Abstract
Pesticide residue analysis in citrus oils is very important for their quality and marketing. This study assessed the reliability and sensitivity of enzyme-linked immunosorbent assays (ELISA) for simazine and cypermethrin screening in orange oil. Simazine was analyzed after extraction of the oil with methanolic phosphate buffer with a limit of quantitation (LOQ) of 40 µg/L for 1-fold and ~100 µg/L for 10-fold oils. Due to matrix effects the immunoanalysis of cypermethrin required hexane–acetonitrile partitioning followed by silica solid phase extraction. The method detected levels higher than 0.5 ppm (mg/L). This LOQ is lower than the U.S. EPA tolerance level (0.9 ppm) for cypermethrin in citrus oils. A good correlation (r2 0.99) between ELISA and LC-MS/MS was observed for the analysis of both analytes in 1-fold orange oil. Immunochemical screening can be used to reduce instrumental analysis costs by its use in preliminary orange oil screening.
Keywords: Immunoassay, orange oil, pyrethroids, triazines
INTRODUCTION
Citrus oils are used in the food, pharmaceutical, and cosmetic industries and in disinfectant formulations. Orange oil is used in perfumes of the cologne type; in many Curacao type liqueurs; for the flavoring (aroma) of food, drinks, confectionery, and drugs; and as cleaning agents, furniture polishes, burner oils, etc.; it also has therapeutic properties. Citrus oils and their products are traded internationally. Lemon, lime, and orange oils represent the largest segment of U.S. essential oil imports in both volume and value. Citrus oils are derived from the outer portion of the fruit, as byproducts in the much more economically important production of citrus juices. Thus, orange oil is extracted from the orange peel by cold-pressing in yields of 0.3–0.5% by weight. It is the outside of the fruit that is exposed to the pesticides applied to protect the citrus crop and trees. The concentration of pesticides in the essential oil is thus much higher than in the fruit; for example, according to the U.S. Environmental Protection Agency (EPA) the processing factor of ζ-cypermethrin for citrus oil is 19× (1). Pesticide residue analysis in citrus oils is very important for their quality and marketing as pesticides that are acceptable in one market may be banned in another. Even in markets where specific pesticides may be allowed, there are increasing restrictions on the permissible levels of chemicals used for treatment because of their impact on public health and the environment. Furthermore, the use of pesticides in citrus cultivation can influence the essential oil composition. The presence of some pesticides is associated with a reduction in the content of aliphatic and terpene aldehydes, the substances that determine the citrus oil olfactory peculiarity, resulting in reduction of the essential oil quality (2).
Simazine (2-chloro-N,N′-diethyl-1,3,5-triazine-2,4-diamine) is a triazine herbicide commonly used in citrus weed control programs (3). Recently the U.S. EPA proposed tolerance levels for simazine in oranges to be 0.25 ppm(4). Pyrethroid insecticides are also among the chemicals currently registered for foliar use in citrus crops (3). According to the U.S. EPA the proposed tolerance level for ζ-cypermethrin in citrus oil is 0.9 ppm and the recommended tolerance level is 4 ppm (5). A “tolerance” represents the maximum level for residues of pesticide chemicals legally allowed in or on raw agricultural commodities and processed foods.
Although many papers have been published detailing methods for the analysis of pesticide residues inmatrices such as water and vegetables, there are specific problems associated with the detection of analytes in citrus oils (6–8).The determination of pesticide residues in citrus oil is a difficult analytical procedure because of its hydrophobic nature and matrix complexity. The chemical properties of the active ingredients of many pesticides, such as polarity, solubility, and retention behavior in chromatography media, are often very similar to those observed for the far more abundant components of the oils. Current methods for analysis of organochlorine and organophosphorus pesticides in citrus oils involve primarily liquid–liquid (LLE) and solid phase extraction (SPE) for sample preparation followed by chromatography (gas and liquid) analysis with either electron capture or mass spectrometry (MS) detection (6–8). The limitations of gas chromatography in the analysis of essential oils that have not undergone cleanup and preconcentration relate to the need to load large amounts of oil components into the systems to detect low levels of pesticide residues. However, the loading of excessive amounts of essential oil components can lead to column damage, resulting in poor resolution, peak tailing, and low recovery of analytes. It is well-known that tandem mass spectrometry (MS/MS) can differentiate between coeluting compounds because specific ions of each one can be studied in a selective way. Although the number of applications using LC-MS/MS in trace analysis of fatty food samples such as olive (9, 10) and vegetable oils (11) is increasing, the technique still has not been applied to citrus oils.
