Abstract
Polymorphisms in the human endothelial nitric oxide synthase (eNOS) gene (NOS3) have been associated with advanced nephropathy in diabetic patients and with decreased expression in tissue culture. However, direct proof that modest genetic decreases in eNOS expression worsen diabetic nephropathy is lacking. To investigate this effect, we took advantage of the hybrid vigor and genetic uniformity of the F1 progeny (eNOS+/+, eNOS+/−, or eNOS−/− with or without diabetes) of a cross between heterozygous 129S6/SvEvTac eNOS+/− inbred females and heterozygous C57BL/6J eNOS+/− inbred males carrying the dominant Akita diabetogenic mutation Ins2C96Y/+. Whereas all C57BL/6J inbred eNOS−/− and eNOS+/− diabetic mice died before 5 mo, almost half of the F1 hybrid eNOS−/− and eNOS+/− diabetic mice lived until killed at 7 mo. Heterozygous eNOS+/− diabetic mice expressed ∼35% eNOS mRNA in the kidney and ∼25% glomerular eNOS protein relative to their eNOS+/+ diabetic littermates. These decreases in eNOS elevated blood pressure (BP) but not blood glucose. Urinary albumin excretion, mesangial expansion, glomerulosclerosis, mesangiolysis, and glomerular filtration rate increased in the order: eNOS+/+ Akita < eNOS+/− Akita < eNOS−/− Akita, independently of BP. Glomerular basement membrane thickening depended on increased BP. Renal expression of tissue factor and other inflammatory factors increased with the nephropathy; Nos2 also increased. Surprisingly, however, decreased eNOS expression ameliorated the increases in oxidative stress and tubulointerstitial fibrosis caused by diabetes. Our data demonstrate that a modest decrease in eNOS, comparable to that associated with human NOS3 variants, is sufficient to enhance diabetic nephropathy independently of its effects on BP.
Keywords: diabetic complications, heterozygotes
Diabetic nephropathy (DN) is the most frequent cause of chronic kidney disease and is a risk factor for stroke and heart attack (1). Clinical studies indicate that 30–40% of diabetic patients develop DN (2). The development and severity of DN vary greatly from one patient to another, and familial clustering suggests that genetic factors play important roles (3). In humans, three variants in the endothelial NOS (eNOS) gene NOS3 (i.e, G894T in exon 7, a different number of tandem repeats in intron 4, and C-786T in the promoter) are associated with DN (4–6). Of these variants, the G894T (Glu298Asp) polymorphism has been particularly well studied, because the frequency of the 894T allele is around 0.3 (4, 7), so that 5–9% of individuals are homozygous for TT. The 894T NOS3 gene produces about 30% of the amount of NO produced by the more common 894G allele when expressed in CHO cells in vitro (4). Although complete absence of eNOS accelerates DN in mice (8–11), this result does not tell us whether production of eNOS at reduced levels comparable to those associated with the NOS3 polymorphisms is sufficient to worsen DN. To investigate this possibility, we first crossed C57BL/6J (B6) female eNOS+/− heterozygous mice with B6 males heterozygous for both the eNOS disruption (eNOS+/−) and the diabetogenic Akita mutation (Ins2C96Y/+). However, this experiment failed because the inbred eNOS−/− and eNOS+/− diabetic males died before the age of 5 mo. We therefore decided to take advantage of the hybrid vigor of F1 mice, which are as genetically uniform as inbred mice but lack most of the detrimental recessive mutations that affect the survival of their inbred parents. Accordingly, we crossed 129S6/SvEvTac (129) inbred females heterozygous for the eNOS disruption (eNOS+/−) with B6 eNOS+/− heterozygous inbred males having the Akita mutation and studied their progeny. The results of studying these hybrid F1 mice demonstrate that a modest decrease in eNOS, comparable to that associated with human NOS3 variants, is sufficient to cause exacerbated DN independent of its effects on BP.
Results
Survival of F1 (B6 × 129) Heterozygous eNOS+/− Akita Mice.
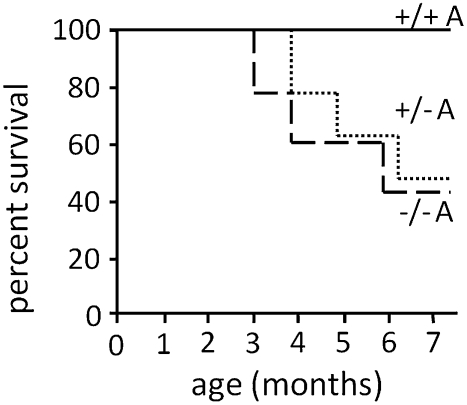
We first attempted to generate eNOS−/− and eNOS+/− Akita diabetic mice on the inbred B6 genetic background, but this experiment failed because all the eNOS−/− and eNOS+/− diabetic males died before age 5 mo. To take advantage of the hybrid vigor of F1 mice, which are as genetically uniform as inbred mice but lack most of the detrimental recessive mutations that affect the survival of inbred strains, we crossed 129 inbred females heterozygous for the eNOS disruption (eNOS+/−) with B6 eNOS+/− heterozygous inbred males also having the Akita mutation (Ins2C96Y/+) and studied their F1 progeny. The progeny include all six possible genotypes: eNOS+/+, eNOS+/−, and eNOS−/−, with or without the dominant Akita diabetogenic mutation. Eleven of 21 eNOS+/− Akita mice (52%), 8 of 17 eNOS−/− Akita mice (47%), and all mice of the other genotypes survived until they were killed at age 7 mo for detailed study (Fig. 1). We were unable to determine the cause of death of the mice that died before age 7 mo, but occasional bleeding within the chest or abdominal cavity was observed, and many of the deceased mice appeared to have been dehydrated.
