Abstract
Shear stress, especially low shear stress (LowSS), plays an important role in vascular remodeling during atherosclerosis. Endothelial cells (ECs), which are directly exposed to shear stress, convert mechanical stimuli into intracellular signals and interact with the underlying vascular smooth muscle cells (VSMCs). The interactions between ECs and VSMCs modulate the LowSS-induced vascular remodeling. With the use of proteomic analysis, the protein profiles of rat aorta cultured under LowSS (5 dyn/cm2) and normal shear stress (15 dyn/cm2) were compared. By using Ingenuity Pathway Analysis to identify protein–protein association, a network was disclosed that involves two secretary molecules, PDGF-BB and TGF-β1, and three other linked proteins, lamin A, lysyl oxidase, and ERK 1/2. The roles of this network in cellular communication, migration, and proliferation were further studied in vitro by a cocultured parallel-plate flow chamber system. LowSS up-regulated migration and proliferation of ECs and VSMCs, increased productions of PDGF-BB and TGF-β1, enhanced expressions of lysyl oxidase and phospho-ERK1/2, and decreased Lamin A in ECs and VSMCs. These changes induced by LowSS were confirmed by using PDGF-BB recombinant protein, siRNA, and neutralizing antibody. TGF-β1 had similar influences on ECs as PDGF-BB, but not on VSMCs. Our results suggest that ECs convert the LowSS stimuli into up-regulations of PDGF-BB and TGF-β1, but these two factors play different roles in LowSS-induced vascular remodeling. PDGF-BB is involved in the paracrine control of VSMCs by ECs, whereas TGF-β1 participates in the feedback control from VSMCs to ECs.
Keywords: mechanotransduction, mechanobiology, bioinformatics, cell biology
Shear stress, caused by the tangential frictional drag force of blood flow, plays an important role in the control of blood vessel growth and functions (1). Clinical and pathological studies have shown that atherosclerotic lesions occur preferentially at vessel branch points, bifurcations, and regions of high curvature, which suggest that low and disturbed shear stress is an important inducer for atherogenesis. In contrast, normal laminar shear stress is atheroprotective (2, 3).
Endothelial cells (ECs), which exist as a monolayer at the intimal surface of the arterial wall, are the primary vascular cell type subjected to the shear stress and convert the mechanical stimuli into intracellular signals (3, 4). There are many kinds of mechanosensors in ECs, including transmembrane proteins such as integrins (4, 5), ion channels (4, 6), junctional proteins (7, 8), growth factor receptors (7), and G protein-coupled receptors (9), as well as membrane lipids (10) and glycocalyx (10, 11). Recent studies have suggested that the envelope of the nucleus might directly sense the mechanical stimuli and regulate gene expression (12). All these molecules are implicated in mechanotransduction of shear stress and subsequently resulted in the cellular functional responses, e.g., proliferation, apoptosis, migration, and permeability.
Vascular smooth muscle cells (VSMCs) are the other major cellular components of the vessel wall. Growing evidence, both in vivo and in vitro, has revealed that the essential pathology of vascular remodeling during atherosclerosis induced by low and oscillating shear stresses involves the proliferation, apoptosis, migration, and phenotype transformation of VSMCs (2, 13, 14). As ECs are directly exposed to shear stress, understanding of their crosstalk with VSMCs under shear flow can help to elucidate the molecular mechanism of the vascular remodeling induced by low shear stress (LowSS). Compared with the knowledge of mechanotransduction in monocultured ECs, the understanding of how ECs transmit the shear stress stimuli to VSMCs is still in its infancy.
In the present study, we investigated the proteomic profiles from vessels cultured under two shear stress levels of 15 dyn/cm2 (normal shear stress; NSS) and 5 dyn/cm2 (LowSS). Analysis of differentially expressed proteins by Ingenuity Pathway Analysis (IPA) revealed signaling networks that are highly associated with mechanotransduction of LowSS. In investigating the crosstalk between ECs and VSMCs during LowSS-induced vascular remodeling, the IPA signaling network analysis led to the identification of two secretory factors—PDGF-BB and TGF-β1—as well as the related molecules lamin A, lysyl oxidase (LOX), and ERK 1/2, and these were selected for further studies. By using an EC/VSMC cocultured parallel-plate flow chamber system, in which ECs and VSMCs are grown on the opposite sides of a porous membrane and shear stress is applied to the ECs, we demonstrated the roles of PDGF-BB and TGF-β1 on the interaction of ECs and VSMCs, as well as the LowSS-induced cellular migration and proliferation.