Immunochemical detection technologies have proven to be fast and sensitive screening methods, as well as quantitative analytical tools for pesticide residue determination in food samples (12–15). The application of enzyme immunoassays for pesticide analysis of edible oils is still very limited; for example, the determination of atrazine (16) and organophosphorus insecticides (17) in olive oil by enzyme-linked immunosorbent assays (ELISA) has been reported. So far, the major drawbacks for the application of pesticide immunoassay in food are related to poor recoveries and the removal of matrix interferences (15, 18, 19). Consequently, sample preparation methods need to provide reliable data using minimum sample processing; otherwise, many of the potential advantages of immunoassays such as high throughput, simplicity, low cost, and sensitivity are lost.
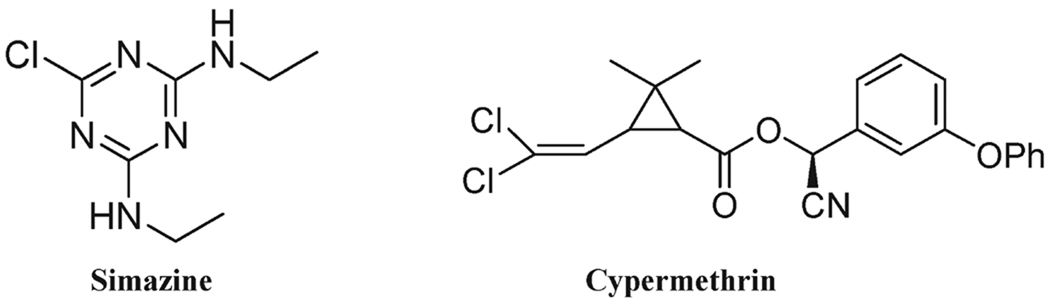
To our knowledge, the determination of pesticide residues in orange and other citrus oils using immunochemical techniques has not been previously explored in the literature. The primary goal of this study was to assess the reliability and sensitivity of ELISA for pesticide detection in orange oil. On the basis of the needs of the orange oil industry, we have selected two target compounds, simazine and cypermethrin (see Figure 1), as representative of triazines and pyrethroids. Our aim was to develop an efficient and simple sample preparation method (if needed) resulting in orange oil extracts that do not interfere with the immunoassay quantification of each target compound. The analytical parameters (sensitivity, accuracy, and precision) of the method in orange oil extracts were evaluated and compared to the corresponding instrumental LC-MS/MS analysis.
Figure 1.
Chemical structures of simazine and cypermethrin.
MATERIALS AND METHODS
Chemicals
Simazine (CAS Registry No. 122-34-9) and cypermethrin (mixture of isomers, CAS Registry No. 52315-07-8) were purchased from Chem Service (West Chester, PA). Stock solutions were prepared in methanol. Tween 20 and bovine serum albumin (BSA) were purchased from Sigma Chemical Co. (St. Louis, MO). PBS (pH 7.5) is phosphate-buffered saline (8 g/LNaCl, 1.15 g/LNa2HPO4, 0.16g/LKH2PO4, 0.2 g/L KCl). PBST is PBS with 0.05% Tween 20 (v/v). α-[Cyclopropane-1-14C/13C]cypermethrin was from BASF, and 14C-simazine (ring labeled) was purchased from Ciba Geigy (Greensboro, NC). CytoScint liquid scintillation cocktail was obtained from Fisher Scientific (Pittsburgh, PA). Solvents (hexane, ethyl acetate, acetonitrile, methanol) used in the cleanup procedures were of HPLC grade. All other reagents were prepared from reagent grade chemicals obtained from Fisher Scientific (Pittsburgh, PA). ELISA experiments were performed in high-binding 96-well microtiter plates (Nunc, Roskilde, Denmark), and the absorbance was read with a SpectramaxPlus microplate reader (Molecular Devices, Sunnyvale, CA). Radiolabel was counted in a Wallac model 1409 liquid scintillation counter (Perkin-Elmer Life Sciences, Downers Grove, IL). An Eppendorf centrifuge 5415D (Brinkmann, Westbury, NY) was used for phase separation after simazine extraction from orange oil, and a Beckman Instruments centrifuge was used for the cypermethrin extraction. Extracts were evaporated on a vacuum centrifugal evaporator (Heto-ATR, Laurel, MD).