Fig. 1.
Survival rates of male F1 (C57BL/6J × 129S6/SvEvTac) Akita (Ins2Akita/+, A) diabetic mice with eNOS genotypes +/+ (solid line), +/− (dotted line), and −/− (dashed line). All nondiabetic mice, regardless of eNOS genotype, survived until the end of the experiment.
The basic parameters of the six groups of mice at age 3 mo and age 7 mo are summarized in Tables S1 and S2. Blood glucose levels, food and water intake, and urine volume were all significantly elevated (P ≤ 0.002) in the diabetic mice compared with their nondiabetic littermates. In contrast, no significant effects of eNOS genotype on these parameters were observed, but tail-cuff blood pressure (BP) increased progressively in the order: eNOS+/+ < eNOS+/− < eNOS−/− at both 3 and 7 mo of age, demonstrating a strong eNOS genotype effect (P < 0.0001). The BPs of the diabetic mice were indistinguishable from those of nondiabetic mice with the same eNOS genotype at age 3 mo. However, at age 7 mo the BP of the diabetic mice of all eNOS genotypes was higher than the BP of nondiabetic mice of the same genotypes, showing an age-dependent effect of diabetes on BP (P < 0.01). Together these results show that an absence or decrease of eNOS in diabetic mice elevates BP and decreases survival without affecting plasma glucose levels.
Expression of Kidney eNOS.
The kidneys from eNOS+/− mice expressed 49 ± 8% eNOS mRNA compared with eNOS+/+ mice when tested by quantitative RT-PCR, whereas kidneys from eNOS−/− mice did not express eNOS mRNA (Fig. 2A). Diabetes decreased eNOS mRNA levels to approximately half those in nondiabetic mice, with the expression in eNOS+/− diabetic kidneys being 35% of that in their eNOS+/+ diabetic littermates (Fig. 2A). Immunostaining against eNOS showed that all glomeruli were positive for eNOS in WT mice and that all glomeruli were negative for eNOS in the eNOS−/− mice (Fig. 2B). Approximately 30% of glomeruli in eNOS+/− mice show no detectable immunoreactivity for eNOS, and the other glomeruli have fewer eNOS-positive cells than glomeruli in WT mice, rather than having half the intensity per cell. We estimate that glomeruli from eNOS+/− mice express 32 ± 6% eNOS protein immunoreactivity relative to glomeruli from eNOS+/+ mice (Fig. 2C). Diabetes decreased glomerular eNOS protein to approximately half that of nondiabetic mice, with the protein expression in eNOS+/− diabetic kidneys being ∼25% of that in their eNOS+/+ diabetic littermates (Fig. 2C). We conclude that the expression levels of eNOS mRNA and protein in the kidneys of heterozygous eNOS+/− mice are ∼35–50% of the levels in WT mice. Diabetes decreases the expression of both mRNA and protein of eNOS in the kidney by about 50%, but in the heterozygous eNOS+/− diabetic mice expression is still ∼25–35% of that in WT diabetic mice.
Fig. 2.
Renal eNOS mRNA and protein expression in 7-mo-old F1 (C57BL/6J × 129S6/SvEvTac) mice. (A) eNOS gene expression in the kidney. (B) Representative immunoreactive eNOS in the glomeruli. (C) Summary of the quantity of immunoreactive eNOS in the glomeruli. A, Ins2Akita/+. +/+, +/−, and −/− designate eNOS genotypes. a, P < 0.05 vs. eNOS+/+; b, P < 0.05 vs. eNOS+/−; c, P < 0.05 vs. eNOS−/−; d, P < 0.05 vs. eNOS+/+ A; e, P < 0.05 vs. eNOS+/− A. Data are mean ± SEM. n ≥ 8. Note that diabetes decreases eNOS mRNA and protein expression. Effects on eNOS mRNA and protein levels of diabetes, eNOS genotype, and their interaction were all significant (P < 0.01, P < 0.0001, and P < 0.05, respectively, for eNOS mRNA; P < 0.01, P < 0.001, and P < 0.02, respectively for eNOS protein).
Renal Function and Morphology.