Results
Networks that Involved PDGF-BB, TGF-β1, Lamin A, LOX, and ERK1/2 Were Disclosed Based on Proteomic Studies on Rat Aorta.
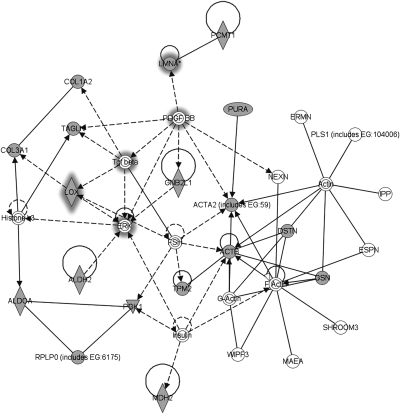
Bu using the comparative proteomic techniques of 2D electrophoresis and MALDI-TOF MS, the protein profiles of rat aorta cultured under NSS (15 dyn/cm2) and LowSS (5 dyn/cm2) were compared (Fig. 1). There were 43 protein spots differentially expressed between NSS and LowSS cultured vessels (Table S1), among which eight proteins were highly expressed in vessels under NSS and 35 proteins were highly expressed in LowSS. Rho dissociation factor α, which was shown to regulate VSMC migration and apoptosis in our previous study (13), did show a lower expression in LowSS in the current study (Table S1), but was not included in the IPA-revealed signaling network because it did not pass the significance level of P < 0.05 (Fig. 2 and Table S2).
Fig. 1.
Typical 2D electrophoresis gel maps of NSS- and LowSS-cultured vessels. The horizontal axis represents the isoelectric focus dimension that separated proteins by their isoelectric point (pH 3–10 NL). The vertical axis represents 12% SDS/PAGE gel, which separated proteins according to their molecular weight (MW). Arrows point to proteins that are differentially expressed between two levels of shear stress and integrated by IPA (nodes with gray background in Fig. 2). Proteins corresponding to numbers 1 to 4 were highly expressed in vessels cultured under NSS, and numbers 5 to 18 were highly expressed in LowSS. The names of these spots are listed in Table S2.
Fig. 2.
Networks revealed by IPA after uploading the global differentially expressed proteins between NSS and LowSS. Nodes with gray background are molecules detected from proteomic analysis, and nodes with white background are molecules forecasted by the IPA. Lines indicate interactions, with the arrowheads indicating directionality. Absence of arrowheads refers to a binding interaction. Dotted line indicates an inferred or indirect interaction. ( indicates enzyme.
indicates enzyme.  indicates group or complex,
indicates group or complex,  indicates transcription regulator,
indicates transcription regulator,  indicates kinase,
indicates kinase,  indicates other,
indicates other,  indicates enzyme binding to itself,
indicates enzyme binding to itself,  indicates group or complex interacting with itself,
indicates group or complex interacting with itself,  indicates group or complex interacting with itself in an inferred or indirect manner, and
indicates group or complex interacting with itself in an inferred or indirect manner, and  indicates molecule binding to itself.) Proteins with grey halation are selected for further research.
indicates molecule binding to itself.) Proteins with grey halation are selected for further research.
These differentially expressed proteins were divided into six groups depending on their functions by Clusters of Orthologous Groups of proteins classification. The first group was components or regulators of the cytoskeleton. The second group was enzymes participating in metabolism. The third group was signal transduction molecules. The fourth group was secretory-type proteins, which are components of extracellular matrix (ECM) or modulators of ECM production. These results suggest that vascular remodeling occurred in cultured rat aorta after exposure to LowSS in the vessel cultured system in vitro for 24 h. The fifth group was proteins involved in DNA damage repair, which are all highly expressed under LowSS. There are some other proteins whose functions cannot be classified into these categories or are still unclear in vascular cells.
To gain further insight into the global network functions between NSS- and LowSS-cultured vessels, differentially expressed proteins were uploaded to the IPA server (http://www.library.ucsf.edu/db/ingenuity-pathways-analysis-ipa). IPA integrates the knowledge on genes, drugs, chemicals, protein families, processes, and pathways based on their interactions and functions derived from the Ingenuity Pathways Knowledge Database Literature (15, 16). The significance values for analyses of network and pathway generation were calculated by comparing the number of proteins that participate in a given function or pathway relative to the total number of occurrences of these proteins in all functional/pathway annotations stored in the Ingenuity Pathways Knowledge Database Literature (16). Pathway networks (Fig. 2 and Table S2) disclosed by IPA that had a significant P value (P < 0.05) were analyzed next.
