Abstract
Hibernation is an energy-conserving behavior consisting of periods of inhibited metabolism (‘torpor’) with lowered body temperature. Torpor bouts are interspersed by arousal periods, in which metabolism increases and body temperature returns to euthermia. In deep torpor, the body temperature typically decreases to 2–10 °C, and major physiological and immunological changes occur. One of these alterations constitutes an almost complete depletion of circulating lymphocytes that is reversed rapidly upon arousal. Here we show that torpor induces the storage of lymphocytes in secondary lymphoid organs in response to a temperature-dependent drop in plasma levels of sphingosine-1-phosphate (S1P). Regulation of lymphocyte numbers was mediated through the type 1 S1P receptor (S1P1), because administration of a specific antagonist (W146) during torpor (in a Syrian hamster at ∼8 °C) precluded restoration of lymphocyte numbers upon subsequent arousal. Furthermore, S1P release from erythrocytes via ATP-binding cassette (ABC)-transporters was significantly inhibited at low body temperature (4 °C) but was restored upon rewarming. Reversible lymphopenia also was observed during daily torpor (in a Djungarian hamster at ± 25 °C), during forced hypothermia in anesthetized (summer-active) hamsters (at ± 9 °C), and in a nonhibernator (rat at ∼19 °C). Our results demonstrate that lymphopenia during hibernation in small mammals is driven by body temperature, via altered plasma S1P levels. S1P is recognized as an important bioactive lipid involved in regulating several other physiological processes as well and may be an important factor regulating additional physiological processes in hibernation as well as in mediating the effects of therapeutic hypothermia in patients.
Keywords: immunology, anesthesiology, sphingolipid, inflammation, suspended animation
Hibernation is an energy-conserving behavior consisting of periods of significantly inhibited metabolism (torpor) that result in a largely reduced heart and ventilation rate (1–3) and body temperature. Torpor bouts are interspersed by arousal periods with durations of 8–24 h, during which metabolism increases and body temperature rapidly returns to euthermia (2, 4). Hibernating mammals display major changes in their physiology that lead (among other changes) to an increased resistance to ischemia/reperfusion (5, 6) and a reduced immune function (7). Remarkably, despite the repetitive cycles of cooling and rewarming, hibernating animals do not show gross signs of organ damage (8). In humans, therapeutic hypothermia is used frequently to limit neuronal injury in cardiac arrest and major surgery of brain and heart (9). However, hypothermia as used during cardiac surgery is associated with increased renal injury postoperatively (10). Therefore, unraveling the mechanisms underlying the changes in physiology of hibernating mammals might be of substantial clinical relevance.
Inflammatory responses induced by hypothermia are implicated in organ injury following therapeutic hypothermia (11–13). Several specific changes occur in the immune system of hibernating animals, such as lower complement levels, phagocytotic capacity, cytokine production, lymphocyte proliferation, antibody production (reviewed in ref. 14), and a profound but readily reversible depletion of circulating leukocytes during torpor (15). Thereby, hibernation affects the function of both the innate and adaptive immune system (14). A reduced innate immune function is demonstrated by the absence of a febrile response to the injection of LPS during torpor (7). Suppression of the adaptive immune system throughout hibernation is demonstrated by the absence of rejection of skin allografts in hibernating ground squirrels until after cessation of their hibernation in spring (16). The mechanisms underlying these specific changes that occur during hibernation still are unresolved. Apart from its applicability in preserving organ function, understanding the immune function in hibernators is of growing importance because large populations of hibernating bats currently are threatened by white-nose syndrome. This condition has a mortality rate of 75–100% and is caused by a psychrophilic (cold-loving) fungus that thrives on bats in torpor (17–21) and is thought to be related to the suppressed immune function during hibernation (14, 22).
To examine mechanisms of immunological alterations in hibernation, changes in the number of circulating leukocytes were examined in different stages of hibernation in hamster species that undergo either deep multiday torpor bouts or shallow daily torpor. Effects were compared with those found in hamsters cooled under anesthesia. Specifically, regulation of lymphocyte numbers was studied because of their importance in adaptive immunity and immunological memory. After confirmation of lymphocyte storage in secondary lymphoid tissue during torpor, we further examined the role of sphingosine-1-phosphate (S1P), a bioactive lipid known to regulate lymphocyte egress from lymph nodes (23–25).
