Abstract
Vascular smooth muscle cell (VSMC) proliferation is an important event in atherosclerosis and other vasculopathies. PDGF signaling is a key mediator of SMC proliferation, but the mechanisms that control its activity remain unclear. We previously identified a mutation in LDL receptor-related protein 6 (LRP6), LRP6R611C, that causes early atherosclerosis. Examination of human atherosclerotic coronary arteries showed markedly increased expression of LRP6 and colocalization with PDGF receptor β (PDGFR-β). Further investigation showed that wild-type LRP6 inhibits but LRP6R611C promotes VSMC proliferation in response to PDGF. We found that wild-type LRP6 forms a complex with PDGFR-β and enhances its lysosomal degradation, functions that are severely impaired in LRP6R611C. Further, we observed that wild-type and mutant LRP6 regulate cell-cycle activity by triggering differential effects on PDGF-dependent pathways. These findings implicate LRP6 as a critical modulator of PDGF-dependent regulation of cell cycle in smooth muscle and indicate that loss of this function contributes to development of early atherosclerosis in humans.
Keywords: human genetics, Wnt, cyclin D1, ubiquitination, atherosclerotic diseases
Vascular smooth muscle cell (VSMC) proliferation is a major pathological component of atherosclerosis and many other vasculopathies. Activation of PDGF signaling is a key driver of VSMC proliferation, and both PDGF and PDGF receptor β (PDGFR-β) show increased expression in atherosclerotic lesions (1). Activation of PDGFR-β in response to endothelial injury induces VSMC proliferation and migration (2, 3). Conversely, inhibition of PDGF signaling reduces neointimal smooth muscle cell accumulation (4–8) and diminishes atherosclerotic burden (9, 10). Recent genetic evidence supports a causal role for increased VSMC proliferation in atherosclerosis (11).
Upon activation, PDGFR-β is phosphorylated, leading to phosphorylation of recombinant activated factor (RAF) (12), ERK1/2 (13), and JAK1/STAT1 (14), which then activate nuclear transcription factors leading to increased expression of cell-cycle regulators including cyclin D1 (15). The detailed mechanisms regulating expression and activation of PDGF and PDGFR-β are incompletely understood.
We recently identified a mutation in LDL receptor-related protein 6 (LRP6), LRP6R611C, that underlies a Mendelian form of early atherosclerosis and metabolic syndrome. Mutation carriers have diffuse coronary artery and cerebrovascular disease and most often die of cardiovascular disease before age 50 y (16); they also show rapid disease progression after percutaneous coronary artery intervention. Although the genetic link between the identified LRP6 mutation and premature atherosclerosis is clear, the mechanisms by which the mutation imparts its effects have not yet been defined.
LRP6 is a member of the LDL receptor family and is known for its function as a membrane coreceptor for canonical Wingless-Int (Wnt) signaling. This protein is expressed abundantly in smooth muscle cells and has been shown to play a role in cell-cycle activity in response to Wnt stimulation (17), raising the possibility that LRP6 normally may play a role in regulation of VSMC proliferation. We report here an investigation of LRP6 and LRP6R611C and their roles in VSMC proliferation.
Results
LRP6 Colocalizes with PDGFR-β and Is Expressed Abundantly in Atherosclerotic Lesions.
