Abstract
Ghrelin is a hunger hormone with gastroprokinetic properties but the factors controlling ghrelin secretion from the stomach are unknown. Bitter taste receptors (T2R) and the gustatory G proteins, α-gustducin (gust) and α-transducin, are expressed in the gut and are involved in the chemosensation of nutrients. This study aimed to investigate whether T2R-agonists affect (i) ghrelin release via α-gustducin and (ii) food intake and gastric emptying via the release of ghrelin. The mouse stomach contains two ghrelin cell populations: cells containing octanoyl and desoctanoyl ghrelin, which were colocalized with α-gustducin and α-transducin, and cells staining for desoctanoyl ghrelin. Gavage of T2R-agonists increased plasma octanoyl ghrelin levels in WT mice but the effect was partially blunted in gust−/− mice. Intragastric administration of T2R-agonists increased food intake during the first 30 min in WT but not in gust−/− and ghrelin receptor knockout mice. This increase was accompanied by an increase in the mRNA expression of agouti-related peptide in the hypothalamus of WT but not of gust−/− mice. The temporary increase in food intake was followed by a prolonged decrease (next 4 h), which correlated with an inhibition of gastric emptying. The delay in emptying, which was partially counteracted by ghrelin, was not mediated by cholecystokinin and GLP-1 but involved a direct inhibitory effect of T2R-agonists on gastric contractility. This study is unique in providing functional evidence that activation of bitter taste receptors stimulates ghrelin secretion. Modulation of endogenous ghrelin levels by tastants may provide novel therapeutic applications for the treatment of weight -and gastrointestinal motility disorders.
Keywords: nutrient sensing, gastrointestinal peptides, appetite
Ghrelin is a 28-amino acid peptide with an octanoyl modification at Ser3, which is mainly produced by the “X/A-like” cells of the oxyntic glands of the stomach (1) in response to conditions of negative energy balance. The octanoylated form of ghrelin is the biological active form, although it represents less than 20% of the circulating ghrelin. Ghrelin signals through the ghrelin receptor, previously known as the growth hormone secretagogue receptor (GHS-R), to stimulate food intake and to decrease fat utilization to stimulate body weight gain (2). Malik et al. (3) showed that even in the absence of caloric deficiency, ghrelin may favor food consumption by enhancing the hedonic and incentive responses to food-related cues. Ghrelin also has important effects on gastrointestinal (GI) motility, which may contribute to appetite signaling. It induces strong “hunger” contractions in the fasted state, originating in the stomach and migrating distally, and accelerates gastric emptying in man and rodents (4–7). Plasma ghrelin levels peak before a meal and decrease shortly after food consumption in humans to dictate the timing of the meals (8). The magnitude of the decrease in plasma ghrelin levels is dependent on the caloric content (9) and the macronutrient composition of the meal (10), but the factors involved in chemosensation of the ghrelin cell are unknown.
The recent identification of taste receptors and their downstream signaling molecules in the GI mucosa suggests a role for these receptors in the functional detection of nutrients in the gut, which may in turn initiate a hormonal or neural cascade pathway (11). Taste receptors are also present in the tongue epithelium and consist of two G protein-coupled receptor families, T1R and T2R. Subtypes of the T1R family heterodimerize to detect sweet (T1R2+T1R3) and l-amino acid tastants (T1R1+T1R3), whereas the T2R receptor family can detect bitter and consists of more than 30 diverse members. Gustducin is the α subunit of a trimeric G-protein complex that is involved in sweet, bitter, and umami taste transduction (12, 13). Not all bitter-sensitive taste cells contain α-gustducin, indicating the existence of other G-protein α-subunits in bitter taste transduction, such as α-transducin and Gi (14).
Gustducin-coupled sweet taste receptors have been demonstrated in endocrine cells in the proximal intestine of mice and humans (15, 16). In mice, α-gustducin was frequently found in L-type cells (GLP-1) but rarely in K cells (GIP) cells. These cells fail to release GLP-1 in response to glucose within the gut lumen in α-gustducin knockout mice (15). The presence of multiple transcripts corresponding to specific bitter taste receptors has been demonstrated in the upper GI tract of rodents and in a mouse GI enteroendocrine cell line, STC-1 (17). It has been reported that the bitter agonists, phenylthiocarbamide (PTC) and denatonium benzoate (DB), induce the release of cholecystokinin (CCK) and GLP-1 from STC-1 cells (11, 18).
This study aimed to investigate whether the ghrelin cell is colocalized with the G proteins of the gustatory complex, α-gustducin and α-transducin, and whether bitter taste receptors coupled to α-gustducin could function as chemosensors for the ghrelin cell. We therefore investigated the effect of bitter taste-receptor agonists on the release of ghrelin in WT and α-gustducin knockout (gust−/−) mice and determined the functional consequences of the altered ghrelin secretion with respect to food intake and gastric emptying.