Results
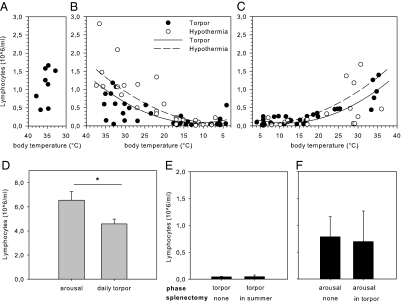
After entrance into deep torpor, the body temperature of the Syrian hamster (Mesocricetus auratus) decreases to 7.7 ± 0.8 °C during the first 24 h (Fig. S1A). During torpor, the total number of circulating white blood cells decreases by 95%, from 1.57 ± 0.22 to 0.07 ± 0.01 × 106 mL. The number of circulating lymphocytes decreases even more dramatically to about 4% of the number in summer-euthermic animals (Fig. 1 A and B). Lymphocyte numbers are restored rapidly upon arousal (Fig. 1C), when body temperature increases to reach euthermia within 2 h (Fig. S1B). The blood lymphocyte count correlates significantly with the body temperature both during entrance into torpor (Pearson R2 = 0.41; P < 0.01) (Fig. 1B) and during arousal following torpor (Pearson R2 = 0.71; P < 0.01) (Fig. 1C). The number of circulating erythrocytes does not change throughout the torpor–arousal cycles (Fig. S2). In an animal showing daily torpor behavior, the Djungarian hamster (Phodophus sungorus), the average body temperature during torpor is 25.2 ± 1.3 °C. During these torpor bouts, the number of circulating leukocytes was reduced by about 50%, whereas the number of circulating lymphocytes decreased by ∼30% (Fig. 1D). To examine the role of body temperature in the decrease of circulating lymphocytes, forced hypothermia was induced in anesthetized (summer-active) hamsters that were not in hibernation to reach a body temperature of 9.1 ± 0.8 °C. Forced hypothermia decreased the number of circulating leukocytes and lymphocytes (Fig. 1B), and the decrease was fully reversed upon rewarming (Fig. 1C). Further, the number of circulating lymphocytes correlated significantly with body temperature during cooling (Pearson R2 = 0.67; P < 0.01) (Fig. 1B) as well as during rewarming following forced hypothermia (Pearson R2 = 0.29; P < 0.01) (Fig. 1C). The number of circulating lymphocytes in summer-euthermic animals that underwent forced hypothermia was, at the start of the experiment, higher than in hibernating animals before entering torpor (Fig. 1B) (P < 0.01). The number of circulating lymphocytes was significantly higher in euthermic (summer-active) animals than in aroused (winter-euthermic) animals (P < 0.05) (Fig. S3). Moreover, in rats (Rattus norvegicus), a nonhibernating animal, forced hypothermia to reach a body temperature of 19.2 ± 0.7 °C did not affect the number of circulating erythrocytes (Fig. S4A) but resulted in a significant decrease in the number of circulating lymphocytes (P < 0.01) (Fig. S4B); this decrease was restored upon rewarming (P < 0.01) (Fig. S4C).
Fig. 1.
Depletion of circulating lymphocytes during torpor is temperature dependent and unaffected by splenectomy. (A) Normal blood lymphocyte count of summer-euthermic Syrian hamster. (B) Body temperature-dependent decrease in blood lymphocytes upon entrance into torpor and to a similar extent in forced hypothermia in nonhibernating animals. (C) Blood lymphocyte counts are restored rapidly during rewarming from torpor and from forced hypothermia. (D) Circulating lymphocyte number is reduced during daily torpor in the Djungarian hamster. (E) Splenectomy before hibernation does not inhibit induction of lymphopenia in torpor. (F) Splenectomy during torpor does not preclude restoration of blood lymphocyte count during the subsequent arousal. Bars represent mean ± SEM of four to eight animals per group. Groups were compared using student's t-test or one-way ANOVA and post hoc least significant difference. *P < 0.05.