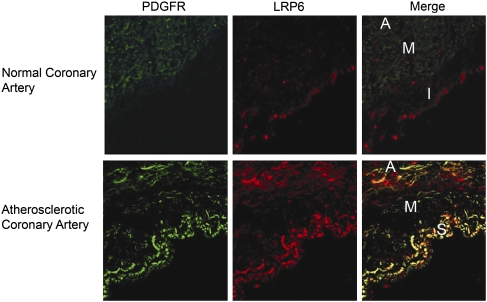
We examined LRP6 expression in human coronary artery specimens from five persons with atherosclerosis and five healthy individuals without atherosclerotic coronary arteries. Immunohistochemical studies showed that in normal coronary arteries LRP6 was expressed primarily at very low levels in the intima. In contrast, LRP6 was much more expressed in the subintima and muscularis layer of all five atherosclerotic arteries than in normal coronary arteries (Fig. 1 and Fig. S1 A and B). Earlier studies of atherosclerotic lesions have shown that smooth muscle cells within these layers exhibit higher expression levels of PDGFR-β than do smooth muscle cells in normal coronary arteries (18, 19). LRP6 and PDGFR-β showed striking colocalization in both subintima and muscularis layers in all atherosclerotic coronary artery specimens. These findings motivated further studies to understand the effect of wild-type LRP6 (LRP6WT) and LRP6R611C on VSMC proliferation.
Fig. 1.
PDGFR-β and LRP6 expression in normal and atherosclerotic coronary arteries. Immunofluorescent staining in a cross-section of a normal coronary artery shows that LRP6 and PDGFR-β are expressed at very low levels in the intima (I) and muscularis (M) layers, respectively (Upper). In the cross-section of the atherosclerotic coronary artery, LRP6 and PDGFR-β are highly expressed and colocalized in the subintima (S) and the muscularis (M) layers (Lower) (n = 5). A, adventicia.
LRP6R611C Attenuates Wnt-Dependent Expression of Cyclin D1 and VSMC Proliferation.
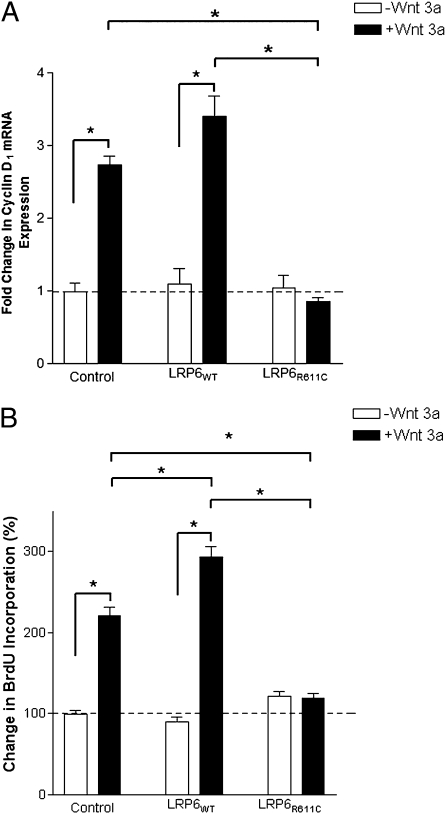
A previous study suggested that LRP6 augments cyclin D1 expression and cell-cycle activity of VSMC in response to Wnt stimulation (17). We examined the impact of the LRP6R611C mutation on these effects. We prepared adenoviral constructs expressing LRP6WT or LRP6R611C from the CMV promoter and showed that these constructs infected nearly 100% of primary human aortic smooth muscle cells (Methods). In response to Wnt3a stimulation, uninfected aortic smooth muscle cell exhibited a more than twofold increase in cyclin D1 expression and cell proliferation (Fig. 2 A and B). Infection with LRP6WT further increased cell proliferation in response to Wnt stimulation. (The effect of LRP6WT overexpression on cyclin D1 expression was not statistically significant.) In contrast, cells expressing LRP6R611C showed no increase in cyclin D1 level and cell proliferation in response to Wnt. This finding is consistent with our previous observation that LRP6R611C impairs Wnt signaling (16) and indicates that the atherosclerosis seen in LRP6R611C mutation carriers cannot be explained by increased Wnt-dependent smooth muscle proliferation.
Fig. 2.