Results
Colocalization of Ghrelin with G Proteins of the Gustatory Complex.
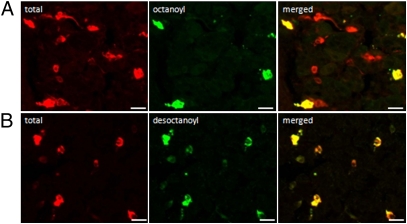
Immunofluorescence studies provided evidence for the existence of two ghrelin cell populations (Fig. 1). The total ghrelin cell population was identified with an antibody, which did not discriminate between octanoyl and desoctanoyl ghrelin (Fig. 1A). Of this total ghrelin cell population, 66 ± 3% of the cells costained for octanoyl ghrelin (Fig. 1A) and 100% were colocalized with desoctanoyl ghrelin (Fig. 1B). These findings suggest that the octanoyl ghrelin cell population was also colocalized with desoctanoyl ghrelin and that there was a second ghrelin cell population that contained only desoctanoyl ghrelin.
Fig. 1.
Identification of ghrelin cell populations. Colocalization between total (octanoyl+desoctanoyl) ghrelin (red) and (A) octanoyl ghrelin (green) or (B) desoctanoyl ghrelin (green) in sections of the mouse stomach. (Scale bars, 20 μm.)
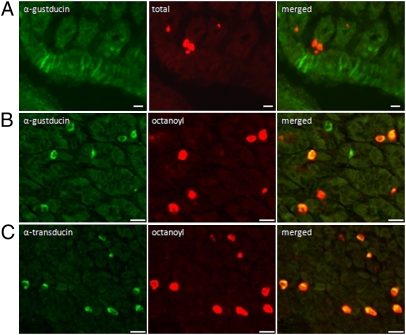
α-Gustducin–positive cells were visualized in the brush cells (Fig. 2A), in the region of the limiting ridge, the boundary between fundus and corpus, and in some endocrine cells (Fig. 2B). In contrast, α-transducin–positive cells were only found in endocrine cells (Fig. 2C). No colocalization was observed between ghrelin and α-gustducin found in the brush cells, although several ghrelin-positive cells were found in close proximity to the α-gustducin–positive cells (Fig. 2A). In contrast, ghrelin was colocalized with G proteins of the gustatory complex present in endocrine cells. Almost 61 ± 12% of the α-gustducin–positive and 98 ± 2% of the α-transducin–positive endocrine cells colocalized with ghrelin. The ghrelin cell population that contained octanoyl and desoctanoyl ghrelin colocalized for 88 ± 2% with α-gustducin (Fig. 2B) and for 85 ± 2% with α-transducin (Fig. 2C). For the cell population that contained only desoctanoyl ghrelin, it was difficult to discern whether these cells colocalized with any of the gustatory G proteins because staining for desoctanoyl ghrelin also stained the cells for octanoyl ghrelin.
Fig. 2.
(A) Double-immunofluorescence study showing α-gustducin (green) staining in brush cells, which does not colocalize with ghrelin staining (red) in endocrine cells. (B) α-Gustducin (green) positive endocrine cells colocalize with octanoyl ghrelin (red) cells. (C) Colocalization of α-transducin (green) and octanoyl ghrelin (red) in endocrine cells. (Scale bars, 20 μm.)
Effect of Bitter Taste-Receptor Agonists on Plasma Ghrelin Secretion.
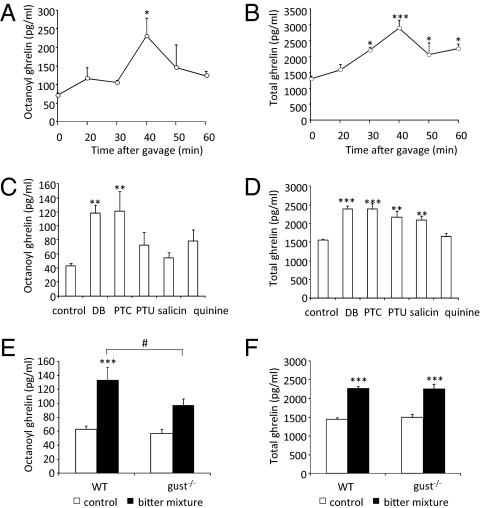
Gavage of a mixture of bitter agonists in WT mice increased plasma concentrations of octanoyl (P < 0.05) and total (P < 0.001) ghrelin in a time-dependent manner (Fig. 3 A and B). Both octanoyl ghrelin and total ghrelin levels peaked 40 min after gavage (octanoyl ghrelin: t0: 72 ± 7 pg/mL vs. t40: 231 ± 49 pg/mL; total ghrelin: t0: 1,295 ± 105 pg/mL vs. t40: 2,892 ± 268 pg/mL). The octanoyl ghrelin peptide content was decreased (P < 0.05) in mouse stomach from 155 ± 19 to 97 ± 5 μg/mg protein (40 min). A similar decrease was observed in total ghrelin content (1,420 ± 127 to 1,133 ± 52 μg/mg protein, P < 0.05). Relative ghrelin mRNA expression was not affected (control: 1.78 ± 0.12; T2R: 1.43 ± 0.34). Of the individual T2R, agonists only DB and PTC increased plasma octanoyl ghrelin levels significantly (Fig. 3C). In contrast, all T2R-agonists, except quinine, significantly increased plasma total ghrelin levels (Fig. 3D). The percent-increase in ghrelin secretion augmented with increasing concentrations of PTC (0.2 mM: 18%; 1 mM: 37%; 10 mM: 74%).
