Abstract
Pax6 is a paired box containing transcription factor that resides at the top of a genetic hierarchy controlling eye development. It continues to be expressed in tissues of the adult eye, but its role in this capacity is unclear. Pax6 is present in the adult corneal epithelium, and we showed that the amount of Pax6 is increased at the migrating front as the epithelium resurfaces the cornea after injury (J. M. Sivak, R. Mohan, W. B. Rinehart, P. X. Xu, R. L. Maas, and M. E. Fini, Dev. Biol. 222:41-54, 2000). We also showed that Pax6 controls activity of the transcriptional promoter for the matrix metalloproteinase, gelatinase B (gelB; MMP-9) in cell culture transfection studies. gelB expression is turned on at the migrating epithelial front in the cornea, and it coordinates and effects aspects of epithelial regeneration (R. Mohan, S. K. Chintala, J. C. Jung, W. V. Villar, F. McCabe, L. A. Russo, Y. Lee, B. E. McCarthy, K. R. Wollenberg, J. V. Jester, M. Wang, H. G. Welgus, J. M. Shipley, R. M. Senior, and M. E. Fini, J. Biol. Chem. 277:2065-2072). We define here two positively acting Pax6 response elements in the gelB promoter. Pax6 binds directly to one of these sites through the paired DNA-binding domain. It binds the second site indirectly by interaction with AP-2α, a transcription factor that also exerts control over eye development. Pax6 control of gelB expression was examined in vivo by using a corneal reepithelialization model in mice heterozygous for a Pax6 paired-domain mutation (Sey+/−). A reduced Pax6 dosage in these mice resulted in a loss of gelB expression at the migrating epithelial front. This effect was correlated with an increase in inflammation and the rate of reepithelialization, a finding consistent with the phenotype of gelB knockout mice. Together, these data indicate that Pax6 controls activity of the gelB promoter through cooperative interactions with AP-2α and support an active role for Pax6 in maintenance and repair of the adult corneal epithelium.
Pax6 is an important regulator residing at the top of a genetic hierarchy controlling eye morphogenesis. The most common form of Pax6 (p46) contains both a paired DNA-binding domain (11, 17), as well as a homeodomain. However, these domains can be combined in multiple ways by differential splicing, allowing for considerable functional flexibility (12, 63). Loss of Pax6 function results in a complete lack of eye formation in the homozygous mouse small eye strain (Sey), and Drosophila eyeless, as well as defects in brain, neural tube, nose, and pancreas (24, 45). Sey heterozygotes are microphthalmic with progressive corneal opacities and lens and iris defects (24). Human diseases displaying a variety of similar problems have also been associated with mutations in Pax6, including Aniridia and Peter's anomaly (18, 21, 55). Interestingly, overexpression of Pax6 in transgenic mice creates phenotypes reminiscent of loss-of-function heterozygotes (48). These observations indicate that correct dosage is critical to proper Pax6 function.
Many of the defects in Pax6 heterozygotes are found in anterior structures of the eye, namely, cornea, lens, and iris. These tissues retain Pax6 expression into adulthood, where it has been postulated to be involved in their maintenance and differentiation (30, 47). In support of this hypothesis is our finding that Pax6 expression is increased in the corneal epithelium during wound healing (53). These observations suggest that Pax6 may be actively involved in coordinating adult tissue organization.
In order to orchestrate the complex events of eye development, Pax6 must ultimately control activity of a large number of downstream genes. Few Pax6 target genes have been identified to date. The list includes other transcription factors, such as the Drosophila genes eyes absent and sine oculus (20, 41), as well as the eye-specific markers crystallins, rhodopsin, and keratin 12 (9, 31, 50). Recently, we reported that the canonical p46 form of Pax6 controls activity of the promoter for the matrix metalloproteinase known as gelatinase B (gelB) or MMP-9 (53). This suggested the existence of a new class of Pax6 target genes, although it remained to be learned whether Pax6 controls gelB expression in vivo.
Matrix metalloproteases (MMPs) are a family of zinc proteases that play a fundamental role as regulators of tissue remodeling in embryonic development, and adult health and disease. Their substrates include structural components of the extracellular matrix, and a wide array of proteins, including cell adhesion molecules, cytokines, and other proteinases. MMPs function as true morphogens with the capacity to control tissue structure dynamics as both effectors and regulators (3, 52, 57). Most MMPs, including gelB, are expressed only upon demand, and expression patterns have provided important clues to understanding function. Control of MMP expression offers currently untapped potential for drug development to control MMP activity in disease.
gelB is expressed in a number of specific sites during embryologic development, including the eye (4, 5, 38, 46). A deficiency of gelB created by gene targeting causes developmental defects in long bone formation due to a delay in vascularization at the growth plate (56). However, other organs appear to form normally. This has enabled the use of gelB knockout mice for investigation of normal and pathological remodeling processes in adult organs and a number of studies have identified critical roles (7, 32, 37).
gelB is induced at the front of the corneal or skin epithelium migrating to resurface a wound and both underexpression or overexpression results in defective repair (14, 16, 37). Thus, like Pax6, the level of gelB expression must be precisely controlled for proper regulation of specific morphogenetic events. Studies from our lab and others have identified several regulatory factors necessary for gelB expression in the migrating epithelium, including AP-1, NF-κB, and Sp1 (15). However, we have found these are not sufficient to direct normal temporal and spatial gelB expression in vivo (38). In particular, the requirement for additional elements within the first 520 bp upstream of the transcription start site was observed (27, 38). Within this region are response elements for Pax6 (53). This region also contains several binding sites for transcription factor AP-2, which was found to have positive regulator influence on promoter activity in cell culture studies (13).
AP-2 denotes a small family of homologous genes that have important roles in eye development (6, 42, 58, 59). Interestingly, mice that are missing the AP-2α gene develop eye defects similar to Pax6 heterozygotes (42, 59). Expression of the AP-2α gene overlaps with Pax6 in corneal and lens epithelia, iris, and ciliary body, neural tube, retina, and brain (23, 42, 59). Like Pax6, AP-2α is constitutively present in adult corneal epithelial cells, where we have shown family members to be increased during wound healing (38).
In the present study we demonstrate that two regions of the gelB promoter respond to Pax6 activity. Pax6 binds the more distal site directly, whereas its mechanism of action at the more proximal site is mediated via an interaction with AP-2. In addition, we provide evidence that gelB is a true target of Pax6 in vivo during corneal reepithelialization.
MATERIALS AND METHODS
EMSA.
Nuclear extracts from corneal epithelial cells were prepared as described previously (38), essentially following the procedure of Dignam et al. (10). Aliquots of protein were frozen at −80°C and thawed just prior to use. The sequences of double-stranded oligonucleotides used for electrophoretic mobility shift assay (EMSA) are delineated in Table 1. Probes containing the canonical DNA-binding sites for transcription factor Sp1 was purchased from Promega (Madison, Wis.). PDcon encodes the optimized Pax6 paired-domain (PD)-binding site as described previously (11). The double-stranded oligonucleotide probes derived from the gelB promoter were synthesized by the Tufts University oligonucelotide facility.
TABLE 1.
Oligonucleotide probes used for EMSA
| Probe | Sequencea |
|---|---|
| GelB promoter probes | |
| GB(−467) | −492CTCCTTCCGCCCAGCTGGAGCCGGGA−467 |
| GB(−487) | −511TCCTGCCTCAGAGAGCCCACTCCT−487 |
| GB(−359) | −385TCAAGGGTGGGCCTGGGGTGGCACTCA−359 |
| GB(−359m) | −385TCAAGGGTGGGTTTGGGGTGGCACTCA−359 |
| Control probes | |
| PDcon (Pax6 PD site) | AATTTTCACGCTTGAGTTCACA |
| SP1con | ATTCGATCGGGGCGGGGCCAGC |
For EMSA radiolabeled DNA was incubated for 20 min at room temperature with equal amounts of nuclear extracts (1 to 2 μg) derived from rabbit corneal epithelium (Pelfreez, Rogers, Ariz.) prepared according to established protocol (53). In competition experiments, a 100-fold molar excess of unlabeled probe was incubated with nuclear lysates for 10 min before the addition of labeled oligonucleotides. For supershifts, protein-DNA complexes were incubated with antibodies for an additional 40 min at room temperature. Rabbit polyclonal NF-κB, Sp1, and pan-specific AP-2 antibodies for supershifts were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). The rabbit polyclonal Pax6 antibody targets the C-terminal region of the canonical p46 isoform and was purchased from Covance Co. (Richmond, Calif.).
