Abstract
Background
The positive co-stimulatory proteins OX40 and OX40L and negative regulatory proteins PD-1, PD-L1 and PD-L2 have emerged as significant regulators of acute rejection in experimental transplantation models.
Methods
We obtained 21 urine specimens from 21 renal allograft recipients with graft dysfunction and biopsy confirmed acute rejection and 25 specimens from 25 recipients with stable graft function and normal biopsy results (stable). Urinary cell levels of mRNAs were measured using real-time quantitative PCR assays, and the levels were correlated with allograft status and outcomes.
Results
Levels of OX40 mRNA (P<0.0001, Mann-Whitney test), OX40L mRNA (P=0.0004) and PD-1 mRNA (P=0.004), but not the mRNA levels of PD-L1 (P=0.08) or PD-L2 (P=0.20) were significantly higher in the urinary cells from the acute rejection group than the stable group. Receiver-operating-characteristic curve analysis demonstrated that acute rejection is predicted with a sensitivity of 95% and a specificity of 92% (AUC=0.98, 95% CI, 0.96 to 1.0, P<0.0001) using a combination of levels of mRNA for OX40, OX40L, PD-1, and levels of mRNA for the previously identified biomarker Foxp3. Within the acute rejection group, levels of mRNA for OX40 (P=0.0002), OX40L (P=0.0004) and Foxp3 (P=0.04) predicted acute rejection reversal, while only OX40 mRNA levels (P=0.04) predicted graft loss following acute rejection.
Conclusion
A linear combination of urinary cell levels of mRNA for OX40, OX40L, PD-1, and Foxp3 was a very strong predictor of acute rejection in human renal allograft biopsies. This prediction model should be validated using an independent cohort of renal allograft recipients.
Keywords: Acute Rejection, OX40, Costimulation, Urinary Biomarkers
Introduction
Acute rejection of organ allografts remains a formidable problem threatening short as well as long-term outcomes (1). The immunosuppressive therapy, used to prevent and treat an episode of acute rejection, is a significant risk factor for life threatening infectious complications and for the development of post-transplant malignancy (2, 3). Thus, a better understanding of mechanisms of acute rejection and safer, preferably noninvasive, approaches to diagnosing acute rejection and predicting its responsiveness to anti-rejection therapy are high priorities for the transplantation community.
T-lymphocytes are central to the acute rejection process (4) and much has been learned in recent years regarding transmembrane signaling requirements including cell surface proteins contributing to antigen recognition, positive costimulation and negative regulation (5, 6). There has also been considerable progress in the area of antigen presentation including the discovery of molecules displayed on antigen presenting cells (APC) that function as counter receptors to cell surface moieties on T-cells and B-cells (7, 8). Elegant pre-clinical studies have brought to our attention the critical role of costimulatory receptors and negative regulatory receptors in allograft rejection and tolerance (9-14).
We investigated the gene expression patterns of positive costimulatory molecules OX40 and its ligand OX40L, and the expression profile of negative regulatory protein programmed death-1 (PD-1) and its ligands PD-L1 and PD-L2 in recipients of deceased donor or live donor renal allografts. Our selection of these molecules for mRNA expression profiling was based on pre-clinical studies demonstrating their participation in allograft rejection and tolerance (9-14) and our reasoning that information regarding their expression patterns in clinical transplantation might be of value from diagnostic, prognostic and therapeutic perspectives.
Our laboratory has developed a urinary cell mRNA profiling protocol and applied it clinically to ascertain the immune status of the renal allograft (15, 16). In a series of investigations, we identified that mRNA encoding cytotoxic attack molecules granzyme B and perforin are over expressed in urinary cells during an episode of acute rejection (15), and that the level of mRNA for the regulatory T-cell specification factor Foxp3 in urinary cells is diagnostic as well as prognostic of acute rejection of human renal allografts (16).
In the current investigation, we tested the diagnostic and prognostic utility of levels of mRNA for OX40, OX40L, PD-1, PD-L1 and PD-L2 in urinary cells. We also investigated whether an algorithm that included our previously identified biomarkers of acute rejection increased the diagnostic accuracy.
We report here that the urinary cell levels of mRNA for OX40, OX40L and PD-1, but not the levels of PDL-1 or PD-L2 are significantly higher during an episode of acute rejection. A model that included levels of mRNA for OX40, OX40L, PD-1 and Foxp3 was an extremely accurate predictor of acute rejection. Moreover, acute rejection reversibility and graft loss following an episode of acute rejection was predicted by urinary cell levels of mRNA for OX40, OX40L and Foxp3. The multi-gene combination resulted in a larger AUC compared to the AUC of OX40 mRNA alone but the difference was statistically significant for predicting acute rejection but not acute rejection reversal or graft loss following an episode of acute rejection.
