Abstract
Genetic analysis of oxygen-sensitive mutants of Cryptococcus neoformans revealed two loci (oxy1 and oxy2) linking hyperoxia sensitivity to production of melanin, a known virulence factor. Hyperoxia-sensitive strain 562 (oxy1 oxy2) is albino and avirulent. oxy2-defective strains lacking the oxy1 defect are melanin deficient but show normal hyperoxia resistance. Mutants defective at three additional mapped melanin loci fail to show hyperoxia sensitivity in the oxy1 background. Revertants of strain 562, which regain the ability to synthesize melanin by mutation at suppressor sites unlinked to oxy2, retain the oxygen sensitivity conferred by their oxy1 and oxy2 defects. These data identify the melanin gene oxy2 as unique in its association of hyperoxia resistance and melanization.
Full text
PDF
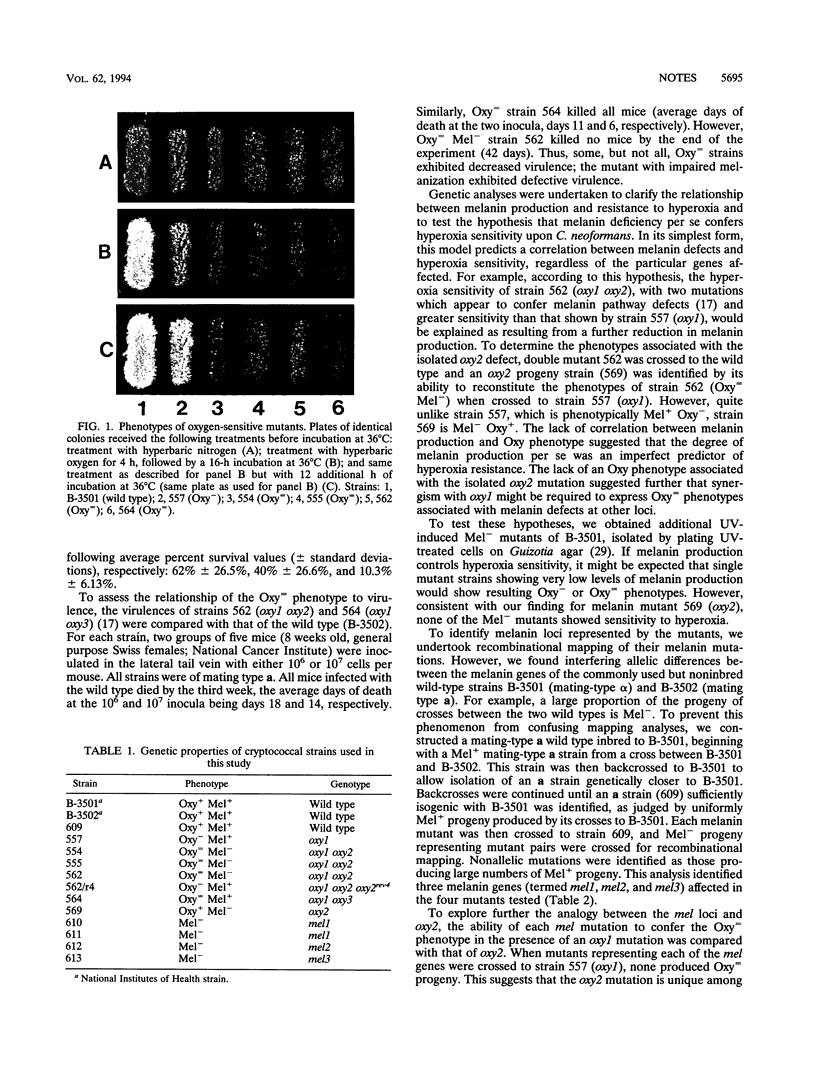

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. R., Golde D. W. Differentiation of macrophages from normal human bone marrow in liquid culture. Electron microscopy and cytochemistry. J Clin Invest. 1978 Jun;61(6):1555–1569. doi: 10.1172/JCI109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. II. Phagocytosis by human leukocytes. J Bacteriol. 1967 Nov;94(5):1480–1483. doi: 10.1128/jb.94.5.1480-1483.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D., Gunn C. M. Cryptococcus neoformans. I. Nonencapsulated mutants. J Bacteriol. 1967 Nov;94(5):1475–1479. doi: 10.1128/jb.94.5.1475-1479.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMONER B., TOWNSEND J., PAKE G. E. Free radicals in biological materials. Nature. 1954 Oct 9;174(4432):689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill J. R., Drutz D. J. Host defense in cryptococcosis. II. Cryptococcosis in the nude mouse. Cell Immunol. 1978 Oct;40(2):263–274. doi: 10.1016/0008-8749(78)90334-9. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Goscin S. A., Fridovich I. Superoxide dismutase and oxygen toxicity in a eukaryote. J Bacteriol. 1974 Feb;117(2):456–460. doi: 10.1128/jb.117.2.456-460.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. S., Ayers D. J., Harrell A. C., Nicholas C. C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. S., Emery H. S. Catecholamine uptake, melanization, and oxygen toxicity in Cryptococcus neoformans. J Bacteriol. 1991 Jan;173(1):401–403. doi: 10.1128/jb.173.1.401-403.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. S., Emery H. S. Temperature regulation of the cryptococcal phenoloxidase. J Med Vet Mycol. 1991;29(2):121–124. doi: 10.1080/02681219180000201. [DOI] [PubMed] [Google Scholar]
- Jacobson E. S., Jenkins N. D., Todd J. M. Relationship between superoxide dismutase and melanin in a pathogenic fungus. Infect Immun. 1994 Sep;62(9):4085–4086. doi: 10.1128/iai.62.9.4085-4086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. S., Tinnell S. B. Antioxidant function of fungal melanin. J Bacteriol. 1993 Nov;175(21):7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D., Chance B., Cadenas E., Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977 Apr;16(1):99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia. 1976 Jul-Aug;68(4):821–833. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Popkin T. J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982 Jun;150(3):1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON H. S., INGRAM D. J., ALLEN B. The free radical property of melanins. Arch Biochem Biophys. 1960 Feb;86:225–230. doi: 10.1016/0003-9861(60)90409-4. [DOI] [PubMed] [Google Scholar]
- Monga D. P. Role of macrophages in resistance of mice to experimental cryptococcosis. Infect Immun. 1981 Jun;32(3):975–978. doi: 10.1128/iai.32.3.975-978.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Polacheck I., Kwon-Chung K. J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982 Jun;36(3):1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A. B., Ajello L. Medium for selective isolation of Cryptococcus neoformans. Science. 1966 Jan 14;151(3707):208–209. doi: 10.1126/science.151.3707.208. [DOI] [PubMed] [Google Scholar]
- Yusa T., Crapo J. D., Freeman B. A. Hyperoxia enhances lung and liver nuclear superoxide generation. Biochim Biophys Acta. 1984 Apr 10;798(2):167–174. doi: 10.1016/0304-4165(84)90299-x. [DOI] [PubMed] [Google Scholar]
- van der Meer J. W., Beelen R. H., Fluitsma D. M., van Furth R. Ultrastructure of mononuclear phagocytes developing in liquid bone marrow cultures. A study on peroxidatic activity. J Exp Med. 1979 Jan 1;149(1):17–26. doi: 10.1084/jem.149.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]