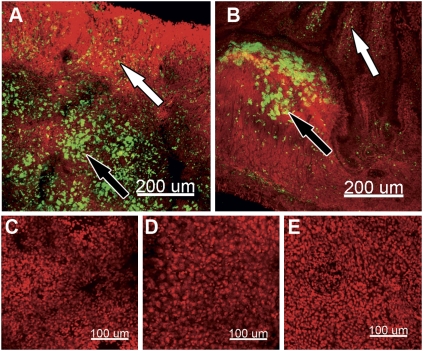
To establish the role of the spleen in the regulation of lymphocyte numbers during hibernation, we measured the expression of markers for lymphocytes and performed splenectomies before and during torpor. mRNA expression of the T-lymphocyte marker CD3ε and the B-lymphocyte marker CD20 is unaffected by torpor (Tables S1 and S2). Although this measurement does not directly demonstrate the number of cells present in the spleen, we have no indication that mRNA expression of these specific markers changes during torpor. Hence, the similar expression levels of these lymphocyte markers during torpor and arousal strongly argue against massive cellular migration into the spleen during torpor or substantial apoptosis of these cells. Surgical removal of the spleen (splenectomy) preceding the hibernation season does not influence the induction of lymphopenia during torpor (Fig. 1E). Conversely, splenectomy during torpor does not preclude restoration of lymphocyte numbers during arousal (Fig. 1F). To detect retention sites of lymphocytes other than the spleen during torpor, fluorescently labeled autologous lymphocytes (from surgically removed spleens) were injected intracardially into splenectomized torpid hamsters. During the subsequent torpor bout, lymphocytes were found in cervical lymph nodes (Fig. 2A) and, to a lesser extent, in gut-associated lymphoid tissue (GALT) (Fig. 2B). Importantly, liver, lung, and kidney did not contain significant numbers of fluorescently labeled lymphocytes (Fig. 2 C–F).
Fig. 2.
Torpor induces storage of lymphocytes in peripheral lymphoid tissues. Panels show representative fluorescent microscopic images of carboxyfluorescein succinimidyl ester (CFSE)-labeled lymphocytes (green) counterstained with TOTO-3 iodide (red) from torpid animals. Animals were splenectomized, and lymphocytes were isolated and labeled with carboxyfluorescein diacetate succinimidyl ester (CFDA-SE), which was injected intracardially into the same animal during torpor. Tissue was harvested during the subsequent torpor bout. (A) Fluorescently labeled lymphocytes in cervical lymph node B-cell follicles (white arrows) and T-cell zones (black arrows). (B) Fluorescently labeled lymphocytes in lamina propria (white arrows) and Peyer's patch (black arrows) of small intestine. (C) Lung tissue. (D) Liver tissue. (E) Kidney tissue.
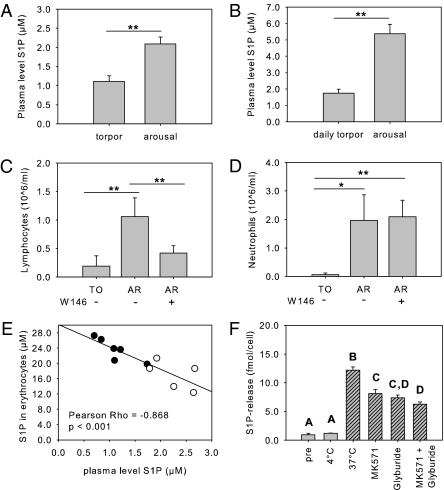
Plasma levels of S1P decreased by ∼50–60% during deep torpor in Syrian hamsters and during daily torpor in Djungarian hamsters (Fig. 3 A and B). During arousal, plasma S1P levels rose rapidly to normal (euthermic) values in both species. Involvement of the S1P system in hibernation-associated lymphopenia was examined further by intracardial injection of a specific type 1 S1P receptor (S1P1) antagonist (W146; 5 mg/kg). No significant difference in the number of lymphocytes was found in torpid animals and aroused animals that were treated with W146, but the number of circulating lymphocytes in the subsequent arousal period was significantly lower following intracardial injection of W146 than in vehicle-treated animals (P < 0.01) (Fig. 3C). In contrast, W146 does not affect the restoration of the number of circulating neutrophils (Fig. 3D).
Fig. 3.