Fig. 3.
(A and B) Time-dependent increase in plasma octanoyl ghrelin and total ghrelin levels in response to oral gavage of T2R-agonists in WT mice (n = 4–6). (C and D) Comparison of the effect of gavage of individual T2R agonists on octanoyl ghrelin and total ghrelin secretion (n = 6–8). (E and F) Octanoyl and total plasma ghrelin levels after gavage of T2R-agonists (40 min) in WT (n = 18–21) and gust−/− (n = 18–19) mice. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05 WT vs. gust−/−.
The role of α-gustducin in the effect of T2R-agonists on ghrelin secretion was determined in gust−/− mice (Fig. 3 E and F). Plasma octanoyl and total ghrelin levels did not differ between both genotypes after gavage of water. The rise in plasma octanoyl ghrelin levels in response to bitter gavage was significantly (P < 0.05) lower in gust−/− mice (97 ± 9 pg/mL) compared with WT mice (133 ± 6 pg/mL). In contrast, the increase in plasma total ghrelin levels remained similar in both genotypes (WT: 2,273 ± 99 pg/mL; gust−/−: 2,261 ± 126 pg/mL).
Effect of Bitter Taste Receptor Agonists on Short-Term Food Intake and Hypothalamic Neuropeptide Expression.
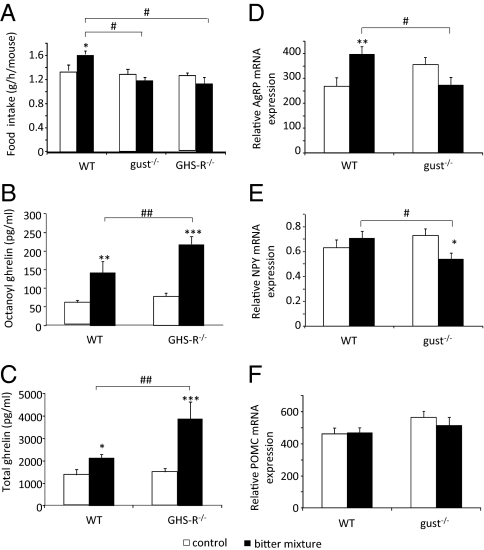
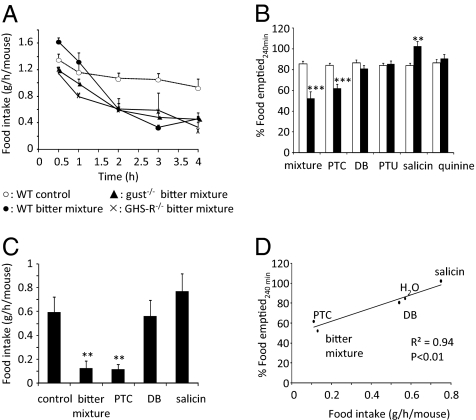
Because bitter agonists stimulate the secretion of hunger hormone ghrelin, we investigated whether this resulted in an increase in food intake. Gavage of T2R-agonists increased food intake in WT mice from 1.35 ± 0.09 to 1.62 ± 0.06 g/h per mouse but not in gust−/− mice and GHS-R−/− mice (Fig. 4A); this was not the result of a lack of effect of T2R-agonists on the release of ghrelin in GHS-R−/− mice because the increase was even significantly (P < 0.01) higher (octanoyl ghrelin: 62%; total ghrelin: 83%) than in WT mice (Fig. 4 B and C).
Fig. 4.
(A) Effect of T2R-agonists on food intake in WT (n = 28–32), gust−/− (n = 26–32), and GHS-R−/− (n = 12–14) mice during the first 30 min after gavage. (B and C) Effect of gavage of T2R-agonists on plasma octanoyl and total ghrelin levels in WT (n = 6–7) and GHS-R−/− mice (n = 5–7). (D–F) Changes in the mRNA expression of AgRP (D), NPY (E), and POMC (F) in the hypothalamus after intragastric gavage of water or T2R-agonists in WT (n = 17–18) and α-gust−/− mice (n = 16–20). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05 WT vs. gust−/− or GHS-R−/−, ##P < 0.01 WT vs. GHS-R−/−.
