Abstract
Cross-ecosystem subsidies to food webs can alter metabolic balances in the receiving (subsidized) system and free the food web, or particular consumers, from the energetic constraints of local primary production. Although cross-ecosystem subsidies between terrestrial and aquatic systems have been well recognized for benthic organisms in streams, rivers, and the littoral zones of lakes, terrestrial subsidies to pelagic consumers are more difficult to demonstrate and remain controversial. Here, we adopt a unique approach by using stable isotopes of H, C, and N to estimate terrestrial support to zooplankton in two contrasting lakes. Zooplankton (Holopedium, Daphnia, and Leptodiaptomus) are comprised of ≈20–40% of organic material of terrestrial origin. These estimates are as high as, or higher than, prior measures obtained by experimentally manipulating the inorganic 13C content of these lakes to augment the small, natural contrast in 13C between terrestrial and algal photosynthesis. Our study gives credence to a growing literature, which we review here, suggesting that significant terrestrial support of pelagic crustaceans (zooplankton) is widespread.
Keywords: allochthony, terrestrial subsidy
External inputs strongly influence ecosystems. Alterations of inputs, such as limiting nutrients or dispersing organisms, can lead to major transformations. Controlling excessive inputs or, in some cases, restoring ancestral inputs are often focal concerns of ecosystem management (1). Nonetheless, ecological theory is just beginning to account for inputs of materials and organisms and the ways in which they subsidize food webs (2–4). Theory is limited in part by the difficulty of measuring the utilization by consumers of allochthonous (or exogenous) inputs. In this context, the open waters of lakes and oceans present special challenges, yet understanding the support of pelagic ecosystems is crucial for understanding their functioning.
Aquatic systems receive organic material from two fundamentally different sources: primary production that occurred within the system's boundaries (autochthonous sources) and primary production imported from the terrestrial watershed (allochthonous sources). In lakes, streams, and rivers, the loading of allochthonous organic material is usually as large to much larger than autochthonous primary production (5), and dissolved compounds of terrestrial origin dominate the standing stock of organic matter in these waters (6, 7). In the past, it was generally assumed that this terrestrial organic matter was mostly refractory and was either buried in sediments or exported. Work on the metabolic balances of aquatic systems has reversed this view. In many aquatic systems, respiration (the degradation of organic C to CO2 by all organisms combined) exceeds gross primary production (the formation of organic matter by photosynthesis; ref. 8). This simple balance demonstrates that at least some portion of the terrestrial input must be actively catabolized in the receiving aquatic system.
That terrestrial material is catabolized suggests that some secondary production of microbes, invertebrates, or fish may be supported directly or indirectly by terrestrial inputs. Using multiple approaches (litter exclusion, gut contents, biomarkers, and stable isotopes), a number of authors have reported that some fishes and benthic invertebrates in streams and the littoral zones of rivers and lakes are indeed supported in part by terrestrial organic matter (e.g., refs. 9–16). Demonstrating a terrestrial influence on pelagic food webs in lakes had been both more difficult and more controversial. Terrestrial organic matter could become available to pelagic organisms by several mechanisms: microbial uptake of terrestrial dissolved organic carbon (DOC) followed by consumption of these microbes by protozoans or zooplankton (17, 18), direct consumption of terrestrial DOC by zooplankton via osmotrophy (19), or by consumption of terrestrially derived particles by zooplankton (20, 21). Terrestrial contributions to zooplankton have been estimated with different methods, predominantly by using ambient 13C and 15N (11, 17, 20, 22–34). Although the majority of these studies suggest significant terrestrial support of zooplankton (Table S1), this interpretation is debatable for three reasons: (i) The pathways outlined above are hard to quantify and gut contents are difficult to determine in zooplankton (35); (ii) approaches using stable isotopes can be problematic because it is difficult to directly measure the isotopic signature of phytoplankton. Suspended particulate organic matter [seston, or particulate organic matter (POM)] is only partially comprised of phytoplankton, and physically isolating the phytoplankton is only possible under certain conditions (36); and (iii) even where measurement or estimation is possible, phytoplankton can be isotopically similar to terrestrial organic matter, especially for carbon (37).
In an attempt to overcome some of these difficulties, we conducted a series of experiments in which we greatly elevated the 13C of dissolved inorganic carbon (DIC) in the surface mixed layer of several small lakes, creating strong contrasts in δ13C between autochthonous primary production and allochthonous inputs. From these experiments and associated models, we calculated that zooplankton could be subsidized from 30 to 70% by terrestrial C in systems that had low phytoplankton biomass (chlorophyll) and high DOC (29, 38). Experimentally elevating primary production with nutrient additions greatly reduced this terrestrial contribution to <10% (20, 27, 29). In a clear water lake low in both phytoplankton and DOC, we found low terrestrial subsidies to a calanoid copepod (2%) and a modest subsidy to a cladoceran (30%; ref. 31 and Table S1).
Whole-lake 13C experiments have several potential biases that may lead to an overestimation of allochthony. Because the 13C was added only to the surface mixed layer, photosynthesis below the mixed layer is not labeled and might be confused with terrestrial C in the analysis. Second, autochthonous primary production that occurred before the 13C addition would not have been labeled, and the resulting autochthonous detritus again could be counted as allochthonous by the analysis. Although these issues were partially addressed (15, 20, 29), Brett et al. (21) recently argued that these 13C addition experiments greatly overestimated allochthony to zooplankton. Here, we estimate allochthony to zooplankton with an independent and unique approach. We used ambient stable isotopes of C, H, and N over depth and time to independently assess terrestrial contributions to zooplankton in two contrasting lakes in which 13C addition experiments had been performed. Further, we present a unique method, based on deuterium (δ2H) to estimate the isotopic signature of phytoplankton without having to separate them physically from seston, and present a comprehensive review of the literature on allochthony to zooplankton.
Results
Study Sites.