In nondiabetic F1 mice, complete absence of eNOS in the eNOS−/− mice significantly increases urinary albumin excretion compared with WT mice; in contrast, the reduction of eNOS seen in the heterozygous eNOS+/− mice has no effect (Fig. 3A). However, when the mice were Akita diabetic, the reduction of eNOS in the heterozygous eNOS+/−Akita mice was sufficient to increase urinary albumin excretion above that in eNOS+/+ Akita mice. Thus, at both 3 and 7 mo of age, urinary albumin excretion in eNOS+/− Akita and eNOS−/− Akita mice was ∼2.5 times higher than in eNOS+/+ Akita mice (186 ± 20 μg/d and 212 ± 21 μg/d vs. 82 ± 15 μg/d at 7 mo) (Fig. 3A).
Fig. 3.
Renal function of F1 mice with different eNOS genotypes and Akita mutation. (A) Daily urinary albumin at age 3 mo and 7 mo. At age 3 mo and 7 mo the effects on daily albumin excretion of diabetes, eNOS genotype, and their interaction were all significant (P < 0.001, P < 0.001, and P < 0.03, respectively). (B) GFR estimated by creatinine clearance at age 3 mo and 7 mo. The effects on GFR of diabetes, eNOS genotype, and their interaction were all significant at age 3 mo (P < 0.001, P < 0.001, and P < 0.05, respectively) but at age 7 mo were P < 0.001, P = 0.1, and P = 0.9, respectively. Data are mean ± SEM. n ≥ 8. A, Ins2Akita/+. +/+, +/−, and −/− designate eNOS genotypes. a, P < 0.05 vs. eNOS+/+; b, P < 0.05 vs. eNOS+/−; c, P < 0.05 vs. eNOS−/−; d, P < 0.05 vs. eNOS+/+ A; e, P < 0.05 vs. eNOS+/− A. *P < 0.05 vs. values in the same mice at age 3 mo.
The glomerular filtration rate (GFR), estimated by endogenous creatinine clearance, in nondiabetic eNOS−/− mice was greater than in WT mice at 3 mo of age but was less than in WT mice at 7 mo (Fig. 3B). The GFR in diabetic mice was increased progressively by both reduction and absence of eNOS at 3 mo (eNOS+/+ Akita < eNOS+/− Akita < eNOS−/− Akita). At age 7 mo the GFR of eNOS+/− Akita mice increased further, although that of eNOS−/− Akita mice was lower than at age 3 mo (Fig. 3B). We conclude that the increase in the GFR caused by diabetes is increased further by the decrease in eNOS that occurs in the eNOS+/− Akita mice.
Kidney sections and morphometric analyses of the glomeruli (Fig. 4) showed no notable effects of the eNOS genotype in nondiabetic mice, but in the diabetic mice the classical changes associated with DN, including mesangial expansion (Fig. 4B), mesangiolysis (Fig. 4C), glomerulosclerosis (Fig. 4D), and glomerular basement membrane (GBM) thickening (Fig. 4E), were progressively more severe in the order: eNOS+/+ Akita < eNOS+/− Akita < eNOS−/− Akita mice. In contrast, as judged by EM morphology, the podocytes and endothelial cells of the eNOS+/− Akita and eNOS−/− Akita mice were unremarkable compared with those of eNOS+/+ Akita mice, although the progressive increases in GBM thickness in the eNOS+/− and eNOS−/− diabetic mice was easily seen (Fig. 4A, Bottom). Not surprisingly, a mild degree of tubulointerstitial fibrosis is apparent in the eNOS+/+ Akita diabetic mice but not in the eNOS+/+ WT nondiabetic mice; surprisingly, however, partial or complete absence of eNOS expression in the eNOS+/− Akita and eNOS−/− Akita mice prevented this fibrosis (Fig. 4F).
Fig. 4.
Histology of F1 mice with different eNOS genotypes (eNOS+/+, eNOS+/−, and eNOS−/−) and Akita mutation. (A) Representative kidney morphology of 7-mo-old diabetic mice with different eNOS genotypes. (Top) PAS stain (100×). Yellow arrow indicates mesangial expansion; open arrows indicate mesangiolysis; yellow arrowhead indicates glomerulosclerosis. (Middle) Glomeruli with Masson Trichrome stain (200×). (Bottom) Transmission electron micrographs with original magnification of 12,500×. (B–F) Quantification of mesangial expansion (B), mesangiolysis (C), glomerulosclerosis (D), GBM thickening (E), and tubulointerstitial fibrosis score (F) of 7 mo-old mice. There was no glomerulosclerosis or mesangiolysis in any nondiabetic mice, regardless of eNOS genotype. A, Ins2Akita/+. +/+, +/−, and −/− designate eNOS genotypes. Data are mean ± SEM. n ≥ 8. a, P < 0.05 vs. eNOS+/+; b, P < 0.05 vs. eNOS+/−; c, P < 0.05 vs. eNOS−/−; d, P < 0.05 vs. eNOS+/+ A; e, P < 0.05 vs. eNOS+/− A. Effects on mesangial expansion and GBM thickness of diabetes, eNOS genotype, and their interaction were all significant (P < 0.001, P < 0.001, and P < 0.05, respectively).