Effect of LRP6WT and LRP6R611C on cyclin D1 expression and cell proliferation. (A) After 12-h stimulation with Wnt3a, mRNA expression levels of cyclin D1 were about threefold lower in cells expressing LRP6R611C than in uninfected cells (P < 0.0001) or cells expressing LRP6WT (P < 0.001). (B) Wnt3a stimulated the proliferation rate in aortic smooth muscle cells overexpressing LRP6WT and LRP6R611C. Percent BrdU incorporation in cells expressing LRP6R611C after stimulation with Wnt3a remained unchanged and was considerably lower than in cells infected with empty vector (Control) (P < 0.001) or cells expressing LRP6WT (P < 0.001).
LRP6WT Inhibits but LRP6R611C Augments Proliferation in Response to PDGF-β.
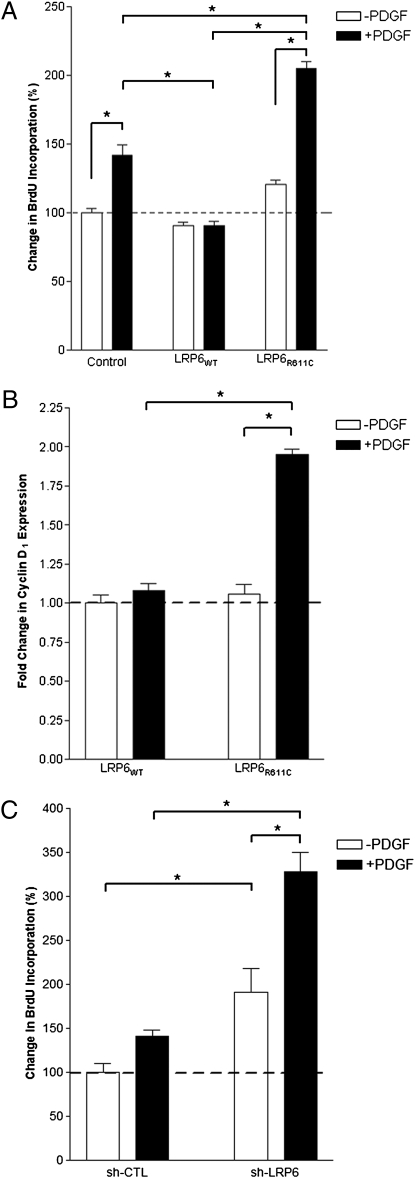
PDGF is a key regulator of VSMC proliferation. Because LRP6 and PDGFR-β colocalize and show increased expression in atherosclerotic lesions versus coronary arteries, we examined the effect of LRP6WT and LRP6R611C on cellular proliferation in response to PDGF-β. Consistent with earlier findings (19), control VSMC (infected with empty adenovirus vector) showed a 45% increase in cellular proliferation after stimulation with PDGF-β. This proliferative response to PDGF-β stimulation was significantly blunted in smooth muscle cells overexpressing LRP6WT (Fig. 3A). In marked contrast, in the presence of LRP6R611C, PDGF increased cellular proliferation by 80%, significantly more than in either LRP6WT or control. This LRP6R611C-induced increase in proliferation is associated with a proportional increase in the expression level of cyclin D1 (Fig. 3B).
Fig. 3.
Increased cell-cycle activity in human aortic smooth muscle cells expressing LRP6R611C or LRP6 shRNA. (A) Percent change in BrdU incorporation is considerably higher in cells expressing LRP6R611C in response to PDGF and is considerably lower in cells expressing LRP6WT cells (P < 0.05) than in cells infected with empty vector (P < 0.001). (B) Fold change in cyclin D1 expression levels. After stimulation with PDGF, cyclin D1 expression levels were significantly increased in cells expressing LRP6R611C (P < 0.01) but remained unchanged in cells expressing LRP6WT. (C) Increased proliferation rate in cells infected with LRP6-specific shRNA (sh-LRP6). RNA interference significantly increased the cell proliferation rate both without (P < 0.05) and with PDGF stimulation compared with scrambled shRNA (sh-CTL) (P < 0.001).