The increase in food intake in WT mice was accompanied by an increase (P < 0.01) in the mRNA expression of agouti-related peptide (AgRP) (49%) but not of neuropeptide Y (NPY) in the hypothalamus (Fig. 4 D and E). In contrast, in gust−/− mice the mRNA expression of NPY was decreased (P < 0.05) but that of AgRP tended to be decreased (−22%, P = 0.09). The effect of T2R-agonists on the expression of NPY and AgRP was therefore significantly (P < 0.05) lower in gust−/− mice than in WT mice. The mRNA expression of proopiomelanocortin (POMC) was not affected in both genotypes by bitter (Fig. 4F).
Effect of Bitter Taste-Receptor Agonists on Long-Term Food Intake.
The initial rise in food intake during the first 30 min after gavage of T2R-agonists was followed by a prolonged decrease in food intake during the next 4 h (Fig. 5A). In WT mice, food intake was 49% lower in mice that were gavaged with T2R-agonists compared with water. A similar decrease in food intake was observed in gust−/− and GHS-R−/− mice, implying that this effect is mediated via mechanisms independent from α-gustducin and ghrelin signaling. We therefore investigated the effect of T2R-agonists on gastric emptying, which is also known to be involved in the regulation of appetite.
Fig. 5.
(A) Time-dependent changes in food intake (g/h per mouse) in WT (n = 28–32), gust−/− (n = 24–32) and GHS-R−/− mice (n = 12–14) after gavage of water or T2R-agonists. (B) Effect of intragastric gavage of water (open bars), the bitter mixture or the individual T2R-agonists (filled bars) on gastric emptying in WT mice (n = 6–8). (C) Effect of water (control), the bitter mixture and the individual T2R-agonists on 4-h food intake in WT mice (n = 16). (D) Correlation between gastric emptying and 4-h food intake (R2 = 0.94, P < 0.01). **P < 0.01, ***P < 0.001 vs. control.
Effect of Bitter Taste-Receptor Agonists on Gastric Emptying.
Intragastric administration of T2R-agonists induced a marked delay in gastric emptying. After 120 min, 52 ± 3% of the solid meal was emptied in water-treated mice compared with 23 ± 4% (P < 0.001) in mice treated with T2R-agonists. Emptying was almost complete 240 min after oral administration of water (85 ± 3%) but not after treatment with T2R-agonists (52 ± 7%; P < 0.001). In addition the efficacy of each individual bitter component to affect gastric emptying was determined and compared with the effect of the bitter mixture (Fig. 5B). Oral administration of PTC mimicked the delay in emptying observed with the mixture. Conversely, in mice gavaged with salicin gastric emptying was significantly (P < 0.01) accelerated (22 ± 4%) compared with water-treated mice. Gastric gavage of DB, propylthiouracil (PTU), and quinine did not alter gastric emptying.
To determine a possible causal relationship between the decrease in gastric emptying and the decrease in food intake, the effect of some of the individual T2R-agonists on 4-h food intake was followed (Fig. 5C). Gavage of PTC induced a marked decrease in 4-h food intake but salicin tended to increase food intake. Intragastric administration of DB failed to affect 4-h food intake, which resulted in a significant (P < 0.01) correlation between the effect of the T2R-agonists on gastric emptying and the decrease in food intake after 4 h (Fig. 5D).
Role of Ghrelin, CCK, and GLP-1 in the Effect of Bitter Taste-Receptor Agonists on Gastric Emptying in WT Mice.
Because intragastric gavage of T2R-agonists stimulated the release of ghrelin partially through α-gustducin, we investigated whether the gastroprokinetic effects of ghrelin could compensate for the T2R-agonists induced delay in gastric emptying. We therefore tested the effect of oral administration of T2R-agonists on gastric emptying in both gust−/− and GHS-R−/− mice (Fig. 6A). The amount of food emptied from the stomach 240 min after gavage was significantly lower in gust−/− (33 ± 2%) and GHS-R−/− (36 ± 3%) mice compared with WT (49 ± 6%) mice.
Fig. 6.
(A) The effect of oral administration of T2R-agonists on gastric emptying in WT (n = 10), gust−/− (n = 12) and GHS-R−/− (n = 8) mice. (B and C) Effect of the CCK antagonist (devazepide) and the GLP-1 antagonist [exendin(9-39)], administered i.p. 15 min before gavage of T2R-agonists, on gastric emptying in WT mice. ***P < 0.001 vs. control; #P < 0.05 WT vs. gust−/− or GHS-R−/−.
The role of CCK and GLP-1, two anorexigenic hormones known to delay gastric emptying, in the effect of T2R-agonists on gastric emptying was investigated by pretreatment of the mice with the antagonists, devazepide or exendin(9-39), respectively (Fig. 6 B and C). None of the antagonists could reverse the delay in gastric emptying induced by the T2R-agonists.