DNA footprinting.
Pax6 PD/glutathione S-transferase (GST) fusions (PD/GST and GST control) were a gift of R. L. Maas (Harvard Medical School) and have been previously described (11). Recombinant protein was purified on glutathione-Sepharose 4B columns as recommended by the manufacturer (Amersham-Pharmacia Biotech, Piscataway, N.J.). The entire gelB promoter was linearized and end labeled with [32P]dATP at the 3′ end. Probe was then purified and footprinting with 10 μg of PD or GST protein was performed according to established protocol (60). A sequencing reaction was run concurrently on the same probe sequence by using the fmol DNA cycle sequencing system (Promega) and run parallel to the footprint lanes in order to identify the protected sequence.
Transient-transfection analysis.
Fresh young rabbit eyes were purchased from Pelfreez, and corneal epithelial cells were isolated according to our standard lab protocol (53). The cells were plated in 35-mm six-well tissue culture dishes. A full-length human Pax6 expression plasmid (canonical p46 form) was a gift from R. Maas and has been previously described (11). Full-length human AP-2α and dominant-negative AP-2α (AP-2αdn) have been previously characterized (61). gelB promoter-reporter DNA constructs used in the present study were derived from the rabbit gelB gene (13). The development of Pr42 was previously described (38). Pr42(−PD) was created by deleting bases −466 to −491, which corresponds to the region of the promoter footprinted by the Pax6 PD. Pr42(mAP-2) has two C-to-T base mutations at −373 and −374 that disrupt an AP-2 binding site (13, 61). Both constructs were created by using a PCR-based site-directed mutagenesis method. The reporter gene used in each construct is the gene for β-galactosidase (β-Gal), and each experiment was cotransfected with a pCAT control plasmid (Promega) for the normalization of transfection data.
In a standard transfection, 0.5 μg of a gelB construct was cotransfected with either 0.5 μg of the Pax6 expression plasmid or control DNA. Each transfection reaction also contained 0.5 μg of chloramphenicol acetyltransferase (CAT) control vector (19). Total DNA transfected was equalized in all cases with empty PCDNA3 expression vector. DNA was introduced into cells by using Lipofectamine Plus reagent according to the procedure recommended by the manufacturer (Invitrogen), and all transfections were done at least three separate times in triplicate. Cells were collected 24 h after treatment, and cell lysates were assayed for β-Gal activity (26). An equivalent volume of each lysate was assayed for CAT activity and then normalized to β-Gal activities.
Pulldown assays and immunoprecipitations.
A six-polyhistidine tag was added to the full-length Pax6 gene plasmid by PCR with the primer 5′-ATACATAGCGGCCGCCATGCATCATCATCATCATCATCAGAACAGTCACAGCGGAGTG-3′. Also, additional Pax6 deletion constructs were made by using primer 5′-ATACGATCTCGAGTGACTAGGAGTGTTGCTGGC-3′ to make a C-terminal deletion at amino acid 297, primer 5′-ATACGATCTCGAGTCGCATCTGAGCTTCATCCG-3′ to make a C-terminal deletion at amino acid 217, and primer 5′-TACATAGCGGCCGCCATGCATCATCATCATCATCATAGCAAAAGCAACAGATGGGCGCA-3′ to make an N-terminal deletion at amino acid 131.
For polyhistidine pulldown assays, 4 μg of tagged Pax6 or control His constructs were each cotransfected with AP-2α expression vector into 10-cm dishes of rabbit corneal epithelial cells, isolated as described above, at ca. 70% confluency. Cells were isolated as described above but plated in this case in 10-cm tissue culture dishes. Transfections were performed with Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's instructions. Cells were collected after 3 days, and lysates were prepared by standard procedures (37). For each reaction, 100 μl of cell lysate was kept and frozen separately from each reaction as input samples. Each pulldown used the total lysate from one 10-cm dish, and all steps were performed at 4°C. Lysate was loaded onto Ni-NTA microcolumns (Qiagen, Valencia, Calif.) and purified according to the manufacturer's instructions. Bound samples were eluted in buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 0.05% NP-40, and 250 mM imidazole and then stored at −80°C. Samples were then Western blotted with monoclonal AP-2α antibody 3B5 supernatant (1:8 dilution; Developmental Studies Hybridoma Bank). Expression of His-tagged proteins was confirmed by blotting with an anti-His antibody (Sigma).
For immunoprecipitation constructs were transfected as described above, but in this case wild-type AP-2α or control plasmid were cotransfected with wild-type Pax6. Cell lysates were prepared as described above. Lysates were precleared by adding 20 μl of protein G plus protein A-agarose beads (Calbiochem, San Diego, Calif.) and 10 μl of rabbit immunoglobulin G. AP-2α antibody was added, and immunoprecipitations were carried out with protein G plus protein A-agarose beads according to standard protocols. Samples were blotted as described above with Pax6 and AP-2α antibodies.
Corneal surgeries.
Adult wild-type or Sey/+ mice (6 to 8 weeks old) were anesthetized by an intraperitoneal injection of 2% Avertin (0.017 ml/g of body weight). Residual eye reflexes were blocked by topical application of 0.5% proparacaine to the corneal surface (Alcon, Fort Worth, Tex.). The epithelium was debrided in the center of each cornea to a 1-mm diameter with an algerbrush by using a 1-mm trephine to first outline the area. Antibiotic ointment was applied to the eyes after surgery. Mice were allowed to recover and then sacrificed 8 h after surgery at a time when the migrating epithelial sheet had partially resurfaced the scrape region. Both reepithelializing and contralateral control eyes were removed and processed for immunohistochemistry or zymography, as described below.
Sey/+ eyes are microophthalmic due to the dosage deficiency of Pax6; therefore, extra care was taken to ensure that the wound areas between wild-type and Sey/+ corneas were no greater than 1 mm in diameter. For unexplained reasons, Sey/+ phenotypes varied in severity within each litter; however, only eyes that had proceeded to eyelid opening were used for wound experiments.
Immunohistochemistry.
Cryostat sections were prepared from control or wounded wild-type and Sey/+ adult mouse eyes. Sections were fixed in 4% paraformaldehyde and then incubated with a rabbit polyclonal antibody to mouse gelB at a 1:1,000 dilution (a gift from B. Senior [2]), and a rat polyclonal antibody to the mouse inflammatory cell marker, Mac-1, at 1:100 (Chemicon, Temecula, Calif.). Anti-rabbit fluorescein isothiocyanate (gelB)- and anti-rat rhodamine (Mac-1)-conjugated secondary antibodies were used at a 1:50 dilution. A Hoechst nuclear stain was included in the final wash (1:1,000 dilution). Pax6 antibody was used at a 1:100 dilution (Covance), followed sequentially by AP-2α at 1:200 (Sigma). Fluorescence staining was visualized on a Nikon E400 microscope, and an image intensity analysis was performed by using Image Pro Plus software (Media Cybernetics, Silver Spring, Md.).
Zymography.
Corneal epithelial defects were created on adult wild-type and Sey/+ mice. After 8 h the epithelium from the wound region was collected by scraping with a scalpel blade and transferred to a microfuge tube containing phosphate-buffered saline. Tissue from two experiments was combined for each tube. The samples were lysed in radioimmunoprecipitation assay buffer containing 1 mM aprotinin and stored at −80° until use. The zymography technique was done according to procedures adapted from Heussen and Dowdle (22). Lysates were then run on a 2% gelatin zymography ready gel (Bio-Rad) and further processed according to a standard protocol.
RESULTS
Two Pax6-binding sites on the gelB promoter.
In a previous study we had identified a positive response region for the canonical p46 form of Pax6 on the gelB promoter located between bases −312 and −522 (53). In the present study we set out to identify the Pax6-binding elements within this region. A series of EMSAs was performed on lysates from rabbit corneal epithelial cells by using oligonucleotide probes to evolutionarily conserved sequences within the positive response region (Table 1). Rabbit corneal epithelial cells in culture express gelB constitutively, much as occurs at the migrating wound edge in vivo (1). Also, they constitutively make low amounts of both Pax6 and AP-2 proteins, which are increased in levels upon wounding in vivo (38, 53).