Orange Oil Samples
Cold pressed 1- and 10-foldValencia orange oil samples were provided by Givaudan. The 10-fold oil is concentrated to of its original weight. In general, folded oils have increased concentration of oxygenated compounds and lower terpene concentration compared to cold-pressed 1-fold oil (22, 23). The samples were stored at 4 °C. According to the LC-MS/MS analysis these oil samples were free of the targeted pesticides [the limit of quantitation (LOQ) for simazine was 20 µg/L and that for cypermethrin was 40 µg/L].
Extraction of Simazine from Orange Oil Samples
Orange oil (0.2 mL) was mixed with hexane (0.2 mL) and extracted with PBS or MeOH/PBS solution by shaking in an orbital shaker for 30 min at room temperature. The layers were separated by centrifugation (5 min at 13000g). The methanolic extract was diluted in PBS to yield 10% MeOH/PBS in order to be measured by the ELISA.
Extraction of Cypermethrin from Orange Oil Samples. Sample Cleanup
LLE with Acetonitrile
Orange oil (0.3mL) was mixed with 1.5 mL of hexane. The organic phase was washed with 0.1 M NaOH in 10% NaCl, and the water phase was discarded. The hexane phase was extracted twice with acetonitrile (2mL each) in an orbital shaker (300 rpm) for 15 min. After centrifugation (10 min at 3000 rpm), the acetonitrile phases were collected, combined, and evaporated until dryness. The residue obtained was reconstituted with 1 mL of hexane.
SPE
A Sep-Pak Plus silica cartridge (55–105 µm) from Waters (Milford, MS) was used. The column was prewashed with 5 mL of hexane. Oil sample (0.3 mL of oil mixed with 1.5 mL of hexane) was then loaded onto the column. The column was washed with 5 mL of hexane, and cypermethrin was eluted with 4 mL of 5% ethyl acetate/hexane. The solvent was evaporated, and 0.3 mL of M containing 0.01%Triton X-100 was added. The extract was dissolved in 0.45mL of PBS. Further dilutions with 40% MeOH/PBS were performed as needed for reliable ELISA analysis.
ELISAs
The simazine ELISA was performed according to the protocol previously reported using coating antigen (XIV-OVA) and antibody 2282 (20). The procedure for the cypermethrin ELISA (4-BSA/antibody 735) was similar to that reported by Lee et al. (21). Calibration standards were prepared in 40% MeOH/PBS. All samples and standards were analyzed in triplicate. For ELISA matrix effect studies simazine (cypermethrin) standard curves were prepared in orange oil extracts, prior to and after dilution with 10% MeOH/PBS (for simazine) and 40% MeOH/PBS (for cypermethrin), and run in the competitive ELISA to compare parallelism with the standard curve prepared in the corresponding methanolic buffer.
LC-MS/MS Analyses
LC-MS/MS analysis of simazine was conducted on an Agilent 1200 LC-API 2000MS/MS system. Separation was carried out on a 150 × 2.0 mm i.d., 3 µm, Luna C18(2) column (Phenomenex, Torrance, CA). Mobile phases A and B were 0.1% HCOOH and 5 mM HCOONH4 in H2O and acetonitrile, respectively. The gradient started with 5% B constant for 1 min, followed by a linear gradient to 95%B in 2min, held for 3min, then returned to 5%B in 2min, and equilibrated for 2 min. The flow rate was 250 µL/min. The MS/MS detection was performed in positive ESI mode, and two MRMs, 202/132 and 202/104, were used for confirmation of simazine; the more intense MRM transition was selected for quantification. A six-point external calibration covering concentrations from 0 to 400 µg/L was determined with an R2 ≥ 0.999. The LOQ was 20 µg/L.