Results
Levels of mRNA in Urinary Cells
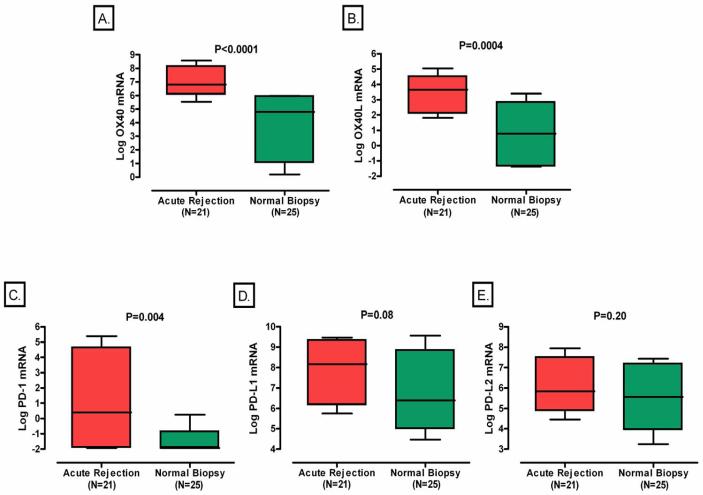
The mean (±SE) log-transformed ratio of OX40 mRNA copies to 18S rRNA copies in urinary cells was 6.98±0.26 in the 21 subjects with acute rejection and was substantially higher than that in the 25 subjects with normal biopsy results (Stable group) (3.88±0.50, P<0.0001, Mann-Whitney test, Fig. 1A). The log-transformed value of 18S-normalized OX40L mRNA (3.11±0.40 vs. 0.72±0.41, P=0.0004, Fig. 1B) was also higher in the acute rejection group compared to the stable group. Levels of mRNA for PD-1 (1.29±0.72 vs. −1.47±0.25, P=0.004, Fig.1C) also differed significantly between the two groups, but not the levels of mRNA for PD-L1 (7.87±0.36 vs. 7.03±0.56, P=0.08, Fig. 1D) or PD-L2 (6.27±0.32 vs.5.49±0.33, P=0.20, Fig.1E).
Figure 1. Levels of mRNA in Urinary Cells.
Boxplots show the 10th, 25th, 50th, 75th, and 90th percentiles of log-transformed 18S-normalized mRNA levels for OX40, OX40L, PD-1, PD-L1, or PD-L2 in urine samples obtained from 21 subjects with graft dysfunction and biopsy-proven acute rejection and 25 subjects with stable graft function and normal biopsy results. The levels of mRNA for OX40 (Panel A), OX40L (Panel B) and PD-1 (Panel C), but not the levels of mRNA for PD-L1 (panel D) or PD-L2 (panel E) were significantly higher in urinary cells from subjects with acute rejection (Acute Rejection) than in the subjects with stable graft function and normal biopsy results (Normal Biopsy). Two-tailed P-values are based on Mann-Whitney test. In all cases, the natural log transformed values of 18S-normalized mRNA levels are shown.