S1P governs lymphocyte dynamics during hibernation through the S1P1 receptor. (A and B) Plasma S1P levels are reduced significantly in (A) deep torpor (Syrian hamster) and (B) daily torpor (Djungarian hamster). (C and D) Upon arousal, the specific S1P1 receptor antagonist W146 precludes the restoration of circulating lymphocytes but not of neutrophils compared with vehicle-treated animals. AR, arousal; TO, torpor. (E) Significant correlation between the level of S1P in erythrocytes and plasma of animals in torpor (closed circles) and arousal (open circles). (F) Temperature governs S1P release from erythrocytes isolated from torpid animals. Erythrocytes were obtained from torpid animals and washed with PBS (pre); continued incubation for 30 min at 4 °C (gray bars) did not induce release of S1P from erythrocytes; ex vivo rewarming for 30 min at 37 °C (hatched bars) induces release of S1P that is blocked by the ABC-transporter inhibitors MK571 (50 μM), glyburide (1 mM), and by MK571 plus glyburide. Bars represent mean ± SEM of four to eight animals per group. Groups were compared using student's t-test or one-way ANOVA and post hoc least significant difference. Different letters above bars represent significant differences at P < 0.05.
Upon arousal, the intracellular level of S1P in erythrocytes decreased (Fig. S5A) without a significant change in the intracellular level of sphingosine (Fig. S5B). Plasma S1P and intracellular S1P levels of erythrocytes correlate negatively in torpor and arousal periods (Pearson R2 = −0.77; P < 0.001; Fig. 3E). To establish the influence of body temperature on the release of S1P from erythrocytes, washed erythrocytes derived from torpid animals were rewarmed to 37 °C. Although erythrocytes maintained at 4 °C do not release S1P into the medium, ex vivo rewarming induces substantial S1P release. Release of S1P from rewarmed erythrocytes is reduced significantly by inhibitors of ATP-binding cassette (ABC)-A1 (glyburide) and ABC-C1 (MK571) transporters and is reduced even further when both inhibitors are combined (Fig. 3F).
Discussion
Our data imply that body temperature is the driving force of lymphopenia during hibernation as shown by (i) the strong correlation between body temperature and blood lymphocyte count, (ii) the occurrence of lymphopenia during both deep and daily torpor, and (iii) the effect of forced cooling of (summer-active) hamsters that were not in hibernation on the number of circulating lymphocytes. At the start of the experiment, however, the number of circulating lymphocytes was significantly higher in animals that underwent forced hypothermia than in hibernating animals that were about to enter torpor. Because no differences were found between summer-euthermic animals that did and did not undergo forced hypothermia, we speculate that this phenomenon is caused by temperature-independent, seasonal changes affecting the number of circulating lymphocytes during euthermia. During daily torpor in the Djungarian hamster, the number of circulating lymphocytes also decreased, in spite of the shorter duration of a torpor bout with a substantial higher body temperature than during deep torpor (2). Although the decrease in the number of circulating lymphocytes during daily torpor was smaller than observed during deep torpor, the fitted curve (Fig. 1B) shows the blood lymphocyte count is ∼40% lower at an average body temperature of ∼25 °C during either natural torpor or forced hypothermia in the Syrian hamster and is about the same as observed during daily torpor in the Djungarian hamster. The temperature dependency of this process is supported further by the fact that hypothermia in rats (nonhibernators) leads to the induction of lymphopenia as well. Because rewarming of severely hypothermic rats affected their breathing, we cooled and rewarmed a second group of ventilated rats to demonstrate the reversibility of lymphopenia induced by hypothermia in rats. In addition, forced hypothermia of anesthetized rats demonstrates that hypothermia-induced lymphopenia is not specific to hibernators but also occurs in nonhibernating animals.
Lymphopenia during hibernation is caused by storage of cells in peripheral lymphoid organs but not in spleen, liver, lung, and kidney. The observation that the number of splenic lymphocytes is stable throughout hibernation and that the lymphopenia during torpor bouts is rapidly reversed upon rewarming strongly favors a storage-and-release mechanism over an apoptosis-and-replenishment mechanism to explain the dynamics of lymphocytes during hibernation. Further, splenectomies preceding the hibernation season demonstrated that the spleen is not necessary for the induction of lymphopenia. Conversely, splenectomy during torpor established that recirculating lymphocytes can be obtained from a source other than spleen. Although we cannot rule out the possibility that (some) lymphocytes are retained in the spleen during torpor, the spleen certainly is not the main organ involved. Indeed, injected fluorescently labeled lymphocytes are found in cervical lymph nodes during the subsequent torpor bout and, to a lesser extent, also in GALT.