The LC-MS/MS method for cypermethrin was based on the same chromatography method of simazine, using an API 5000MS/MS analyzer operated in positive ESI mode for better sensitivity. Two MRMs, 433/191 and 433/127, were used for confirmation of cypermethrin, and the more intense MRM transition was selected for quantification. A five-point external calibration covering concentrations from 0 to 1500 µg/L was determined with an R2 ≥ 0.999. The LOQ was 40 µg/L.
Validation Studies
For the validation studies 1-fold orange oil samples were fortified with the target analytes and were split into two fractions for ELISA and LC-MS/MS analyses. The results obtained with both methods were used to perform correlation studies using a linear regression analysis.
RESULTS AND DISCUSSION
Immunobased analytical methods generally are performed in aqueous media with different tolerances to water miscible organic solvents and to real sample components (fat, hydrocarbons, etc.) controlled by the specific antibody in any given immunoassay. Cold-pressed orange oils are mixtures of volatile components such as terpene hydrocarbons (d-limonene ~ 95%; myrcene ~ 2.5%), oxygenated compounds (linalool, neral, citronellal, geranial, etc.), and nonvolatile compounds such as pigments and waxes (23). Our target analytes (Figure 1) represent examples of two different types of compounds in terms of their physicochemical properties: water solubilities are 5 ppm (mg/L) for simazine and 50–80 ppb for cypermethrin; log Kow values are 2.3 and 5.2 for simazine and cypermethrin, respectively. Some form of extraction from the hydrophobic oil matrix was therefore necessary. It should be noted that the major orange oil component, d-limonene, has physicochemical properties (water solubility = 7.6 ppm, log Kow = 4.5) similar to those of cypermethrin, which makes the separation of the analyte from that matrix difficult. Commonly used solvents in immunoassay are MeOH(14, 24) and DMSO(25). Our approach was to analyze those pesticides in orange oil (1- and 10-fold) with the minimum sample preparation needed to reduce possible matrix effects using first simple dilution, then progressing to solvent extraction and cleanup as necessary prior to immunoassay analysis.
Simazine Immunoanalysis
The ELISA for the detection of simazine is a competitive (inhibition) immunoassay in indirect format (20). The assay exhibits high selectivity for simazine (100%) and atrazine (76%). The immunoassay can tolerate up to 10% methanol in PBS. The parameters of the standard curve in 10% MeOH/PBS are as follows: Amax = 1.17 ± 0.29; Amin = 0.21 ± 0.08; slope = 0.93 ± 0.1; R2 = 0.995; midpoint IC50 = 0.49 ± 0.12 µg/L; and dynamic range between 0.11 ± 0.03 and 2.18 ± 0.67 µg/L simazine. These data correspond to 19 standard curves run on different days during 3 months.