Urinary albumin excretion and mesangial score were highly correlated with BP (R2 = 0.68, P < 0.001, and R2 = 0.56, P = 0.002, respectively) but not with blood glucose levels (R2 = 0.5, P = 0.3, and R2 = 0.05, P = 0.94, respectively) (Fig. S1). The strong correlations between these indicators of DN and increased BP raise questions about the degree to which the enhanced DN is a direct effect of reduced eNOS or is secondary to the increased BP resulting from decreased eNOS. Accordingly, we used partial regression leverage plots to separate statistically the effects of BP and of genotype on six classical parameters of DN (urinary albumin excretion, mesangial expansion, GBM thickening, glomerulosclerosis, mesangiolysis, and GFR). The results of this analysis are presented in Table S3, which shows that at age 7 mo the overall effects of the reduced expression of eNOS with the change in eNOS genotype (eNOS+/+ versus eNOS+/−) are highly significant for all parameters (P ≤ 0.005), except that the effect on GFR is detectable only at age 3 mo (P < 0.04). The mesangial expansion is caused in part indirectly by the effects of decreased eNOS on BP (P = 0.01) and in part by direct effects of this decrease (P = 0.02). The GBM thickening is caused mainly by the indirect effects of increased BP (P = 0.008). The increases in albumin excretion (P = 0.01), glomerulosclerosis (P = 0.01), and mesangiolysis (P < 0.001) are caused mainly by the direct effects of the decreased eNOS. Together these results demonstrate that the decrease of eNOS seen in eNOS+/− heterozygous diabetic mice is sufficient to exacerbate the functional and morphological changes of DN, in part directly and in part indirectly through increased BP.
Fibrin, Tissue Factor, and Inflammation in the Kidney.
NO inhibits coagulation (12), and diabetes leads to fibrin deposition in glomeruli (13). eNOS−/− mice made diabetic by low doses of streptozotocin have increased fibrin deposition and tissue factor (TF, F3) expression in their glomeruli (11). Accordingly, we examined fibrin deposition and TF expression in the kidneys of Akita diabetic mice. As expected, the eNOS+/− Akita and eNOS−/− Akita mice had more immunoreactive fibrin in their glomeruli than did Akita mice having WT eNOS (Fig. 5A). eNOS+/− Akita mice and eNOS−/− Akita mice also had increased renal TF (F3) mRNA levels and activities as compared with eNOS+/+ Akita mice (Fig. 5 B and C). Expression of F4/80 (Emr1), a macrophage marker, paralleled the TF expression (Fig. 5D). The glomeruli of the diabetic eNOS+/− Akita mice and eNOS−/− Akita mice showed prominent TF immunoreactivity colocalized with monocytes/macrophages (Fig. 5E). Although the kidneys of the nondiabetic mice were negative for immunoreactive TF irrespective of their eNOS genotype, all cells in the diabetic kidneys that were positive for TF were monocytes/macrophages, as judged by monocyte/macrophage marker (MOMA-2) reactivities. All MOMA-2+ cells were positive for TF.
Fig. 5.
Fibrin deposition, TF (F3) expression, and macrophage infiltration in the kidneys of 7-mo-old mice. (A) Immunostaining against fibrin in kidney glomeruli from diabetic mice (100×). Kidneys from all nondiabetic mice, regardless of eNOS genotype, were negative for fibrin. (B and C) Kidney TF (F3) mRNA levels relative to eNOS+/+ mice (B) and kidney TF activity (C). (D) Expression of a macrophage marker, F4/80 (Emr1), in the kidney relative to eNOS+/+ A. Effects on TF expression of diabetes, eNOS genotype, and their interaction were all significant (P < 0.004, P < 0.001, and P < 0.02, respectively). A, Ins2Akita/+. +/+, +/−, and −/− designate eNOS genotypes. a, P < 0.05 vs. eNOS+/+; b, P < 0.05 vs. eNOS+/−; c, P < 0.05 vs. eNOS−/−; d, P < 0.05 vs. eNOS+/+ A; e, P < 0.05 vs. eNOS+/− A. (E) Immunostaining of kidney glomeruli against TF and monocytes/macrophages (MOMA-2) in diabetic mice (100×). DIC, differential interference contrast. There was no significant immunostaining of TF or MOMA-2 outside glomeruli (in the tubulointerstitial compartment).
NO is able to attenuate inflammation (12). Inflammation increases expression of TF, and TF induces inflammation (14). It also is well known that diabetes leads to inflammation and fibrosis (15, 16). We therefore studied the renal expression of inflammatory and fibrogenic genes (Fig. 6 and Table S4). In the eNOS+/− Akita kidneys the expression of many of these genes, including Tnf, Il-6, monocyte chemotactic protein 1 (Mcp1), intercellular adhesion molecule 1 (Icam1), vascular cell adhesion molecule 1 (Vcam1), Tgfb, and type 4 collagen (Col4), was significantly higher than in the eNOS+/+ Akita kidneys. The expression of Il-6, Mcp1, Icam1, Vcam1, Tgfb, and Col4 in the eNOS+/− Akita kidneys was as great as in the eNOS−/− Akita kidneys (Fig. 6).