Inkovaara et al. (26) previously showed that number of leukocytes increases in lung (mainly neutrophils) and gut (mainly lymphocytes) during torpor compared with summer euthermia or arousal in hibernating hedgehogs (Erinaceus europaeus). The number of intraepithelial lymphocytes and lamina propria leukocytes increases about threefold, whereas the number of lymphocytes in Peyer's patches increases only slightly compared with summer euthermia in the thirteen-lined ground squirrel (Ictidomys tridecemlineatus) (27). Because T lymphocytes in the blood are mainly T-cell receptor (TCR)αβ-positive, influx and retention of circulating T lymphocytes into GALT would result in an altered TCRαβ+:TRCγδ+ ratio. Logically, the authors speculate that the unaffected TCRαβ+:TRCγδ+ ratio in GALT reflects absence of influx, and thus that the increased lymphocyte count in GALT is caused by local expansion of cells (27). However, our results suggest that there is some influx of circulating lymphocytes into GALT during torpor. Influx of circulating lymphocytes might not alter the TCRαβ+:TRCγδ+ ratio as long as the number of cells that migrate into GALT remains relatively small compared with the number of cells already present in GALT. Therefore, the increased number of lymphocytes in GALT might well result from a combination of influx and local expansion of cells. Taken together, these observations show that secondary lymphoid tissue is the main site for the retention of lymphocytes during torpor.
In our study, we demonstrate direct effects of S1P on the restoration of the number of circulating lymphocytes. Because lymphocytes continuously recirculate among various lymphoid organs via the blood, the number of circulating lymphocytes depends on the balance between influx from the blood into peripheral lymphoid organs (homing) and egress out of these organs (as mediated by S1P). Logically, a decreased S1P plasma level, such as occurs during torpor, leads to a reduced egress from peripheral lymphoid organs and consequently to a lymphopenic state. Our data demonstrate that the retention of lymphocytes in peripheral lymphoid organs is regulated by plasma S1P levels acting via S1P1. Although the effect of S1P on lymphocyte egress from peripheral lymphoid organs has been demonstrated previously in knockout models or by using synthetic agonists and antagonists (23–25, 28), our study demonstrates the importance of S1P in regulating lymphocyte numbers in the absence of pharmacological interventions or genetic changes. Lymphocyte egress from peripheral lymphoid organs normally is favored when the S1P concentration is low in lymphoid tissue interstitium and is high at exit sites, i.e., in blood (23).
Although the extent of lymphopenia is larger during deep torpor, the relative decrease in S1P is larger during daily torpor. Other factors, such as time, blood flow velocity, and the level of expression of S1P receptors, which might differ among species, influence the egress of lymphocytes. Therefore, levels cannot be compared directly between species. However, during both deep and daily torpor the number of circulating lymphocytes correlated significantly with the S1P plasma level. Involvement of the S1P system is substantiated by (i) the rapid change in plasma S1P levels, (ii) the correlation between plasma S1P level and blood lymphocyte count, and (iii) the observation that blockade of S1P1 during arousal completely blocks the restoration of the number of circulating lymphocytes. Thus, the blockade of lymphocyte egress by antagonism of the S1P1 indicates that the increase in plasma S1P levels is a causative mechanism in the restoration of circulating lymphocyte numbers during arousal. Because blockade of S1P1 did not affect the restoration of the number of circulating neutrophils and monocytes or change erythrocyte counts, a nonspecific action of S1P1 blockade seems highly unlikely. Together, these data suggest that S1P acting via S1P1 has a major role in regulating lymphocyte number in peripheral blood during hibernation.