ROC-Curve Analysis of mRNA Levels for Predicting Acute Rejection
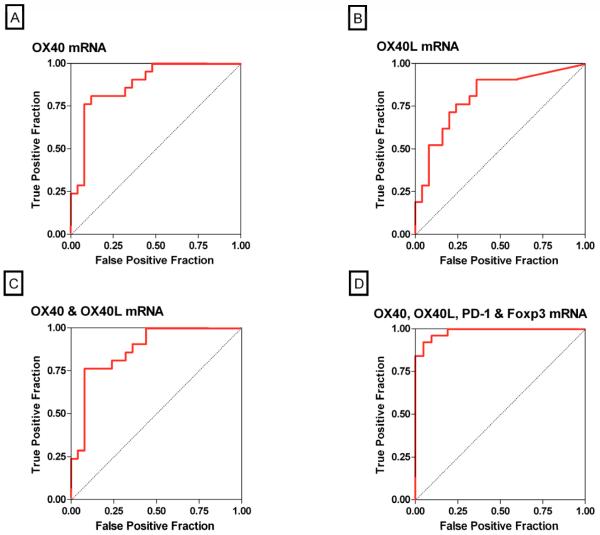
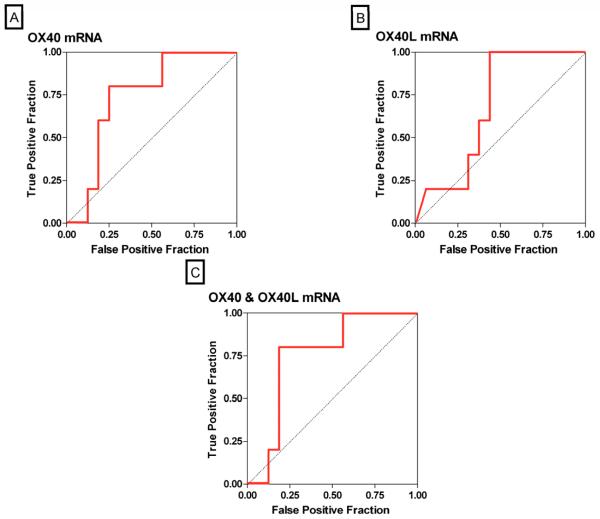
The ROC curves (Fig 2) show the fraction of true positive results (sensitivity) and false positive results (1-specificity) for various cutoff levels of an individual mRNA or a combination of mRNAs. The log-transformed cutoff value that gave the maximum combined sensitivity and specificity for OX40 was 5.98; at this cutpoint, the sensitivity was 81% and the specificity was 88% (AUC: 0.88, 95% confidence interval [CI]: 0.78 to 0.98, P<0.0001) (Fig. 2A). The cutoff value for OX40L of 1.79 was diagnostic of acute rejection with a sensitivity of 90% and a specificity of 64% (AUC: 0.81, 95% CI: 0.68 to 0.94, P<0.0001) (Fig. 2B), and the AUC for the combination of OX40 and OX40L was 0.87 (95% CI: 0.77 to 0.98, P<0.0001) (Fig. 2C).
Figure 2. Receiver-Operating-Characteristic Curves of mRNA Levels for Predicting Acute Rejection.
The fraction of true positive results (sensitivity) and false positive results (1-specificity) for levels of mRNA, each normalized for 18S rRNA, are displayed as predictors of acute rejection. The calculated area under the curve (AUC) was 0.88 (95 percent confidence interval, 0.78 to 0.98) for OX40 mRNA levels (P<0.0001, Panel A), 0.81 (95 percent confidence interval, 0.68 to 0.94) for OX40L mRNA levels (P<0.0001, Panel B), 0.87 (95 percent confidence interval, 0.77 to 0.98) for the best linear combination of OX40 and OX40L (P<0.0001, Panel C) and 0.98 (95 percent confidence interval, 0.96 to 1.0) for the best linear combination of levels of OX40, OX40L, PD-1 and Foxp3 (P<0.0001, Panel D). An AUC value of 0.5 is considered no better than predicted by chance (null hypothesis), while a value of 1.0 is considered a perfect test.
Using a stepwise logistic regression analysis, we investigated whether a linear combination of the log-transformed, 18S-normalized levels of urinary cell mRNAs improved the diagnostic accuracy. Our analysis showed that a combination of urinary cell levels of mRNA for OX40, OX40L, PD-1 and Foxp3 increased the AUC to 0.98 (95% CI: 0.96 to 1.00, P<0.0001) and predicted acute rejection with a sensitivity of 95% and a specificity of 92% (Fig. 2D). Inclusion of the remaining 4 mRNA measures (PD-L1, PD-L2, granzyme B and perforin) resulted in an AUC of 1.00 (100% sensitivity and 100% specificity), but this model likely reflects overfitting. Importantly, whereas no significant difference was found between the AUC for the combination of OX40 and OX40L compared to the AUC for OX40 alone (Fig. 2A vs. 2C) (P=0.60), the AUC for the combination of OX40, OX40L, PD-1 and Foxp3 was significantly greater than the AUC for OX40 alone (Fig. 2A vs. 2D) (P=0.03).
Acute Rejection Severity and Urinary Cell mRNA Levels
Within the acute rejection group (N=21), we examined whether acute rejection severity, as reflected by serum creatinine levels, was associated with urinary cell levels of mRNA. Our analysis showed that none of the mRNAs were significantly associated with serum creatinine levels: OX40, Spearman’s correlation coefficient [rs] = −0.24, P=0.29; OX40L, rs = − 0.05, P=0.83; PD-1, rs = − 0.07; P=0.78; PD-L1, rs = 0.16, P=0.50; PD-L2, rs = 0.02, P=0.95; granzyme B, rs = 0.07, P=0.78; perforin, rs = 0.05, P=0.84; and Foxp3, rs = −0.04, P=0.85.