We sampled Paul and Crampton lakes monthly (May–August 2009) to measure isotopic pools and background conditions (Table S2). Paul Lake (L.) is small (1.7 ha) and has a brown color because of significant concentrations of chromophoric dissolved organic matter. The lake is mesotrophic (chlorophyll-a 2–4 μg·liter−1 in the oxic zone), sharply stratified with a steep thermocline beginning at ≈3.5 m, and anoxic <5.5 m; Crampton is larger (25 ha), clear, oligotrophic (chlorophyll a 1–2 μg·liter−1), oxic throughout its water column, with a broad thermocline. Both lakes had slight chlorophyll maxima in oxic waters. The dominant crustacean zooplankton in Paul L. were cladocerans including Daphnia (D. rosea and D. pulex) and Holopedium gibberum as well as cyclopoid copepods. Crampton L. had Holopedium gibberum, small cyclopoid copepods, and a calanoid copepod, Leptodiaptomus minutus.
Isotopic Composition of Endmembers.
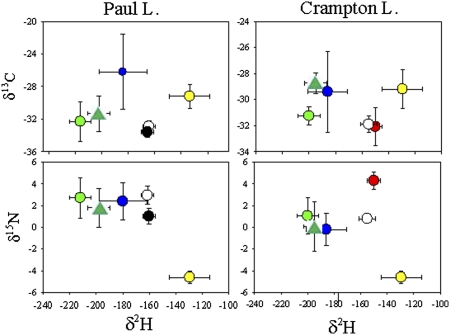
The isotopic composition of benthic and pelagic primary producers in the lake and in the watershed was distinct for some of the isotopes (Fig. 1 and Table S3). Dilution-regrowth cultures of phytoplankton (Methods) from these lakes confirmed the large fractionation for δ2H between water and algae reported (34, 39), and there was little difference between deep and surface water phytoplankton. In the surface waters of both lakes, phytoplankton had a δ2H of near −200‰ with low variance (Table S3). In contrast, terrestrial vegetation was much heavier than phytoplankton (by 65‰) averaging −129‰. Suspended POM in both lakes had δ13C midway between that of phytoplankton and terrestrial vegetation (Table S3). Assuming that POM is a mixture of terrestrial vegetation and phytoplankton, we calculated the C and N isotopic signatures of phytoplankton at each depth (Table S3 and Methods). In Paul L., the calculated δ13C of phytoplankton was lower compared with terrestrial sources, averaging −31.3 ± 2.2 in surface water and ≈1‰ lower at depth (Fig. 1 and Table S3) In Crampton L., surface phytoplankton (−28.8 ± 0.08) was very close to that of terrestrial organic C and was lower by ≈4‰ at depth (Fig. 1 and Table S3). In both lakes, the calculated δ15N of phytoplankton increased with depth and was higher than terrestrial values (Fig. 1 and Table S3).
Fig. 1.
Isotope biplots for Paul (Left) and Crampton lakes (Right) for 2009. Zooplankton [Daphnia (Paul; filled black circles); Holopedium (both lakes, open circles); and Leptodiaptomus (Crampton, filled red circle] are shown in relation to possible food sources: phytoplankton in the upper mixed layer (green filled circle); deep water phytoplankton (dark green triangle); littoral benthic algae (filled blue circle), and terrestrial vegetation (filled yellow circle). Means and SDs are shown; in some cases, the symbols are larger than the SDs. Zooplankton are averages for all depths and dates taken. For surface water phytoplankton, SDs combine variance over time and the depths within the upper mixed layer; for periphyton and deep water phytoplankton, the SDs are temporal only. POM, not shown here, is shown in Fig. S1.
Isotopic Signatures of Zooplankton and Seston.
In both lakes, POM and zooplankton had isotopic signatures that varied little over depth and were intermediate between potential aquatic and terrestrial sources (Fig. S1). In both lakes, there were some significant differences among taxa in isotopic composition. In Paul L., Daphnia and Holopedium differed significantly in δ2H and δ15N but not in δ13C. In Crampton L., Leptodiaptomus and Holopedium differed significantly in all three isotopes. For Paul L., there was no significant difference in the zooplankton isotopes over depth (each pair, t test). In Crampton, only δ15N was significantly (t test, P < 0.05) higher at 7 m relative to the other depths. Because the differences among depths were small and inconsistent, and because zooplankton can move throughout the water column, we kept the taxa separate in the following analyses but combined data over depth and date to increase the sample size.
Zooplankton were distinct from their basal food resources (i.e., terrestrial and algal end members) for δ2H in all cases (Fig. 1). Littoral benthic algae and both surface and deep phytoplankton were significantly lower in δ2H compared with zooplankton (Fig. 1). The cladoceran zooplankton in both Paul and Crampton lakes were higher in δ15N compared with terrestrial sources by 4–6‰ suggesting that, with the expected trophic enrichment, terrestrial N was a likely partial source of food for zooplankton. In contrast, cladocerans were similar to or slightly lower than algal δ15N sources, suggesting algae are not sole N sources for these zooplankton (Fig. 1). Leptodiaptomus in Crampton L. was higher by 2–3‰ in δ15N compared with phytoplankton and by as much as 8‰ compared with terrestrial sources. Depending on the extent to which Leptodiaptomus is a primary consumer or an omnivore (i.e., feeding partially on other zooplankton), either terrestrial or algal N are possible food sources. The δ13C of zooplankton in Paul L. was lower than benthic algal sources and close to, but slightly lower than, either deep or surface phytoplankton sources. It is likely that some lower δ13C source (e.g., methanotrophic bacteria; ref. 40) is used by zooplankton in this lake (see below).