VSMC express endogenous LRP6. If endogenous LRP6 inhibits the response to PDGF, knock down of LRP6 with shRNA-containing lentivirus should result in increased proliferation. This treatment resulted in a significantly higher proliferation rate than seen in cells that express scrambled control shRNA (Fig. 3C). These findings collectively demonstrate that LRP6 negatively regulates cellular proliferation in response to PDGF signaling and that LRP6R611C has dominant-negative effects on this inhibition.
LRP6WT and LRP6R611C Have Opposite Effects on PDGFR-β Expression and Activation.
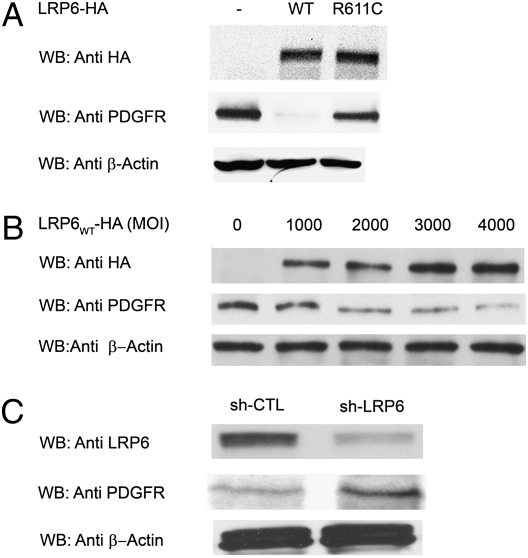
To investigate the mechanism of the different effects of LRP6WT and LRP6R611C, we examined PDGFR-β expression and activation in human aortic smooth muscle cells infected with empty adenovirus vectors and vectors expressing LRP6WT and LRP6R611C. Cells expressing LRP6WT exhibited a dramatic decrease in levels of PDGFR-β protein; in contrast, LRP6R611C induced little if any decrease in PDGFR-β (Fig. 4A). This wild-type effect was dose dependent (Fig. 4B) and was absent in the presence of LRP6R611C (Fig. S2A). In contrast, knocking down LRP6 levels by RNA interference raised PDGFR-β levels (Fig. 4C). The dramatic decrease in PDGFR-β protein level with increased LRP6WT was not accompanied by a change in PDGFR-β mRNA level (Fig. S2B). These results demonstrate that LRP6WT expression causes reduction of PDGFR-β and that LRP6R611C impairs this effect.
Fig. 4.
Expression levels of PDGFR-β in human aortic smooth muscle cells expressing LRP6WT and LRP6R611C. (A) LRP6WT significantly reduces PDGFR-β expression levels (P = 0.001). This effect is diminished significantly in the presence of LRP6R611C. (B) Dose effect of LRP6WT on endogenous PDGFR-β expression. There is an inverse relationship between the multiplicity of infection of viral particles expressing LRP6WT-HA2 and PDGFR-β expression. (C) LRP6 knock down by RNA interference (shLRP6). Knocking down LRP6 by RNA interference raised PDGFR-β levels (P = 0.01).
LRP6 Forms a Complex with PDGFR-β and Promotes Its Degradation.
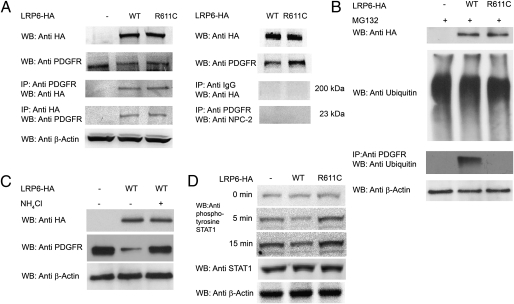
To determine whether LRP6 and PDGFR-β form a complex, we performed coimmunoprecipitation in cells infected with a modest multiplicity of infection of virus expressing LRP6 tagged with HA (1,000 virus particles per cell). Proteins from cell lysates were immunoprecipitated with anti-HA or anti–PDGFR-β antibodies followed by Western blotting with anti–PDGFR-β or anti-HA antibodies, respectively. Immunoprecipitation in both directions showed that LRP6WT and LRP6R611C are in a complex with PDGFR-β (Fig. 5A). The specificity of this interaction was demonstrated by the lack of coimmunoprecipitation of PDGFR-β with IgG or Niemann–Pick disease, type C2 (NPC2).