Effect of Bitter Taste-Receptor Agonist on in Vitro Contractility of Antral and Duodenal Smooth-Muscle Strips.
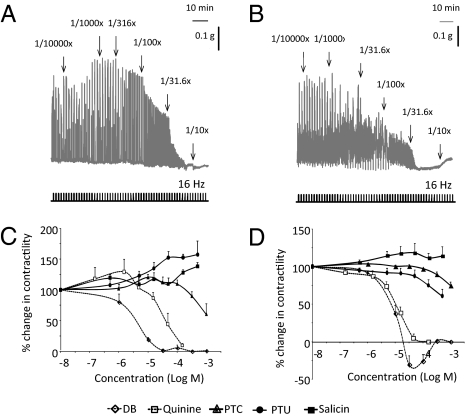
A possible direct effect of the bitter receptor agonists on antral and duodenal contractility was investigated. The representative tracings in Fig. 7 A and B show that the bitter agonist mixture inhibited the electrical field stimulation-induced neural responses in both antral and duodenal smooth-muscle strips in a concentration-dependent manner. The order of potency to inhibit neural responses in antral smooth-muscle strips was: DB (pEC50: 5.52 ± 0.19) > quinine (pEC50: 4.40 ± 0.18) > PTC (pEC50: 2.84 ± 0.40) (Fig. 7C). Salicin and PTU were without effect. In duodenal smooth-muscle strips the potency of DB (pEC50: 4.98 ± 0.05) and PTC (pEC50: 2.52 ± 0.12) was decreased but that of quinine (pEC50: 5.02 ± 0.06) and PTU (pEC50: 2.75 ± 0.36) was increased (Fig. 7D).
Fig. 7.
(A and B) Representative tracings of the effect of different dilutions of T2R-agonists on neural responses in antral (A) and duodenal (B) smooth-muscle strips of WT mice in vitro. (C and D) Cumulative concentration-response curves of T2R-agonists in antral (C) or duodenal (D) smooth muscle strips (n = 3–4).
Discussion
The discovery that taste receptors and their downstream effectors exist in the gut raised the intriguing possibility that the gut is able to “taste” luminal contents (19). Sensing of the luminal content is important to initiate the appropriate response of digestion and absorption of nutrients or neutralization and expulsion of drugs, toxins, and microorganisms but also for food control, which is regulated via the gut-brain axis.
Our data are unique in providing evidence that bitter taste-receptor agonists stimulate the secretion of the hunger hormone ghrelin via the gustatory G protein, α-gustducin. As previously described (20), α-gustducin staining was detected in the brush cells which are arranged in a continuous band at the “limiting ridge,” which represents the boundary between the fundus and the corpus. The brush cells may represent an important chemosensory monitoring system because they have immediate contact to the lumen, at the place where food passes from the reservoir compartment (fundus) to the part where digestive processes are initiated (corpus). The results of the present study show that ghrelin cells were visualized in close proximity to α-gustducin–containing brush cells but were not colocalized. In contrast, we observed coexpression of α-gustducin and ghrelin in the endocrine cells. Besides α-gustducin, the perception of bitter and sweet is also based on the activation of another taste receptor-linked G protein, α-transducin (14). Our colocalization studies revealed that 89% of the α-transducin expressing endocrine cells stained for ghrelin. By using specific antibodies directed against octanoyl and desoctanoyl ghrelin, we could distinguish two ghrelin cell populations. One population consisted of cells immunoreactive for octanoyl ghrelin and desoctanoyl ghrelin, representing 66 ± 3% of the total ghrelin population. This population was colocalized with α-gustducin and α-transducin. The other cell population was only immunoreactive for desoctanoyl ghrelin but, for technical reasons, it was difficult to discern whether these cells contained the gustatory G proteins.
Intragastric administration of T2R agonists increased plasma octanoyl and total ghrelin levels with a maximal effect after 40 min. The increase in plasma octanoyl ghrelin levels was mediated via α-gustducin because the effect was partially blunted in α-gust−/− mice. This in agreement with our immunofluorescence studies, which confirmed that cells containing octanoyl ghrelin colocalized for 91 ± 5% with α-gustducin. In contrast, the increase in total plasma ghrelin levels did not differ between both genotypes. It is therefore likely that the release of desoctanoyl ghrelin is mediated via T2R receptors coupled to α-transducin, which is also colocalized with cells containing desoctanoyl ghrelin. Whether these transduction pathways also involve effects on the enzyme responsible for the octanoylation of ghrelin [ghrelin-O-acyltransferase (GOAT)], in which activation of α-gustducin and α-transducin may selectively stimulate or inhibit GOAT, is unclear.
