The posttranslational modification of proteins with ubiquitin and ubiquitin-like proteins (collectively referred to as UBLs) has emerged as a major regulatory mechanism in eukaryotes. UBLs are characterized by a core β-grasp fold and an essential carboxy terminal glycine within a di-glycine motif (1). These features are also found in several prokaryotic sulfur carriers, suggestive of an evolutionary relationship to UBLs (2–5). A case in point is the UBL Urm1 (ubiquitin-related modifier 1). Urm1 is known to be conjugated to the peroxiredoxin Ahp1 (6), but its sequence and structure more closely resembles bacterial sulfur carrier proteins (7). In PNAS, work by Van der Veen et al. provides important insight into the mechanism of Urm1 conjugation that highlights its position as an ancient UBL in eukaryotes and solidifies its roles as a posttranslational protein modifier involved in oxidative stress response mechanisms (8).
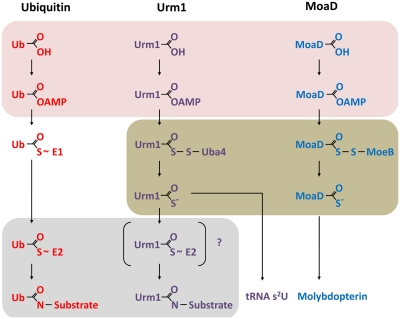
All UBLs require ATP-dependent activation through their cognate E1 enzyme before conjugation onto target proteins (Fig. 1). After the initial formation of a UBL-adenylate in the nucleotide binding pocket of the E1 through its carboxy terminal glycine, the UBL is transferred onto an acceptor sulfhydryl—the E1 catalytic cysteine—to form a high-energy thiolester intermediate (9, 10). The ultimate covalent transfer of the UBL onto its target proteins involves additional specialized enzymes—E2s (conjugating enzymes) and sometimes E3s (ligases)—which receive the UBL through a trans-thiolation reaction using other active site cysteines, thereby providing additional layers of specificity and regulation. UBLs are usually transferred, via their carboxyl terminus, onto the ε-amine of lysine side chains of their substrates in a site-specific manner to form a covalent iso-peptide bond.
Fig. 1.
Juxtaposition of Urm1 as a prokaryotic sulfur carrier and a eukaryotic UBL. The shaded areas indicate overlapping aspects of the pathways, illustrating that Urm1 seems to use features common to both ubiquitin and MoaD for its activation and conjugation. The Urm1–E2 intermediate indicated in brackets is still hypothetical but may account for the hydroxylamine sensitivity of Urm1–protein conjugates formed in response to oxidative stress.
The discovery of Urm1 in 2000 relied on the observation that prokaryotic sulfur carrier proteins, such as MoaD and ThiS, contain the characteristic UBL di-glycine motif and require ATP-dependent activation through a mechanism similar to E1 enzymes (7) (Fig. 1). These proteins function as sulfur donors in biosynthetic pathways, with MoaD involved in molybdenum cofactor (Moco) synthesis and ThiS in thiamine biosynthesis (2, 5, 11, 12). In contrast to the mechanism described above for UBLs, ATP-dependent activation promotes the formation of an acyl disulfide with MoeB and ThiS, respectively (Fig. 1). This leads to the formation of a thiocarboxylate that then provides sulfur necessary for subsequent reactions.
How is Urm1 activated? Whereas the Urm1 E1 (Uba4 in budding yeast, MOCS3 in humans) was originally proposed to form a thiolester linkage to the carboxyl terminus of Urm1 (7), more recent in vitro studies determined that Urm1 forms an acyl disulfide through its E1, leading to the formation of thiocarboxylated Urm1 (ref. 13; Fig. 1). This mechanism requires two cysteine residues: one that has sulfurtransferase activity and another with adenylyltransferase activity. This is not entirely surprising, given that MOCS3 is also involved in the biosynthesis of Moco in humans (13–15). Thus, these observations suggest that Urm1 activation is more similar to that of a prokaryotic sulfur carrier protein than that of a UBL. This function has been linked to the downstream thiolation of certain tRNAs during oxidative stress, where their modification alters their decoding specificity (16–19).
Urm1 also functions as a UBL to covalently modify proteins. Unlike other UBL systems, however, enzymes other than an E1 have not been identified; thus, it remained unclear how activated Urm1 is transferred onto proteins, the nature of these conjugates, and how residues on these proteins are selected for modification. Although previous studies detected covalent Urm1 conjugates in budding yeast, only a single substrate, the peroxiredoxin Ahp1, has been identified (6, 7). Genetic studies implicated Urm1 and Ahp1 in oxidative stress response mechanisms (6, 20).