As the key point of the present study is the crosstalk between ECs and VSMCs in response to different levels of shear stress, secretary molecules in the IPA-revealed network were considered first. PDGF-BB and TGF-β1, which are known to be involved in cellular communication and function modulation (17–19), and three other related molecules, lamin A, LOX and ERK1/2, were selected for further studies.
LowSS Modulated Expression of PDGF-BB, TGF-β1, Lamin A, LOX, and Phospho-ERK1/2 and Induced Migration and Proliferation of ECs and VSMCs in Coculture Flow Chamber System.
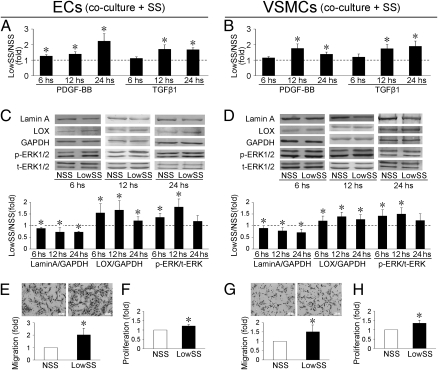
To demonstrate the crosstalk between ECs and VSMCs, an EC/VSMC cocultured parallel-plate flow chamber system was used. The concentrations of PDGF-BB and TGF-β1 were quantified by ELISA kit respectively. In the media from the ECs, the concentrations of PDGF-BB increased at 6 h after shear stress application (Fig. 3A). The concentrations of TGF-β1 in VSMCs and PDGF-BB in ECs and VSMCs were not changed significantly after 6 h of shear stress application (Fig. 3 A and B). The concentrations of both PDGF-BB and TGF-β1 in EC and VSMC sides were significantly higher with LowSS than NSS at 12 h and 24 h after shear stress application (Fig. 3 A and B). The expressions of lamin A, LOX, and phospho-ERK1/2 were detected by Western blotting. In both ECs and VSMCs, the expression of lamin A decreased significantly after LowSS (compared with NSS) at 6 h, 12 h, and 24 h (Fig. 3 C and D). The expressions of LOX increased in ECs and VSMCs following LowSS, i.e., showing opposite changes to lamin A (Fig. 3 C and D). The differences of lamin A and LOX between LowSS and NSS found by Western blotting correspond well with the proteomic analysis, which revealed that lamin A was highly expressed in vessels cultured under NSS (pointed spot 4, Fig. 1), whereas LOX was highly expressed under LowSS (pointed spot 7, Fig. 1). In ECs and VSMCs, the phosphorylation of ERK1/2 was up-regulated by LowSS at 6 h and 12 h, but not at 24 h (Fig. 3 C and D).
Fig. 3.
In ECs and VSMCs, LowSS induced the secretion of PDGF-BB and TGF-β1 at 12 and 24 h (A and B) and decreased the expression of lamin A and LOX at 6, 12, and 24 h (C and D). The phosphorylation of ERK1/2 was up-regulated by LowSS at 6 and 12 h in ECs and VSMCs, but not at 24 h (C and D). Twelve hours of application of of LowSS induced migration and proliferation of ECs (E and F) and VSMCs (G and H). Values shown are the mean ± SD for each condition from three independent experiments for ELISA analysis, and four for the others. _ _ _ indicates the values of NSS standardized to 1 (*P < 0.05 vs. NSS).
Treatment with LowSS for 12 h induced migration (analyzed by Transwell; Fig. 3 E and G) and proliferation (analyzed by BrdU ELISA Kit; Fig. 3 F and H) of ECs (Fig. 3 E and F) and VSMCs (Fig. 3 G and H).
Differential Effects of PDGF-BB and TGF-β1 on Cell Migration and Proliferation and Expressions of Lamin A, LOX, and Phospho-ERK1/2 of ECs and VSMCs in Static Condition.
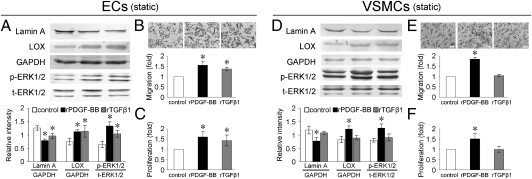
Under static conditions, the recombinant proteins of PDGF-BB (rPDGF-BB) and TGF-β1 (rTGF-β1) decreased the expression of lamin A and increased the expression of LOX and phospho-ERK1/2 in ECs cultured alone (Fig. 4A). rPDGF-BB and rTGF-β1 also increased the migration and proliferation of ECs (Fig. 4 B and C).
