Abstract
Population outbreaks in tundra rodents have intrigued scientists for a century as a result of their spectacular appearances and their general lessons in ecology. One outstanding question that has led to competing hypotheses is why sympatric lemmings and voles differ in regularity and shape of their outbreaks. Lemming outbreaks may be lost for decades while vole populations maintain regular population cycles. Moreover, when lemming populations eventually irrupt, they do so more steeply than the vole populations. Norwegian lemmings exhibited a large-scale outbreak synchronously with gray-sided voles in Finnmark, northern Fennoscandia, during 2006 to 2007 for the first time in two decades. Analyses of spatial variability of this outbreak across altitudinal gradients allowed us to identify determinants of the contrasting lemming and vole dynamics. The steeper lemming outbreak trajectories were caused by breeding and population growth during winter, when nonbreeding vole populations consistently declined. The differently shaped lemming and vole outbreaks appear to result from a particular demographic tactic of lemmings that evolved as an adaptation to the long and cold Arctic–Alpine winters. The lemming outbreak amplitude increased with altitude and vole density, indicating that lemming outbreaks are jointly facilitated by low temperatures and apparent mutualism with voles mediated by shared predators. High sensitivity to variation in climate and predation is likely to be the reasons why lemmings have more erratic population dynamics than sympatric voles. The combination of continued climatic warming and dampened vole cycles is expected to further decrease the frequency, amplitude, and geographic range of lemming outbreaks in tundra ecosystems.
Keywords: arctic tundra, climate impact, density dependence, spatial population dynamics
For centuries, lemmings have caught the attention of naturalists and scientists as a result of their spectacular population dynamics and keystone functioning in tundra ecosystems (1–4). Charles Elton (5) was the first to recognize the cyclic nature of lemming population outbreaks—a discovery that initiated a lasting research tradition that aims to identify causes of multiannual cycles in animal populations (6).
Current research on lemmings has been fueled by two recent discoveries made in the case of the Norwegian lemming Lemmus lemmus, the most renowned lemming species (3). The first is that its outbreak trajectory is differently shaped from that of the gray-sided vole (Myodes rufocanus), the often codominant rodent species, which always exhibits population peaks synchronously with the lemming. Specifically, the lemming population erupts more steeply than the vole population. This discrepancy in “outbreak topology” has been suggested to result from different trophic interactions; the “sharp” lemming outbreaks from an interaction with food plants, the “blunt” vole peaks from an interaction with predators (7, 8). However, there are still contrasting views on which factors regulate lemming populations (4, 6, 9).
The other issue renewing ecologists’ search for circumstances causing cyclic population outbreaks is the recent emergence of collapsed cycles in several species (10). One well documented case of a recent absence of outbreaks is that of a local Norwegian lemming population in alpine southern Norway, where cyclic outbreaks at regular 3- to 4-y intervals prevailed until the past 15 y (11). However, this recent incident is not unprecedented. In large tracts of sub- and low-Arctic Fennoscandian tundra, the Norwegian lemming population has erupted only two times since the 1970s, during which time, interestingly, the sympatric gray-sided vole has maintained a regular 5-y population cycle (7, 8). Thus, the northern Fennoscandian tundra offers opportunities to elucidate why lemming and vole outbreak trajectories differ and why lemming outbreaks may get lost for long time periods.
Besides the possibility that lemming–plant interactions are more prone to irregularities (including a more variable outbreak amplitude) than vole–predator interactions (8), there are two other hypotheses explaining why cyclic lemming outbreaks are impeded while sympatric voles maintain cycling. One assumes that lemmings are more sensitive than voles to climate variation (10, 12). The other emphasizes community processes and predicts that lemming outbreaks are limited by indirect interaction with voles mediated by shared predators (13–15). To our knowledge, no previous study has evaluated the relative merits of these (not necessarily mutually exclusive) hypotheses.