Van der Veen et al. significantly extend these observations by demonstrating that oxidative stress specifically induces the formation of Urm1–protein conjugates in both yeast and human cells (8). How do these conjugates form? In the absence of oxidative stress and under steady-state conditions, ∼60% of Urm1 is in its thiocarboxylate form. Thus, Urm1 activation is necessary but not sufficient for its conjugation. Through a clever series of biochemical experiments, the authors demonstrate that in vitro-generated Urm1 thiocarboxylate—but not Urm1 carboxylate—is conjugated to proteins in HeLa cell extracts only in the presence of the thiol oxidizer diamide. Intriguingly, conjugate formation also seems to require a thiolester linkage because treatment of cells with hydroxylamine, a cell-permeable compound that disrupts thiolester linkages, reduces oxidative-stress–dependent Urm1 conjugation. Because the conjugates are largely resistant to the reducing agent hydroxylamine, Urm1 modification involves a covalent iso-peptide linkage, which the authors confirm is through lysine residues of target proteins.
Collectively, these experiments suggest that Urm1’s function as a sulfur carrier is directly coupled to its functions as a UBL, linking both of these to oxidative stress response. Moreover, they imply that Urm1 modification involves a unique “noncanonical” mechanism for a UBL. Formation of the Urm1 thiocarboxylate by its E1 allows for oxidative-stress–dependent sulfur transfer and presumably the formation of a Urm1 thiolester before covalent transfer onto substrate lysine residues. Given that Urm1 activation is through an unusual mechanism not involving a thiolester linkage on the E1 active site cysteine and other “canonical” E1s or E2s have not been identified, the mechanism that allows for specific urmylation currently remains unresolved. Presumably, such E2s exist because, as the present study shows, a thiocarboxylate on the C terminus of eGFP is not sufficient to enable it to engage in conjugate formation. Likewise, an E2–Urm1 thiolester intermediate could explain the hydroxylamine sensitivity of protein urmylation.
In addition to confirming that Ahp1 is a Urm1 substrate in yeast (6), the authors
Urm1's function as a sulfur carrier is directly coupled to its functions as a UBL.
identified by mass spectrometry a variety of human proteins modified in response to oxidative stress (8). These include proteins involved in Urm1 functions (MOCS3, ATPD3, and CTU2), the deubiquitylating enzymes USP15 and USP47, proteins involved in nuclear transport, and RNA-processing proteins. Several of these were studied in more detail, and in all cases oxidative stress induced a molecular weight shift of the protein consistent with a single Urm1 modification. Thus, multiple Urm1 attachments or Urm1 polymers do not seem to be synthesized on proteins in cells. These modifications seem to be restricted to a specific lysine side chain, but exactly how substrate and site specificity are defined remains a significant unresolved question. Unlike most UBLs, Urm1 is not synthesized as a precursor that needs to be trimmed at the carboxyl terminus to reveal the essential glycine, but by analogy to all other UBL modifications characterized to date, Urm1 modification is also likely to be reversible through an as-of-yet unknown “deurmylating” enzyme.
What are the functions of Urm1 modifications? As a UBL, Urm1 is anticipated to alter a protein's function, such as targeting it for degradation, changing its subcellular localization, modulating its interactions with other proteins, or inducing conformational changes. On the basis of the subset of proteins analyzed by Van der Veen et al. (8) and previous studies on Ahp1 (6), Urm1 does not seem to have a role in targeting proteins for degradation. Exactly why proteins involved in Urm1 functions, such as its E1 MOCS3, would be modified in response to oxidative stress is unclear, although it is tempting to speculate that these may prevent subsequent sulfur mobilization through Urm1 (8). The observation that two deubiquitylating enzymes are modified suggests a connection between these posttranslational modification systems; however, the authors were unable to uncover any changes in the activity of these enzymes in response to Urm1 modification (8). The discovery that CAS (cellular apoptosis susceptibility) protein is modified in the cytosol suggests a potential role in preventing its translocation to the nucleus (8). However, this remains unanswered, as does the question of why this would be important for oxidative stress response. Nevertheless, it is now abundantly clear that Urm1 is activated and conjugated onto proteins through a mechanism that emphasizes its roles both as a sulfur transfer protein and as a UBL.
Footnotes
The authors declare no conflict of interest.
See companion article on page 1763.