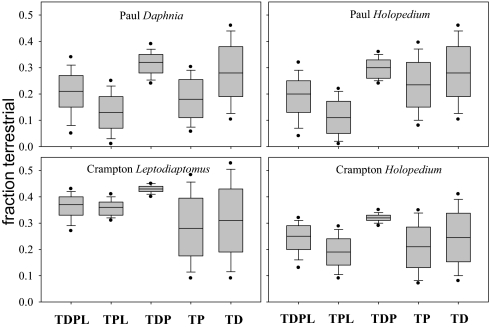
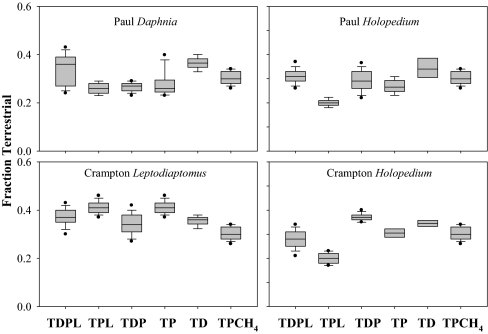
In our modeling analysis, we treat all zooplankton as primary consumers that feed on some mixture of algal and terrestrial resources. We use 15% as an estimate of dietary water (Methods) for consumers. We calculated the δ2H in food (e.g., corrected for dietary water) of each zooplankton taxa and used these corrected values for the isotope modeling with δ2H alone and for δ2H in combination of δ13C and δ15N. Using δ2H alone, all models suggest zooplankton are comprised in part of terrestrial organic matter with median estimates ranging from 10 to 30% depending on the model (Fig. 2). However, the δ2H-alone models are not well constrained with 5 and 95 percentiles ranges from near 0 to >40% (Fig. 2). Adding δ13C and δ15N to the models greatly reduced the range of possible values of the percentage of terrestrial organic matter (ϕT) in zooplankton biomass (Fig. 3). With the three isotope models, none include zero ϕT at the extremes, and the medians range from ≈25 to 39% (Fig. 3). Further, no models that excluded terrestrial sources fit within a tolerance of 3‰ (Methods). Collectively, these models provide strong evidence that zooplankton are significantly supported by terrestrial organic matter.
Fig. 2.
Box-whisker plots of the fraction of zooplankton biomass from terrestrial organic matter estimated from IsoSource models by using only one stable isotope ratio (δ2H) in Paul and Crampton lakes. The box boundaries represent the 25th and 75th percentile, the horizontal line is the median, and the whiskers mark the 10th and 90th percentiles. The dots denote the 5 and 95% values of the distribution. The models include combinations of possible sources: T, terrestrial; P, phytoplankton in the upper mixed layer; D, phytoplankton at the chlorophyll max in oxic water; L, benthic algae.
Fig. 3.
Box-whisker plots of the fraction of zooplankton biomass from terrestrial organic matter estimated from IsoSource models by using three stable isotopes (δ2H, δ15N, and δ13C) in Paul and Crampton lakes. The box boundaries represent the 25th and 75th percentile, the horizontal line is the median, and the whiskers mark the 10th and 90th percentiles. The dots denote the 5 and 95% values of the distribution. The models include combinations of possible sources: T, terrestrial; P, phytoplankton in the upper mixed layer; D, phytoplankton at the chlorophyll maximum in oxic water; L, benthic algae; CH4, metanotrophic bacteria.
Methanotrophic bacteria are a possible food source to zooplankton, and even modest consumption of these bacteria results in significant 13C depletion (41). We do not have direct measurements of the isotopes of methanotrophs but provide an estimate based on assumptions from the literature for 13C and δ2H (ref. 42 and Table S3), with the additional assumption that methanotrophic bacteria, as primary autototrophs, have the same δ15N as phytoplankton. Adding methanotrophs has little effect on the estimate of ϕT in the models (Fig. 3).
Discussion
Terrestrial Support of Zooplankton.
Based on analysis of ambient stable isotopes, zooplankton in the two study lakes are comprised in part of allochthonous organic matter. Although the estimates of the magnitude are uncertain and vary with lake, taxon, and the sources used in each model, ϕT exceeded 10% in all cases and was between 20 and 40% for most of the source combinations we tried. In no case using multiple isotopes could we explain the composition of zooplankton from any combination of surface and deep water phytoplankton in the absence of some terrestrial material. The mean values of multiple models are in agreement with those obtained from the whole lake 13C additions for the same lakes for cladocerans and substantially higher for the copepod Leptodiaptomus (ref. 31 and Table S1). The 13C addition experiments have two potential biases that could result in overestimates of allochthony: deep feeding by vertically migrating zooplankton and consumption of detritus of algal origin that was produced before the addition of the label (15, 21). However, the ambient isotope approach used here did not give substantially lower estimates of ϕT than the 13C additions, indicating that neither of these potential biases were of major importance for zooplankton in these lakes.
The isotopic modeling indicates zooplankton are comprised of multiple sources in these lakes, but distinguishing among some of these sources is difficult. For example, the autotrophic components are not well separated from each other isotopically. Thus, models often cannot distinguish among two or more of these sources in terms of their support of zooplankton. In Paul L., although there was not strong isotopic separation between surface and deep phytoplankton, 13C in periphyton was lower than in phytoplankton. In models that included phytoplankton and periphyton, periphyton was a minor possible source to zooplankton, <10% for either Daphnia or Holopedium. In Crampton L., none of the autochthonous components were well separated and all sources were likely in some models. If we sum the three possible autochthonous sources in each model, we can compare ϕT to support by total autochthonous production, and this comparison revealed a consistent pattern. Autotrophic production (some combination of benthic algae plus surface and deep phytoplankton) accounted for >60% (at the median of the IsoSource distributions) of zooplankton biomass in all cases.