During 2006 to 2007 a large-scale Norwegian lemming outbreak arose in Finnmark, in sub- and low-Arctic Fennoscandia, for the first time in at least two decades. Based on spatially extensive monitoring of rodent populations at 109 tundra sites spanning an area of 10 000 km2, we were able to encompass substantial spatial variation in outbreak amplitude along replicated climatic (i.e., altitudinal) gradients in lemmings and gray-sided voles. Here we provide an analysis of this variation that sheds light on the differences and interrelations between lemming and vole dynamics and which factors may impede lemming outbreaks for decades.
Results
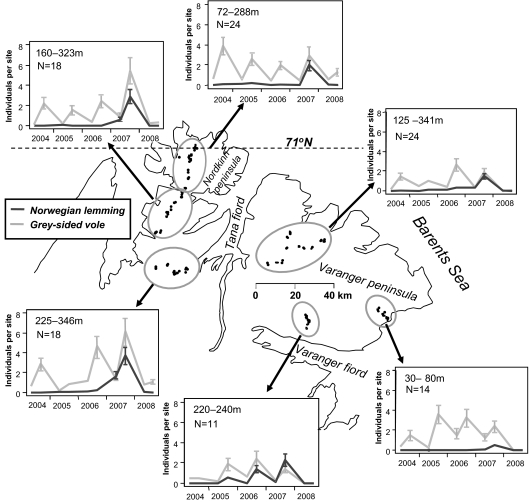
To visually illustrate the spatiotemporal features of the lemming outbreak compared with the dynamics of the gray-sided vole in the entire monitoring area, we display spatially averaged outbreak trajectories for six separate subregions (Fig. 1). Although all rodent populations simultaneously had converged on very low postoutbreak densities by spring 2008, the incipient stage of the lemming outbreak differed markedly from that of the gray-sided vole. The onset of the lemming irruption was delayed compared with the onset of the increase phase of voles. From its onset, the lemming outbreak arose steeply to reach sharp peaks simultaneously across the study region in fall 2007, although with large spatial variation in outbreak amplitude (i.e., peak densities; Fig. 1). Peak densities also varied in voles, but were reached more gradually, and the dynamics were more spatially asynchronous than in lemmings.
Fig. 1.
Population trajectories of Norwegian lemmings (L. lemmus) and gray-sided voles (M. rufocanus) displayed for six separate tundra subregions in eastern Finnmark, northernmost Fennoscandia. Population trajectories are based on the mean number of individuals caught per site (with SE bars) in spring and fall in each year. One individual/site corresponds to 4.17 individuals and 100 trap-nights. Small squares on the map denote the position of the trapping sites in each region. The number of trapping sites per subregion and the altitude range they spanned are provided in the panel for each subregion.
To provide a quantitative, comparative assessment of population trajectories and their potential determinants in lemmings and voles, we analyzed site-specific seasonal (winter and summer) growth rates during the lemming outbreak period (fall 2006 to fall 2007). On average, the Norwegian lemming had positive winter growth rates, whereas they were consistently negative in the gray-sided vole (Table 1 and Fig. 1). Summer growth rates in lemmings and voles were positive, although somewhat lower in the lemmings (Table 1). The degree of spatial coherence in growth rates was assessed by computing Moran I statistics. Also, this spatial aspect of the seasonal dynamics differed between the lemming and the vole. Lemming growth rates exhibited spatial autocorrelation in winter, but not in summer, whereas the gray-sided vole had the opposite seasonal difference (Table 1).
Table 1.