Fig. 6.
mRNA expression of inflammatory and fibrogenic genes in the kidney of 7-mo-old diabetic mice. A, Ins2Akita/+. +/+, +/−, and −/− designate eNOS genotypes. Values are relative to +/+ A. d, P < 0.05 vs. eNOS+/+ A; e, P < 0.05 vs. eNOS+/− A. The expression of the genes of six groups of mice, including nondiabetic mice, is shown in Table S4.
We next tested whether parameters of DN and levels of gene expression are associated. Urinary albumin excretion and mesangial score were highly correlated with the expression of F3 and Tnf (Fig. S2 A–D). The expression of F3 and Tnf and BP also were highly correlated with each other (Fig. S2 E–G). The expression of other inflammatory markers (Il-6, Mcpt1, Icam1, and Vcam1) also was highly correlated with urinary albumin excretion and mesangial score (not shown for clarity). We conclude that decreases in eNOS increase renal expression of TF (F3) and inflammatory genes, in parallel with an exacerbation of DN.
Oxidative Stress and Inducible NOS.
Localized increases in oxidative stress have been postulated to be key components in the development of DN (17). Glutathione (GSH) plays a critical role in cellular defense against oxidative stress in tissues and cells. In our nondiabetic mice, the level of reduced GSH and the ratio of GSH to its oxidized form (GSH/GSSG) in the kidneys of eNOS+/− mice were indistinguishable from those in eNOS+/+ mice, although GSH level and GSH/GSSG ratio were decreased in eNOS−/− mice (Fig. S3). Not surprisingly, these parameters were decreased in the WT mice made diabetic by the Akita mutation. Surprisingly, however, the kidneys of eNOS+/− Akita and eNOS−/− Akita diabetic mice had significantly higher levels of GSH (P = 0.003 and 0.001, respectively) and higher GSH/GSSG ratios (P = 0.002 and 0.001, respectively) than did kidneys in eNOS+/+ Akita mice. Because increases in GSH levels and GSH/GSSG ratios are indicators of reduced oxidative stress, these data show that reduced eNOS actually decreases the oxidative stress induced by diabetes (Fig. S3). We therefore exclude increased oxidative stress as a factor in the exacerbation of DN resulting from reduced eNOS.
We also tested whether changes in renal inducible NOS (iNOS; Nos2) expression are involved in the pathogenesis of DN when the expression of eNOS decreases. We find that renal expression of Nos2 is greater in the eNOS+/− Akita mice than in the eNOS+/+ Akita mice (P = 0.03) (Fig. S4A). However, urinary NO metabolites (NOx) excretion, an indicator of total NO production in an animal, is significantly less in eNOS+/− Akita mice than in eNOS+/+ Akita mice (P = 0.003) (Fig. S4B). We conclude that the increased expression of iNOS that occurs in the eNOS+/− Akita mice is not sufficient to prevent an overall decrease in NO production.
Discussion
Three NOS3 polymorphisms are associated with the development of severe DN and with a decrease in NO production in some tissue culture experiments (4–6). Several previous studies using homozygous eNOS−/− diabetic mice, including ours, have demonstrated that absence of eNOS worsens DN (8–11). However, there have been no reports of humans homozygous for absence of eNOS. Consequently the phenotype of eNOS−/− diabetic mice does not tell us whether reduced NO production, as associated with the NOS3 polymorphisms, is sufficient to exacerbate DN. Our experience with mice shows that in some cases, even when homozygous knockouts (−/−) have a profound phenotype, the heterozygous knockouts (+/−) and WT (+/+) can be indistinguishable, as occurs with disruption of the furosemide-sensitive NaK2Cl cotransporter NKCC2 (18, 19) and of renin (Ren1c) (20). However, in other cases, such as the natriuretic peptide receptor A (Npr1), the phenotype of heterozygous knockout mice is readily distinguishable from that of WT (21). Our present study accordingly was aimed at removing uncertainly about whether the mild genetic decrease in eNOS expression which occurs in eNOS+/− mice is sufficient to cause DN.
