting but led to subsequent dose holds and reductions. Mucositis (grade 1–3) was seen in the phase I study of erlotinib at 150 mg/day, and also occurred in NO16966 at a rate of 35% grade 1–2 and 2% grade 3. Thus, it is not surprising that it was dose limiting at the 150 mg/day dose level, and occurred in 71% (grade 1–3) of patients overall.
The 100-mg dose of erlotinib at the MTD is lower than 3 published studies of erlotinib at 150 mg/day with 5-FU and oxaliplatin. The mFOLFOX6/bevacizumab/erlotinib trial treated 35 patients at the 150 mg erlotinib dose level without any dose exploration.18 mFOLFOX6 consists of a slightly lower dose of oxaliplatin of 85 mg/m2 compared to FOLFOX6, and the day 2 5-FU bolus is omitted (with a 46-hour infusion, instead of two 22-hour infusions with FOLFOX4). The authors note that “the toxic effects of the regimen limited any conclusions regarding efficacy,” with 20% of patients coming off of study before the first restaging scan and 77% removed for toxicity or withdrawal of consent. The second CAPOX/erlotinib study also had excessive gastrointestinal toxicity, but rather than lower the dose of erlotinib, the dose of capecitabine was lowered from the standard 2000 mg/m2/day down to 1500 mg/m2/day after the first 13 patients.16 A phase Ib study of FOLFOX4/erlotinib by Hanauske et al in patients with solid tumors (72% CRC) explored 3 6-patient cohorts, starting with a dose reduction of both erlotinib and FOLFOX4.39 However, 2 of 6 DLTs were seen at the 100-mg cohort (with full-dose FOLFOX-4) and at the 150 mg cohort (including grade 5 sepsis), which would typically trigger a de-escalation to the previous dose level in a phase I study. Instead, per protocol the investigators expanded to 9-patient cohorts. Thus, according to the typical 3 + 3 design used here, the Hanauske et al study would have resulted in a lower dose determination of erlotinib 50 mg. It is interesting to note, however, that 17 patients (82% CRC) were treated at the 150-mg dose level (with full-dose FOLFOX4) and the rate of grade ≥ 3 events remained < 25%, with anorexia (24%), fatigue (12%), and diarrhea (12%) predominating as grade 3/4 events. There were 3 toxic deaths.
In our study, a difference in erlotinib pharmacokinetics was noted when administered alone after a single dose or in combination after multiple doses. The differences were not observed when the single-dose data were corrected with the accumulation ratio. There was no difference in 5-FU pharmacokinetics. The lack of pharmacokinetic interactions between these agents was expected, given an absence of interactions in the Hanauske et al study and our previously published study of FOLFIRI/erlotinib.24,39
Correlative pharmacodynamic studies included in this protocol failed to show a demonstrable effect in this limited patient population. Although a phase I study of gefitinib in selected tumor types showed impressive downregulation of activated EGFR and MAPK from serial skin biopsies,40 a subsequently published phase I study failed to show a significant difference in activated EGFR in the skin.41 Moreover, in a biologic trial of patients with breast cancer, serial skin and buccal mucosal biopsies failed to show any modulation of EGFR, phosphorylated EGFR, phosphorylated MAPK, and phosphorylated AKT.42 Paradoxically, there was a statistically significant upregulation of pEGFR in patients with EGFR-negative tumors, perhaps because of some compensatory mechanism. Similarly, a biologic study of gefitinib in CRC with 17 paired tumor biopsies failed to demonstrate any significant modulation of activated and total EGFR, MAPK, and Akt.43 As for soluble EGFR, the finding that soluble levels increased after initiation of an EGFR inhibitor is consistent with preclinical data showing an upregulation of EGFR mRNA and protein following erlotinib exposure, perhaps through some type of regulatory feedback mechanism.44 It has also been shown that in patients with lung cancer treated with gefitinib, higher levels of baseline serum EGFR were associated with response, and increasing levels at day 28 were an indicator of disease progression.45 In our study, there was a great deal of variability in serum EGFR and no consistent patterns were seen.