Twelve of the 21 acute rejection biopsies were classified as T-cell mediated acute rejection Banff grade IA and the remaining 9 as Banff grade 1B. Neither serum creatinine level (mean [±SE], 2.76±0.48 vs. 3.27±0.46, respectively; P=0.36, Mann-Whitney test) nor levels of mRNA for OX40 (P=0.46), OX40L (P=0.32), PD-1 (P=0.33), PD-L1 (P=0.27), PD-L2 (P=0.08), granzyme B (P=0.19), perforin (P=0.50) or Foxp3 (P=0.41) were significantly different between the two Banff grades.
Acute Rejection Reversibility and Urinary Cell mRNA Levels
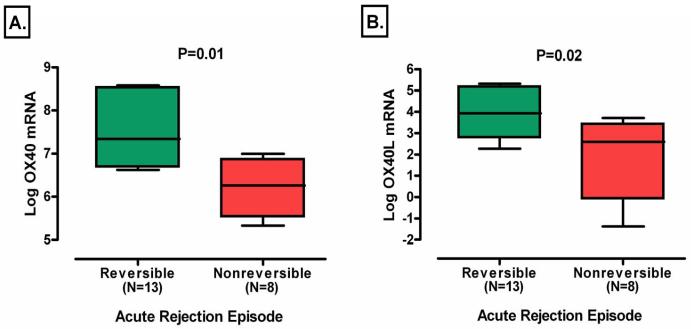
Thirteen of the 21 acute rejections qualified as successfully reversed (16). The mean (±SE) log transformed level of OX40 mRNA in urinary cells was higher in those with reversible acute rejection than those without reversal (7.47±0.31 vs. 6.18±0.28, P=0.01, Fig. 3A). The levels of OX40L mRNA were also higher in those with reversal than those without reversal (3.88±0.34 vs. 1.87±0.74, P=0.02, Fig. 3B). We also examined whether a relationship exists between log transformed OX40 and OX40L mRNA levels and percent improvement in 1/Creatinine, and this analysis showed that there was no significant linear relationship between the log-transformed levels of OX40 (rs=−0.40, P=0.07) and OX40L mRNA (rs=−0.21, P=0.36) and percent improvement in 1/creatinine.
Figure 3. Levels of mRNA in Urinary Cells in Subjects with Reversible or Nonreversible Acute Rejection.
Successful reversal of acute rejection was defined as a return in creatinine to within 15 percent of baseline within 4 weeks of initiating anti-rejection treatment (16), and 13 of the 21 acute rejections qualified as reversed using this criterion. Boxplots show the 10th, 25th, 50th, 75th, and 90th percentiles of log-transformed 18S-normalized mRNA levels for OX40 and OX40L, in urine samples obtained from the 13 subjects with a return in creatinine to within 15 percent of baseline within 4 weeks of initiating anti-rejection treatment (Reversible) and the 8 subjects without a return in creatinine to within 15 percent of baseline within 4 weeks of initiating anti-rejection treatment (Nonreversible). The levels of mRNA for OX40 (Panel A) and OX40L (Panel B) were significantly higher in urinary cells from subjects with reversible acute rejection than in the subjects with nonreversible acute rejection. Two-tailed P-values are based on Mann-Whitney test. In all cases, the natural log transformed values of 18S-normalized mRNA levels are shown.
Acute rejection reversibility was also predicted by the level of mRNA for Foxp3 (P=0.04). In contrast, levels of mRNA for PD-1 (P=0.80), PD-L1 (P=0.33), PD-L2 (P=0.33), granzyme B (P=0.41) and perforin (P=0.21) did not predict acute rejection reversal. In a stepwise logistic regression analysis, Foxp3 was a marginally significant predictor controlling for OX40, and none of the other 6 mRNA measures was independently predictive of acute rejection reversibility.