The release of octanoyl ghrelin may also come from the ghrelin cells found in close proximity of the α-gustducin–containing brush cells. These cells may function as input cells and may detect chemical stimuli present in the stomach lumen and convey the signal to the associated closed ghrelin cells, which may function as output cells (20). A similar system has been described in the taste buds (21).
More than 36 bitter taste-receptor genes and 7 pseudogenes have been reported in mouse (22). For example, DB is the known ligand for T2R108, which has been detected in mouse stomach and duodenum (22), but another potential T2R that recognizes DB includes T2R120 (23) and T2R8 (24). PTC activates T2R138, which is also expressed in mouse intestine (22). Salicin exclusively activates T2R16 (25) and PTU activates T2R1, T2R4, and T2R38 in humans (26). A specific taste receptor for quinine has yet to be identified (27). The heterogeneity in bitter taste receptors together with the existence of two ghrelin cell populations, of which at least one is likely to express two gustatory G proteins, suggest that complex mechanisms are involved in the effect of bitter agonists on ghrelin secretion. PTC and DB induced the secretion of both octanoyl and desoctanoyl ghrelin, whereas PTU and salicin selectively stimulated the release of desoctanoyl ghrelin. It is therefore likely that DB and PTC may couple to both G proteins but that PTU, DB, and salicin do not, thereby discriminating octanoyl ghrelin from desoctanoyl ghrelin as secreted proteins.
The finding that bitter taste-receptor agonists, which are considered as toxic, induce the secretion of a hunger hormone with gastroprokinetic effects seems somewhat counterintuitive. However, in herbal medicine bitter herbs are being used to stimulate appetite and improve digestion. The before-dinner drink, or aperitif, had its origin in the Roman practice of drinking wine infused with bitter herbs to process food with maximum efficiency and to counteract the effects of overeating. We found that food intake during the first 30 min after gavage of the bitter mixture was significantly increased. The lack of effect on food intake in α-gust−/− and GHS-R−/− mice suggests that in WT mice, ghrelin released via α-gustducin may act on the ghrelin receptor to stimulate food intake. Ghrelin is known to stimulate food intake by increasing the mRNA expression of AgRP and to a lesser degree of NPY in the hypothalamus (28). In agreement with these studies we observed an enhanced mRNA expression of AgRP in WT mice but not in gust−/− mice. The temporary increase in food intake was followed by a long-term decrease in food intake. The magnitude of the decrease in food intake was similar in all genotypes, suggesting that it occurs independently of α-gustducin and ghrelin.
During the ingestion of food, the brain also receives important information from the GI tract. Mechanoreceptors, quantitating stretch, and chemoreceptors, activated by nutrients, present in the GI tract send information to the brain via the vagus. In addition, the well-controlled process of gastric emptying also plays a crucial role in the regulation of satiety. Persistent delayed gastric emptying prolongs the presence of food within the stomach and has been associated with debilitating upper GI symptoms and less food intake. In the present study, we investigated whether the distinct reduction in food intake could be associated with an effect of the T2R agonists on gastric emptying. Intragastric administration of some but not all T2R-agonists induced a marked delay in gastric emptying in WT mice, which correlated perfectly with the decrease in food intake. Furthermore, the delay in gastric emptying was even more pronounced in gust−/− and GHS-R−/− mice than in WT mice, indicating that the release of octanoyl ghrelin, known to stimulate gastric emptying (4, 6, 29), can counteract part of the delay in gastric emptying. Because rodents lack the vomiting reflex, we hypothesize that they have developed multiple mechanisms for protecting them against toxic food, such as slowing down gastric emptying to prevent the absorption of the toxic components in the duodenum. This process will prolong the feeling of satiation and will reduce further food intake. Nevertheless, our data do not provide functional proof for an exclusive mediatory role of gastric emptying in the decrease of food intake. The release of ghrelin by bitter agonists may induce taste aversion by signaling to the brain, either indirectly via activation of the vagus nerve or directly via the blood stream, where ghrelin can directly gain access to nuclei known to mediate the response to aversive stimuli, such as the area postrema. Bitter tastants also affect the secretion of CCK and GLP-1 in the enteroendocrine cell line STC-1 (11, 18). These anorexigenic hormones, which are known to delay gastric emptying, were not involved in the delay in emptying induced by the T2R-agonists because the CCK antagonist, devazepide, and the GLP-1 antagonist, exendin(9-39), could not reverse the inhibition of emptying. It is therefore likely that bitter taste-receptor agonists may have direct effects on gastric contractility. DB, quinine, and PTC indeed inhibited neural contractions in antral and duodenal smooth-muscle strips. The effect and the potency of the compounds were dependent on the tissue studied. The mechanisms involved are currently unclear. Quinine is a wide-spectrum channel blocker affecting voltage-sensitive K+ channels as well as Ca2+-activated ones (30–32), and DB may block the delayed-rectifier K+ channels (32). A discrepancy was observed between the in vivo and in vitro contractility effects of the bitter agonists. DB and quinine did not affect gastric emptying in vivo but affected contractility in vitro, whereas PTC was almost ineffective in vitro but delayed emptying in vivo. Both DB and PTC are known to activate vagal afferent neurons via different neuronal pathways (33). It is likely that the central effects of DB on gastric emptying may be counterbalanced by its direct peripheral effects, but those of PTC are not. However, it cannot be excluded that in vivo the bitter agonists are more susceptible to degradation to inactive metabolites than in vitro.