References
- 1.Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009;458:422–429. doi: 10.1038/nature07958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lake MW, Wuebbens MM, Rajagopalan KV, Schindelin H. Mechanism of ubiquitin activation revealed by the structure of a bacterial MoeB-MoaD complex. Nature. 2001;414:325–329. doi: 10.1038/35104586. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan KV. Biosynthesis and processing of the molybdenum cofactors. Biochem Soc Trans. 1997;25:757–761. doi: 10.1042/bst0250757. [DOI] [PubMed] [Google Scholar]
- 4.Taylor SV, et al. Thiamin biosynthesis in Escherichia coli. Identification of ThiS thiocarboxylate as the immediate sulfur donor in the thiazole formation. J Biol Chem. 1998;273:16555–16560. doi: 10.1074/jbc.273.26.16555. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Xi J, Begley TP, Nicholson LK. Solution structure of ThiS and implications for the evolutionary roots of ubiquitin. Nat Struct Biol. 2001;8:47–51. doi: 10.1038/83041. [DOI] [PubMed] [Google Scholar]
- 6.Goehring AS, Rivers DM, Sprague GF., Jr Attachment of the ubiquitin-related protein Urm1p to the antioxidant protein Ahp1p. Eukaryot Cell. 2003;2:930–936. doi: 10.1128/EC.2.5.930-936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furukawa K, Mizushima N, Noda T, Ohsumi Y. A protein conjugation system in yeast with homology to biosynthetic enzyme reaction of prokaryotes. J Biol Chem. 2000;275:7462–7465. doi: 10.1074/jbc.275.11.7462. [DOI] [PubMed] [Google Scholar]
- 8.Van der Veen AG, et al. Role of the ubiquitin-like protein Urm1 as a noncanonical lysine-directed protein modifier. Proc Natl Acad Sci USA. 2011;108:1763–1770. doi: 10.1073/pnas.1014402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciechanover A, Heller H, Katz-Etzion R, Hershko A. Activation of the heat-stable polypeptide of the ATP-dependent proteolytic system. Proc Natl Acad Sci USA. 1981;78:761–765. doi: 10.1073/pnas.78.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas AL, Warms JV, Hershko A, Rose IA. Ubiquitin-activating enzyme. Mechanism and role in protein-ubiquitin conjugation. J Biol Chem. 1982;257:2543–2548. [PubMed] [Google Scholar]
- 11.Iyer LM, Burroughs AM, Aravind L. The prokaryotic antecedents of the ubiquitin-signaling system and the early evolution of ubiquitin-like beta-grasp domains. Genome Biol. 2006;7:R60. doi: 10.1186/gb-2006-7-7-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi J, Ge Y, Kinsland C, McLafferty FW, Begley TP. Biosynthesis of the thiazole moiety of thiamin in Escherichia coli: Identification of an acyldisulfide-linked protein-protein conjugate that is functionally analogous to the ubiquitin/E1 complex. Proc Natl Acad Sci USA. 2001;98:8513–8518. doi: 10.1073/pnas.141226698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz J, et al. The sulfurtransferase activity of Uba4 presents a link between ubiquitin-like protein conjugation and activation of sulfur carrier proteins. Biochemistry. 2008;47:6479–6489. doi: 10.1021/bi800477u. [DOI] [PubMed] [Google Scholar]
- 14.Leimkühler S, Rajagopalan KV. A sulfurtransferase is required in the transfer of cysteine sulfur in the in vitro synthesis of molybdopterin from precursor Z in Escherichia coli. J Biol Chem. 2001;276:22024–22031. doi: 10.1074/jbc.M102072200. [DOI] [PubMed] [Google Scholar]
- 15.Matthies A, Rajagopalan KV, Mendel RR, Leimkühler S. Evidence for the physiological role of a rhodanese-like protein for the biosynthesis of the molybdenum cofactor in humans. Proc Natl Acad Sci USA. 2004;101:5946–5951. doi: 10.1073/pnas.0308191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B, Lu J, Byström AS. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14:2183–2194. doi: 10.1261/rna.1184108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leidel S, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 18.Nakai Y, Nakai M, Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J Biol Chem. 2008;283:27469–27476. doi: 10.1074/jbc.M804043200. [DOI] [PubMed] [Google Scholar]
- 19.Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci USA. 2008;105:18255–18260. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goehring AS, Rivers DM, Sprague GF., Jr Urmylation: A ubiquitin-like pathway that functions during invasive growth and budding in yeast. Mol Biol Cell. 2003;14:4329–4341. doi: 10.1091/mbc.E03-02-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]