That zooplankton consume more algae (probably phytoplankton) than terrestrial detritus agrees with our assessments of ϕT from whole lake 13C additions and with most estimates from the literature (Table S1). That zooplankton are formed in part from terrestrial detritus also agrees with the whole lake 13C additions in these lakes and with most prior studies that have used a variety of methods. The 13C additions suggest that ϕT for cladocerans was ≈30% in Crampton L. (31) and 20–37% in Paul (20, 38). This result agrees well with the estimates developed here by using only ambient isotope measurements. The 13C addition analysis for Crampton L. suggested that Leptodiaptomus was almost entirely supported (98%) by autochthonous primary production, whereas the ambient isotope approach leads to a higher estimate of support by allochthonous sources (30–40%). The ambient isotope approach treats all zooplankton as primary consumers. This assumption is reasonable for cladocerans but may not apply to Leptodiaptomus, which consumes small zooplankton, heterotrophic protists (e.g., ciliates), phytoplankton, and detritus (30, 35, 43, 44). The higher δ15N of Leptodiaptomus is the result of feeding on a higher trophic level than Holopedium, feeding on different basal food sources, or both (Fig. 1). Thus, our simple analysis may overestimate ϕT for Leptodiaptomus, and a more sophisticated approach may be needed for secondary consumers or those, like Leptodiaptomus, which probably feed at multiple tropic levels (13).
Literature Review.
At least 15 prior studies have attempted, using a variety of methods, to estimate allochthony in zooplankton (Table S1). Of these, four provided only qualitative assessments of which two (22, 23) describe allochthony as being low while two others (11, 24) describe allochthony as large or very large. All of the studies that provide quantitative estimates suggest that allochthony for some zooplankton taxa in the systems studied is >20%, and many of the studies estimate much higher values (50–70%; e.g., refs. 26 and 28). Most of the published studies used ambient stable isotopes (largely 13C, some in combination with 15N) as the basis of the estimate. One intriguing study used specific fatty acid biomarkers and traced the C supporting whitefish (Coregonus 11) to terrestrial material that transferred to fish via copepods. Another study that used ambient 14C, which can help distinguish materials based on their age found high allochthony for zooplankton, in the Hudson River where old allochthonous carbon is important (34).
Isotopic Signatures of Phytoplankton.
We used δ2H both as a food web tracer and, because of its high contrast between terrestrial and algal photosynthesis, as a way to estimate the isotopic signature of phytoplankton (Methods and Table S3). The calculated values of δ13C for phytoplankton using the δ2H approach returned values of −31.3‰ in Paul L. and −29‰ in Crampton, which are comparable to estimates we obtained from the 13C addition experiments (31, 38) and imply phytosynthetic fractionation (13C-CO2 minus 13C-phytoplankton) values in the range previously observed in freshwaters (−12 to −20‰; refs. 31 and 45). Using δ2H, we estimate that POM is highly allochthonous, >80% in both lakes. This estimate is somewhat higher than we estimated from the whole-lake 13C additions for Paul L. and much higher than that for Crampton L; this discrepancy may reflect differences among years, methods, or both. However, if we assumed a lower 13C value for phytoplankton, terrestrial organic C would have to be an even larger fraction of the POM in both lakes. The δ2H approach estimated δ15N values for phytoplankton of 1.8‰ in Paul and 0.08‰ in Crampton. These values are higher than the measured terrestrial 15N by 4–6‰. Similar contrasts were reported by France (46) for benthic algae in forested lakes. Our estimated values of δ15N in phytoplankton were slightly lower (by 0.3–0.6‰) compared with our measured values in benthic algae. Thus, δ2H may be a promising tool in estimating the 13C and 15N isotopic signature of phytoplankton, and more work needs to be done to refine its use.
Role of Methane.
Many studies, including this one, find that zooplankton have lower 13C than measurable sources (22, 47). Hypotheses advanced to explain this discrepancy include the following: a lower 13C signature in the phytoplankton consumed by zooplankton (30, 48); feeding deep in the water column where 13C is sometimes lower than in surface waters (22, 30) and the consumption of methanotrophic bacteria, which can be have extremely low 13C signatures (42). For our study, neither the seston nor our estimates of the phytoplankton 13C signature showed marked 13C depletion over depth in oxic water (Table S3), and we have accounted for the possibility of feeding deep in the water column in the mixing models. In Paul L., but not Crampton L., it is conceivable that there is a source of food in anoxic water that we did not measure (e.g., purple or green sulfur bacteria). Using this source would require zooplankton to feed in anoxic water. If the zooplankton are restricted to oxic water, the consumption of methanotrophic bacteria is quite likely. These lakes have measureable CH4 concentrations at all depths including surface waters (49) and measurable but low rates of CH4 oxidation in oxic waters (50). Including methanotrophic bacteria in the mixing, models provided a better fit for 13C, but has little effect on the estimate of ϕT for any of the taxa-lake combinations.
How Do Zooplankton Access Terrestrial Organic Matter.
The standing stock of DOM in most lakes in this region is heavily dominated by terrestrial sources (7, 20). The importance of terrestrial organic matter to POM is more variable. In experimentally fertilized lakes, POM was almost entirely derived from autochthonous primary production (27, 29). Based on the 13C addition to Crampton L., Pace et al. (31) estimated that POM was highly autocthonous (31). In contrast in this study, consistent with the Leptodiaptomus results, we calculate that POM was highly allochthonous. Although methodological differences cannot be ruled out, year-to-year variability is a possibility. Because Crampton L. is dilute, small differences in either terrestrial loading or primary production could account for this discrepancy. Given the allochthonous nature of combined dissolved and particulate organic matter in these lakes, it is striking that terrestrial organic matter comprises only 25–30% of zooplankton biomass. The relatively high reliance on algal material demonstrates that zooplankton are quite selective in keeping with physiological studies (51, 52) and some field studies (22, 23).