Parameters of seasonal population growth rates for Norwegian lemming and gray-sided vole during the lemming outbreak period
| Moran I |
Coefficients of linear state-space models |
|||||
| Species/growth season | Mean | 0–10 km | 20–50 km | Density dependence, βdd | Altitude | Vole density |
| Lemming | ||||||
| Winter (RW) | 0.07 | 0.26* | 0.054† | −0.207‡ (−1.05, 0.578) | 1.270‡ (0.409, 2.157) | 0.613‡ (0.032, 1.301) |
| Summer (RS) | 0.68 | 0.015 | 0.007 | 0.117‡ (−0.089, 0.361) | 0.629‡ (0.370, 0.915) | 0.059 (−0.288, 0.426) |
| Gray-sided vole | ||||||
| Winter (RW) | −0.66 | 0.00 | 0.003 | 0.865 (0.426, 1.373) | 0.181 (−0.565, 0.178) | NA |
| Summer (RS) | 0.85 | 0.13† | 0.14* | 0.320‡ (0.081, 0.615) | 0.018 (−0.270, 0.226) | NA |
Growth season means are based on all site-specific growth rates (N = 109) in the study region (Fig. 1) taken as RW = log(nspring 2007 + 1) – log(nfall_2006 + 1) and RS = log(nfall_2007 + 1) − log(nspring_2007 + 1), where n is the number of individuals per trapping site per season (spring and fall) and year (2006 and 2007). Moran's I statistics quantify spatial autocorrelation in growth rates among trapping sites at two spatial scales. Coefficients of linear state-space models (with 95% credible intervals in brackets) quantify the partial effects of density dependence, altitude and density of voles (only applicable in case of lemmings) on seasonal growth rates. Note that negative density-dependence implies βdd < 1.
Significant autocorrelation at *P < 0.0001 and †P < 0.001.
‡Significant coefficient.
We used linear state-space models (Methods) to estimate the effect of the following potential predictors of seasonal growth rates (all model coefficients are provided in Table 1): direct density dependence, altitude (as a proxy of climatic variation), and density of voles (to estimate the effect of community interactions). Winter and summer growth rates were negatively density dependent in the lemming, although more strongly so in winter than in summer. In the vole, only summer growth rates were density dependent. Population growth in the vole was generally unaffected by altitude. In contrast, lemming populations grew more steeply with increasing altitude, causing the outbreak to reach the highest amplitude at the highest altitudes (Fig. 2). The altitude effect was strongest during the winter (Table 1). Indirect community interactions were indicated by a positive effect of density of voles on lemming growth rate during the winter.
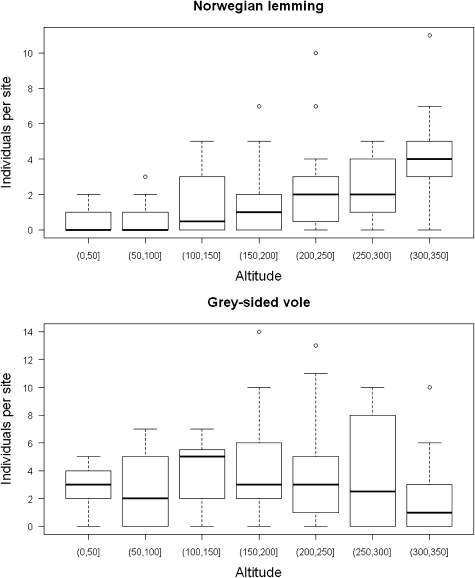
Fig. 2.
Norwegian lemming outbreak amplitude as a function of altitude. Box plots give the distribution of number of individuals caught per site in fall 2007 in the 109 tundra sites binned in intervals of 50 m above sea level. Equivalent plots for the gray-sided vole are given for comparison.
Discussion
Our spatially extensive seasonal monitoring, which happened to encompass the now rare event of a proper lemming outbreak in northern Fennoscandia, allowed us to provide a detailed comparison of the topology of the lemming outbreak with the simultaneous dynamics of the gray-sided vole. In accordance with previous studies (7, 8), the lemming exhibited a steeper increase phase than that of the vole. However, the previous studies based their analysis of population growth rates taken at an annual time scale (fall to fall); i.e., the population dynamics were not separated into seasonal components. In contrast, we analyzed growth rates for summers (spring to fall) and winters (fall to spring) separately and thereby can provide unique insights into the basis for the lemming–vole dichotomy.