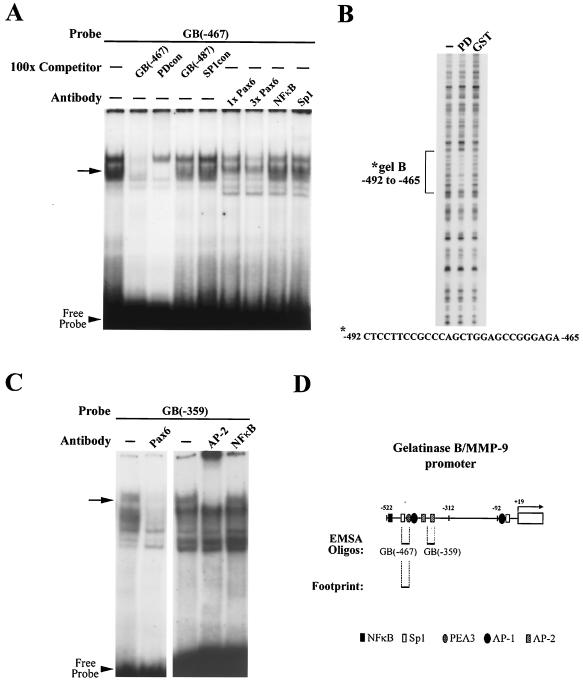
Figure 1A shows a representative EMSA with the GB(−467) oligonucleotide as a probe to bases −467 to −492 see (also Fig. 1D). A shifted band was effectively competed away by the addition of 100-fold unlabeled competitor matching the Pax6 PD-binding consensus sequence (PDcon) and also by increasing amounts of Pax6 antibody (1× and 3× Pax6, Fig. 1A, arrow). An upper band in Fig. 1A is also competed away by the antibody but not by the PD probe. Since the antibody is targeted to the Pax6 C-terminal region, this upper band may consist of a Pax6 splice variant, such as the slightly larger 5a form, or it may be simply another higher mobility complex containing p46 Pax6. In contrast, neither an irrelevant gelB promoter sequence (GB-487) nor an SP1 binding consensus probe (SP1con) had any competitive effect. Likewise, antibodies to NF-κB and SP1 created no supershift. These results support the identification of the GB(−467) probe region as a specific Pax6-binding site.
FIG. 1.
Pax6 binding is found at two sites on the gelB promoter. (A) EMSA with GB(−467) as a probe. The arrow indicates a shifted band that is removed by a Pax6 PD consensus sequence (PDcon) and Pax6 antibody but not control oligonucleotides [GB(−487) and SP1con] or control antibodies (NF-κB and SP1). (B) DNase footprinting assay with recombinant Pax6/GST PD protein (PD) protected bases −492 to −465 of the gelB promoter that were not protected by GST alone. This sequence corresponds to the GB(−467) oligonucleotide sequence. (C) EMSA with GB(−359) as a probe. The arrow indicates a shifted band that is removed by antibody to Pax6 and AP-2, but not NF-κB. (D) Representation of the gelB promoter showing previously identified transcription factor binding sites and the locations of the new Pax6 elements.
The second binding site at bases −359 to −385 overlaps with a previously characterized binding sequence for AP-2 transcription factors (13) (Fig. 1D). Figure 1C shows an EMSA with probe GB(−359) to this site. In this case a shifted band was removed by the addition of Pax6 antibody and supershifted by a panspecific AP-2 antibody but was not altered by an antibody to NF-κB. The presence of Pax6 and AP-2 in a complex at this binding site introduced the possibility of interactions between the two proteins that were investigated in further experiments.
Both the GB(−467) and GB(−359) sequences have partial homology to the PD-binding consensus. To learn whether Pax6 binds these DNA elements directly, a DNase protection footprinting assay was performed on the gelB promoter by using purified GST-Pax6 PD. The PD protein protected a region spanning bases −465 to −492, which was not protected by GST alone (Fig. 1B). This sequence corresponds almost identically to the GB(−467) probe, which indicates that this site does indeed bind Pax6. The promoter sequence corresponding to probe GB(−359) did not footprint, which led us to conclude that the PD does not bind alone to this sequence.
In summary, the EMSA search produced two sites which bound to complexes containing Pax6; one at bases −467 to −492, and the other between −359 and −385 [oligonucleotides GB(−467) and GB(−359), respectively]. A stylized map of the gelB promoter showing the locations of the probes and footprinted region is presented in Fig. 1D. The locations of previously characterized response elements for NF-κB, SP-1, AP-1, and PEA3 transcription factors have also been included. Note that these transcription factors are probably present in the cell lysates, and their binding sites formed the basis for the control oligonucleotides used for gel shifts. Both of the Pax6-binding sites were subsequently investigated in detail to determine the mechanisms of the Pax6 response.
Pax6 PD partially controls gelB promoter activity.
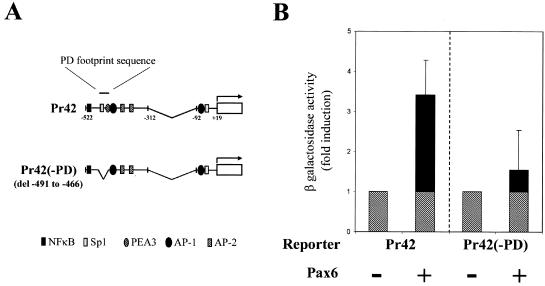
In order to test the functionality of the GB(−467) PD binding site, cotransfection experiments were conducted by using a minimal gelB promoter construct, Pr42. Pr42 contains bases −312 to −522 of the gelB promoter driving a LacZ reporter. This construct was known from earlier study to respond positively to Pax6 stimulus (53). In a typical experiment Pr42 was transfected into cultured corneal epithelial cells, which are normally quiescent but have the capacity to be induced to make gelB (13, 52). Cotransfection with a human p46 Pax6 expression vector increases reporter β-Gal activity by three- to fourfold (after normalization to a constitutive chloramphenicol acetyl-transferase expression vector: cytomegalovirus [CMV]-CAT). For this experiment the GB(−467) PD-binding site was deleted from Pr42, creating construct Pr42(−PD) (Fig. 2A). When Pr42(−PD) was cotransfected as before the response to Pax6 was reduced to only 1.5-fold (Fig. 2B). Taken together, these data indicate that bases −465 to −492 of the gelB promoter contain a functional Pax6 PD-binding site, but that this site accounts for only part of the promoter response. We therefore turned our attention to the second Pax6 response element we had identified.
FIG. 2.
Pax6 directly controls gelB promoter activity through the PD-binding site. (A) Diagram depicting the two gelB promoter constructs used in panel B: Pr42, which is a minimal gelB promoter construct that responds to Pax6, and Pr42(−PD), which has the PD-binding sequence from −491 to −466 deleted. (B) Transfection results with Pr42 and Pr42(−PD) in corneal epithelial cells. Cotransfection of Pax6 with Pr42 induces a typical increase in β-Gal reporter activity that is partially lost when the PD-binding site is deleted in Pr42(−PD).
Pax6 also controls the gelB promoter through interactions at an AP-2 binding site.
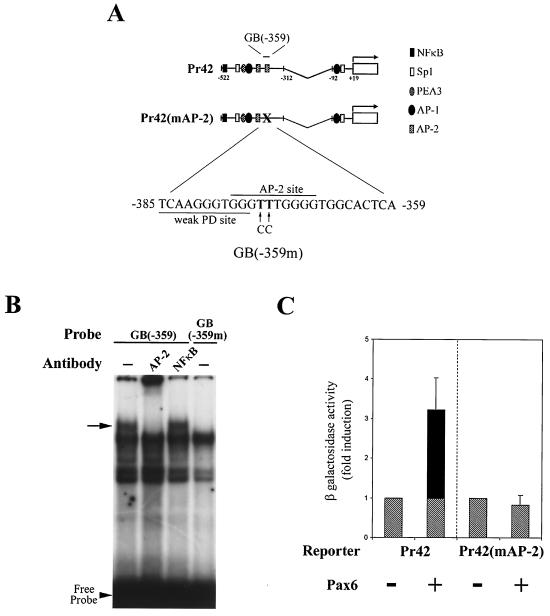
The second Pax6-binding element identified in the EMSA screen, located at bases −359 to −385, overlaps a previously characterized binding site for the transcription factor AP-2 (13). This juxtaposition led us to investigate whether the two proteins interact with each other. As an initial test of this hypothesis, AP-2 binding was blocked at the site by mutation of two key bases in the Pr42 construct, labeled Pr42(mAP-2) (Fig. 3A). The substitution of two thymines for cytosines at bases −373 and −374 mutates two critical residues and renders the site inactive to bind AP-2 proteins (60). To verify that AP-2 binding had been blocked by the mutation, an EMSA was performed by using the corresponding probe GB(−359m), which contains the same changes. A band supershifted by pan-specific AP-2 antibody when the unaltered GB(−359) probe is used disappears when GB(−359m) is used (Fig. 3B).