This study does not address the pathways that provide zooplankton with terrestrial organic matter; some of our prior work does. Bacterial uptake of DOM, and subsequent consumption by zooplankton, is likely only part of the story. From the 13C experiments, we estimate for Paul L. that this pathway provides <10% of the terrestrial C that zooplankton consume (20). This low supply is the result of low rates of bacterial production compared with zooplankton demand and that bacteria assimilate DOM of both algal and terrestrial origin in about equal proportion (53). There are a number of reports of invertebrates that take up DOC directly (54, 55) but very few for crustacean zooplankton. Speas and Duffy (19) suggest the process occurs in Daphnia but is not significant to its C balance. It is likely, therefore, that zooplankton are consuming particles that either entered the lake from land or formed by flocculation of DOM. Because direct aeolian inputs appear to provide only a small fraction (<10%) of the total zooplankton demand (56), flocculation of DOM is the most likely mechanism for a large source of terrestrial particles (57).
There is ample evidence that some zooplankton, especially cladocerans, will ingest numerous kinds of particles (52). Our isotopic evidence suggests that particles of terrestrial origin must also be assimilated by zooplankton. Recently, Brett et al. (21) measured assimilation of terrestrial organic matter by Daphnia magna in laboratory experiments. D. magna grew and reproduced poorly on diets of terrestrial particles alone (red alder leaves), but growth and reproduction were positive on mixtures of algae and alder leaves even when the algal component was as low as 20% of the total (21). On mixtures approaching what we estimate here for cladocerans (30% terrestrial, 70% algae), growth and reproduction were not different from diets using nutritious laboratory algae that produced maximal growth (21). Hence, despite the arguments of Brett et al. (21) that zooplankton are not supported by terrestrial organic matter, their laboratory results are consistent with the field analyses reported here and elsewhere (Table S1). Zooplankton do not grow simply on nutritious algae but subsist on algae of variable quality (58) and on organic matter derived from terrestrial sources.
Conclusions.
Using a unique method that can be applied to a wide range of ecosystems, we found significant terrestrial support of pelagic zooplankton. Our findings support previous findings of allochthony in pelagic systems and counter arguments that allochthony is an artifact of methods. The literature reports a range in ϕT for zooplankton among systems and taxa (Table S1), and some patterns are consistent with the feeding ecology of zooplankton. Zooplankton are selective feeders, some taxa more than others, and phytoplankton is usually a preferred food. It is only when the concentration of particles of terrestrial origin (or bacteria that consumed terrestrial DOM) of an appropriate particle-size is considerably larger than the concentration of edible phytoplankton that we would expect significant ϕT. Accordingly, ϕT should be highest in humic lakes with low phytoplankton biomass, and lowest in either eutrophic lakes, or clear-water lakes with limited terrestrial inputs. This pattern is supported by our findings and existing literature (Table S1). As methods improve and more studies are conducted, we expect considerable variation in support of consumers by allochthonous resources, which should lead to the development of models that explain this variation among ecosystems. Further, improved isotope mixing models that better incorporate uncertainty in sources (48, 59) will likely aid in producing better models.
Methods
Sample Collection.
Samples were taken at four depths in each lake four to six times during the ice-free season of 2009. Zooplankton were collected with an open diaphragm bilge pump where the inlet hose was set at the desired sampling depth, and the outlet hose pumped water through an 80-μm mesh net. Samples for seston were collected by the same method without filtering the water (i.e., whole water samples). Zooplankton samples were sorted by taxa under a dissecting microscope, dried (40 °C), and desiccated pending isotope analysis. Seston samples were filtered in the laboratory shortly after collection. For 13C and 15N samples, seston was collected on 25-mm glass fiber filters (Whatman GF/F) and dried. For δ2H, samples were filtered through 47-mm MicronSep Cellulosic. The accumulated seston was back-rinsed into a small volume of water and then dried. This separate procedure for δ2H was used because glass fiber filters interfere with the δ2H analysis. From the same samples as the isotopes, aliquots were taken for the analysis of chlorophyll-a by fluorometry. Because the filters clog as particles accumulate, bacteria are included in the seston but we cannot separately estimate the isotopic signature of bacteria. Profiles of dissolved oxygen and temperature were taken by using a model YSI Professional Plus meter.
Isotope Analysis.
Stable isotope ratios of organic samples were measured on isotope ratio mass spectrometers at the University of Alaska (δ13C and δ15N) and the University of Northern Arizona (δ2H). Methods for δ2H analysis followed those of Doucett et al. (39) and Finlay et al. (60), including a benchtop equilibration to correct for exchange of H atoms between samples and ambient water vapor (61–63). Water samples were analyzed for δ2H via cavity-ring-down laser spectroscopy. The δ2H is the nonexchangeable fraction (39).
Isotopic Signatures of Phytoplankton.
We obtained the δ2H values of phytoplankton by performing dilution-regrowth experiments in the surface waters of each lake. Water was collected and filtered through Whatman GF/F filters (4 liters) and mixed with small inocula of unfiltered water. The samples were incubated under ambient light at 22 °C with aeration. By taking frequent samples for chlorophyll-a, we could assess when enough growth (≈5 μg of chl-a·liter−1) had occurred so that enough new particulate material could be easily collected, which took from 4 to 10 d depending on lake and time. The δ2H of phytoplankton was estimated from the δ2H of the new material. These experiments provide εH (the contrast between δ2H in phytoplankton and water), which allows the calculation of phytoplankton δ2H at any time and depth. We assumed that POM is comprised of a mixture of phytoplankton and terrestrial material and solved for the 13C and 15N of phytoplankton algebraically (Table S2 for details). The consistency of εH in these lakes and in the literature (5, 15, 39, 60) justifies this approach.
Isotope Modeling.
We used the multiple polygon model of Phillips and Gregg (ref. 64; IsoSource) to analyze source contributions to zooplankton. We chose IsoSource, which solves iteratively for feasible mixing solutions, for several reasons. The model is designed to handle situations where there are more possible sources than isotopes, which is the case for some of our model runs. More importantly, IsoSource is a well-tested model, available to all (www.epa.gov/wed/pages/models/stableIsotopes/isosource/isosource.htm), and widely cited in the literature.