Our data suggest that S1P release from erythrocytes constitutes an important regulatory mechanism in S1P plasma levels. This proposal is consistent with previous observations in sphingosine kinase-deficient mice that the plasma level of S1P is derived mainly from erythrocytes (23). Indeed, erythrocytes derived from torpid animals release S1P upon rewarming ex vivo, thus demonstrating that body temperature is the primary factor that can stimulate release. Furthermore, the strong negative correlation between plasma S1P levels and intracellular S1P content of erythrocytes suggests a prominent role for erythrocytes in regulating the plasma level of S1P. The fact that release of S1P can be reduced by inhibitors of ABC-A1 (glyburide) and ABC-C1 (MK571) transporters and is reduced even further when both inhibitors are combined demonstrates a role for ABC transporters in regulating S1P release from erythrocytes upon rewarming. The combination of both inhibitors demonstrates a role for both ABC-A1 and ABC-C1 transporters in the release of S1P from erythrocytes. Incomplete inhibition, however, might suggest the involvement of other (yet unknown) transporters in the release of S1P from erythrocytes as well. Taken together, the data show that, in the absence of potential regulating factors from plasma, an increase in body temperature is sufficient to induce a rapid and substantial release of S1P from erythrocytes of torpid animals.
Our study identifies the reduction of body temperature resulting from metabolic suppression in torpor as the major driving force in modulating lymphocyte egress from lymphoid tissue that constitutes a versatile system of a rapidly reversible reduction of immune function. The induction of lymphopenia by low body temperature is widely conserved, because it seems to be a common response of mammals (shared by hibernating hamsters, hypothermic hamsters, and hypothermic, nonhibernating rats). Although this response seems to be common among mammals rather than an adaptation specific for hibernators, it may be beneficial during hibernation. The induction of an immune response during arousal increases the time before animals go back into torpor (7). Because periodic arousals account for 80–90% of the energy used throughout hibernation, an immune response might increase the energy costs of hibernation (29). Because, in general, no massive death of hibernating animals occurs, and most microbes do not proliferate well at low temperatures, we speculate that the energy benefits of a reduced immune function outweigh the risk of infection. However, some pathogens, such as the psychrophilic fungus Geomyces destructans that causes white-nose syndrome in bats, grow well at low temperatures (14, 17–19, 22). Hence, immune suppression during torpor may be detrimental. Unfortunately, reports on leukocyte dynamics during hibernation in bats are lacking. Thus, whether immune suppression might be involved in the etiology of white-nose syndrome remains to be elucidated.
In this study we show that release of S1P from erythrocytes is reduced significantly by low body temperature. S1P may serve a key function during the induction of torpor by regulating additional aspects of the immune system as well as other physiological changes that are not related primarily to immune function (30). Not only does S1P regulate the function of other types of leukocytes (31); it also is involved in other biological processes (32) and is implicated in governing protection against ischemia/reperfusion-induced injury in brain, heart, liver, and kidney (33–36). Therapeutic hypothermia is used in patients with clinical conditions such as cardiac arrest and brain trauma and in patients undergoing cardiac or brain surgery because reducing cerebral metabolism has neuroprotective properties in periods of low oxygen supply (9). However, hypothermia is associated with increased renal injury postoperatively (10). Given that the plasma level of S1P in humans also is regulated mainly by transport of S1P from erythrocytes (37, 38) and the S1P-system exerts protective effects following ischemia/reperfusion in different organs (33–36), our results may be of direct consequence in understanding the benefits and detriments of therapeutic hypothermia.
Conclusion
Our study identifies the reduction of body temperature resulting from metabolic suppression in torpor as the major driving force in modulation of lymphocyte egress from lymphoid tissue because of decreased S1P plasma levels caused by inhibition of S1P release from erythrocytes (Fig. S6). Understanding the mechanisms of specific physiological alterations involved in the induction of torpor increases our knowledge of natural hibernation and has relevance for therapeutic hypothermia as well as for pharmacologically induced suspended animation.
Materials and Methods
Hibernation.
To induce hibernation in Syrian hamsters (Mesocricetus auratus), the light:dark (L:D) cycle was shortened to 8 h:16 h for ∼10 wk followed by continuous dim light (<5 lux) at an ambient temperature of 5 °C. Movement detectors connected to a computer allowed us to determine the animals’ hibernation pattern. In the Djungarian hamsters (Phodophus sungorus), hibernation was induced by shortening the L:D cycle to 8 h:16 h for ∼14 wk at an ambient temperature of 21 ± 1 °C. Daily torpor was determined by observation at the start of the light phase (usual torpor phase) and a single body temperature measurement at the time of decapitation. All experiments were approved by the Institutional Animal Ethical Committees of the University Medical Center Groningen and University of Aberdeen.