FIG. 3.
Pax6 also controls gelB promoter through interactions at an AP-2 binding site. (A) Diagram depicting the two gelB promoter constructs used in panel C: Pr42 minimal gelB promoter and Pr42(mAP-2), which has a nonfunctioning AP-2 binding site. (B) EMSA with GB(−359) and GB(−359m) as probes. GB(−359m) contains mutations corresponding to those in Pr42(mAP-2). The shifted band indicated by the arrow is removed by an AP-2 antibody when the GB(−359) probe is used. The same band is absent when the probe GB(−359m) is used. (C) Transfection results with Pr42, which is induced in response to cotransfection with Pax6 in corneal epithelial cells. Mutation of the AP-2 binding sequence in Pr42(mAP-2) eliminates the Pax6 response.
In experiments similar to those described above, Pr42 and Pr42(mAP-2) were cotransfected with or without Pax6 into corneal epithelial cells. Strikingly, use of the Pr42(mAP-2) reporter resulted in a complete loss of response to Pax6 (Fig. 3C). These data show that AP-2 binding is necessary for Pax6 activity at the −359 to −385 site and may also influence Pax6 activation at the more distal site.
The AP-2α gene interacts with Pax6 in corneal epithelial cells.
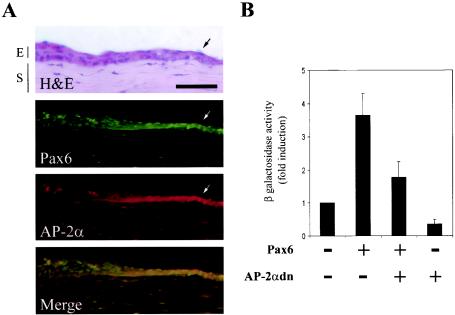
The AP-2 gene family currently consists of four separate genes: α, β, γ, and δ. Although each gene is highly homologous, they have separate functions, as evidenced by distinct expression patterns (23). As discussed, the AP-2α gene has overlapping expression with Pax6 in many tissues. However, due to availability we have in the past used a pan-specific AP-2 antibody for experiments in the cornea. In order to confirm AP-2α as a candidate protein for interactions with Pax6 in the corneal epithelium, we used a monoclonal AP-2α antibody to stain sections of reepithelializing cornea. We observed that staining was present in the basal epithelial cells and was upregulated during corneal reepithelialization (Fig. 4A, AP-2α panel). This expression pattern correlates with the increase seen in a similar section stained for Pax6 (Fig. 4A, Pax6 panel). Figure 4A also shows an adjacent section stained with hematoxylin and eosin to outline the relevant corneal epithelial and stromal layers and wound edge.
FIG. 4.
gelB promoter response to Pax6 is reduced by blocking AP-2α activity. (A) Immunohistochemical analysis of reepithelializing mouse corneas. Sections were either stained with hematoxylin and eosin or with AP-2α and Pax6. The arrows point to the increased Pax6 and AP-2α staining at the migrating wound edge. The bottom panel shows a merged image indicating Pax6 and AP-2α staining overlap in yellow. Bar, 50 μm; E, epithelium; S, stroma. (B) Transfection results in which the Pr42 gelB promoter construct was cotransfected with Pax6 and dominant-negative AP-2αdn. The inductive response to Pax6 is reduced upon cotransfection with AP-2αdn.
In order to test for functional interactions with Pax6 in corneal epithelial cells, we used an interferring or “dominant-negative” form of the human AP-2α gene to block endogenous AP-2 activity. This construct, labeled AP-2αdn, is missing its N-terminal activation domain, but it retains a DNA-binding and dimerization domain (61). In our experiment, the gelB promoter construct Pr42 was cotransfected with either Pax6 alone or Pax6 and AP-2αdn. The addition of AP-2αdn reduced the normal Pax6 response seen with Pr42 (Fig. 4B). These results confirm those observed in Fig. 3C, where mutation of the AP-2 response element also blocked Pax6 response in Pr42. Additional experiments cotransfecting full-length AP-2α and Pax6 were performed; however, there was no significant increase in reporter activity over each factor alone (data not shown). This latter result is difficult to interpret, however, since corneal epithelial cells already constitutively express low levels of AP-2α. Also, previous work has shown that the gelB promoter in culture does not respond robustly to AP-2α unless the response elements are used in conjunction with only the minimal basal promoter (38).
Pax6 and AP-2α proteins interact physically.
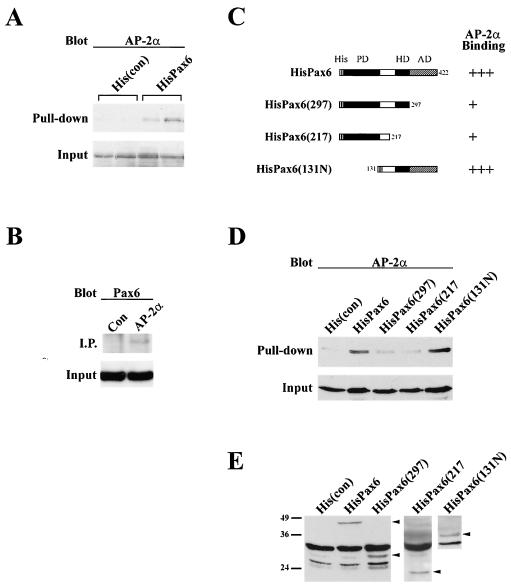
Transfection and gel shift experiments provided functional evidence for interactions between Pax6 and AP-2α. Therefore, we proceeded to test their ability to bind to each other in a series of immunoprecipitation and pull-down experiments (Fig. 5). For immunoprecipitations, human Pax6 and AP-2α expression vectors were cotransfected into corneal epithelial cells. A monoclonal antibody to AP-2α was used to immunoprecipitate lysates and, after sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the mixture was blotted with a polyclonal antibody to Pax6. A Pax6 band could be seen in the AP-2α transfected lysates but not in transfected controls (Fig. 5B).
FIG. 5.
Interactions between Pax6 and AP-2α proteins. (A) Duplicate pull-down experiments with polyhistidine-tagged Pax6 (HisPax6), followed by blotting with a monoclonal antibody to AP-2α. AP-2α protein is pulled down from lysates containing HisPax6 but not from those transfected with control His vector [His(con)]. (B) Immunoprecipitation with AP-2α monoclonal antibody blotted for Pax6. A Pax6 band is present in the AP-2α-transfected lysate but not the control lysate. (C) Diagram depicting the various Pax6 deletion constructs used in panel D and their AP-2α binding activities. Abbeviations: His, N-terminal polyhistidine tag; HD, homeodomain; AD, activation domain. Constructs which deleted the AD and HD removed AP-2α binding [HisPax6(297) and HisPax6(217)], whereas constructs that retained those domains continued to bind AP-2α [HisPax6 and HisPax6(131N)]. (D) Representative His pull-down results from panel C showing decreases in AP-2α binding to HisPax6(297) and HisPax6(217). (E) Anti-His blot showing comparable expression levels of truncated proteins in cell lysates.
There were technical difficulties with the reverse pull down because the 50-kDa size of AP-2α is similar to the size of the antibody light chain. Therefore, human Pax6 expression constructs were polyhistidine tagged at their N termini and cotransfected into corneal epithelial cells with AP-2α. Lysates were then pulled down by using a nickel column and Western blotted to determine the presence or absence of AP-2α. Figure 5A shows duplicate samples transfected with either control His vector [His(con)] or HisPax6. AP-2α protein is clearly present in the eluates from cells transfected with His Pax6 but not in the control.
In order to narrow down the region of Pax6 responsible for binding AP-2α, a series of Pax6 deletion constructs were created. Each construct broadly removes a functional region of the Pax6 protein: HisPax6(297) is missing the C-terminal activation domain, HisPax6(217) has no activation domain or homeodomain, and HisPax6(131N) has a deletion at its N terminus that removes the PD. Representations of these constructs are shown in Fig. 5C. Each vector was cotransfected into corneal epithelial cells with AP-2α, pulled down, and blotted as described above. Lysates were also blotted with anti-His antibodies to ensure comparable expression of truncated proteins (Fig. 5E). Deletion of either the activation domain [HisPax6(297)] or the activation and homeodomain [HisPax6(217)] removed most AP-2α binding; however, deletion of the PD [HisPax6(131N)] had no effect (Fig. 5C and D). These data taken together indicate that Pax6 binds to AP-2α through its C-terminal domains.