IsoSource addresses variability in source isotope signatures by using a tolerance parameter that allows models to fit within a certain range of the mean (64). We used tolerance parameters of 1–3‰, which are similar to the range among replicate field samples and reflect the uncertainty in sources (Table S3). For each source, IsoSource computes a frequency distribution of the proportion of organic matter that the source contributes to the consumer. In most cases, this distribution has a single well-defined peak. We express the IsoSource results as box-and-whisker plots of these distributions.
Dietary Water and Trophic Fractionation.
To model food sources to zooplankton, we needed to estimate trophic fractionation in 15N and for dietary water for δ2H. Solomon et al. (65) showed that trophic fractionation for δ2H was negligible. For 15N, we made the standard assumption that consumers are higher by 3‰ than their food sources, recognizing that there is variability around this mean (66). A fraction of an organism's nonexchangeable H comes from water rather than assimilated food. Because we are interested in the food web, we need to estimate the fraction of dietary water and correct for it. We created a series of models in IsoSource (64) in which we used one isotope (2H), the δ2H of water in each lake, and the possible food sources (phytoplankton, deep phytoplankton, benthic algae, and terrestrial vegetation). We fit a range of possibilities for dietary water in 20 models with various combinations of these sources (Fig. S2). The medians of these models ranged from 17 to 12%, and the box-whisker plots of the full distribution are reasonably narrow. None of the models fit with dietary water <10%; a large majority (13 of 20) fit with dietary water between 10 and 15%; and only 1 model fit >25% (Fig. S2). In the analysis presented in the text, we used 15% for dietary water, which is in agreement with both these model runs and the recent review of the literature by Solomon et al. (65).
We tested the effect of different values of dietary water on the outcome in models that included multiple isotopes and sources. Decreasing the estimate of dietary water to 10% increased ϕT and increasing it to 20% decreased ϕT; models with dietary water >25% had no solution. For example, for Daphnia in Paul L., the three isotope models that included all sources (terrestrial, surface phytoplankton, deep phytoplankton, and benthic algae) with diet water at 10, 15, and 20% had decreasing means (± SD) for ϕT of 0.4 (±0.036); 0.324 (±0.036), and 0.146 (±0.008), respectively. At diet water of 22% or above, no solution was obtained within a tolerance of 3‰. Clearly, uncertainty in dietary water leads to uncertainty in the magnitude of ϕT. A final caveat concerns the possible alteration δ2H (or the other isotopes) in organic matter as it decomposes. Large differences in the isotopic signatures of living phytoplankton and detritus derived from phytoplankton, for which there is no evidence, could complicate this analysis. Because the residence time for particles in these water columns is short (days), it is unlikely to see a large diagenetic effect.
Supplementary Material
Acknowledgments
We thank Jim Coloso and Laura Smith who collected much of the data shown here and Jim Hodgson, Jon Frum for inspiration in writing this paper. The work took place at the University of Notre Dame Environmental Research Center, and we are grateful for the assistance from Dr. Gary Belovsky and his staff. We thank the National Science Foundation for funding.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012807108/-/DCSupplemental.
References
- 1.Lake PS, Bond N, Reich P. Linking ecological theory with stream restoration. Freshw Biol. 2007;52:597–615. [Google Scholar]
- 2.Polis GA, Anderson WB, Holt RD. Toward and integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu Rev Ecol Syst. 1997;28:289–316. [Google Scholar]
- 3.Leroux SJ, Loreau M. Subsidy hypothesis and strength of trophic cascades across ecosystems. Ecol Lett. 2008;11:1147–1156. doi: 10.1111/j.1461-0248.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- 4.Power ME. Prey exchange between a stream and its forested watershed elevates predator densities in both habitats. Proc Natl Acad Sci USA. 2001;98:14–15. doi: 10.1073/pnas.98.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caraco NF, Cole JJ. In: Food Webs at the Landscape Level. Polis GA, Power ME, Huxley GR, editors. Chicago: Univ of Chicago Press; 2004. pp. 301–316. [Google Scholar]
- 6.Wetzel RG. Limnology: Lake and River Ecosystems. San Diego, CA: Academic; 2001. [Google Scholar]
- 7.Bade DL, et al. Sources and fates of dissolved organic carbon in lakes as determined by whole-lake carbon isotope additions. Biogeochem. 2007;84:115–129. [Google Scholar]
- 8.Jansson M, Persson L, De Roos AM, Jones RI, Tranvik LJ. Terrestrial carbon and intraspecific size-variation shape lake ecosystems. Trends Ecol Evol. 2007;22:316–322. doi: 10.1016/j.tree.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JB, Eggert SL, Meyer JL, Webster JR. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science. 1997;277:102–104. [Google Scholar]
- 10.Hershey AE, et al. Stable isotope signatures of benthic invertebrates in arctic lakes indicate limited coupling to pelagic production. Limnol Oceanogr. 2006;51:177–188. [Google Scholar]