ROC-Curve Analysis of mRNA Levels for Predicting Acute Rejection Reversal
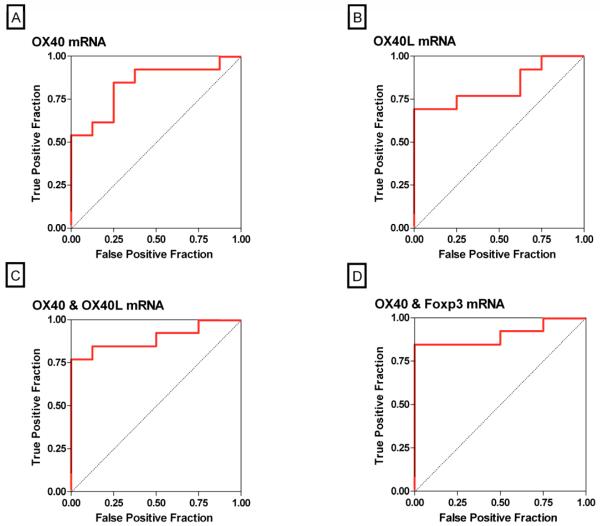
The log-transformed threshold that had the maximum combined sensitivity and specificity for OX40 mRNA was 6.68, and using this threshold reversibility of acute rejection was predicted with a sensitivity of 85% and a specificity of 75% (AUC: 0.84, 95% CI: 0.66 to 1.0, P=0.0002) (Fig. 4A). At a cutoff value of 3.79 for OX40L mRNA, reversibility of acute rejection was predicted with a sensitivity of 69% and a specificity of 100% (AUC: 0.83, 95% CI: 0.65 to 1.0, P=0.0004) (Fig. 4B). The AUC for the combination of OX40 and OX40L was 0.89 (95% CI: 0.75 to 1.0, P<0.0001) (Fig. 4C), and the AUC for the combination of OX40 and Foxp3 was 0.90 (95% CI: 0.77 to 1.0, P<0.0001) (Fig. 4D). The AUC for the combination of all 8 mRNA measures (again an overfitted model) was 0.93 (95% CI: 0.80 to 1.0, P<0.0001). Although the AUCs for the combination of OX40 and OX40L, the combination of OX40 and Foxp3, and the combination of all 8 genes were each numerically greater than the AUC of OX40 alone, a statistical comparison of the ROC curves showed that none was significantly greater than the AUC for OX40 alone (all P>0.20).
Figure 4. Receiver-Operating-Characteristic Curves of mRNA Levels for Predicting Acute Rejection Reversibility.
The fraction of true positive results (sensitivity) and false positive results (1-specificity) for levels of mRNA, each normalized for 18S rRNA, are displayed as predictors of reversal of acute rejection. The calculated area under the curve was 0.84 (95 percent confidence interval, 0.66 to 1.0) for OX40 mRNA levels (P=0.0002, Panel A), 0.83 (95 percent confidence interval, 0.65 to 1.0) for OX40L mRNA levels (P=0.0004, Panel B), 0.89 (95 percent confidence interval, 0.75 to 1.0) for the best linear combination of levels of mRNA for OX40 and OX40L (P<0.0001, Panel C) and 0.90 (95 percent confidence interval, 0.77 to 1.0) for the best linear combination of levels of mRNA for OX40 and Foxp3 (P<0.0001, Panel D). A value of 0.5 is considered no better than predicted by chance and a value of 1.0 is considered a perfect indicator.
Whereas levels of urinary cell mRNAs for OX40, OX40L and Foxp3 predicted reversibility of an episode of acute rejection, age (mean [±SD], 41±12.0 years vs. 51±12.7 years, respectively; P=0.06), gender (8 men, 5 women vs. 3 men, 5 women; P=0.39), race (2 white, 4 black, and 7 other race vs. 2 white, 3 black, 3 other race; P=0.75), donor type (9 live and 4 deceased vs. 4 live and 4 deceased; P=0.65), or the type of acute rejection therapy (9 with single modality of treatment and 4 with combined modalities vs. 7 with single modality of treatment and 1 with combined modalities; P=0.61) were not predictive. Also, the creatinine level at the time of biopsy was not predictive of acute rejection reversal (2.79±1.19 vs. 3.28±2.05, P=0.64).
Graft Loss and Urinary Cell mRNA Levels
Of the 21 subjects with acute rejection, 5 lost their grafts within six months of biopsy-proven acute rejection. The mean creatinine [±SE] at the time of biopsy of the five patients with graft loss was 4.14±0.98 mg/dL and higher than those without graft loss (2.61±0.29 mg/dL) but this difference was not significant (P=0.12).
In the logistic regression analysis, OX40 was the mRNA measure that had the strongest association, albeit not statistically significant (P=0.17), with graft loss; those with lower OX40 were more likely to lose their graft. The log-transformed threshold that had the maximum combined sensitivity and specificity for OX40 mRNA was 6.68, and using this threshold graft loss was predicted with a sensitivity of 80% and a specificity of 75% (AUC: 0.74, 95% CI: 0.51 to 0.97, P=0.04) (Fig. 5A), and the AUC for OX40L was 0.68 (95% CI: 0.44 to 0.92, P=0.14) (Fig. 5B). The AUC for the combination of OX40 and OX40L was 0.75 (95% CI: 0.52 to 0.98, P=0.04) (Fig. 5C). There was no significant difference however between the AUC for OX40 alone and the AUC for the combination of OX40 and OX40L (Fig. 5A vs. 5C) (P=0.48) but the difference between the AUC for OX40 alone and the AUC for the combination of all 8 genes was marginally significant (P=0.09) but this prediction model is confounded by the inclusion of a relatively large number of mRNA measures for the small number of outcome events observed.