In conclusion, our study is unique in showing that the release of octanoyl ghrelin by bitter tastants is under control of α-gustducin and results into functional effects, such as stimulation of food intake early after administration and counteraction of the delay in gastric emptying further on. Our study also shows that the behavioral effects of bitter compounds may be the consequence of a complex integration of stimuli, not only initiated at the taste buds but also at the level of the gastrointestinal tract, where bitter taste agonists may delay emptying by directs effects on gastric contractility to prevent intake of toxic foods.
It remains to be investigated whether other tastants control ghrelin secretion through the taste-receptor signaling system in the gut. This finding may provide important information for exploiting tastant-induced endogenous release of ghrelin for therapeutic intervention.
Materials and Methods
Animals.
Male C57BL/6 WT mice were obtained from Janvier, gust−/− mice were kindly provided by R. Margolskee (Mount Sinai School of Medicine, NY), and GHS-R−/− mice were obtained from Janssen Pharmaceutica (34). All mice (10–14 wk of age) were housed (20–22 °C) under a 14-h:10-h light-dark cycle and had ad libitum access to food and drinking water. All experimental procedures were approved by the Ethical committee for Animal Experiments of the Catholic University of Leuven.
Experimental Design.
Overnight-fasted WT, gust−/−, or GHS-R−/− mice were intragastrically gavaged with either 150 μL sterile H2O (control) or bitter taste-receptor agonist mixture [DB 10 mM, PTC 10 mM, PTU 5 mM, quinine 1.5 mM, and d-[-]salicin 5 mM (Sigma Aldrich)]. Mice were habituated to intragastric gavage before the start of the experiment. Mice were killed, the stomach and hypothalamus were removed, and blood was collected by cardiac puncture.
Immunohistochemistry.
Stomach sections (12 μm) from WT mice were double-stained at 4 °C overnight with a combination of the following primary antibodies: rabbit anti-octanoyl ghrelin [1-8] (Ab5004, in-house developed), rabbit anti-desoctanoyl ghrelin [1-17] (gift from C. Tomasetto, Université Louis Pasteur, Strasbourg, France), goat anti-total ghrelin (sc-10368), rabbit anti-α-gustducin (sc-395), or rabbit anti-α-transducin (sc-390) (Santa Cruz Biotechnology). Donkey anti-goat Alexa488 or donkey anti-rabbit/goat Alexa594 were used as secondary antibodies. For detection of two primary antibodies of the same host species, an additional blocking step with normal serum and an excess of unconjugated Fab antibody against the host species of the primary antibody was included before staining with the second primary antibody.
Radioimmunoassay for N-octanoyl and Total Ghrelin.
Blood samples (2 mg/mL EDTA and 500 kIU/mL aprotinin) were centrifuged and acidified (10%) with 1 N HCl. Samples were extracted on a Sep-Pak C18 cartridge (Waters Corporation) and vacuum-dried. The radioimmunoassay was performed with 125I[Tyr24] human ghrelin [1-23] as a tracer and with a rabbit antibody raised against human ghrelin [14-28] (Ab2066, final dilution 1:3,000), which recognizes both octanoylated and desoctanoylated ghrelin. For the determination of octanoylated ghrelin, a rabbit antibody against human ghrelin [1-8] was used (Ab5004, final dilution 1:100.000), which did not cross-react with desoctanoyl ghrelin. For Ab5004 and Ab2066, the ED-50 values were 128 ± 7 pg/mL and 1,015 ± 34 pg/mL, the lower detection limits were 16 and 112 pg/mL; the intra-assay coefficients of variation were 5% and 6%; the interassay coefficients of variation were 15% and 12%; the linearities after dilution (r2) were 0.98 and 0.99; and the peptide recoveries were 93% and 104%, respectively.
Quantitative Real-Time PCR.
Real-time PCR was performed as previously described (34). Primers sequences used were as follows: NPY: forward CCgCTCTgCgACACTACAT, reverse TgTCTCAgggCTggATCTCT; AgRP: forward gCggAggTgCTAgATCCA, reverse AggACTCgTgCAgCCTTA; POMC: forward ACCTCACCACggAgAgCA, reverse gCgAgAggTCgAgTTTgC; ghrelin: forward CCAgAggACAgAggACAAgC, reverse ACATCgAAgggAgCATTgAA.
Breath Test for Gastric Emptying.