Proximately, the more rapid increase in the lemming was a result of population increase in winter, when the vole populations steeply decreased. Population increase during winter in lemmings results from winter reproduction (4, 16)—a demographic trait virtually absent in the gray-sided vole (14). The recruitment component of lemming winter growth can also explain why only lemmings exhibited density-dependent growth rate in winter, as recruitment in northern rodents appears to be more sensitive to density than adult survival (17). In essence, our analysis suggests that the contrasting topologies of the population peaks in lemmings and voles are more likely caused by different intrinsic demographic tactics than different trophic interactions. Winter reproduction in lemmings, the only truly arctic small rodent taxon, is likely to have evolved as an adaptation to the 9- to 10-mo winter at high latitudes and altitudes. Thus, it appears to us that a taxon-specific demographic tactics is the most parsimonious explanation for the propensity for irruptive outbreak dynamics across all lemming species and a range of food web contexts in the circumpolar Arctic (8).
The variable outbreak amplitude across the extensive tundra region encompassed by our monitoring program allowed us to use a spatial approach to test hypotheses about what impedes lemming outbreaks. First, the climate hypothesis predicts that lemming outbreaks depend on low temperatures caused by snow cover properties (11, 18) or improved food quality (19). The monitoring program was specifically designed to include replicate altitudinal gradients as proxies for spatial climatic variation among tundra sites. Consistent with the expectation from a climate effect, we found that lemming population growth increased steeply with altitude. The most elevated tundra sites at 300 to 350 m above sea level were associated with lemming outbreak densities that were approximately three times higher than sites 200 m lower in the altitudinal gradients (Fig. 2). Interestingly, similar altitudinal gradients in outbreak amplitude have recently been demonstrated for cyclically outbreaking subalpine populations of moths (20, 21).
The lack of any effect of altitude on the dynamics of the gray-sided vole is consistent with the hypothesis that lemmings are the most climate-sensitive among northern rodents (10, 12). The altitude effect on lemmings was strongest in winter, thus also supporting previous findings that climate impacts through snow cover properties (11). The more modest summer effect indicates that some additional mechanism also contributes, for instance, temperature-dependent quality of mosses (19). Consequently, lemmings may be more sensitive to high temperatures than voles as a result of different diets (i.e., reliance on mosses; ref. 1) and intrinsic demographic tactics (i.e., winter breeding before outbreaks; ref. 16).
It has been hypothesized that Norwegian lemmings do not reach high abundances at low altitudes because of apparent competition with voles mediated by shared predators (8, 15). However, the results of our analysis do not support this hypothesis. First, voles were not more abundant at low altitudes (Fig. 2). Second, we found evidence for a positive effect of vole density on lemming population growth. As shown by theoretical modeling (22), community dynamics driven by cyclic predator–prey interactions is sensitive to the (empirically unknown) shape of predator functional and numerical responses and may also imply positive indirect interactions between prey species, termed apparent mutualism. Empirically, such an outcome has been found to be operating between arctic birds and rodents, in that case termed an alternative prey mechanism (23). Here we suggest that an alternative prey mechanism may be operating among species of northern rodents. Consequently, although Norwegian lemming outbreaks depend foremost on long and cold winters, providing a favorable environment for winter breeding, high vole abundance appears to contribute to lemming population increase, probably by providing a short-term release from a “predator pit” (24). Coincidences of these two conditions may have become rarer in northern Fennoscandia during recent decades as to cause a paucity of lemming outbreaks in this region. The climate has become warmer and the vole cycle has changed in terms of dampened amplitude (10) and longer intervals between cyclic peaks (25).
In conclusion, our study points to the particular adaptations and sensitivities of lemmings to conditions during the Alpine–Arctic winters, including climate and predation, as the main reason for why lemmings differ from voles in terms of topology and regularity of their outbreaks. Continued climatic warming and dampening of vole cycles in tundra ecosystems can be expected to decrease the frequency, amplitude, and geographic range of lemming outbreaks in the future.
Methods
Study Area and Monitoring Design.