- 11.Perga ME, Bec A, Anneville O. Origins of carbon sustaining the growth of whitefish Coregonus lavaretus early larval stages in Lake Annecy: Insights from fatty-acid biomarkers. J Fish Biol. 2009;74:2–17. doi: 10.1111/j.1095-8649.2008.02105.x. [DOI] [PubMed] [Google Scholar]
- 12.Francis TB, Schindler DE. Shoreline urbanization reduces terrestrial insect subsidies to fishes in North American lakes. Oikos. 2009;118:1872–1882. [Google Scholar]
- 13.Rasmussen JB. Estimating terrestrial contribution to stream invertebrates and periphyton using a gradient-based mixing model for delta13C. J Anim Ecol. 2010;79:393–402. doi: 10.1111/j.1365-2656.2009.01648.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman JC, Bronk DA, Olney JE. Contribution of allochthonous carbon to American shad production in the Mattaponi River, Virginia, using stable isotopes. Estuaries Coasts. 2007;30:1034–1048. [Google Scholar]
- 15.Solomon CT, Carpenter SR, Cole JJ, Pace ML. Support of benthic invertebrates by detrital resources and current autochthonous primary production: Results from a whole-lake C-13 addition. Freshw Biol. 2008;53:42–54. [Google Scholar]
- 16.Weidel B, et al. Carbon sources supporting fish growth in a north temperate lake. Aquat Sci. 2008;70:446–458. [Google Scholar]
- 17.Berggren M, et al. Lake secondary production fueled by rapid transfer of low molecular weight organic carbon from terrestrial sources to aquatic consumers. Ecol Lett. 2010;13:870–880. doi: 10.1111/j.1461-0248.2010.01483.x. [DOI] [PubMed] [Google Scholar]
- 18.Lennon JT, Pfaff LE. Source and supply of terrestrial organic matter affects aquatic microbial metabolism. Aquat Microb Ecol. 2005;39:107–119. [Google Scholar]
- 19.Speas DW, Duffy WG. Uptake of dissolved organic carbon (DOC) by Daphnia pulex. J Freshwat Ecol. 1998;13:457–463. [Google Scholar]
- 20.Cole JJ, et al. Differential support of lake food webs by three types of terrestrial organic carbon. Ecol Lett. 2006;9:558–568. doi: 10.1111/j.1461-0248.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 21.Brett MT, Kainz MJ, Taipale SJ, Seshan H. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proc Natl Acad Sci USA. 2009;106:21197–21201. doi: 10.1073/pnas.0904129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Giorgio PA, France RL. Ecosystem-specific patterns in the relationship between zooplankton and POM or microplankton delta C-13. Limnol Oceanogr. 1996;41:359–365. [Google Scholar]
- 23.Sobczak WV, Cloern JE, Jassby AD, Müller-Solger AB. Bioavailability of organic matter in a highly disturbed estuary: The role of detrital and algal resources. Proc Natl Acad Sci USA. 2002;99:8101–8105. doi: 10.1073/pnas.122614399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson J, Jonsson A, Meili M, Jansson M. delta N-15 of zooplankton species subarctic lakes in northern Sweden: Effects in s of diet and trophic fractionation. Freshw Biol. 2004;49:526–534. [Google Scholar]
- 25.Meili M, et al. Sources and partitioning of organic matter in a pelagic microbial food web inferred from the isotopic composition δ13 C and δ15 N) of zooplankton species. Arch Hydrobiol Spec Issues Advanc Limnol. 1996;48:53–61. [Google Scholar]
- 26.Jones RI, Grey J, Sleep D, Quarmby C. An assessment, using stable isotopes, of the importance of allochthonous organic carbon source to the pelagic food web in Loch Ness. Proc Biol Sci. 1998;265:105–111. [Google Scholar]
- 27.Cole JJ, Carpenter SR, Kitchell JF, Pace ML. Pathways of organic carbon utilization in small lakes: Results from a whole-lake C-13 addition and coupled model. Limnol Oceanogr. 2002;47:1664–1675. [Google Scholar]
- 28.Karlsson J, Jonsson A, Meili M, Jansson M. Control of zooplankton dependence on allochthonous organic carbon in humic and clear-water lakes in northern Sweden. Limnol Oceanogr. 2003;48:269–276. [Google Scholar]
- 29.Carpenter SR, et al. Ecosystem subsidies: Terrestrial support of aquatic food webs from C-13 addition to contrasting lakes. Ecology. 2005;86:2737–2750. [Google Scholar]
- 30.Matthews B, Mazumder A. Habitat specialization and the exploitation of allochthonous carbon by zooplankton. Ecology. 2006;87:2800–2812. doi: 10.1890/0012-9658(2006)87[2800:hsateo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Pace ML, et al. Does terrestrial organic carbon subsidize the planktonic food web in a clear-water lake? Limnol Oceanogr. 2007;52:2177–2189. [Google Scholar]
- 32.Taipale S, Kankaala P, Tiirola M, Jones RI. Whole-lake dissolved inorganic 13C additions reveal seasonal shifts in zooplankton diet. Ecology. 2008;89:463–474. doi: 10.1890/07-0702.1. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed MN, Taylor WD. Relative contribution of autochthonous and allochthonous carbon to limnetic zooplankton: A new cross-system approach. Fund Appl Limnol. 2009;175:113–124. [Google Scholar]
- 34.Caraco N, Bauer JE, Cole JJ, Petsch S, Raymond P. Millennial-aged organic carbon subsidies to a modern river food web. Ecology. 2010;91:2385–2393. doi: 10.1890/09-0330.1. [DOI] [PubMed] [Google Scholar]
- 35.Rautio M, Vincent WF. Benthic and pelagic food resources for zooplankton in shallow high-latitude lakes and ponds. Freshw Biol. 2006;51:1038–1052. [Google Scholar]
- 36.Hamilton SK, Sippel SJ, Bunn SE. Separation of algae from detritus for stable isotope or ecological stoichiometry studies using density fractionation in colloidal silica. Limnol Oceanogr Methods. 2005;3:149–157. [Google Scholar]