Gastric emptying was measured with a noninvasive 13C octanoic acid breath test as previously described (6, 34). The percent of food emptied was calculated after 120 and 240 min. The role of CCK and GLP-1 in the effect of T2R-agonists on gastric emptying was investigated by intraperitoneal administration of the antagonists, devazepide (100 μg/kg) (Tocris Bioscience) or exendin(9-39) (25 nmol/kg) (Bachem) 15 min before intragastric administration of T2R-agonists. Control mice were injected with saline. The mice were trained to adapt to the experimental conditions and the injection before the start of the experiments.
In Vitro Contractility Studies with Smooth-Muscle Strips.
Smooth-muscle strips from the antrum and duodenum from WT mice were suspended along their circular axis in a tissue bath filled with Krebs solution, and neural responses were elicited by continuous electrical field stimulation, as described previously (35). When stable responses were obtained either several dilutions (1/10–1/10,000) of the agonist mixture or different concentrations (0.1 μM–1 mM) of the individual bitter agonists were added to the tissue bath in a cumulative manner and the mean response during the EFS-period was determined and expressed as grams per square millimeter.
Statistical Analysis.
Results are presented as means ± SEM. Data (food intake, gastric emptying) obtained from repeated measurements performed at different time points in the same mice were analyzed with a repeated-measures ANOVA. All other data were analyzed with a factorial analysis of variance followed by a Newmann-Keuls post hoc test (STATISTICA 9.0; StatSoft). Significance was accepted at the 5% level.
Acknowledgments
The authors thank Linda Nys and Anneleen Geuzens for their skilful technical assistance. This work was supported in part by grants from the Flemish Foundation for Scientific Research (FWO G.0670.10) and by a Methusalem grant from the University of Leuven for research on “The Brain-Gut Axis in Health and Disease: from Mucosal Integrity to Cortical Processing.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 3.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Tack J, et al. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- 5.Tack J, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL. Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut. 2005;54:1078–1084. doi: 10.1136/gut.2005.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujino K, et al. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–240. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 9.Callahan HS, et al. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89:1319–1324. doi: 10.1210/jc.2003-031267. [DOI] [PubMed] [Google Scholar]
- 10.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–850. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 11.Chen MC, Wu SV, Reeve JR, Jr, Rozengurt E. Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: Role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol. 2006;291:C726–C739. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 12.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 13.He W, et al. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci. 2004;24:7674–7680. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sainz E, et al. Functional characterization of human bitter taste receptors. Biochem J. 2007;403:537–543. doi: 10.1042/BJ20061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang HJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margolskee RF, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SV, et al. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon TI, Zhu B, Larson JL, Osborne TF. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest. 2008;118:3693–3700. doi: 10.1172/JCI36461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hass N, Schwarzenbacher K, Breer H. A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol. 2007;128:457–471. doi: 10.1007/s00418-007-0325-3. [DOI] [PubMed] [Google Scholar]
- 21.Roper SD. Cell communication in taste buds. Cell Mol Life Sci. 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 23.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 24.Chandrashekar J, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 25.Meyerhof W, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 26.Kaji I, Karaki S, Fukami Y, Terasaki M, Kuwahara A. Secretory effects of a luminal bitter tastant and expressions of bitter taste receptors, T2Rs, in the human and rat large intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296:G971–G981. doi: 10.1152/ajpgi.90514.2008. [DOI] [PubMed] [Google Scholar]
- 27.Brasser SM, Mozhui K, Smith DV. Differential covariation in taste responsiveness to bitter stimuli in rats. Chem Senses. 2005;30:793–799. doi: 10.1093/chemse/bji071. [DOI] [PubMed] [Google Scholar]
- 28.Kamegai J, et al. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- 29.Depoortere I, et al. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005;515:160–168. doi: 10.1016/j.ejphar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Cummings TA, Kinnamon SC. Apical K+ channels in Necturus taste cells. Modulation by intracellular factors and taste stimuli. J Gen Physiol. 1992;99:591–613. doi: 10.1085/jgp.99.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- 32.Spielman AI, et al. A method for isolating and patch-clamping single mammalian taste receptor cells. Brain Res. 1989;503:326–329. doi: 10.1016/0006-8993(89)91684-3. [DOI] [PubMed] [Google Scholar]
- 33.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2008;294:R33–R38. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- 34.Verhulst PJ, et al. Role of ghrelin in the relationship between hyperphagia and accelerated gastric emptying in diabetic mice. Gastroenterology. 2008;135:1267–1276. doi: 10.1053/j.gastro.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 35.De Smet B, Thijs T, Peeters TL, Depoortere I. Effect of peripheral obestatin on gastric emptying and intestinal contractility in rodents. Neurogastroenterol Motil. 2007;19:211–217. doi: 10.1111/j.1365-2982.2006.00883.x. [DOI] [PubMed] [Google Scholar]