Figure 5. Receiver-Operating-Characteristic Curves of mRNA Levels for Predicting Graft Loss.
The fraction of true positive results (sensitivity) and false positive results (1-specificity) for levels of mRNA, each normalized for 18S rRNA, are displayed as predictors of graft loss within 6 months of biopsy proven acute rejection. The calculated area under the curve was 0.74 (95 percent confidence interval, 0.51 to 0.97) for OX40 mRNA levels (P=0.04, Panel A), 0.68 (95 percent confidence interval, 0.44 to 0.92) for OX40L mRNA levels (P=0.14, Panel B), 0.75 (95 percent confidence interval, 0.52 to 0.98) for the best linear combination of levels of mRNA for OX40 and OX40L (P=0.04, Panel C). A value of 0.5 is considered no better than predicted by chance and a value of 1.0 is considered a perfect indicator.
Discussion
We have identified that acute rejection of human renal allografts, an important post-transplant complication, is associated with the levels of mRNA for OX40, OX40L, and PD-1 in urinary cells, and that a diagnostic algorithm that included urinary cell levels of mRNA for OX40, OX40L, PD-1, and Foxp3 is an excellent predictor of acute rejection of human renal allografts. Our urinary cell mRNA profiling strategy also indicates that acute rejection reversal and perhaps graft loss following an episode of acute rejection are predictable with the use of levels of mRNAs in urinary cells, but these results need to be confirmed in a larger study. It is noteworthy that OX40 was the strongest predictor of all three outcomes, and that Foxp3 was the second strongest predictor of both acute rejection and acute rejection reversal.
OX40 is a member of the tumor necrosis factor receptor superfamily, expressed primarily by CD4+ T-cells and some CD8+ T-cells. The ligand for OX40 is typically expressed by APCs, and is also a member of the tumor necrosis factor superfamily. Stimulation via the OX40/OX40L pathway propagates T-cell expansion, cytokine production, and generation of memory T-cells. OX40 costimulation typically evokes a Th2 response signal; however, it is also capable of a Th1 response (17-21). Elegant pre-clinical studies showed that early OX40 stimulation results in acute rejection whereas delayed stimulation leads to chronic rejection of experimental allografts (9). The new observation from the current study that the levels of transcripts for OX40 and OX40L are higher during an episode of acute rejection compared to stable allograft status is consistent with the pre-clinical findings that the OX40/OX40L pathway facilitates acute rejection. Very importantly, our novel observations demonstrate for the first time that noninvasively ascertained levels of mRNA for OX40 and OX40L are positively associated with an episode of acute rejection in humans.
The OX40/OX40L proteins have also been implicated in regulating the emergence of regulatory T-cells (Treg cells) and modulating their suppressive activity (17, 21). Our observation that acute rejection reversal is associated with high rather than low levels of OX40 appears to conflict with the notion that OX40 signaling may impair Treg cell associated down regulation of the anti-allograft response. It appears however that the role of OX40 in Treg cell associated processes is complex and not unidirectional. Griseri et al. recently reported that the presence of OX40 on the surface of Treg cells is an obligatory requirement for the Treg cells to accumulate at the site of inflammation and control colitogenic effector T-cells (22). In their experimental model of colitis, OX40 was also required for the effector response. This dual role for OX40 positive cells, one protective and the other pathogenetic, may account for our finding that high OX40 is associated not only with acute rejection but also with acute rejection reversal. Our new finding is also reminiscent of our earlier observation that Foxp3 is over expressed during an episode of acute rejection and that acute rejection reversal is associated with high rather than low levels of Foxp3 (16).
A novel T-cell inhibitory pathway involves PD-1 and its ligands, PD-L1 and PD-L2 (23-25). The PD-1/PD-L interaction is reported to arrest the cell cycle in the G0-G1 phase (25) but PD-1 activation resulting in T-cell proliferation has also been reported (26). It has also been reported that blockade of PD-1 or PD-L1 results in accelerated acute rejection in pre-clinical models (14, 25, 27). Our findings suggest that the urinary cell levels of PD-1, PD-L1, and PD-L2 are less informative of allograft status compared to the levels of mRNA for the positive costimulatory proteins OX40 and OX40L.