Since spring 2004, we have used a large-scale, permanent system for monitoring rodent populations in the tundra of eastern Finnmark, Norway (70°N to 71°N). High-amplitude Norwegian lemming outbreaks are so conspicuous that they are reliably reported in local newspapers (1, 12). The last recorded high-amplitude outbreak in eastern Finnmark before the onset of our monitoring was in 1978. At a more southwestern site in Finnmark, where a local trapping-based census of tundra rodent populations has been maintained since 1977, lemming outbreaks were recorded in 1978 and 1988 (7, 8). Thus, lemming outbreaks in Finnmark occur at irregular intervals, sometimes with decades between consecutive outbreaks. However, during the same three decades, peak years in the gray-sided vole have occurred regularly according to a 5-y cycle (10). The last vole peak year before our monitoring commenced was in 2002 (26).
The monitoring system encompasses 109 permanent census sites distributed in treeless tundra easily accessible from roads. At each site a trapping unit (a 15-m × 15-m small quadrate) with 12 snap traps is activated for 2 d shortly after snow melt in late June (spring) and in mid-September (fall) every year (ref. 27 provides details). We used snap trapping and a fairly low sampling intensity per site to be able to complete the spatially extensive sampling within a short period (8 d) in each season. The sites within the different altitudinal gradient (i.e., subregions; Fig. 1) were trapped simultaneously. Accessibility together with natural topographical features such as peninsulas, fjords, and valleys cause the sites to be somewhat aggregated in six subregions (Fig. 1). To include spatial variation in climate as a key aspect of the monitoring design, census sites are distributed along altitudinal gradients within the subregions (whenever topography and accessibility allowed). The orographic effect in this region typically amounts to a decrease of approximately 0.6 °C per 100 m (28). The lower end of these gradients is usually set by the presence of subalpine forest, whereas the upper end is set where the tundra vegetation becomes discontinuous. The sites included an approximately equal proportion of xeric and mesic tundra vegetation and care was taken not to obtain any altitudinal or other spatial biases in tundra type.
Statistical Analysis.
We assessed autocorrelation in raw seasonal growth rates (as defined in the legend to Table 1) as well as residuals from statistical models (as detailed later) by means of Moran I statistics (29). We computed Moran I statistics for two nonoverlapping spatial scales defined by neighborhood graphs; i.e., 0 to 10 km and 20 to 50 km. We confirmed that we had not missed any spatial structure in the data beyond the spatial scales used in the Moran I analysis by also inspecting spline-based correlograms (30).
To assess potential determinants of spatial variation in seasonal growth rates of lemming and gray-sided vole, we used linear state-space models (31) with seasonal population density (Xt estimated by using the number of individuals caught per site Nt, as detailed later) as the response variable. The baseline model included population density in the preceding season (Xt−1) as a predictor, as this model implicitly represents the growth rate as follows:
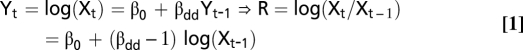 |
and because direct density dependence (i.e., βdd < 1 implies negative density dependence) is notoriously a strong predictor of lemming and gray-sided vole population dynamics (11, 14). Delayed density dependence (i.e., dependence on Xt−2) was not considered as we analyzed growth rates only during the lemming outbreak period (fall 2006 to fall 2007). The model considering dynamics during the outbreak summer (i.e., RS) had Xt = Fall_2007 as response and Xt−1 = Spring_2007 as predictor, whereas the model of winter dynamics (i.e., Rw) had Xt = Spring_2007 as response and Xt−1 = Fall_2006 as predictor.
Because the values for the response and the predictor are affected by stochastic sampling variability (i.e., “measurement error”) that will affect estimates of density dependence, we used a statistical model assuming that observed numbers were the realization of a Poisson process (31), Nt ∼ Poisson(μt) and Nt−1 ∼ Poisson(μt−1):
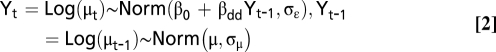 |
This baseline model was fitted with site-specific altitude and vole density (in case of the lemming) as additional predictor terms. Altitude served as a proxy for spatial variation in climate (as detailed earlier). Density of voles was predicted to affect lemming growth rate only indirectly through shared predators, as lemmings are competitively dominant to voles (13). We tried the effect of vole density separately in the preceding spring and fall (14), but because this gave similar results, we present only the results for vole spring densities (Table 1). The measurement errors in vole density were handled as detailed earlier.