For advanced CRC, FOLFOX is a standard first- or second-line regimen, and bevacizumab has shown at least a progression-free survival benefit in both settings.37,46,47 Although this combination therapy has shown considerable activity, there remains an acute need for improved combination regimens to improve both PFS and OS, and the proportion of patients with advanced CRC amenable to metastasectomy. Though it was expected that cetuximab and panitumumab would obviate the need for further development of the small-molecule inhibitors in CRC, unfortunately 2 studies have shown that “double-biologic” strategies combining cytotoxic chemotherapy such as FOLFOX with either bevacizumab/panitumumab7 or bevacizumab/cetuximab8 result in higher toxicity and lower PFS. The N0147 study in which cetuximab was added to FOLFOX in the adjuvant setting has also been closed because of lack of efficacy, even in the KRAS wild-type setting. The explanation for these unexpected results is unclear. Because combination studies of oral EGFR inhibitors with cytotoxic chemotherapy (with and without bevacizumab) have shown promising efficacy results, there now may be renewed interest in using small-molecule EGFR TK inhibitors rather than antibodies. There is an ongoing study (DREAM-OPTIMOX3) examining the activity of erlotinib as a maintenance therapy following combination chemotherapy, after the combination in the feasibility phase was found to be too toxic.27 In the future, to avoid seeing unexpected excessive toxicity in phase II studies, it may be advisable to perform dose-escalation trials such as this one before simply combining full doses of agents.
Conclusion
Erlotinib combined with FOLFOX4 (and bevacizumab in the final cohort) is fairly well tolerated in advanced colorectal cancer patients at an erlotinib dose of 100 mg daily, although additional gastrointestinal toxicities and rash were noted compared to previous trials of FOLFOX4 alone. There were no drug to drug pharmacokinetic drug interactions detected. Although there were a limited number of patients, the regimen was noted to be active with a response rate of 78% and a median OS of 30 months. With the failure of “double-biologic” regimens in several phase III trials, there may be renewed interest in combining EGFR TK inhibitors with bevacizumab and cytotoxic chemotherapy.
Acknowledgments
This trial was supported by U01 CA70095. Dr. Messersmith receives funding support from 523CA115500. Pharmacology-based correlative studies were also supported by P30CA069773. The authors thank Sharyn Baker for her scientific input during the study.
Footnotes
Disclosures
Dr. Messersmith has served as a consultant for OSI Pharmaceuticals. Dr. Carducci has served as a consultant for Genentech, Inc. and sanofi-aventis U.S.; and has received research funding from sanofiaventis U.S. All other authors have no relevant relationships to report.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 6.Messersmith WA, Ahnen DJ. Targeting EGFR in colorectal cancer. N Engl J Med. 2008;359:1834–6. doi: 10.1056/NEJMe0806778. [DOI] [PubMed] [Google Scholar]
- 7.Hecht ME, Mitchell E, Jr, Chidiac T, et al. An updated analysis of safety and efficacy of oxaliplatin/bevacizumab ± panitumumab for first-line treatment of mCRC from a randomized, controlled trial (PACCE). Presented at: the 2008 Gastrointestinal Cancers Symposium; January 25–27, 2008; Orlando, FL. Abstract 273. [Google Scholar]
- 8.Punt CJ, Tol J, Rodenburg CJ, et al. Randomized phase III study of capecitabine, oxaliplatin, and bevacizumab with or without cetuximab in advanced colorectal cancer, the CAIRO2 study of the DCCG. J Clin Oncol. 2008;26(15 suppl):180s. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JR, Cohen M, Sridhara R, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005;11:6414–21. doi: 10.1158/1078-0432.CCR-05-0790. [DOI] [PubMed] [Google Scholar]