Pax6 controls gelB expression in vivo.
So far, the evidence presented for Pax6 control of gelB expression has been largely from in vitro studies and correlative data. A useful tool for studying the regulation of gelB expression in vivo is a well-established corneal wound-healing model. In these experiments, the epithelial layer is removed by abrasion in a circumscribed region in the center of the cornea. The epithelium is then allowed to resurface the corneas until the defect is partially closed, at which time the animals are sacrificed and the corneas are removed for analysis. In normal animals the corneal epithelial cells at the migrating wound edge begin to express high levels of gelB within an hour of injury, whereas expression in the unwounded contralateral cornea remains undetectable (34, 52).
An ideal experiment for our study would be simply to compare the levels of gelB induction in wild-type mouse corneas versus corneas without Pax6. Unfortunately, homozygous deletions of Pax6, such as in the Small eye mouse (Sey), leads to a complete lack of eye formation. We did have at our disposal, however, heterozygous Sey mice for our studies. In these mice, eye development proceeds, but they are microophthalmic, and defects are evident in the cornea, lens, iris, and ciliary body (25). Based on past observations, we predicted that the reduced Pax6 dosage might be sufficient to observe an effect on gelB induction in the corneal scrape model.
In these experiments the epithelium was removed from a 1- mm diameter circle in the center of wild-type and heterozygous Sey (Sey/+) corneas. This smaller wound size was chosen so that the wound region was small enough to produce a minimum of disturbance to stem cells in the corneal periphery in both wild-type corneas and the smaller Sey/+ corneas. Initially, the eyes were allowed to heal for 20 h, which typically allows wild-type eyes to almost resurface. We found that after 20 h the Sey/+ eyes had completely resurfaced, and so no scrape region could be defined (data not shown). We therefore switched to a much-shorter 8-h healing period in order to catch the still-healing wound edge. Corneas were then sectioned for immunohistochemistry or, alternatively, the epithelium was collected for zymography (Fig. 6 and 7).
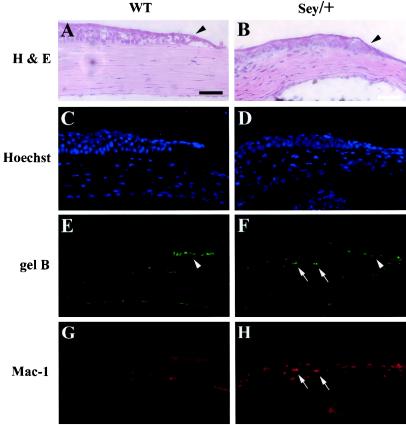
FIG. 6.
Pax6-deficient mice make little gelB and have increased inflammation during corneal reepithelialization. Epithelial defects (∼1 mm long) were produced on the corneas of wild-type (WT) and Pax6 heterozygous (Sey/+) mice. After 8 h, the eyes were sectioned and stained with hematoxylin and eosin (A and B) or triple stained with Hoechst nuclear stain (C and D), antibodies to gelB (E and F), or inflammatory cell marker Mac-1 (G and H). Sey/+ eyes showed a lack of gelB staining at the wound edge (F, arrowhead) and a dramatic increase in Mac-1 staining cells (H, arrows). Bar, 50 μm.
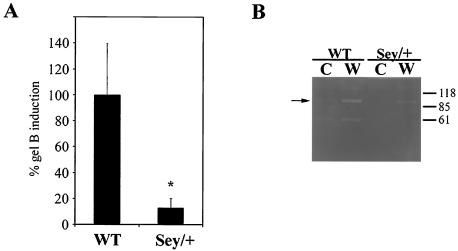
FIG. 7.
Quantification of gelB reduction in Sey/+ reepithelialization experiments. (A) Fluorescence images from three corneal epithelial scrape experiments were analyzed for intensity of increased gelB staining at the wound edge after 8 h. Wild-type eyes (WT) showed a characteristic increase in gelB antibody staining at the wound edge, arbitrarily set to 100%. In comparison, Pax6-deficient eyes (Sey/+) had only a 16% increase over background (P < 0.002). (B) Gelatin zymography of lysates scraped from unwounded (C) or wounded (W) corneas from either wild-type (WT) or Pax6-deficient (Sey/+) eyes. White bands indicate gelatinolytic activity on the gel. Wild-typlysates had no gelatinolytic activity in unwounded control, but wounded samples show an increase in activity at 92 kDa as expected. Sey/+ lysates also showed no activity in unwounded controls; however, there was also little increase in wound samples (arrow).
The corneal epithelia of the Sey/+ mice were thinner, with fewer stratified layers than the epithelia of wild-type mice (Fig. 6A and B). Nevertheless, wound reepithelialization occurred much as in the wild-type mice. Interestingly, although wound reepithelialization was normal, our clear impression was that it occurred considerably faster (data not shown), similar to the phenotype of gelB knockout mice. In wild-type mice, the wound edge stained brightly with an antibody to gelB as expected (Fig. 6E, arrowhead). In dramatic contrast, Sey/+ mice showed no increase in anti-gelB staining at the wound edge (Fig. 6F, arrowhead), a finding consistent with the gelB-deficient reepithelialization phenotype. Unwounded wild-type and Sey/+ mice did not stain (data not shown).
Sey/+ mice did show increased gelB staining, however, in select cells of the underlying corneal stromal layer (Fig. 6F, arrows). Upon closer inspection these cells appeared as if they might be inflammatory cells invading the wound site. Therefore, sections were costained with antibodies to the inflammatory cell marker Mac-1 (Fig. 6G and H). Wild-type scrape corneas showed no Mac-1 staining, as would be expected (Fig. 6G), but the Sey/+ scrapes showed a marked increase in Mac-1 staining (Fig. 6H). These Mac-1-positive cells proved to be the same as the gelB-staining stromal cells seen in Fig. 6F. The increased inflammation in Sey/+ corneas is important in two ways. First, since the inflammatory cells in the corneal stroma still make gelB, this serves as an internal control and indicates that the loss of gelB induction at the epithelial wound edge is a specific regulatory phenomenon. Second, the increased inflammatory cell invasion appears to be similar to what we recently reported seeing in corneal wound healing in gelB knockout mice (37). This similar inflammatory phenotype supports the observed lack of gelB staining in Sey/+ corneas.
Quantification of gelB absence in Sey/+ corneal wounds.
Two different approaches were taken to quantify the difference in gelB induction between wild-type and Sey/+ corneas (Fig. 7). First, image intensity analysis of three separate gelB staining experiments was performed by using the increase in gelB staining at the wound edge compared to the background. Sey/+ corneal scrapes displayed a significant decrease of 87% in gelB induction compared to the wild type (P < 0.002 [Student t test]) (Fig. 7A). A second way to quantify the loss of gelB induction was the use of zymography analysis. This technique allows for the separation and activation of gelB on a gelatin substrate included in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. In our experience this method is an extremely sensitive way to compare changes in amounts of proteases in a tissue. Epithelial lysates from the central region of either control or scraped corneas were isolated and run on a gelatin zymogram gel. After reactivation and incubation, a 92-kDa gelatinolytic band corresponding to gelB could be detected in wound lysates from wild-type mice. The same band was markedly decreased in wounded Sey/+ corneas (Fig. 7B). Lysates from unwounded wild-type and Sey/+ mice did not create a band. The lower 60-kDa band in Fig. 7B could be a degradation product of gelB but is probably GelA, a related MMP that recognizes similar substrates. The absence of this band in the Sey lanes is probably not due to Pax6 control over its transcription, since GelA is constitutively found throughout the body and is unique among MMPs in that it does not seem to be transcriptionaly regulated (15). Note that the 60-kDa band is characteristically present in both wounded and unwounded control corneas. The lack of these bands in the Sey lanes may be due to the lack of tear film or aqueous humour in which wild-type corneas are normally bathed. Therefore, we were able to confirm by zymography analyses the lack of gelB induction in Sey/+ mice observed immunohistochemically.
DISCUSSION
Pax6 is a powerful morphogenic regulator whose mechanisms of activity and downstream targets remain poorly understood. An intriguing question is how relatively global expression of Pax6 in the eye can be translated into tissue-specific effects on patterning and differentiation. We had previously reported that the common p46 form of Pax6 activates the promoter for gelB, an MMP directly involved in the formation and maintenance of tissue architecture (53). Here we have presented evidence that this regulation occurs through both direct PD binding and cooperative interactions with AP-2α, and we show its importance in the adult in vivo by using a corneal wound healing model.