Fig. 4.
rPDGF-BB and rTGF-β1 decreased expression of lamin A and increased expression of LOX and phospho-ERK1/2 (A) and induced migration (B) and proliferation (C) of ECs. rPDGF-BB showed similar effects on VSMCs as in ECs (D–F). However, rTGF-β1 had no significant effect on protein expression (D), migration (E), and proliferation (F) in VSMCs. Values shown are the mean ± SD for each condition from four independent experiments (*P < 0.05 vs. control).
In VSMCs cultured alone under static condition, rPDGF-BB repressed the expression of lamin A and enhanced the expression of LOX and phospho-ERK1/2 (Fig. 4D), as well as VSMC migration and proliferation (Fig. 4 E and F). However, rTGF-β1 had no specific effects on migration (Fig. 4E), proliferation (Fig. 4F), or the expression of lamin A, LOX, or phospho-ERK1/2 in VSMCs (Fig. 4D).
As shown in Fig. 3, LowSS increased the secretion of PDGF-BB and TGF-β1 in ECs and VSMCs, but the effects of rPDGF-BB and rTGF-β1 on ECs and VSMCs were different. Hence, we hypothesized that PDGF-BB and TGF-β1 might play different roles in the crosstalk between ECs and VSMCs during LowSS-induced vascular remodeling.
ECs Provided Paracrine Control of PDGF-BB, but Not TGF-β1, on LowSS-Induced VSMC Migration and Proliferation in Coculture Flow Chamber System.
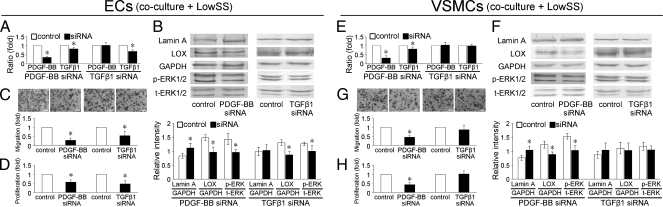
Under the static condition, transfection of PDGF-BB target siRNA significantly decreased the secretion of not only PDGF-BB from ECs, but also TGF-β1 (Fig. S1A). These results correspond with the IPA-revealed networks based on proteomic analysis that PDGF-BB modulates the expression of TGF-β1. Interestingly, the PDGF-BB knockdown in ECs also markedly repressed the secretion of PDGF-BB and TGF-β1 from cocultured VSMCs (Fig. S1B), suggesting that the PDGF-BB secreted from ECs plays a crucial role in regulating the production of PDGF-BB and TGF-β1 in VSMCs in close proximity. Under LowSS, PDGF-BB knockdown in ECs revealed similar effects as under static condition (Fig. 5 A and E), indicating that target siRNA transfection of PDGF-BB in ECs blocked, at least partly, the effects of LowSS on production of PDGF-BB and TGF-β1 from ECs and VSMCs.
Fig. 5.
PDGF-BB target siRNA transfection significantly decreased the secretions of PDGF-BB and TGF-β1 from ECs under LowSS conditions (A). PDGF-BB knockdown in ECs also markedly repressed the secretion of PDGF-BB and TGF-β1 from cocultured VSMCs (E). PDGF-BB target siRNA transfection in ECs increased the expression of lamin A, decreased expression of LOX and phospho-ERK1/2 (B and F), and repressed migration (C and G) and proliferation (D and H) of ECs and coculutred VSMCs. TGF-β1 target siRNA transfection in ECs had no specific effect on PDGF-BB production from ECs (A), nor on PDGF-BB and TGF-β1 production from VSMCs (E). TGF-β1 knockdown suppressed the expressions of LOX and phospho-ERK1/2 (B), migration (C), and proliferation (D) of ECs, but had no significant effects on cocultured VSMCs (F–H). Values shown are the mean ± SD for each condition from three independent experiments for ELISA analysis, and four for the others (*P < 0.05 vs. control).
Accompanying the suppressed secretion of PDGF-BB in ECs, the expression of lamin A was increased and the expression of LOX and phospho-ERK1/2 in ECs and cocultured VSMCs were decreased (Fig. S1 C and D and Fig. 5 B and F), and migration and proliferation of ECs and VSMCs were suppressed (Fig. S1 E–H and Fig. 5 C, D, G, and H) under both static and LowSS conditions.