The state space models were fit using a Bayesian approach and parameter posterior densities were numerically estimated by using Markov chain Monte Carlo simulations (32, 33). We used standard methods to assess the convergence of numerical chains, such as the Gelman and Rubin (34) convergence diagnostic (convergence was considered as good when we had scale reduction of R < 1.005 for all parameters). Spatial autocorrelation in the residuals was checked with the use of Moran I statistics, but was not found to be present in any of the models. Statistical significance was assessed in terms of 95% credibility intervals. All analyses were done in R (35).
Acknowledgments
We thank Dorothee Ehrich, John-André Henden, and two reviewers for providing comments on the manuscript. We thank Ingrid Jensvoll, Arne-Petter Sarre, and Alfred Ørjebu for their important contributions in running the spatially extensive rodent monitoring program in eastern Finnmark. The Directorate for Nature Management provides financial funds for the monitoring. Our research on population cycles is currently funded through the Ecocycles project in the European Union program BioDiversa.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.B. is a guest editor invited by the Editorial Board.
References
- 1.Stenseth NC, Ims RA, editors. The Biology of Lemmings. London: Academic Press; 1993. [Google Scholar]
- 2.Ims RA, Fuglei E. Trophic interaction cycles in tundra ecosystems and the impact of climate change. Bioscience. 2005;55:311–322. [Google Scholar]
- 3.Coulson T, Malo A. Population biology: Case of the absent lemmings. Nature. 2008;456:43–44. doi: 10.1038/456043a. [DOI] [PubMed] [Google Scholar]
- 4.Krebs CJ. Of lemmings and snow shoe hares: The ecology of northern Canada. Proc R Soc Lond. 2010 doi: 10.1098/rspb.2010.1992. 10.1098/rspb.2010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elton CS. Periodic fluctuations in the numbers of animals: Their causes and effects. J Exp Biol. 1924;2:119–163. [Google Scholar]
- 6.Hudson PJ, Bjørnstad ON. Ecology. Vole stranglers and lemming cycles. Science. 2003;302:797–798. doi: 10.1126/science.1092366. [DOI] [PubMed] [Google Scholar]
- 7.Turchin P, Oksanen L, Ekerholm P, Oksanen T, Henttonen H. Are lemmings prey or predators? Nature. 2000;405:562–565. doi: 10.1038/35014595. [DOI] [PubMed] [Google Scholar]
- 8.Oksanen T, Oksanen L, Dahlgren J, Olofsson J. Arctic lemmings, Lemmus spp. and Dicrostonyx spp.: integrating ecological and evolutionary perspectives. Evol Ecol Res. 2008;10:415–434. [Google Scholar]
- 9.Gauthier G, Berteaux D, Krebs CJ, Reid D. Arctic lemmings are not simply food limited—a comment. Evol Ecol Res. 2009;11:483–484. [Google Scholar]
- 10.Ims RA, Henden J-A, Killengreen ST. Collapsing population cycles. Trends Ecol Evol. 2008;23:79–86. doi: 10.1016/j.tree.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Kausrud KL, et al. Linking climate change to lemming cycles. Nature. 2008;456:93–97. doi: 10.1038/nature07442. [DOI] [PubMed] [Google Scholar]
- 12.Angerbjörn A, Tannerfeldt M, Lundberg H. Geographical and temporal patterns of lemming population dynamics in Fennoscandia. Ecography. 2001;24:298–308. [Google Scholar]
- 13.Hanski I, Henttonen H. Predation on competing rodent species: A simple explanation of complex patterns. J Anim Ecol. 1996;65:220–232. [Google Scholar]