Through an EMSA screen, we identified two possible Pax6-binding elements on the gelB promoter and proceeded to investigate the regulatory mechanisms at these sites. One site, located between bases −467 and −492 binds the Pax6 PD directly, as shown by DNase footprinting analysis. However, loss of the PD-binding sequence at this site only partially removes responsiveness to Pax6 (Fig. 2). The other site, located between bases −359 and −385, works indirectly through novel interactions with the transcription factor AP-2α. At this site, mutagenesis of the AP-2 binding sequence completely removed Pax6 response (Fig. 3). Cotransfection with a dominant-negative AP-2α construct was similarly able to reduce the Pax6 response (Fig. 4). Finally, immunoprecipitation and pull-down assays confirmed that Pax6 physically interacts with AP-2α through its C terminus (Fig. 5). These data support a model in which Pax6 controls gelB promoter activity through a combination of direct PD binding and cooperative interactions with AP-2α. In this scenario, AP-2α binding to its site may also reinforce or recruit Pax6 to an overlapping promoter sequence. Future analyses of the DNA-binding mechanisms involved will be needed to clarify whether the two binding elements cooperate or act independently of each other.
Interactions between Pax6 and AP-2α.
At present the AP-2α family consists of four highly homologous genes: AP-2α, AP-2β, AP-2γ, and AP-2δ (36, 39, 43, 65). Each gene has both overlapping and distinct expression patterns; however, they all bind similar promoter sequences as homodimers (40, 62). Expression of the AP-2α gene shares a number of similarities to Pax6. AP-2α is found during the development of the neural tube, forebrain, facial prominences, lens and corneal epithelia, and retina (23, 42, 59). In adult eyes AP-2α is found in the basal layer of the corneal epithelium, the lens epithelium, the iris and ciliary body, and the retinal ganglion and inner nuclear layers (59). AP-2α knockout mice exhibit a failure in cranial neural tube closure, exencephaly, and a variety of craniofacial skeletal deformities and develop eye defects similar to those of Pax6 heterozygotes. In an effort to isolate distinct roles of AP-2α, mice chimeric for knockout cells were studied. When the chimeric cells were found in the eye, these mice developed isolated lens and corneal defects (42, 59).
Although Pax6 and AP-2α exhibit overlapping expression patterns in many tissues, they also have distinct differences. For example AP-2α is found in the developing limb bud mesenchyme, and Pax6 is found in pancreatic acini (35, 47). Combinatorial interactions between multiple transcription factors are increasingly found to direct specific transcriptional events not specified by either factor alone. For example, interactions between AP-2 proteins and a number of other transcription factors have been implicated in controlling differentiation of the epidermis (51). We propose that interactions between Pax6 and AP-2α may modulate a specific subset of genes in particular tissues such as the cornea. In support of this model is a recent report from of a mouse strain harboring a Pax6 mutation that truncates the C-terminal end of the protein (33). These mice develop milder defects than Sey mice (in which the entire protein is deleted) that are limited to progressive corneal opacifications. It is interesting that the C-terminal domain of Pax6 deleted in these mice is the same region that interacts with AP-2α. It may be that cooperative interactions between Pax6 and AP-2α are necessary for normal corneal epithelial development and maintenance.
In recent years a number of studies have found evidence of interactions between Pax6 and cooperative partner proteins to influence regional gene expression. For example, Pax6 interacts with the transcription factor SOX2 to synergistically induce the γ crystallin promoter during lens placode formation (29). Also in the lens, Pax6 has been shown to bind to the retinoblastoma protein (8). In pancreas, Pax6 has been shown to interact with Maf and cdx-2 transcription factors to cooperatively activate the glucagon gene promoter (28, 44). Interestingly, the Pax6/Maf study was unable to find a similar combinatorial effect on the αA-crystallin promoter, even though both proteins are present during lens development and transactivate αA-crystallin separately (44). Here we have presented evidence for cooperative interactions between Pax6 and AP-2α in the cornea. To our knowledge the present study is the first to propose a mechanism for the regulation of specific corneal genes by Pax6 or which provides an understanding of the interaction between Pax6 and AP-2α. Taken together, all of these studies suggest an evolved mechanism whereby Pax6 controls tissue-specific gene expression through interactions with a variety of local proteins.
Pax6 as a regulator of gelB expression.
Recent years have seen revisions in our understanding of MMP function. Whereas previously they were thought to be simply degradative in nature, MMPs are now understood to be deeply integrated into the regulatory processes underlying tissue formation and maintenance. MMPs have been implicated in cytokine activation, cleavage of cell adhesion molecules, and the creation of biologically active fragments (3, 57). For example, besides basement membrane components, gelB is also capable of proteolytically activating latent TGFβ on the cell surface (64). In the reepithelializing corneas of gelB knockout mice, we observed an accumulation of the provisional matrix component fibrin(ogen). However, we also noted an increase in the rate of epithelial sheet migration and cell replication, as well as premature infiltration of the wound by inflammatory cells. These changes were correlated with a delay in activation of Smad-2 transcription factor and a premature increase in the levels of the inflammatory cytokine interleukin-1α (37). In light of these newly appreciated functions it is doubly important to understand the factors regulating gelB expression.
We were able to test the influence of Pax6 dosage on gelB expression with heterozygous Sey mice (Sey/+). Unlike homozygotes, which do not form eyes, in Sey/+ mice, eye development proceeds, although the eyes are microphthalmic. Also, these animals develop corneal opacities characterized by an epithelium that fails to properly stratify (25). We used a corneal reepithelialization model to determine whether we could observe changes in the normal gelB induction at the wound edge in Sey/+ mouse corneas. An equal region of epithelium was removed from wild-type and Sey/+ corneas. After an 8-h healing period little, if any, increase in gelB was detectable in Sey/+ corneas by either immunohistochemistry or zymography compared to the normal wild-type increase (Fig. 6 and 7).
Two additional observations correlated the lack of gelB activation seen in Sey/+ corneas with the phenotypes observed in reepithelializing gelB knockout mice. First, in gelB−/− mice we observed increased inflammatory cell invasion after scraping, as observed by staining with the neutrophil marker Mac-1. In the present study we saw a similar increased inflammatory cell invasion in Sey/+ mice. Interestingly, these Sey/+ inflammatory cells still stained as in the wild-type animals with gelB antibody, whereas the wound edge did not. This observation supports the conclusion that Pax6 control over gelB expression is corneal epithelium specific. A second, indirect, observation is that in initial experiments, eyes were allowed to heal for 20 h, a time that in wild-type mice typically allows the cornea to almost reepithelialize. However, after this time we were unable to detect a wound edge in Sey/+ mice since their corneas had completely healed over. Since care was taken to ensure that the wound size remained constant between control and experimental animals, this observation correlates with the increased reepithelialization rate seen in gelB knockout mice (37). Further experiments directed at migration and proliferation rates of corneal epithelial cells from Sey/+ or chimeric mice will help clarify the influence of Pax6 over these processes. The similarities between Sey/+ and gelB knockout corneas support our conclusion that proper dosage of Pax6 in these cells is necessary for gelB promoter activation.
There are a number of other transcription factors known to induce gelB expression, such as NF-κB, SP1, and AP-1 (15). These other factors, however, are much more broadly and generally expressed than Pax6. We propose that Pax6 and AP-2α cooperate to regulate gelB expression in a tissue-specific manner, acting in this case to localize and/or induce gelB expression in the reepithelializing cornea. This interpretation is supported by our observation in Pax6-deficient mice that gelB is still made by inflammatory cells, but not the corneal epithelium (Fig. 6). This mechanism helps explain how local control of gelB expression is maintained in the presence of more global signals. It will be interesting to test whether other cornea-specific genes are controlled in a similar fashion.
A great deal of interest has been directed at discovering therapeutic targets to control pathological gelB expression and activity. In the eye, excessive, inappropriate, or reduced gelB expression has been linked to improper corneal wound healing, formation of chronic corneal ulcers, cataracts, glaucoma, and proliferative retinopathies (16, 37, 49, 52, 54). It is interesting that at later developmental stages and into adulthood both Pax6 and gelB expression in the eye become refined to the cornea and lens epithelia, iris and ciliary body, and the retinal ganglion cell layer (30, 53). Furthermore, Pax6 deficiencies in both heterozygous Sey mice and humans are characterized by progressive corneal opacities, as well as iris and lens defects. If changes in gelB expression facilitate these phenotypes, then it may be that Pax6 could be used as a regulatory target to control expression of gelB in the eye. Alternatively, our demonstrated interaction between Pax6 and AP-2α could be exploited in therapeutic strategies to control gelB expression in the cornea.