Transfection of TGF-β1 target siRNA significantly decreased the secretion of TGF-β1 from ECs under static and LowSS conditions (Fig. S2A and Fig. 4A). The secretion of TGF-β1 from cocultured VSMCs was not affected (Fig. S2B and Fig. 4B). The TGF-β1 knockdown in ECs had no specific effects on the production of PDGF-BB from ECs and cocultured VSMCs under static and LowSS conditions (Fig. S2 A and B and Fig. 4 A and B).
Under static condition, TGF-β1 knockdown did not change significantly the expressions of lamin A, LOX, and phospho-ERK1/2 in ECs and cocultured VSMCs (Fig. S2 A and B). The slight decreases of migration and proliferation of ECs are not statistically significant (Fig. S2 E and G). The migration and proliferation of cocultured VSMCs were also not affected (Fig. S2 F and H).
Under LowSS condition, TGF-β1 knockdown in ECs suppressed the expressions of LOX and phospho-ERK1/2, but had no effect on expression of lamin A. The migration and proliferation of ECs were also decreased after target TGF-β1 siRNA transfection (Fig. 5 C and D). In cocultured VSMCs, TGF-β1 knockdown in ECs had no significant effects on the expression of lamin A, LOX, and phospho-ERK1/2, or on cellular migration and proliferation (Fig. 5 F–H).
VSMCs Provided Positive Feedback of PDGF-BB and TGF-β1 to ECs on LowSS-Induced Cell Migration and Proliferation in Coculture Flow Chamber System.
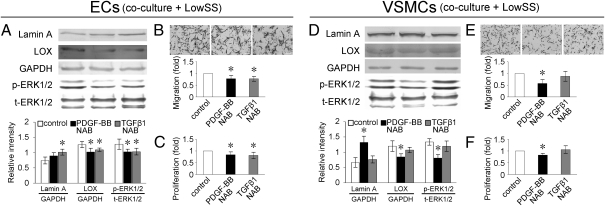
To analyze the effects of PDGF-BB and TGF-β1 secreted from VSMCs under LowSS on the migration and proliferation of cocultured ECs, the neutralizing antibodies (NABs) of PDGF-BB and TGF-β1 were used to block the bioactivity of PDGF-BB and TGF-β1, respectively, in VSMCs before they were subjected to shear stress. The expression of lamin A in cocultured ECs was increased by TGF-β1 NAB, but not PDGF-BB NAB (Fig. 6A). The expressions of LOX and phospho-ERK1/2 in cocultured ECs were decreased (Fig. 6A), and migration and proliferation of cocultured ECs were repressed by both NABs (Fig. 6 B and C).
Fig. 6.
Incubation of TGF-β1 NAB in VSMCs increased the expression of lamin A in cocultured ECs, but PDGF-BB had no significant effect (A). NABs of both PDGF-BB and TGF-β1 incubation in VSMCs decreased expressions of LOX and phospho-ERK1/2 (A) and suppressed migration (B) and proliferation (C) of cocultured ECs. Incubation of NAB of PDGF-BB increased expression of lamin A, decreased expression of LOX and phospho-ERK1/2 (D), and suppressed migration (E) and proliferation (F) of VSMCs, but TGF-β1 NAB had no notable effects (D–F). Values shown are the mean ± SD for each condition from four independent experiments (*P < 0.05 vs. control).
In VSMCs, PDGF-BB NAB enhanced the expression of lamin A (Fig. 6D), down-regulated the expressions of LOX and phospho-ERK1/2 (Fig. 6D), and suppressed migration and proliferation (Fig. 6 E and F). TGF-β1 NAB showed no notable effects on these factors in VSMCs (Fig. 6 D–F).
Discussion
There is ample evidence for the linkage between the localization of atherogenesis and the regional variations of flow patterns in the vascular system (1–4). Atherosclerotic lesions occur preferentially at sites of curvature, branching, or expansion of cross-section, where the flow patterns are complex. Recent investigations have shown that low and disturbed shear stress is an inducer in atherogenesis, whereas the normal level and laminar shear stress is atheroprotective. The present research is focused on vascular remodeling modulated by the level of shear stress (i.e., NSS vs. LowSS) and the intercellular communication between ECs and VSMCs.
ECs and VSMCs are the major cellular components of the wall of arteries. ECs in the intima of the arterial wall, which are constantly exposed to the shear stress caused by the tangential frictional drag force of blood flow, convert the mechanical stimuli into intracellular signals (4) and interact with the underlying VSMCs. It has been reported that ECs induce gene expressions of PDGF-AA, PDGF-BB, and TGF-β in cocultured VSMCs (17) and that shear stress modulates VSMC migration, apoptosis, proliferation, and gene expressions in an EC-dependent manner (1, 20, 21). Shear stress acting on ECs changes the phenotype of the cocultured VSMCs, with the subsequent alterations in the EC release of proinflammatory cytokines, as well as proliferation, apoptosis, and gene expression that induce atherogenesis (22, 23). These studies revealed that the interactions between ECs and VSMCs play an important role in vascular remodeling induced by LowSS.