Measurement of OX40 mRNA and OX40L mRNA in urinary cells offers a noninvasive means of diagnosing acute rejection and accurately predicting the outcome of an episode of acute rejection. The current investigation confirmed and extended our earlier finding that urinary cell levels of Foxp3 predict acute rejection reversal (16). We also found that a combination of levels of mRNA for OX40 and Foxp3 resulted in a larger AUC compared to the AUC of OX40, or OX40 and OX40L but the null hypothesis that the improvement in AUC was due to chance alone could not be rejected in this study.
In the current study, urinary cell level of OX40 mRNA was the strongest predictor of graft loss and none of the other mRNAs were significant predictors. Inclusion of all 8 mRNAs in a prediction model resulted in the largest AUC that was marginally better than the AUC of OX40 alone. This result however should be interpreted with due caution in view of the small sample size and the potential for model overfitting.
In summary, our findings demonstrate that measurement of mRNA for OX40, OX40L and PD-1 in urine offers a noninvasive means of diagnosing acute rejection of human renal allografts, and a combination of urinary cell levels of mRNA for OX40, OX40L, PD-1 and Foxp3 is the best predictor of biopsy confirmed acute rejection. Among the mRNAs measured, OX40 mRNA appeared to outperform others with respect to predicting acute rejection reversibility and graft loss following an episode of acute rejection and studies to validate the biomarkers of renal allograft status discovered in this study merit further consideration.
Materials & Methods
Study Cohorts
We examined urine samples from 46 kidney transplant recipients. There were 21 subjects with graft dysfunction (mean [±SE] creatinine level, 2.98±0.34 mg/dL) and biopsy-confirmed acute rejection (mean[±SD] age, 45±13 years; 11 men and 10 women; 4 white, 7 black, and 10 other race; 8 deceased donor and 13 living donor grafts; median time from transplantation to for-cause biopsy, 180 days) and 25 subjects with stable graft function (creatinine level, 1.40±0.06 mg/dL) and normal biopsy results (age, 47±13 years; 14 men and 11 women; 9 white, 5 black, and 11 other race; 5 deceased donor and 20 living donor grafts; time from transplantation to protocol biopsy, 51 days). Forty-four of the 46 urine specimens were collected on the day of the biopsy. The remaining 2 samples were from the normal biopsy group and collected before the biopsy procedure.
Formalin-fixed, paraffin-embedded renal-biopsy specimens were stained with hematoxylin and eosin, periodic acid–Schiff, and Masson’s trichrome stains and were scored on the Banff classification (28) by a pathologist blinded to the results of the gene expression. There was no evidence of BKV or CMV on light microscopy.
Additional information regarding the study subjects is provided as Supplemental Digital Content.
Quantitation of mRNA by Real Time Quantitative PCR assays
Total RNA was isolated from urine cell pellets, quantified, and reverse transcribed to cDNA. PCR analysis, quantification of mRNA copies, and the sequence of the gene specific oligonucleotide primers and TaqMan probes used in this investigation are described in Supplemental Table 1 (Supplemental Digital Content).
Statistical Analysis
The levels of 18S-normalized mRNA for OX40, OX40L, PD-1, PD-L1, PD-L2, granzyme B, perforin and Foxp3 deviated from a normal distribution (P<0.0001) and natural log-transformation substantially reduced the deviation. All statistical calculations were performed using GraphPad Prism v.4.0. Using 18S-normalized levels, we used the Mann-Whitney test to test for differences between the group with acute rejection and the group with normal biopsy results. Categorical variables were compared using Fisher’s Exact Test or Chi-Square Analysis. We used Spearman’s rank-order correlations to test for monotonic associations with the 18S-adjusted mRNA transcript levels, and supplemental Table 2 (Supplemental Digital Content) shows the relationships among the levels of mRNAs measured in this investigation. We generated ROC curves for individual mRNA levels as well as a linear combination of mRNA levels to determine the cutoff points that yielded the highest combined sensitivity and specificity for predicting the diagnosis, reversibility of an episode of acute rejection, and graft loss following acute rejection. Successful reversal of acute rejection was defined as a return in creatinine to within 15 percent of baseline within 4 weeks of initiating anti-rejection treatment (16). A second end point was the loss of the graft during the first six months after the diagnosis of acute rejection.
Supplementary Material
Acknowledgments
This work was supported in part by an award (R37 AI051652) from NIAID, NIH, and an award (NPRP 08-503-3-111) from the Qatar National Research Foundation to Manikkam Suthanthiran and by grant ULI RR 024996 of the Clinical and Translational Science Center at Weill Cornell Medical College.