In conclusion, we present evidence that gelB is a true target of Pax6, controlling promoter activity in the adult corneal epithelium both directly and through novel interactions with AP-2α. Since Pax6 function has been largely studied during developmental processes, these findings provide the first demonstration of Pax6 function in the adult mammalian eye. Further studies are needed to elaborate the role of Pax6 in the adult cornea.
Acknowledgments
We thank Brian Stramer and Ajay Kumar for helpful comments and suggestions, and we gratefully acknowledge Richard Maas (Harvard Medical School) for providing the Pax6 constructs. We thank Robert Senior (Washington University School of Medicine) for the gift of gelB antibody.
This study was supported by National Institutes of Health grants EY12651 and EY13078 (M.E.F.), EY11910 (J.A.W.-M.), CA77833 and DE12728 (T.W.), and GM44634 and CA94187 (A.Y.) and by Army grant BC990538 (A.Y.). This study was also supported by Research to Prevent Blindness and the Massachusetts Lions Eye Research Fund, Inc. JMS was the recipient of a student fellowship from Fight For Sight. M.E.F. was a Jules and Doris Stein Research to Prevent Blindness Professor and currently holds the Walter G. Ross Chair in Ophthalmic Research. T.W. is the Timpte/Brownlie Chair in Craniofacial/Molecular Biology.
REFERENCES
- 1.Bargagna-Mohan, P., K. J. Strissel, and M. E. Fini. 1999. Regulation of gelatinase B production in corneal cells is independent of autocrine IL-1α. Investig. Ophthalmol. Vis. Sci. 40:784-789. [PubMed] [Google Scholar]
- 2.Betsuyaku, T., Y. Fukuda, W. C. Parks, J. M. Shipley, and R. M. Senior. 2000. Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin. Am. J. Pathol. 157:525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudreau, N., and M. J. Bissell. 1998. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr. Opin. Cell Biol. 10:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canete Soler, R., Y. H. Gui, K. K. Linask, and R. J. Muschel. 1995. MMP-9 (gelatinase B) mRNA is expressed during mouse neurogenesis and may be associated with vascularization. Brain Res. Dev. Brain Res. 88:37-52. [DOI] [PubMed] [Google Scholar]
- 5.Canete-Soler, R., Y. H. Gui, K. K. Linask, and R. J. Muschel. 1995. Developmental expression of MMP-9 (gelatinase B) mRNA in mouse embryos. Dev. Dyn. 204:30-40. [DOI] [PubMed] [Google Scholar]
- 6.Chen, T. T., R. L. Wu, F. Castro-Munozledo, and T. T. Sun. 1997. Regulation of K3 keratin gene transcription by Sp1 and AP-2 in differentiating rabbit corneal epithelial cells. Mol. Cell. Biol. 17:3056-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chintala, S. K., X. Zhang, J. S. Austin, and M. E. Fini. 2002. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J. Biol. Chem. 277:47461-47468. [DOI] [PubMed] [Google Scholar]
- 8.Cvekl, A., F. Kashanchi, J. N. Brady, and J. Piatigorsky. 1999. Pax6 interactions with TATA-box-binding protein and retinoblastoma protein. Investig. Ophthalmol. Vis. Sci. 40:1343-1350. [PubMed] [Google Scholar]
- 9.Cvekl, A., and J. Piatigorsky. 1996. Lens development and crystallin gene expression: many roles for Pax6. Bioessays 18:621-630. [DOI] [PubMed] [Google Scholar]
- 10.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein, J., J. Cai, T. Glaser, L. Jepeal, and R. Maas. 1994. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J. Biol. Chem. 269:8355-8361. [PubMed] [Google Scholar]
- 12.Epstein, J. A., T. Glaser, J. Cai, L. Jepeal, D. S. Walton, and R. L. Maas. 1994. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 8:2022-2034. [DOI] [PubMed] [Google Scholar]
- 13.Fini, M. E., J. D. Bartlett, M. Matsubara, W. B. Rinehart, M. K. Mody, M. T. Girard, and M. Rainville. 1994. The rabbit gene for 92-kDa matrix metalloproteinase. Role of AP1 and AP2 in cell type-specific transcription. J. Biol. Chem. 269:28620-28628. [PubMed] [Google Scholar]
- 14.Fini, M. E., J. R. Cook, and R. Mohan. 1998. Proteolytic mechanisms in corneal ulceration and repair. Arch. Dermatol. Res. 290(Suppl.):S12-S23. [DOI] [PubMed] [Google Scholar]
- 15.Fini, M. E., J. R. Cook, R. Mohan, and C. E. Brinckerhoff. 1998. Regulation of matrix metalloproteinase gene expression, p. 300-339. In W. C. Parks and R. P. Mecham (ed.), Matrix metalloproteinases. Academic Press, Inc., San Diego, Calif.
- 16.Fini, M. E., W. C. Parks, W. B. Rinehart, M. T. Girard, M. Matsubara, J. R. Cook, J. A. West-Mays, P. M. Sadow, R. E. Burgeson, J. J. Jeffrey, M. B. Raizman, R. R. Krueger, and J. D. Zieske. 1996. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am. J. Pathol. 149:1287-1302. [PMC free article] [PubMed] [Google Scholar]
- 17.Frigerio, G., M. Burri, D. Bopp, S. Baumgartner, and M. Noll. 1986. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell 47:735-746. [DOI] [PubMed] [Google Scholar]
- 18.Glaser, T., L. Jepeal, J. G. Edwards, S. R. Young, J. Favor, and R. L. Maas. 1994. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet. 7:463-471. [DOI] [PubMed] [Google Scholar]
- 19.Gorman, C. M., L. F. Moffat, and B. H. Howard. 1982. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halder, G., P. Callaerts, S. Flister, U. Walldorf, U. Kloter, and W. J. Gehring. 1998. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development 125:2181-2191. [DOI] [PubMed] [Google Scholar]
- 21.Hanson, I. M., J. M. Fletcher, T. Jordan, A. Brown, D. Taylor, R. J. Adams, H. H. Punnett, and V. van Heyningen. 1994. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat. Genet. 6:168-173. [DOI] [PubMed] [Google Scholar]
- 22.Heussen, C., and E. B. Dowdle. 1980. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 102:196-202. [DOI] [PubMed] [Google Scholar]
- 23.Hilger-Eversheim, K., M. Moser, H. Schorle, and R. Buettner. 2000. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell cycle control. Gene 260:1-12. [DOI] [PubMed] [Google Scholar]
- 24.Hill, R. E., J. Favor, B. L. Hogan, C. C. Ton, G. F. Saunders, I. M. Hanson, J. Prosser, T. Jordan, N. D. Hastie, and V. van Heyningen. 1991. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354:522-525. [DOI] [PubMed] [Google Scholar]
- 25.Hogan, B. L., G. Horsburgh, J. Cohen, C. M. Hetherington, G. Fisher, and M. F. Lyon. 1986. Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J. Embryol. Exp. Morphol. 97:95-110. [PubMed] [Google Scholar]
- 26.Holcomb, G. N., and T. J. Silhavy. 1972. Synthesis of l-(p-iodobenzenesulfonyl)-3,5-di-n-propyl isocyanurate. J. Org. Chem. 37:3357-3358. [DOI] [PubMed] [Google Scholar]
- 27.Huhtala, P., A. Tuuttila, L. T. Chow, J. Lohi, J. Keski-Oja, and K. Tryggvason. 1991. Complete structure of the human gene for 92-kDa type IV collagenase: divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J. Biol. Chem. 266:16485-16490. [PubMed] [Google Scholar]
- 28.Hussain, M. A., and J. F. Habener. 1999. Glucagon gene transcription activation mediated by synergistic interactions of pax-6 and cdx-2 with the p300 co-activator. J. Biol. Chem. 274:28950-28957. [DOI] [PubMed] [Google Scholar]
- 29.Kamachi, Y., M. Uchikawa, A. Tanouchi, R. Sekido, and H. Kondoh. 2001. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 15:1272-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koroma, B. M., J. M. Yang, and O. H. Sundin. 1997. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Investig. Ophthalmol. Vis. Sci. 38:108-120. [PubMed] [Google Scholar]