By using comparative proteomic analysis, the differentially expressed proteins between vessels cultured under NSS and LowSS were globally characterized, and further studied by IPA. The customized pathways (Fig. 2) disclosed by IPA have important implications for the understanding of the pathogenesis of vascular remodeling induced by LowSS. Two secretory molecules involved in the pathways, PDGF-BB and TGF-β1, were selected for further investigation on the interaction between ECs and VSMCs under different levels of shear stress.
To demonstrate the roles of PDGF-BB and TGF-β1 on the crosstalk between ECs and VSMCs under shear stress, an EC/VSMC cocultured parallel-plate flow chamber system was used in which ECs and VSMCs are grown on the opposite sides of a 10-μm-thick polyethylene terephthalate membrane and shear stress was applied to the ECs. There are 1.6 million pores/cm2 on the membrane, and each pore is 0.4 μm in diameter. The porous structure permitted the interaction between ECs and VSMCs and allowed the respective analysis on these two kinds of cells (20–22).
In the present research, application of LowSS to the ECs increased the migration and proliferation of ECs and VSMCs, which is in agreement with previous reports that LowSS is a pathogenic factor for the vascular wall by inducing the migration and proliferation of ECs and VSMCs (1–4). The induced productions of PDGF-BB and TGF-β1 in VSMCs by LowSS suggested that the VSMCs were drifting to the synthetic/proliferative phenotype, rather than contractile, and hence our results represent primarily the interaction of intimal (rather than medial) VSMCs with the ECs, and the present findings would be more relevant to the intimal origin of atherosclerosis.
Studies were also carried out on three other molecules—lamin A, LOX, and ERK1/2—that were found by IPA to be linked with PDGF-BB and TGF-β1 in the network. Lamin A is the common component of the nuclear matrix that plays a major role in the maintenance of nuclear shape, stability, and structure (12, 24). It has been reported that lamin A combines with emerin, another component of the nuclear matrix, to regulate the activity of transcription regulators, i.e., BAF, GCL, and Btf, thus increasing the expression of their respective target genes (24, 25). LOX plays a critical role in the formation and repair of the extracellular matrix by initiating the formation of covalent cross-linkages that could participate in vascular remodeling (26, 27). These two molecules have been reported to regulate various cellular functions (25, 27–29) such as migration, apoptosis, and proliferation. ERK1/2 is a widely investigated member of the MAPK family. It has been reported that TGF-β1 is involved in the mechanotransduction in ECs through intracellular ERK1/2 pathways (28).
In the present research, PDGF-BB was involved in the regulation of lamin A, LOX, and phospho-ERK1/2 in ECs and VSMCs under static and shear stress, whereas TGF-β1 had similar effects as PDGF-BB on expressions of lamin A, LOX, and phospho-ERK1/2 in ECs, but had no specific effect in VSMCs. Based on these data, IPA disclosed two supplementary interactions for the networks: first, PDGF-BB modulates the phosphorylation of ERK1/2 in both ECs and VSMCs; and second, TGF-β1 regulates the expression of lamin A in ECs.
The in vitro models of the ex vivo vessel culture system and the in vitro EC/VSMC cocultured flow chamber system were chosen to mimic the physical and organizational environments of ECs and VSMCs. The ex vivo vessel culture preserves the organizational features of the vessel in terms of the EC-VSMC relation and provides a system closer to the physiological condition. The in vitro system also simulates the in vivo organization of ECs and VSMCs separated by a porous membrane, although it is more artificial. The in vitro system, however, provides advantages in that it makes possible the precise control of the physicochemical environment, determination of molecular alterations in the signaling system, measurements of cellular functions, modulations of the molecular entities, and quantization of molecular expression profiles in the cocultured ECs and VSMCs. These results obtained from the in vitro system allow the use of the IPA to identify a protein–protein interaction network based on the genes chosen from the ex vivo vessel culture system. Thus, the combination of the ex vivo and in vitro systems allow the generation of the results of the present study that contribute to the understanding of the roles of mechanotransduction and cellular communication in vascular remodeling during atherosclerosis.