The mice used in our study were F1 mice obtained from crosses between inbred B6 doubly heterozygous males (eNOS +/− and Ins2Akita/+) and inbred 129 heterozygous females (eNOS +/−). F1 progeny usually are more vigorous than inbred mice, because they lack many of the detrimental recessive mutations that can affect inbred strains, and we found that the B6/129 F1 eNOS+/− and eNOS−/− diabetic mice survived much longer than the equivalent inbred mice. Thus, although the life spans of the eNOS+/− diabetic F1 mice were reduced compared with their diabetic siblings with WT eNOS (Fig. 1), sufficient numbers survived to age 7 mo, at which time they were killed for study. It is important to note that F1 mice generated by crossing two different inbred mouse lines are as genetically uniform as human monozygotic twins. Note also that by bringing in the dominant Akita diabetogenic allele from the male parent, problems associated with fetal development in a hyperglycemic environment are avoided. Another advantage of this breeding scheme is that it avoids using potentially debilitated homozygous mutants as breeders, but the progeny include all six possible combinations of the genes of interest (eNOS+/+, eNOS+/−, and eNOS−/− with and without the dominant Akita allele), including homozygous mutants and WT controls. This complete coverage, combined with the genetic uniformity of the progeny, enables the detection of subtle or unexpected differences, as discussed below, some which can be missed when only a subset of the possible genotypes is studied.
Using these F1 mice, we showed that the expression of eNOS mRNA and protein in the kidneys of heterozygous eNOS+/− nondiabetic and diabetic mice was 25–50% that of their eNOS+/+ nondiabetic and diabetic littermates (Fig. 2). These levels of renal eNOS expression in the eNOS+/− nondiabetic and diabetic mice are comparable to the NO production observed in some tissue culture experiments with NOS3 894T and NOS3 894G transgenes (∼30%). Thus, our eNOS+/− mice provide an excellent model with which to test whether a mild decrease in NO production comparable to that observed in the NOS3 G894T polymorphism can exacerbate DN.
Our results demonstrate that six primary parameters of DN—mesangial expansion, mesangiolysis, glomerulosclerosis, GBM thickening, albuminuria, and changes in GFR—were progressively enhanced in the order: eNOS+/+ Akita < eNOS+/− Akita < eNOS−/− Akita (Figs. 3 and 4). The modest decrease of eNOS seen in eNOS+/− heterozygotes is sufficient to exacerbate all these functional and morphological indicators of DN.
Among the parameters of DN, GFR deserves particular comment. Typically diabetes first increases GFR and later decreases GFR to below normal. The Akita diabetic mutation in combination with complete absence of eNOS shows this trend (Fig. 3B). Thus, GFR is markedly increased in the eNOS−/− Akita mice at age 3 mo but is decreased at age 7 mo. GFR in the eNOS+/− Akita mice increases between age 3 mo and 7 mo, as might be expected when DN is developing but is not yet full blown, and is significantly greater at both ages than in their eNOS+/+ Akita littermates.
Our data with F1 hybrid mice show that the decreased expression of eNOS in the eNOS+/− mice and its absence in the eNOS−/− mice have marked effects on inflammatory determinants, as indicated by significant increases in renal expression of TF and several other inflammatory genes (Figs. 5 and 6). Furthermore, in a previous study of B6 mice made diabetic by low-dose streptozotocin, we showed that anti-TF neutralizing antibody decreases renal expression of inflammatory and fibrogenic genes (11). This increase in TF in the eNOS+/− Akita mice may be one of the ways whereby decreased expression of eNOS worsens DN.
In this context, it is notable that B6 mice made diabetic by streptozotocin develop variable degrees of tubulointerstitial fibrosis (11, 22). In agreement with this result, we find that our eNOS+/+ Akita diabetic mice developed mild tubulointerstitial fibrosis at age 7 mo (Fig. 4F). Surprisingly, however, there was less tubulointerstitial fibrosis in eNOS+/− Akita mice and eNOS−/− Akita mice than in the eNOS+/+ Akita mice and indeed was less than in the nondiabetic eNOS WT mice. We conclude that a decrease in eNOS does not enhance the tubulointerstitial fibrosis induced by diabetes.
Oxidative stress has been suggested as contributing to DN (17). Not-surprisingly, therefore, we find that diabetes in the eNOS+/+ Akita mice is accompanied by a decrease in GSH levels and GSH/GSSG ratios, indicating increased oxidative stress. Surprisingly, however, we find that the increased oxidative stress caused by diabetes in the eNOS+/+ Akita mice is decreased by reduced expression of eNOS (Fig. S3), indicating that a decrease in eNOS is protective in this aspect of DN. Indeed, as judged by these indicators, the oxidative stress of the diabetic mice with a decreased level or absence of eNOS is less than that of the completely WT nondiabetic mice. This observation emphasizes the value of having a comprehensive set of genetically uniform mice to study the effects of genotype on a deleterious condition. We therefore exclude increased oxidative stress as a factor in the exacerbation of DN resulting from reduced expression of eNOS. Renal expression of Nos2 is greater in the eNOS+/−Akita mice than in the eNOS+/+Akita mice. However, these increases are not sufficient to prevent decreased production of NO in the eNOS+/−Akita mice, as judged by the reduced urinary excretion of NOx.