- 31.Liu, J. J., W. W. Kao, and S. E. Wilson. 1999. Corneal epithelium-specific mouse keratin K-12 promoter. Exp. Eye Res. 68:295-301. [DOI] [PubMed] [Google Scholar]
- 32.Liu, Z., J. M. Shipley, T. H. Vu, X. Zhou, L. A. Diaz, Z. Werb, and R. M. Senior. 1998. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J. Exp. Med. 188:475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyon, M. F., D. Bogani, Y. Boyd, P. Guillot, and J. Favor. 2000. Further genetic analysis of two autosomal dominant mouse eye defects, Ccw and Pax6(coop). Mol. Vis. 6:199-203. [PubMed] [Google Scholar]
- 34.Matsubara, M., M. T. Girard, C. L. Kublin, C. Cintron, and M. E. Fini. 1991. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodeling cornea. Dev. Biol. 147:425-439. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell, P. J., P. M. Timmons, J. M. Hebert, P. W. Rigby, and R. Tjian. 1991. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 5:105-119. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell, P. J., C. Wang, and R. Tjian. 1987. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell 50:847-861. [DOI] [PubMed] [Google Scholar]
- 37.Mohan, R., S. K. Chintala, J. C. Jung, W. V. Villar, F. McCabe, L. A. Russo, Y. Lee, B. E. McCarthy, K. R. Wollenberg, J. V. Jester, M. Wang, H. G. Welgus, J. M. Shipley, R. M. Senior, and M. E. Fini. 2002. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 277:2065-2072. [DOI] [PubMed] [Google Scholar]
- 38.Mohan, R., W. B. Rinehart, P. Bargagna-Mohan, and M. E. Fini. 1998. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J. Biol. Chem. 273:25903-25914. [DOI] [PubMed] [Google Scholar]
- 39.Moser, M., A. Imhof, A. Pscherer, R. Bauer, W. Amselgruber, F. Sinowatz, F. Hofstadter, R. Schule, and R. Buettner. 1995. Cloning and characterization of a second AP-2 transcription factor: AP-2β. Development 121:2779-2788. [DOI] [PubMed] [Google Scholar]
- 40.Moser, M., J. Ruschoff, and R. Buettner. 1997. Comparative analysis of AP-2α and AP-2β gene expression during murine embryogenesis. Dev. Dyn. 208:115-124. [DOI] [PubMed] [Google Scholar]
- 41.Niimi, T., M. Seimiya, U. Kloter, S. Flister, and W. J. Gehring. 1999. Direct regulatory interaction of the eyeless protein with an eye-specific enhancer in the sine oculis gene during eye induction in Drosophila. Development 126:2253-2260. [DOI] [PubMed] [Google Scholar]
- 42.Nottoli, T., S. Hagopian-Donaldson, J. Zhang, A. Perkins, and T. Williams. 1998. AP-2-null cells disrupt morphogenesis of the eye, face, and limbs in chimeric mice. Proc. Natl. Acad. Sci. USA 95:13714-13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oulad-Abdelghani, M., P. Bouillet, C. Chazaud, P. Dolle, and P. Chambon. 1996. AP-2.2: a novel AP-2-related transcription factor induced by retinoic acid during differentiation of P19 embryonal carcinoma cells. Exp. Cell Res. 225:338-347. [DOI] [PubMed] [Google Scholar]
- 44.Planque, N., L. Leconte, F. M. Coquelle, S. Benkhelifa, P. Martin, M. P. Felder-Schmittbuhl, and S. Saule. 2001. Interaction of Maf transcription factors with Pax-6 results in synergistic activation of the glucagon promoter. J. Biol. Chem. 276:35751-35760. [DOI] [PubMed] [Google Scholar]
- 45.Quiring, R., U. Walldorf, U. Kloter, and W. J. Gehring. 1994. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265:785-789. [DOI] [PubMed] [Google Scholar]
- 46.Reponen, P., C. Sahlberg, C. Munaut, I. Thesleff, and K. Tryggvason. 1994. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J. Cell Biol. 124:1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sander, M., A. Neubuser, J. Kalamaras, H. C. Ee, G. R. Martin, and M. S. German. 1997. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 11:1662-1673. [DOI] [PubMed] [Google Scholar]
- 48.Schedl, A., A. Ross, M. Lee, D. Engelkamp, P. Rashbass, V. van Heyningen, and N. D. Hastie. 1996. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell 86:71-82. [DOI] [PubMed] [Google Scholar]
- 49.Sethi, C. S., T. A. Bailey, P. J. Luthert, and N. H. Chong. 2000. Matrix metalloproteinase biology applied to vitreoretinal disorders. Br. J. Ophthalmol. 84:654-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheng, G., E. Thouvenot, D. Schmucker, D. S. Wilson, and C. Desplan. 1997. Direct regulation of rhodopsin 1 by Pax-6/eyeless in Drosophila: evidence for a conserved function in photoreceptors. Genes Dev. 11:1122-1131. [DOI] [PubMed] [Google Scholar]
- 51.Sinha, S., L. Degenstein, C. Copenhaver, and E. Fuchs. 2000. Defining the regulatory factors required for epidermal gene expression. Mol. Cell. Biol. 20:2543-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivak, J. M., and M. E. Fini. 2002. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin. Eye Res. 21:1-14. [DOI] [PubMed] [Google Scholar]
- 53.Sivak, J. M., R. Mohan, W. B. Rinehart, P. X. Xu, R. L. Maas, and M. E. Fini. 2000. Pax-6 expression and activity are induced in the reepithelializing cornea and control activity of the transcriptional promoter for matrix metalloproteinase gelatinase B. Dev. Biol. 222:41-54. [DOI] [PubMed] [Google Scholar]
- 54.Tamiya, S., I. M. Wormstone, J. M. Marcantonio, J. Gavrilovic, and G. Duncan. 2000. Induction of matrix metalloproteinases 2 and 9 following stress to the lens. Exp. Eye Res. 71:591-597. [DOI] [PubMed] [Google Scholar]
- 55.Ton, C. C., H. Hirvonen, H. Miwa, M. M. Weil, P. Monaghan, T. Jordan, V. van Heyningen, N. D. Hastie, H. Meijers-Heijboer, M. Drechsler, et al. 1991. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell 67:1059-1074. [DOI] [PubMed] [Google Scholar]
- 56.Vu, T. H., J. M. Shipley, G. Bergers, J. E. Berger, J. A. Helms, D. Hanahan, S. D. Shapiro, R. M. Senior, and Z. Werb. 1998. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vu, T. H., and Z. Werb. 2000. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 14:2123-2133. [DOI] [PubMed] [Google Scholar]
- 58.West-Mays, J. A., B. M. Coyle, J. Piatigorsky, S. Papagiotas, and D. Libby. 2002. Ectopic expression of AP-2α transcription factor in the lens disrupts fiber cell differentiation. Dev. Biol. 245:13-27. [DOI] [PubMed] [Google Scholar]
- 59.West-Mays, J. A., J. Zhang, T. Nottoli, S. Hagopian-Donaldson, D. Libby, K. J. Strissel, and T. Williams. 1999. AP-2α transcription factor is required for early morphogenesis of the lens vesicle. Dev. Biol. 206:46-62. [DOI] [PubMed] [Google Scholar]
- 60.Williams, T., A. Admon, B. Luscher, and R. Tjian. 1988. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 2:1557-1569. [DOI] [PubMed] [Google Scholar]
- 61.Williams, T., and R. Tjian. 1991. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 5:670-682. [DOI] [PubMed] [Google Scholar]
- 62.Williams, T., and R. Tjian. 1991. Characterization of a dimerization motif in AP-2 and its function in heterologous DNA-binding proteins. Science 251:1067-1071. [DOI] [PubMed] [Google Scholar]
- 63.Wilson, D., G. Sheng, T. Lecuit, N. Dostatni, and C. Desplan. 1993. Cooperative dimerization of paired class homeo domains on DNA. Genes Dev. 7:2120-2134. [DOI] [PubMed] [Google Scholar]
- 64.Yu, Q., and I. Stamenkovic. 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 14:163-176. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao, F., M. Satoda, J. D. Licht, Y. Hayashizaki, and B. D. Gelb. 2001. Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2δ, with unique DNA binding and transactivation properties. J. Biol. Chem. 276:40755-40760. [DOI] [PubMed] [Google Scholar]