In the in vivo vascular system, the atherosclerosis-prone areas such as branch points are exposed to not only LowSS but also disturbed flow with recirculation, reattachment, and unsteady flow. The present study addresses only the LowSS, and further studies are needed to elucidate the roles of disturbed flow as well as pulsatility. It would also be valuable to extend the present investigation to in vivo remodeling and proteomic profiling in arteries following ligation, thus shedding more insights into the roles of modification of shear flow patterns on gene interaction network in such a pathophysiological state.
In summary, LowSS was a pathological inducer for vascular remodeling by up-regulated migration and proliferation of ECs and VSMCs. LowSS increased the paracrine secretion of PDGF-BB and TGF-β1 from ECs, but these two factors play different roles in the LowSS-induced vascular remodeling. The LowSS-induced secretion of PDGF-BB from ECs up-regulated the production of PDGF-BB and TGF-β1 in VSMCs, both of which subsequently provided a feedback control to the ECs. The production of TGF-β1 from ECs induced by LowSS was not involved in the paracrine control of VSMCs, but might participate in regulating migration and proliferation of ECs via paracrine or autocrine effect from the VSMCs. The activation of ERK 1/2 and expressions of LOX and lamin A might participated in the effects of PDGF-BB and/or TGF-β1 on cellular migration and proliferation.
Materials and Methods
Materials.
Immobilized pH gradient (IPG) dry strips [pH 3–10, nonlinear (NL)] and IPG buffer (pH 3–10 NL) were purchased from GE Healthcare. Polyclonal antibodies against lamin A, LOX, and GAPDH were purchased from Santa Cruz Biotechnology. Rabbit polyclonal antibodies directed against total-ERK1/2 and phospho-ERK1/2 were purchased from Cell Signaling Technologies. Recombinant proteins of PDGF-BB and TGF-β1, NABs of PDGF-BB and TGF-β1, and Quantikine ELISA Kit for PDGF-BB and TGF-β1 were purchased from R&D Systems. The colorimetric BrdU KIT was purchased from Roche Diagnostics. All other antibodies and chemicals of reagent grade were obtained from Sigma or GE Healthcare.
Vessel Culture Protocol.
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication no. 85–23, revised 1996), and the protocol was approved by the Animal Research Committee of Shanghai Jiao Tong University. The thoracic aorta of adult male Sprague–Dawley rats, 220 to 260 g, was rapidly dissected with ligation of all branches under an operation microscope. The vascular segment was mounted in a vessel perfusion culture system and subjected to NSS and LowSS for 24 h (13).
Comparative Proteomic Analysis and IPA.
The sample solution was applied onto 24 cm IPG dry strips (pH 3–10 NL) of 2D electrophoresis. Gels were stained by blue-silver method (30) and analyzed with Image Master Platinum software (GE Healthcare). Proteins with expression variance of a factor of at least three between vessels cultured under NSS and LowSS were identified by MALDI-TOF MS.
After uploading differentially expressed protein lists to the IPA server, the pathway networks with significant P values (P < 0.05) were disclosed by IPA.
Cell Culture and Coculture Parallel-Plate Flow Chamber System.
Primary rat aortic ECs were obtained by digestive method (31) and were characterized by immunohistochemical staining for von Willebrand factor. VSMCs were obtained by the explant technique (13) and were characterization by smooth muscle-specific α-actin. ECs with passages two through four and VSMCs with passages four through seven were used. For shear stress subjection, ECs were first seeded onto the outer side of the PET membrane at a density of 2 × 105 cells per cup. After 8 h for attachment, the membrane was placed with the EC side down in the six-well plate, and VSMCs were seeded on the inside of the membrane at a density of 2 × 105 cells per cup. NSS and LowSS were subjected to ECs in the flow chamber system (20–22).
Western Blotting Analysis and ELISA.
For protein expression levels detection, cells lysates were separated with 10% SDS/PAGE. The concentration of PDGF-BB and TGF-β1 were analyzed by Quantikine ELISA Kit (R&D Systems).
Cell Migration and Proliferation.
Cell migration assay was performed with the Transwell system (Costar). Cell proliferation was analyzed by using a colorimetric BrdU kit (Roche Diagnostics).
Statistical Analysis.
Each experiment was performed at least in triplicate, and all values are expressed as mean ± SD. The one-way ANOVA was used to compare two groups. P values lower than 0.05 were accepted as statistically significant.
Supplementary Material
Acknowledgments
This research was supported by National Natural Science Foundation of China Grants 10732070 (to Z.-L.J.), 10702043 (to Y.-X.Q.), 10972140 (to Y.-X.Q.), and 30470432 (to Z.-L.J.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019219108/-/DCSupplemental.