Our present data from F1 mice show, in agreement with previous observations (23), that BP is markedly affected by changes in the expression of eNOS, and that the BP of the F1 mice correlates strongly with many of the measures of DN, including albumin excretion, mesangial score, and renal expression of F3 and Tnf. Furthermore, in human patients, BP is a major determinant of the risk of developing DN (20). Additionally, decreasing BP using hydralazine ameliorates the DN that develops in eNOS−/− mice made diabetic by streptozotocin (24). These observations raised the possibility that the highly significant effects of modestly decreased eNOS expression on DN that we have documented here could be mainly the consequence of increased BP. To evaluate this possibility, we tabulated the overall effects at age 7 mo of the eNOS+/− genotype on six classical indicators of DN (urinary albumin excretion, mesangial expansion, GBM thickening, glomerulosclerosis, mesangiolysis, and GFR) and by statistically separating the effects of BP and of genotype using partial regression leverage plot analysis (Table S3). The results of this statistical analysis are unequivocal: At age 7 mo the overall effects of the reduced eNOS are highly significant for all indicators, except that the effect on GFR is detectable only at age 3 mo. The mesangial expansion is caused in part indirectly by the effects of decreased eNOS expression on BP but also is caused in part by the direct effects of this decrease in eNOS. The GBM thickening is caused mainly by the indirect effects of increased BP. The increases in albumin excretion are caused mainly by direct effects of the decreased eNOS, as are glomerulosclerosis (P = 0.01) and mesangiolysis (P < 0.001).
Thus, by using genetically uniform and robust B6/129 hybrid F1 mice, we have shown that a modest decrease in eNOS, comparable to that associated with polymorphisms in the human NOS3 gene, markedly enhances the development of diabetic nephropathy. Most of the damaging effects are independent of changes in blood pressure.
Methods
Animals.
All animal experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. Mice having the null allele for the eNOS gene were developed originally in 129/Ola ES cells (25) and transferred to a C57BL/6J genetic background by backcrossing to WT C57BL/6J at least six times. Subsequently the mutation was transferred to the 129S6/SvEvTac background by serial backcrossing for at least 12 generations. By mating eNOS+/− Akita male mice on a C57BL/6J background with eNOS+/− female mice on a 129S6/SvEvTac background, we generated F1 eNOS+/+, eNOS+/−, and eNOS−/− mice with and without the Akita diabetogenic mutation. Animals were fed a regular chow, Prolab IsoproRMH3000 5P00 (14% calories from fat), and were maintained without insulin treatment for 7 mo. At age 3 mo and age 7 mo, individual mice were placed in metabolic cages designed by Thomas Coffman (Duke University, Durham, NC), and 24-h urine was collected for two consecutive days (11, 20, 23). Body weight, food and water intake, and urine volume were measured every 24 h. Usually, when the study is carried out in the room in which the mice have been housed in regular cages, or when mice are acclimatized in the study room for 1 wk before being housed in metabolic cages, there is no significant difference in urine volume and daily urinary albumin excretion between the first and second day of collection. We measured daily urinary albumin excretion and creatinine clearance using the second 24-h sample and blood drawn at the end of 48-h study. If there was a change in body weight >10%, samples were excluded from the study.
Measurement of BP and GFR.
BP was measured in 3- and 7-mo-old mice by the computerized tail-cuff method we have developed in the laboratory (Visitech) (20, 26). All measurements were taken in the morning, and the first 10 of the 60 measurements taken each day were discarded to obtain data after the mice were acclimatized to the equipment. BP was measured for 5 d, and the mean values of individual mice were used for analysis. GFR was estimated by creatinine clearance. Plasma and urinary creatinine concentrations were measured using LC-MS/MS (27).
Biochemical Measurements.
Urinary albumin was determined using Albuwell-M kits (Exocell). Kidney GSH and GSSH levels were measured by GSH assay kit (Cayman Chemical). TF activity was measured as previously described (11, 28).
Quantitative RT-PCR.
Gene expression in the kidney cortex was quantified with TaqMan real-time quantitative RT-PCR (Applied Biosystems) with β-actin as a reference gene, as we previously described (11, 20). Primers and probes used are listed in Table S5.
Kidney Morphometry and Immunohistochemistry.
Cross-sections of kidneys containing papilla (5-μm-thick paraffin) were stained with periodic acid-Schiff (PAS) or with Masson's Trichrome and analyzed (11, 20). Monoclonal rat anti-mouse TF antibody (1H1) was kindly provided by Daniel Kirchhofer (Genentech, South San Francisco, CA) (29). eNOS protein levels were determined by immunostaining using rabbit anti-mouse eNOS antibody (Thermo Fisher Scientific) and semiquantified using Image J (11, 2).
Statistical Analysis.
Data are expressed as mean ± SEM. Multifactorial ANOVA with the program JMP 8.0 (SAS Institute) was used to compare the effects of eNOS genotype and diabetes. The Tukey–Kramer Honestly Significant Difference (HSD) test was used for post hoc comparisons between each group. P < 0.05 was considered statistically different. Partial regression lineage plot analyses to separate the direct effects of eNOS genotype on DN from indirect effects caused by changes in BP were performed with the program JMP 8.0 (SAS Institute).