Forced Hypothermia.
Summer-euthermic Syrian hamsters and Wistar rats housed at an L:D cycle of 12 h:12 h were anesthetized by injecting 200 mg/kg ketamine and 1.5 mg/kg diazepam i.p. Spontaneously breathing rats were cooled. Spontaneously breathing hamsters and ventilated rats were cooled and rewarmed. Animals were cooled by applying ice-cold water to their fur and were rewarmed using a water-based heating mattress; both processes were performed at a rate of ∼1 °C of body temperature per 3 min. A catheter was inserted into the jugular vein for blood sampling, rectal temperature was measured continuously, and heart rate (ECG) was monitored on the anesthesia monitor Cardiocap S/5 (Datex Ohmeda).
Splenectomies.
Splenectomies were performed on summer-euthermic and torpid Syrian hamsters. Immediately after induction of anesthesia (2–2.5% isofluorane/O2), a blood sample was drawn by cardiac puncture, and 4 mg/kg flunixin-meglumin (Finadyne; Schering-Plough) was given s.c. as analgesia. Summer-euthermic animals that underwent splenectomy recovered in a warm room (L:D cycle 8 h:16 h). After induction of hibernation, animals were killed during their third torpor bout, which was 60.3 ± 8.1 d following splenectomy. During surgery in the third torpor bout, torpid animals were kept at <10 °C with ice-packs and were recovered in the climate-controlled room. Animals were killed upon reaching euthermia.
Statistical Analysis and Data Presentation.
Data are presented as mean ± SEM. Statistical analysis was performed by student's t-test or one-way ANOVA with post hoc least significant difference (SPSS 16.0 for Windows), with P < 0.05 considered significantly different. Correlations were calculated using Pearson's correlations. Sigmaplot 11.0 was used to produce the graphs shown in this article.
Supplementary Material
Acknowledgments
Costs of measurements were funded in part by a grant from the Jan Kornelis de Cock Foundation (to H.R.B., F.G.K., and R.H.H).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008823108/-/DCSupplemental.
References
- 1.Hampton M, Nelson BT, Andrews MT. Circulation and metabolic rates in a natural hibernator: An integrative physiological model. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1478–R1488. doi: 10.1152/ajpregu.00273.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Milsom WK, Zimmer MB, Harris MB. Regulation of cardiac rhythm in hibernating mammals. Comp Biochem Physiol A Mol Integr Physiol. 1999;124:383–391. doi: 10.1016/s1095-6433(99)00130-0. [DOI] [PubMed] [Google Scholar]
- 4.Hut RA, Barnes BM, Daan S. Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J Comp Physiol B. 2002;172:47–58. doi: 10.1007/s003600100226. [DOI] [PubMed] [Google Scholar]
- 5.Lindell SL, et al. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am J Physiol Gastrointest Liver Physiol. 2005;288:G473–G480. doi: 10.1152/ajpgi.00223.2004. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz CC, Lindell SL, Mangino MJ, Carey HV. Hibernation confers resistance to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2006;291:G895–G901. doi: 10.1152/ajpgi.00155.2006. [DOI] [PubMed] [Google Scholar]
- 7.Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1054–R1062. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- 8.Zancanaro C, Malatesta M, Mannello F, Vogel P, Fakan S. The kidney during hibernation and arousal from hibernation. A natural model of organ preservation during cold ischaemia and reperfusion. Nephrol Dial Transplant. 1999;14:1982–1990. doi: 10.1093/ndt/14.8.1982. [DOI] [PubMed] [Google Scholar]
- 9.Arrich J, Holzer M, Herkner H, Müllner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;(4):CD004128. doi: 10.1002/14651858.CD004128.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Kourliouros A, et al. Low cardiopulmonary bypass perfusion temperatures are associated with acute kidney injury following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2010;37:704–709. doi: 10.1016/j.ejcts.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Zheng JH, Gao BT, Jiang ZM, Yu XQ, Xu ZW. Evaluation of early macrophage activation and NF-kappaB activity in pulmonary injury caused by deep hypothermia circulatory arrest: An experimental study. Pediatr Cardiol. 2010;31:215–221. doi: 10.1007/s00246-009-9586-z. [DOI] [PubMed] [Google Scholar]