Fig. 5.
LRP6WT and LRP6R611C form complexes with PDGFR-β. (A) Proteins from cell lysates were immunoprecipitated with either anti-HA or anti–PDGFR-β antibodies followed by Western blotting with either anti–PDGFR-β or anti-HA antibodies, respectively. LRP6WT and LRP6R611C but not IgG or NPC2 (used as control) coimmunoprecipitated with PDGFR-β. (B) LRP6WT ubiquitinates PDGFR-β. Ubiquitination of PDGFR-β following MG132 treatment in cells overexpressing LRP6R611C or LRP6WT and in cells infected with empty vector was compared. LRP6WT but not LRP6R611C ubiquitinates PDGFR-β. (C) Lysosomal degradation of PDGFR-β. The lysosomal inhibitor NH4Cl rescues reduced expression of PDGFR-β (P = 0.001). (D) LRP6WT reduces and LRP6R611C increases STAT1 tyrosine phosphorylation. After PDGF stimulation, tyrosine phosphorylation of STAT1 is significantly higher in cells expressing LRP6R611C (P < 0.001) and is significantly lower in cells expressing LRP6WT (P < 0.001) than in cells transfected with empty plasmid.
PDGFR-β levels are regulated by ubiquitination (20) and proteosomal or lysosomal degradation (21). Accessory proteins, such as LRP1, have been shown to target PDGFR-β for degradation (22, 23), suggesting that LRP6 might play a similar role. Cells expressing LRP6WT exhibit significantly higher PDGFR-β ubiquitination following incubation with the proteasome inhibitor MG132 than cells expressing LRP6R611C or infected with empty vector (Fig. 5B).
Reduced levels of PDGFR-β in the presence of LRP6WT were rescued only partially by the proteosomal inhibitor MG132 (Fig. S3A). In contrast, when cells were treated with the lysosomal inhibitor NH4Cl, we observed nearly complete rescue of PDGFR-β levels (Fig. 5C). This result suggests that lysosomal degradation plays a greater role in degradation of PDGFR-β in these cells. Immunostaining revealed that expression of LRP6WT but not LRP6R611C led to redistribution of a substantial fraction of PDGFR-β to an intracellular compartment that costains with early endosomal antigen 1 (EEA1) and the lysosomal marker lysosomal-associated membrane protein 2 (LAMP2), consistent with a substantial fraction of this intracellular PDGFR-β being in the lysosomal pathway (Fig. S3 B–D). Together these findings indicate that LRP6 is in a complex with PDGFR-β and promotes its degradation.
LRP6WT and LRP6R611C Have Differential Effects on PDGF Signaling Peptides.
PDGF exerts its proliferative effects on VSMC largely through activation of ERK1/2 (24) and STAT1 (25). Investigation of the PDGF signaling components revealed that the antiproliferative effect of LRP6 is accounted for by reduced activation of ERK1/2 (p42, and p44) and the JAK–STAT pathway (Fig. S4 A–C). LRP6WT reduces phosphorylation of ERK1/2, JAK1, and STAT1 after stimulation with PDGF-β. In contrast, LRP6R611C significantly increases JAK1 and STAT1 serine and tyrosine phosphorylation (Fig. 5D).