- 37.France RL. Stable carbon and nitrogen isotopic evidence for ecotonal coupling between boreal forests and fishes. Ecol Freshwat Fish. 1997;6:78–83. [Google Scholar]
- 38.Pace ML, et al. Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature. 2004;427:240–243. doi: 10.1038/nature02227. [DOI] [PubMed] [Google Scholar]
- 39.Doucett RR, Marks JC, Blinn DW, Caron M, Hungate BA. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology. 2007;88:1587–1592. doi: 10.1890/06-1184. [DOI] [PubMed] [Google Scholar]
- 40.Deines P, Bodelier PLE, Eller G. Methane-derived carbon flows through methane-oxidizing bacteria to higher trophic levels in aquatic systems. Environ Microbiol. 2007;9:1126–1134. doi: 10.1111/j.1462-2920.2006.01235.x. [DOI] [PubMed] [Google Scholar]
- 41.Kankaala P, et al. Experimental delta C-13 evidence for a contribution of methane to pelagic food webs in lakes. Limnol Oceanogr. 2006;51:2821–2827. [Google Scholar]
- 42.Deines P, Wooller MJ, Grey J. Unraveling complexities in benthic food webs using a dual stable isotope (hydrogen and carbon) approach. Freshw Biol. 2009;54:2243–2251. [Google Scholar]
- 43.Bundy MH, Vanderploeg HA, Lavrentyev PJ, Kovalcik PA. The importance of microzooplankton versus phytoplankton to copepod populations during late winter and early spring in Lake Michigan. Can J Fish Aquat Sci. 2005;62:2371–2385. [Google Scholar]
- 44.Rautio M, Vincent WF. Isotopic analysis of the sources of organic carbon for zooplankton in shallow subarctic and arctic waters. Ecography. 2007;30:77–87. [Google Scholar]
- 45.Bade DL, Pace ML, Cole JJ, Carpenter SR. Can algal photosynthetic inorganic carbon isotope fractionation be predicted in lakes using existing models? Aquat Sci. 2006;68:142–153. [Google Scholar]
- 46.France RL. Scope for use of stable carbon isotopes in discerning the incorporation of forest detritus into aquatic foodwebs. Hydrobiol. 1996;325:219–222. [Google Scholar]
- 47.Jones RI, Grey J, Arvola L. Stable isotope analysis of zooplankton carbon nutrition in humic lakes. Oikos. 1999;86:97–104. [Google Scholar]
- 48.Solomon CT, et al. Terrestrial, benthic, and pelagic resource use in lakes: Results from a three-isotope Bayesian mixing model. Ecology. 2011 doi: 10.1890/10-1185.1. doi:10.1890/10-1185.1. [DOI] [PubMed] [Google Scholar]
- 49.Bastviken D, Cole J, Pace M, Tranvik L. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeo Cyc. 2004;18 GB3010. [Google Scholar]
- 50.Bastviken D, Cole JJ, Pace ML, van de Bogert MC. Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. J Geophys Res-Biogeosci. 2008;113 G02024. [Google Scholar]
- 51.DeMott WR. Utilization of a cyanobacterium and a phosphorus-deficient green alga as complementary resources by daphnids. Ecology. 1998;79:2463–2481. [Google Scholar]
- 52.Kirk KL. Suspended clay reduces daphnia feeding rate – behavioral mechanisms. Freshw Biol. 1991;25:357–365. [Google Scholar]
- 53.Kritzberg ES, Cole JJ, Pace ML, Graneli W, Bade DL. Autochthonous versus allochthonous carbon sources of bacteria: Results from whole-lake C-13 addition experiments. Limnol Oceanogr. 2004;49:588–596. [Google Scholar]
- 54.Baines SB, Fisher NS, Cole JJ. Dissolved organic matter and persistence of the invasive zebra mussel (Dreissena polymorpha) under low food conditions. Limnol Oceanogr. 2007;52:70–78. [Google Scholar]
- 55.Barnard C, Martineau C, Frenette JJ, Dodson JJ, Vincent WF. Trophic position of zebra mussel veligers and their use of dissolved organic carbon. Limnol Oceanogr. 2006;51:1473–1484. [Google Scholar]
- 56.Preston ND, Carpenter SR, Cole JJ, Pace ML. Airborne carbon deposition on a remote forested lake. Aquat Sci. 2008;70:213–224. [Google Scholar]
- 57.von Wachenfeldt E, Sobek S, Bastviken D, Tranvik LJ. Linking allochthonous dissolved organic matter and boreal lake sediment carbon sequestration: The role of light-mediated flocculation. Limnol Oceanogr. 2008;53:2416–2426. [Google Scholar]
- 58.DeMott WR, Tessier AJ. Stoichiometric constraints vs. algal defenses: Testing mechanisms of zooplankton food limitation. Ecology. 2002;83:3426–3433. [Google Scholar]
- 59.Ward EJ, et al. Including source uncertainty and prior information in the analysis of stable isotope mixing models. Environ Sci Technol. 2010;44:4645–4650. doi: 10.1021/es100053v. [DOI] [PubMed] [Google Scholar]
- 60.Finlay JC, Doucett RR, McNeely C. Tracing energy flow in stream food webs using stable isotopes of hydrogen. Freshwat Biol. 2010;55:941–951. [Google Scholar]
- 61.DeNiro MJ, Epstein S. Hydrogen isotope ratios of mouse tissues are influenced by a variety of factors other than diet. Science. 1981;214:1374–1376. doi: 10.1126/science.7313700. [DOI] [PubMed] [Google Scholar]
- 62.Wassenaar LI, Hobson KA. Stable-carbon and hydrogen isotope ratios reveal breeding origins of red-winged blackbirds. Ecol Appl. 2000;10:911–916. [Google Scholar]
- 63.Wassenaar LI, Hobson KA. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes Environ Health Stud. 2003;39:211–217. doi: 10.1080/1025601031000096781. [DOI] [PubMed] [Google Scholar]
- 64.Phillips DL, Gregg JW. Source partitioning using stable isotopes: Coping with too many sources. Oecologia. 2003;136:261–269. doi: 10.1007/s00442-003-1218-3. [DOI] [PubMed] [Google Scholar]
- 65.Solomon CT, et al. The influence of environmental water on the hydrogen stable isotope ratio in aquatic consumers. Oecologia. 2009;161:313–324. doi: 10.1007/s00442-009-1370-5. [DOI] [PubMed] [Google Scholar]
- 66.Post DM. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.