- 12.Moore FD, Jr., Warner KG, Assousa S, Valeri CR, Khuri SF. The effects of complement activation during cardiopulmonary bypass. Attenuation by hypothermia, heparin, and hemodilution. Ann Surg. 1988;208:95–103. doi: 10.1097/00000658-198807000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Woude FJ, Schnuelle P, Yard BA. Preconditioning strategies to limit graft immunogenicity and cold ischemic organ injury. J Investig Med. 2004;52:323–329. doi: 10.1136/jim-52-05-32. [DOI] [PubMed] [Google Scholar]
- 14.Bouma HR, Carey HV, Kroese FG. Hibernation: The immune system at rest? J Leukoc Biol. 2010;88:619–624. doi: 10.1189/jlb.0310174. [DOI] [PubMed] [Google Scholar]
- 15.Bouma HR, et al. Blood cell dynamics during hibernation in the European ground squirrel. Vet Immunol Immunopathol. 2010;136:319–323. doi: 10.1016/j.vetimm.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Shivatcheva TM. Survival of skin allografts in European ground squirrels, Spermophilus citellus L., during hibernation. Folia Biol (Krakow) 1988;36:213–221. [PubMed] [Google Scholar]
- 17.Zimmerman R. Ecology. Biologists struggle to solve bat deaths. Science. 2009;324:1134–1135. doi: 10.1126/science.324_1134. [DOI] [PubMed] [Google Scholar]
- 18.Buchen L. Disease epidemic killing only US bats. Nature. 2010;463:144–145. doi: 10.1038/463144a. [DOI] [PubMed] [Google Scholar]
- 19.Blehert DS, et al. Bat white-nose syndrome: An emerging fungal pathogen? Science. 2009;323:227. doi: 10.1126/science.1163874. [DOI] [PubMed] [Google Scholar]
- 20.Puechmaille SJ, et al. White-nose syndrome fungus (Geomyces destructans) in bat, France. Emerg Infect Dis. 2010;16:290–293. doi: 10.3201/eid1602.091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlow A, Ford S, Green R, Morris C, Reaney S. Investigations into suspected white-nose syndrome in two bat species in Somerset. Vet Rec. 2009;165:481–482. doi: 10.1136/vr.165.16.481-a. [DOI] [PubMed] [Google Scholar]
- 22.Wibbelt G, Moore MS, Schountz T, Voigt CC. Emerging diseases in Chiroptera: Why bats? Biol Lett. 2010;6:438–440. doi: 10.1098/rsbl.2010.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 24.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 25.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 26.Inkovaara P, Suomalainen P. Studies on the physiology of the hibernating hedgehog. 18. On the leukocyte counts in the hedgehog's intestine and lungs. Ann Acad Sci Fenn [Biol] 1973;200:1–21. [PubMed] [Google Scholar]
- 27.Kurtz CC, Carey HV. Seasonal changes in the intestinal immune system of hibernating ground squirrels. Dev Comp Immunol. 2007;31:415–428. doi: 10.1016/j.dci.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Cabrera PJ, et al. Full pharmacological efficacy of a novel S1P1 agonist that does not require S1P-like headgroup interactions. Mol Pharmacol. 2008;74:1308–1318. doi: 10.1124/mol.108.049783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayser C. In: Physiological Mammalogy. Mayer W, van Gelder R, editors. New York: Academic; 1965. pp. 180–296. [Google Scholar]
- 30.Nelson CJ, Otis JP, Carey HV. Global analysis of circulating metabolites in hibernating ground squirrels. Comp Biochem Physiol Part D Genomics Proteomics. 2010;5:265–273. doi: 10.1016/j.cbd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 33.Bajwa A, et al. Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:955–965. doi: 10.1681/ASN.2009060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann U, et al. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- 35.Park SW, et al. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P1 receptor activation. Lab Invest. 2010;90:1209–1224. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Köck K, et al. Expression of adenosine triphosphate-binding cassette (ABC) drug transporters in peripheral blood cells: Relevance for physiology and pharmacotherapy. Clin Pharmacokinet. 2007;46:449–470. doi: 10.2165/00003088-200746060-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.