Discussion
LRP6 is well known as a coreceptor with frizzled proteins in the Wnt signaling pathway; however, its Wnt-independent roles have been largely unexplored. LRP6 is abundantly expressed in human VSMC and, as we show here, attenuates PDGF signaling by promoting ubiquitination and degradation of PDGFR-β, thereby reducing expression of cyclin D1 and VSMC proliferation. Knock down of endogenous LRP6 by RNA interference shows the opposite effect, indicating that the effect of LRP6 on PDGFR ubiquitination is specific. PDGF is a key regulator of VSMC proliferation (1), which plays a critical role in the pathogenesis of atherosclerosis in both human and animal models (11). The increased expression of both PDGFR-β and LRP6 and their colocalization in atherosclerotic lesions is consequently of considerable interest, suggesting that LRP6 plays an inhibitory and potentially beneficial role in these lesions. Impairment of this function probably contributes to accelerated atherosclerosis in carriers of the LRP6R611C mutation.
These effects of LRP6 are similar to those of LRP1 (26). Although LRP1’s effect on PDGF signaling is believed to be regulated by ligand interactions, the mechanisms by which LRP6’s effects are regulated, as well as the factors inducing increased LRP6 expression in atherosclerotic lesions, are unknown and will be of interest in further investigations.
Of particular interest is our demonstration that the LRP6R611C mutation linked to a Mendelian form of premature coronary artery disease has dominant-negative effects on the inhibition of PDGFR-β. Thus, there is greater proliferation in the presence of this mutation than in normal cells. In conjunction with the increased LRP6 expression in atherosclerotic lesions, this observation can account for the observed dominant transmission of the trait and a contribution of this mutation to atherosclerosis via increased VSMC proliferation.
These findings do not directly explain the metabolic consequences in patients harboring this mutation; however, the observed promiscuity of LRP6 interactions raises the possibility of effects not only on the Wnt signaling pathway but with other signaling pathways involved in metabolic functions. We anticipate that further studies of the effects of wild-type and mutant LRP6 on metabolism will be illuminating.
Materials and Methods
Immunohistochemical Studies of Normal and Sporadic Atherosclerotic Coronary Arteries.
Sectioning and staining of the specimens with antibodies against LRP6 (Abgent) and PDGFR-β (Santa Cruz) were carried out by the Surgical Pathology Service of the Yale University Department of Pathology using 1:100 diluted LRP6 antibody, 1:50 diluted PDGFR-β antibody, and 1:500 diluted Alexa Fluor 488 and Alexa Fluor 568 fluorescence-conjugated secondary antibodies.
Viral Infection of Aortic Smooth Muscle Cells with Adenovirus.
Human aortic smooth muscle cells were cultured in M199 medium containing 20% bovine serum to 90% confluence and were infected with replication-defective cytomegalovirus promoter LRP6-HA2, LRP6R611C-HA2, or empty adenovirus vectors created by Viraquest. Efficiency of gene transfer was assessed by concomitant infection with Adv-GFP and by epifluorescence microscopy.
Cyclin D1 Expression Levels.
Cells were infected with HA-LRP6WT, HA-LRP6R611C, or empty adenovirus as described. Seventy-two hours after infection cells were subjected to starvation (DMEM + 0.2% FBS) for 24 h. Wnt3a (25 ng/mL) or PDGF-BB (30 ng/mL) was added for 12 h. Then RNA was extracted using TRIzol (Invitrogen), and cDNA was produced using SuperScript III reverse transcription kits (Invitrogen). Expression levels were reexamined by real-time PCR experiments as described (27).
Analysis of the Expression and Activation of PDGFR-β and Its Downstream Peptides in Human Aortic Smooth Muscle Cells.
PDGF-BB (Millipore) was added to the medium at a final concentration of 30 ng/mL At 5, 15, and 30 min following stimulation, cell extracts were prepared with RIPA buffer containing protease inhibitor (Roche) and Halt Phosphatase Inhibitor Mixtures (Thermo Scientific) and were subjected to immunoblotting with antibodies against PDGFR-β (Cell Signaling), HA(Cell Signaling), and total and phospho-ERK1/2, JAK1, p-JAK1, STAT1, phosphoserine STAT1, and phosphotyrosine STAT1 (Cell Signaling).