Supplementary Material
Acknowledgments
We thank Dr. Daniel Kirchhofer (Genentech) for kindly providing the monoclonal rat anti-mouse TF antibody (1H1); Longquan Xu, Victoria J Madden, Leonard Collins, Sara Emamian, and David Babitt for technical assistance; and Drs. Hirofumi Makino, Naoki Kashihara, Sadayoshi Ito, Hiroshi Sato, and Ron Korstanje for critical reading of the manuscript. This work was supported by Grants 0265464U and 0855335E from the American Heart Association; Grants U01 DK076131, P30DK56350, and P30 ES10126 from the National Institutes of Health; and by Grant-in-Aid 22890016 from The Japan Society of Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018766108/-/DCSupplemental.
References
- 1.Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: An update. J Clin Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins BA, Krolewski AS. Early nephropathy in type 1 diabetes: A new perspective on who will and who will not progress. Curr Diab Rep. 2005;5:455–463. doi: 10.1007/s11892-005-0055-7. [DOI] [PubMed] [Google Scholar]
- 3.Bowden DW. Genetics of diabetes complications. Curr Diab Rep. 2002;2:191–200. doi: 10.1007/s11892-002-0080-8. [DOI] [PubMed] [Google Scholar]
- 4.Noiri E, et al. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension. 2002;40:535–540. doi: 10.1161/01.hyp.0000033974.57407.82. [DOI] [PubMed] [Google Scholar]
- 5.Ezzidi I, et al. Association of endothelial nitric oxide synthase Glu298Asp, 4b/a, and -786T>C gene variants with diabetic nephropathy. J Diabetes Complications. 2008;22:331–338. doi: 10.1016/j.jdiacomp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Sandrim VC, de Syllos RW, Lisboa HR, Tres GS, Tanus-Santos JE. Endothelial nitric oxide synthase haplotypes affect the susceptibility to hypertension in patients with type 2 diabetes mellitus. Atherosclerosis. 2006;189:241–246. doi: 10.1016/j.atherosclerosis.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Thaha M, et al. Association of endothelial nitric oxide synthase Glu298Asp polymorphism with end-stage renal disease. Clin Nephrol. 2008;70:144–154. doi: 10.5414/cnp70144. [DOI] [PubMed] [Google Scholar]
- 8.Zhao HJ, et al. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa T, et al. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 10.Kanetsuna Y, et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol. 2007;170:1473–1484. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, et al. Elevated tissue factor expression contributes to exacerbated diabetic nephropathy in mice lacking eNOS fed a high fat diet. J Thromb Haemost. 2010;8:2122–2132. doi: 10.1111/j.1538-7836.2010.03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman JE, et al. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 13.Ozüyaman B, et al. Endothelial nitric oxide synthase plays a minor role in inhibition of arterial thrombus formation. Thromb Haemost. 2005;93:1161–1167. doi: 10.1160/TH03-09-0588. [DOI] [PubMed] [Google Scholar]
- 14.Hooper WC. The relationship between inflammation and the anticoagulant pathway: The emerging role of endothelial nitric oxide synthase (eNOS) Curr Pharm Des. 2004;10:923–927. doi: 10.2174/1381612043452857. [DOI] [PubMed] [Google Scholar]
- 15.Rivero A, et al. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond) 2009;116:479–492. doi: 10.1042/CS20080394. [DOI] [PubMed] [Google Scholar]
- 16.Ninichuk V, Kulkarni O, Clauss S, Anders HJ. Tubular atrophy, interstitial fibrosis, and inflammation in type 2 diabetic db/db mice. An accelerated model of advanced diabetic nephropathy. Eur J Med Res. 2007;12:351–355. [PubMed] [Google Scholar]
- 17.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–1454. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi N, et al. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc Natl Acad Sci USA. 2000;97:5434–5439. doi: 10.1073/pnas.090091297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi N, et al. Posttranscriptional compensation for heterozygous disruption of the kidney-specific NaK2Cl cotransporter gene. J Am Soc Nephrol. 2002;13:604–610. doi: 10.1681/ASN.V133604. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi N, et al. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol. 2005;16:125–132. doi: 10.1681/ASN.2004060490. [DOI] [PubMed] [Google Scholar]
- 21.Oliver PM, et al. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA. 1998;95:2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimoto H, Grahovac G, Zeisberg M, Kalluri R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes. 2007;56:1825–1833. doi: 10.2337/db06-1226. [DOI] [PubMed] [Google Scholar]
- 23.Ito M, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosugi T, et al. Lowering blood pressure blocks mesangiolysis and mesangial nodules, but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am J Pathol. 2009;174:1221–1229. doi: 10.2353/ajpath.2009.080605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Boysen G, Li F, Li Y, Swenberg JA. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71:266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 28.Wang JG, Manly D, Kirchhofer D, Pawlinski R, Mackman N. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. 2009;7:1092–1098. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aikawa M, et al. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 1999;100:1215–1222. doi: 10.1161/01.cir.100.11.1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.