CA was supported by an award (T32 HL08382401) from NIH to Todd Evans.
ML was supported in part by the Empire Clinical Research Investigator Program (ECRIP) New York State award.
Footnotes
CA: Participated in research design, writing of the paper, in the performance of the research, and in data analysis, no conflict of interest
TM: Participated in research design, writing of the paper, in the performance of the research, and in data analysis, no conflict of interest
ML: Participated in research design, performance of the research, and in data analysis, no conflict of interest
RD: Participated in research design and contributed new reagents or analytic tools, no conflict of interest
CS: Participated in the performance of the research, no conflict of interest
VS: Participated in research design, no conflict of interest
SS: Participated in the performance of the research, no conflict of interest
DD: Participated in the performance of the research, no conflict of interest
JES: Participated in the writing of the paper and in data analysis, no conflict of interest
MS: Participated in research design, writing of the paper, and in data analysis, no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 2.Bustami RT, Ojo AO, Wolfe RA, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4(1):87. doi: 10.1046/j.1600-6135.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 3.Jamil B, Nicholls K, Becker GJ, Walker RG. Impact of acute rejection therapy on infections and malignancies in renal transplant recipients. Transplantation. 1999;68(10):1597–603. doi: 10.1097/00007890-199911270-00027. [DOI] [PubMed] [Google Scholar]
- 4.Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008;3:189. doi: 10.1146/annurev.pathmechdis.3.121806.151508. [DOI] [PubMed] [Google Scholar]
- 5.Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol Rev. 2003;196:85. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 6.Simeoni L, Lindquist JA, Smida M, Witte V, Arndt B, Schraven B. Control of lymphocyte development and activation by negative regulatory transmembrane adapter proteins. Immunol Rev. 2008;224:215. doi: 10.1111/j.1600-065X.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- 7.Bharat A, Mohanakumar T. Allopeptides and the alloimmune response. Cell Immunol. 2007;248(1):31. doi: 10.1016/j.cellimm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10(2-3):101. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 9.Burrell BE, Lu G, Li XC, Bishop DK. OX40 costimulation prevents allograft acceptance induced by CD40-CD40L blockade. J Immunol. 2009;182(1):379. doi: 10.4049/jimmunol.182.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Xiao X, Demirci G, Li XC. OX40 controls islet allograft tolerance in CD154 deficient mice by regulating FOXP3+ Tregs. Transplantation. 2008;85(11):1659. doi: 10.1097/TP.0b013e3181726987. [DOI] [PubMed] [Google Scholar]
- 11.Demirci G, Amanullah F, Kewalaramani R, et al. Critical role of OX40 in CD28 and CD154-independent rejection. J Immunol. 2004;172(3):1691. doi: 10.4049/jimmunol.172.3.1691. [DOI] [PubMed] [Google Scholar]
- 12.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10(11):1185. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003;76(6):994. doi: 10.1097/01.TP.0000085010.39567.FB. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Carper K, Malone F, et al. PD-L1/PD-1 signal deficiency promotes allogeneic immune responses and accelerates heart allograft rejection. Transplantation. 2008;86(6):836. doi: 10.1097/TP.0b013e3181861932. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Hartono C, Ding R, et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344(13):947. doi: 10.1056/NEJM200103293441301. [DOI] [PubMed] [Google Scholar]
- 16.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353(22):2342. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 17.Demirci G, Li XC. Novel roles of OX40 in the allograft response. Curr Opin Organ Transplant. 2008;13(1):26. doi: 10.1097/MOT.0b013e3282f3def3. [DOI] [PubMed] [Google Scholar]
- 18.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165(6):3043. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 19.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229(1):271. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 20.Takeda I, Ine S, Killeen N, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172(6):3580. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 21.Xiao X, Kroemer A, Gao W, Ishii N, Demirci G, Li XC. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J Immunol. 2008;181(5):3193. doi: 10.4049/jimmunol.181.5.3193. [DOI] [PubMed] [Google Scholar]
- 22.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207(4):699. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.del Rio ML, Buhler L, Gibbons C, Tian J, Rodriguez-Barbosa JI. PD-1/PD-L1, PD-1/PD-L2, and other co-inhibitory signaling pathways in transplantation. Transpl Int. 2008;21(11):1015. doi: 10.1111/j.1432-2277.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 24.Kitazawa Y, Fujino M, Wang Q, et al. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83(6):774. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- 25.Sandner SE, Clarkson MR, Salama AD, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174(6):3408. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 26.Tamura H, Dong H, Zhu G, et al. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97(6):1809. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Ueno T, Clarkson MR, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 28.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713.29. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.