Cells were infected with incremental doses of viral particles containing LRP6 (0, 1,000, 2,000, 3,000, and 4,000 virus particles per cell) to investigate the dose effect of LRP6 on PDGFR-β expression. Lysates were prepared and subjected to immunoblotting with PDGFR-β antibody as described.
LRP6 shRNA (SMARTvector 2.0 Lentiviral shRNA technology) were purchased from Dharmacon and were reverse-infected according to the manufacturer's instructions.
Investigation of PDGFR-β Degradation by LRP6.
Cells were infected with HA-LRP6WT, HA-LRP6R611C, or empty adenovirus as described. Cells were lysed in a nondenaturing cell lysis buffer (Cell Signaling) and were subjected to immunoprecipitation with PDGFR or HA antibodies. Precipitated proteins were subjected to immunoblot analysis with anti-HA, anti-NPC2 (Santa Cruz Biotechnology), and immobilized mouse IgG (Cell Signaling) antibodies.
For PDGFR-β mRNA expression levels, RNA was extracted from infected cells, and real-time PCR experiments were carried out as described.
For proteosomal degradation studies, proteosomal inhibitor MG132 (Sigma) was added to the cell medium 72 h after infection to a final concentration of 2 μM for 2 h. Cell extracts were subjected to immunoprecipitation with PDGFR antibody, and precipitated proteins were subjected to immunoblot analysis with anti-ubiquitin (Cell Signaling). For lysosomal degradation studies, NH4Cl was added to the cell medium to a final concentration of 20 mM for 12 h.
For immunofluorescent studies, cells were placed in 24-well plates containing coverslips and were infected as described. Seventy-two hours later, cells were fixed by 4% paraformaldehyde and were permeabilized in 0.1% Triton X-100 and blocked with 3% BSA in PBS after appropriate washes and incubated with 1:100 diluted PDGFR-β, HA, EEA1 (Abcam), and LAMP2 (Abcam) overnight at 4 °C. Cells subsequently were incubated with 1:500 diluted Alexa Fluor 488 and Alexa Fluor 568 fluorescence-conjugated secondary antibodies (Invitrogen) at room temperature and were mounted with Vectashield (Vector Laboratories). Specimens were examined by a Zeiss LSM510 confocal microscope using excitation and emission filters at 488 nm and 522 nm, respectively.
Examination of Cellular Proliferation.
Cell proliferation rates were assessed by BrdU incorporation. Seventy-two hours after infection, cells were subjected to starvation (DMEM + 0.2% FBS) for 24 h. After 1 h treatment with PDGF-BB (30 ng/mL) or Wnt3a (25 ng/mL), cells were pulsed with BrdU (10 μmol/L) for 12 h and were fixed and labeled using the BrdU Labeling and Detection Kit III (Roche Applied Science).
Statistical Analysis.
All experimental data represent results from four independent experiments. Data are expressed as means ± SE. Comparisons between two groups were performed using the unpaired t test (two-tailed). For multiple comparisons, Tukey's test in conjunction with ANOVA was carried out using Graphpad Prism. Protein expression levels were quantified by densitometry of Western blots. Statistical significance was defined as P < 0.05.
Supplementary Material
Acknowledgments
We thank Fred S. Gorelick and Dr. Michael Simons for valuable discussions on the scientific approach. This work was supported, in part, by National Institutes of Health Grants R01HL094784-01 and R01HL094574-03 (to A.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019443108/-/DCSupplemental.