References
- 1.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 3.Caro CG. Discovery of the role of wall shear in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:158–161. doi: 10.1161/ATVBAHA.108.166736. [DOI] [PubMed] [Google Scholar]
- 4.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Goldfinger LE, et al. Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circ Res. 2008;103:177–185. doi: 10.1161/CIRCRESAHA.108.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoger JH, Ilyin VI, Forsyth S, Hoger A. Shear stress regulates the endothelial Kir2.1 ion channel. Proc Natl Acad Sci USA. 2002;99:7780–7785. doi: 10.1073/pnas.102184999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83:131–151. doi: 10.1016/s0079-6107(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 11.Florian JA, et al. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 12.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi YX, et al. Rho-GDP dissociation inhibitor alpha downregulated by low shear stress promotes vascular smooth muscle cell migration and apoptosis: A proteomic analysis. Cardiovasc Res. 2008;80:114–122. doi: 10.1093/cvr/cvn158. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto N, Ohashi T, Sato M. Effect of fluid shear stress on migration of vascular smooth muscle cells in cocultured model. Ann Biomed Eng. 2006;34:408–415. doi: 10.1007/s10439-005-9043-y. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, et al. Proteome alteration of U251 human astrocytoma cell after inhibiting retinoic acid synthesis. Mol Cell Biochem. 2009;323:185–193. doi: 10.1007/s11010-008-9978-z. [DOI] [PubMed] [Google Scholar]
- 16.Dai L, Li C, Shedden KA, Misek DE, Lubman DM. Comparative proteomic study of two closely related ovarian endometrioid adenocarcinoma cell lines using cIEF fractionation and pathway analysis. Electrophoresis. 2009;30:1119–1131. doi: 10.1002/elps.200800505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heydarkhan-Hagvall S, et al. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J Cell Biochem. 2003;89:1250–1259. doi: 10.1002/jcb.10583. [DOI] [PubMed] [Google Scholar]
- 18.Baker AB, et al. Endothelial cells provide feedback control for vascular remodeling through a mechanosensitive autocrine TGF-beta signaling pathway. Circ Res. 2008;103:289–297. doi: 10.1161/CIRCRESAHA.108.179465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CN, et al. Synergistic roles of platelet-derived growth factor-BB and interleukin-1beta in phenotypic modulation of human aortic smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:2665–2670. doi: 10.1073/pnas.0510973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu JJ, Chen LJ, Chen CN, Lee PL, Lee CI. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J Biomech. 2004;37:531–539. doi: 10.1016/j.jbiomech.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Wang HQ, et al. Shear stress protects against endothelial regulation of vascular smooth muscle cell migration in a coculture system. Endothelium. 2006;13:171–180. doi: 10.1080/10623320600760282. [DOI] [PubMed] [Google Scholar]
- 22.Chiu JJ, et al. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood. 2007;110:519–528. doi: 10.1182/blood-2006-08-040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YH, et al. Normal shear stress and vascular smooth muscle cells modulate migration of endothelial cells through histone deacetylase 6 activation and tubulin acetylation. Ann Biomed Eng. 2010;38:729–737. doi: 10.1007/s10439-009-9896-6. [DOI] [PubMed] [Google Scholar]
- 24.Holaska JM. Emerin and the nuclear lamina in muscle and cardiac disease. Circ Res. 2008;103:16–23. doi: 10.1161/CIRCRESAHA.108.172197. [DOI] [PubMed] [Google Scholar]
- 25.Meaburn KJ, Misteli T. Cell biology: Chromosome territories. Nature. 2007;445:379–781. doi: 10.1038/445379a. [DOI] [PubMed] [Google Scholar]
- 26.Bank RA, van Hinsbergh VW. Lysyl oxidase: New looks on LOX. Arterioscler Thromb Vasc Biol. 2002;22:1365–1366. doi: 10.1161/01.atv.0000033935.66786.de. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez C, et al. Regulation of lysyl oxidase in vascular cells: Lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vora SR, et al. Lysyl oxidase propeptide inhibits FGF-2-induced signaling and proliferation of osteoblasts. J Biol Chem. 2010;285:7384–7393. doi: 10.1074/jbc.M109.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candiano G, et al. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 31.Kwan HY, Leung PC, Huang Y, Yao X. Depletion of intracellular Ca2+ stores sensitizes the flow-induced Ca2+ influx in rat endothelial cells. Circ Res. 2003;92:286–292. doi: 10.1161/01.res.0000054625.24468.08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.