- 10.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 11.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 12.Ogino S, Meyerhardt JA, Cantor M, et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin Cancer Res. 2005;11:6650–6. doi: 10.1158/1078-0432.CCR-05-0738. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg ML, LaFleur B, Levy DE, et al. Randomized phase II trial of the clinical and biological effects of two dose levels of gefitinib in patients with recurrent colorectal adenocarcinoma. J Clin Oncol. 2005;23:9265–74. doi: 10.1200/JCO.2005.03.0536. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie MJ, Hirte HW, Glenwood G, et al. A phase II trial of ZD1839 (Iressa) 750 mg per day, an oral epidermal growth factor receptor-tyrosine kinase inhibitor, in patients with metastatic colorectal cancer. Invest New Drugs. 2005;23:165–70. doi: 10.1007/s10637-005-5862-9. [DOI] [PubMed] [Google Scholar]
- 15.Townsley CA, Major P, Siu LL, et al. Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer. 2006;94:1136–43. doi: 10.1038/sj.bjc.6603055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyerhardt JA, Zhu AX, Enzinger PC, et al. Phase II study of capecitabine, oxaliplatin, and erlotinib in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:1892–7. doi: 10.1200/JCO.2005.05.3728. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Verslype C, Beale P, et al. A phase Ib dose-escalation study of erlotinib, capecitabine and oxaliplatin in metastatic colorectal cancer patients. Ann Oncol. 2008;19:332–9. doi: 10.1093/annonc/mdm452. [DOI] [PubMed] [Google Scholar]
- 18.Meyerhardt JA, Stuart K, Fuchs CS, et al. Phase II study of FOLFOX, bevacizumab and erlotinib as first-line therapy for patients with metastatic colorectal cancer. Ann Oncol. 2007;18:1185–9. doi: 10.1093/annonc/mdm124. [DOI] [PubMed] [Google Scholar]
- 19.Fisher GA, Kuo T, Ramsey M, et al. A Phase II Study of Gefitinib, 5-Fluorouracil, Leucovorin, and Oxaliplatin in Previously Untreated Patients with Metastatic Colorectal Cancer. Clin Cancer Res. 2008;14:7074–9. doi: 10.1158/1078-0432.CCR-08-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo T, Cho CD, Halsey J, et al. Phase II study of gefitinib, fluorouracil, leucovorin, and oxaliplatin therapy in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:5613–9. doi: 10.1200/JCO.2005.08.359. [DOI] [PubMed] [Google Scholar]
- 21.Cascinu S, Berardi R, Salvagni S, et al. A combination of gefitinib and FOLFOX-4 as first-line treatment in advanced colorectal cancer patients. A GISCAD multicentre phase II study including a biological analysis of EGFR overexpression, amplification and NF-kB activation. Br J Cancer. 2008;98:71–6. doi: 10.1038/sj.bjc.6604121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zampino MG, Magni E, Massacesi C, et al. First clinical experience of orally active epidermal growth factor receptor inhibitor combined with simplified FOLFOX6 as first-line treatment for metastatic colorectal cancer. Cancer. 2007;110:752–8. doi: 10.1002/cncr.22851. [DOI] [PubMed] [Google Scholar]
- 23.Gelibter AJ, Gamucci T, Pollera CF, et al. A phase II trial of gefitinib in combination with capecitabine and oxaliplatin as first-line chemotherapy in patients with advanced colorectal cancer. Curr Med Res Opin. 2007;23:2117–23. doi: 10.1185/030079907X226113. [DOI] [PubMed] [Google Scholar]
- 24.Messersmith WA, Laheru DA, Senzer NN, et al. Phase I trial of irinotecan, infusional 5-fluorouracil, and leucovorin (FOLFIRI) with erlotinib (OSI-774): early termination due to increased toxicities. Clin Cancer Res. 2004;10:6522–7. doi: 10.1158/1078-0432.CCR-04-0746. [DOI] [PubMed] [Google Scholar]
- 25.Veronese ML, Sun W, Giantonio B, et al. A phase II trial of gefitinib with 5-fluorouracil, leucovorin, and irinotecan in patients with colorectal cancer. Br J Cancer. 2005;92:1846–9. doi: 10.1038/sj.bjc.6602569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoro A, Comandone A, Rimassa L, et al. A phase II randomized multicenter trial of gefitinib plus FOLFIRI and FOLFIRI alone in patients with metastatic colorectal cancer. Ann Oncol. 2008;19:1888–93. doi: 10.1093/annonc/mdn401. [DOI] [PubMed] [Google Scholar]