References
- 1.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts) N Engl J Med. 1976;295:369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 4.Ferns GA, et al. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 5.Lewis CD, Olson NE, Raines EW, Reidy MA, Jackson CL. Modulation of smooth muscle proliferation in rat carotid artery by platelet-derived mediators and fibroblast growth factor-2. Platelets. 2001;12:352–358. doi: 10.1080/09537100120071013. [DOI] [PubMed] [Google Scholar]
- 6.Leppänen O, et al. Intimal hyperplasia recurs after removal of PDGF-AB and -BB inhibition in the rat carotid artery injury model. Arterioscler Thromb Vasc Biol. 2000;20:E89–E95. doi: 10.1161/01.atv.20.11.e89. [DOI] [PubMed] [Google Scholar]
- 7.Banai S, et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97:1960–1969. doi: 10.1161/01.cir.97.19.1960. [DOI] [PubMed] [Google Scholar]
- 8.Giese NA, et al. The role of alpha and beta platelet-derived growth factor receptor in the vascular response to injury in nonhuman primates. Arterioscler Thromb Vasc Biol. 1999;19:900–909. doi: 10.1161/01.atv.19.4.900. [DOI] [PubMed] [Google Scholar]
- 9.Sano H, et al. Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation. 2001;103:2955–2960. doi: 10.1161/01.cir.103.24.2955. [DOI] [PubMed] [Google Scholar]
- 10.Kozaki K, et al. Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice. Am J Pathol. 2002;161:1395–1407. doi: 10.1016/S0002-9440(10)64415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visel A, et al. Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature. 2010;464:409–412. doi: 10.1038/nature08801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy JB, Perrimon N. The torso pathway in Drosophila: Lessons on receptor tyrosine kinase signaling and pattern formation. Dev Biol. 1994;166:380–395. doi: 10.1006/dbio.1994.1324. [DOI] [PubMed] [Google Scholar]
- 13.Puglianiello A, et al. Expression and role of PDGF-BB and PDGFR-beta during testis morphogenesis in the mouse embryo. J Cell Sci. 2004;117:1151–1160. doi: 10.1242/jcs.00981. [DOI] [PubMed] [Google Scholar]
- 14.Wen Z, Zhong Z, Darnell JE., Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 15.Xiong W, et al. Cyclin D1 is required for S phase traversal in bovine tracheal myocytes. Am J Physiol. 1997;272:L1205–L1210. doi: 10.1152/ajplung.1997.272.6.L1205. [DOI] [PubMed] [Google Scholar]
- 16.Mani A, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Adhikari N, Li Q, Hall JL. LDL receptor-related protein LRP6 regulates proliferation and survival through the Wnt cascade in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;287:H2376–H2383. doi: 10.1152/ajpheart.01173.2003. [DOI] [PubMed] [Google Scholar]
- 18.Katsuda S, Boyd HC, Fligner C, Ross R, Gown AM. Human atherosclerosis. III. Immunocytochemical analysis of the cell composition of lesions of young adults. Am J Pathol. 1992;140:907–914. [PMC free article] [PubMed] [Google Scholar]
- 19.Katsuda S, Coltrera MD, Ross R, Gown AM. Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol. 1993;142:1787–1793. [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake S, Lupher ML, Jr., Druker B, Band H. The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc Natl Acad Sci USA. 1998;95:7927–7932. doi: 10.1073/pnas.95.14.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddi AL, et al. Binding of Cbl to a phospholipase Cgamma1-docking site on platelet-derived growth factor receptor beta provides a dual mechanism of negative regulation. J Biol Chem. 2007;282:29336–29347. doi: 10.1074/jbc.M701797200. [DOI] [PubMed] [Google Scholar]
- 22.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 23.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 24.Zhan Y, et al. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- 25.Simon AR, Takahashi S, Severgnini M, Fanburg BL, Cochran BH. Role of the JAK-STAT pathway in PDGF-stimulated proliferation of human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1296–L1304. doi: 10.1152/ajplung.00315.2001. [DOI] [PubMed] [Google Scholar]
- 26.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, et al. Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ Res. 2008;103:1280–1288. doi: 10.1161/CIRCRESAHA.108.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.