- 14.Hansen TF, Stenseth NC, Henttonen H, Tast J. Interspecific and intraspecific competition as causes of direct and delayed density dependence in a fluctuating vole population. Proc Natl Acad Sci USA. 1999;96:986–991. doi: 10.1073/pnas.96.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oksanen T. Does predation prevent Norwegian lemmings from establishing permanent populations in lowland forests? In: Stenseth N, Ims RA, editors. The Biology of Lemmings. New York: Academic Press; 1993. pp. 425–437. [Google Scholar]
- 16.Krebs CJ. Are lemmings large Microtus or small reindeer? A review of lemming cycles after 25 years and recommendations for future work. In: Stenseth N, Ims RA, editors. The Biology of Lemmings. New York: Academic Press; 1993. pp. 247–260. [Google Scholar]
- 17.Aars J, Ims RA. Intrinsic and climatic determinants of population demography: The winter dynamics of tundra voles. Ecology. 2002;83:3449–3456. [Google Scholar]
- 18.Gilg O, Sittler B, Hanski I. Climate change and cyclic predator-prey population dynamics in the high Arctic. Glob Change Biol. 2009;15:2634–2652. [Google Scholar]
- 19.Tast J. Will the Norwegian lemming become endangered if climate becomes warmer? Arct Alp Res. 1991;23:53–60. [Google Scholar]
- 20.Johnson DM, et al. Climatic warming disrupts recurrent Alpine insect outbreaks. Proc Natl Acad Sci USA. 2010;107:20576–20581. doi: 10.1073/pnas.1010270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schott T, Hagen SB, Ims RA, Yoccoz NG. Are population outbreaks in sub-arctic geometrids terminated by larval parasitoids? J Anim Ecol. 2010;79:701–708. doi: 10.1111/j.1365-2656.2010.01673.x. [DOI] [PubMed] [Google Scholar]
- 22.Abrams PA, Holt RD, Roth JD. Apparent competition or apparent mutualism? Shared predation when populations cycle. Ecology. 1998;79:201–212. [Google Scholar]
- 23.Gilg O, Yoccoz NG. Ecology. Explaining bird migration. Science. 2010;327:276–277. doi: 10.1126/science.1184964. [DOI] [PubMed] [Google Scholar]
- 24.Krebs CJ. Population cycles revisited. J Mammal. 1996;77:11–24. [Google Scholar]
- 25.Henden JA, Ims RA, Yoccoz NG. Nonstationary spatio-temporal small rodent dynamics: Evidence from long-term Norwegian fox bounty data. J Anim Ecol. 2009;78:636–645. doi: 10.1111/j.1365-2656.2008.01510.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoccoz NG, Ims RA. Spatial population dynamics of small mammals: Some methodological and practical issues. Anim Biodivers Conserv. 2004;27:427–435. [Google Scholar]
- 27.Killengreen ST, et al. Structural characteristics of a low Arctic tundra ecosystem and the retreat of the Arctic fox. Biol Conserv. 2007;13:459–472. [Google Scholar]
- 28.Karlsen SR, et al. MODIS-NDVI-based mapping of the length of the growing season in northern Fennoscandia. Int J Appl Earth Obs Geoinf. 2008;10:253–266. [Google Scholar]
- 29.Bivand R, Pebesma EJ, Gómez-Rubio V. Applied Spatial Data Analysis with R. New York: Springer; 2008. [Google Scholar]
- 30.Bjørnstad ON, Falck W. Nonparametric spatial covariance functions: Estimation and testing. Environ Ecol Stat. 2001;8:53–70. [Google Scholar]
- 31.Stenseth NC, et al. Seasonality, density dependence, and population cycles in Hokkaido voles. Proc Natl Acad Sci USA. 2003;100:11478–11483. doi: 10.1073/pnas.1935306100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge: Cambridge Univ Press; 2007. p. 648. [Google Scholar]
- 33.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002;64:583–639. [Google Scholar]
- 34.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7:457–472. [Google Scholar]
- 35.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2010. [Google Scholar]