- 27.Tournigand CL, Lledo G, Delord J, et al. Modified Folfox7/bevacizumab or modified Xelox/bevacizumab with or without erlotinib (E) in first-line metastatic colorectal cancer: Results of DREAM-OPTIMOX3 study. J Clin Oncol. 2007;25(18 suppl):187s. (abstract 4097) [Google Scholar]
- 28.Meyerhardt JA, Clark JW, Supko JG, et al. Phase I study of gefitinib, irinotecan, 5-fluorouracil and leucovorin in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2007;60:661–70. doi: 10.1007/s00280-006-0411-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M, He P, Rudek MA, et al. Specific method for determination of OSI-774 and its metabolite OSI-420 in human plasma by using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;793:413–20. doi: 10.1016/s1570-0232(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 30.Frohna P, Lu J, Eppler S, et al. Evaluation of the absolute oral bioavailability and bioequivalence of erlotinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in a randomized, crossover study in healthy subjects. J Clin Pharmacol. 2006;46:282–90. doi: 10.1177/0091270005284193. [DOI] [PubMed] [Google Scholar]
- 31.D’Argenio DZ, Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979;9:115–34. doi: 10.1016/0010-468x(79)90025-4. [DOI] [PubMed] [Google Scholar]
- 32.Lu JF, Eppler SM, Wolf J, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80:136–45. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Forrest AHJ, Egorin MJ. Evaluation of a new program for population PK/PD analysis applied to simulated phase I data. Clin Pharmacol Ther. 1991;49:153. [Google Scholar]
- 34.Zufia L, Aldaz A, Giraldez J. Simple determination of capecitabine and its metabolites by liquid chromatography with ultraviolet detection in a single injection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;809:51–8. doi: 10.1016/j.jchromb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 36.Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–12. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 37.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–9. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 38.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI- 774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–79. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 39.Hanauske AR, Cassidy J, Sastre J, et al. Phase 1b dose escalation study of erlotinib in combination with infusional 5-Fluorouracil, leucovorin, and oxaliplatin in patients with advanced solid tumors. Clin Cancer Res. 2007;13:523–31. doi: 10.1158/1078-0432.CCR-06-1627. [DOI] [PubMed] [Google Scholar]
- 40.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 41.Herbst RS, Maddox AM, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002;20:3815–25. doi: 10.1200/JCO.2002.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Tan AR, Yang X, Hewitt SM, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol. 2004;22:3080–90. doi: 10.1200/JCO.2004.08.189. [DOI] [PubMed] [Google Scholar]
- 43.Daneshmand M, Parolin DA, Hirte HW, et al. A pharmacodynamic study of the epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 in metastatic colorectal cancer patients. Clin Cancer Res. 2003;9:2457–64. [PubMed] [Google Scholar]
- 44.Jimeno A, Rubio-Viqueira B, Amador ML, et al. Epidermal growth factor receptor dynamics influences response to epidermal growth factor receptor targeted agents. Cancer Res. 2005;65:3003–10. doi: 10.1158/0008-5472.CAN-04-3586. [DOI] [PubMed] [Google Scholar]
- 45.Gregorc V, Ceresoli GL, Floriani I, et al. Effects of gefitinib on serum epidermal growth factor receptor and HER2 in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:6006–12. doi: 10.1158/1078-0432.CCR-03-0770. [DOI] [PubMed] [Google Scholar]
- 46.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 47.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]