Extraction Procedure and Matrix Effect Studies
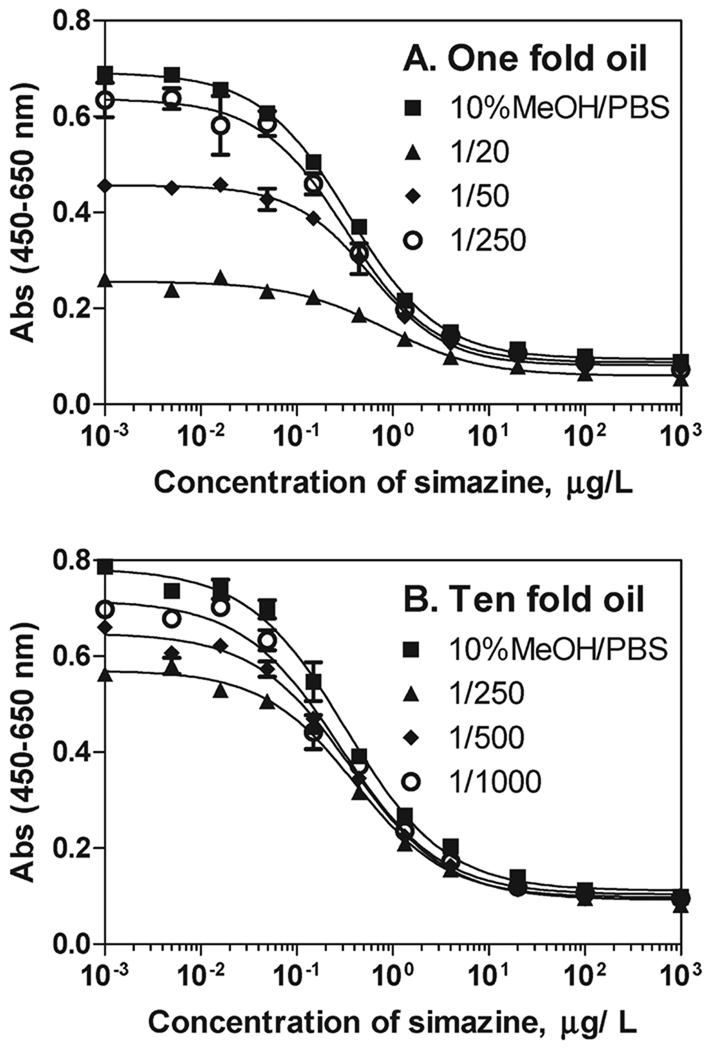
Direct analysis of simazine in emulsions formed by 1-fold orange oil and 10% MeOH/PBS resulted in severe immunoassay inhibition. Therefore, we focused on the extraction of simazine with water (and/or PBS) and MeOH/PBS from orange oil. Initially, water (PBS) extraction of simazine from 1- and 10-fold Valencia orange oil was tested using radiolabeled simazine. Orange oil (0.2 mL) was spiked with 1 ppm 14C-simazine (hexane standard solution); 0.2 mL of hexane was added to the oil and extracted with water (PBS) by shaking in an orbital shaker for 10–30 min. About 45% of the simazine was recovered in the aqueous fraction. Similar recoveries were obtained by agitation by vortex or sonication. No effect of temperature was observed on the recovery (tested up to 55 °C). Although simazine is more soluble at lower pH(pKa = 1.7), acidification did not improve recoveries in the water phase (data not shown). Simazine extraction efficiency was improved by increasing the percentage of MeOH in the aqueous (PBS) phase. Thus, simazine recoveries using 50% MeOH/PBS, 70% MeOH/PBS, and 90% MeOH/PBS in the oil extraction were 77, 88, and 91%, respectively. However, increasing the methanolic content in the aqueous (PBS) phase led to an increase in the amount of orange oil transferred to the aqueous phase due to the miscibility of oil with MeOH. Greater oil residue in the aqueous phase resulted in higher interference in the assay and, therefore, an increased dilution of the extract was required to eliminate the inhibitory oil matrix effect on the ELISA performance, thus increasing the limit of quantitation of the analysis. ELISA calibration curves were run in different dilutions of methanolic extracts of 1- and 10-fold orange oils. Orange oils were mixed with hexane (oil–hexane = 1:1) and extracted with 50% MeOH/PBS (oil–hexane/MeOH–PBS = 1:1). The extracts obtained were diluted with PBS to reduce the amount of methanol to 10%, which is tolerated by the ELISA. The extracts were further diluted with 10% MeOH/PBS (from 1:20 to 1:1000). As seen in Figure 2 the presence of oil in the final extract caused significant interference in the assay. However, this effect could be eliminated after a 1:250 dilution of 1-fold oil and a 1:1000 dilution for 10-fold oil. If 70% MeOH/PBS was used in the extraction, a 1:700 total oil dilution of 1-fold oil and a 1:1400 dilution of 10-fold oil were required to eliminate the matrix effects. In both cases, 10-fold oil extracts have a stronger inhibitory effect on the immunoassay. This could be attributed to the fact that the oil residue in 10-fold oil extracts is greater than that in 1-fold oil extracts due to the larger content of polar compounds. Folded oils have better solubility in alcohol as compared to the original cold-pressed oils due to increased content of oxygenated compounds (aldehydes and alcohols) (22, 23). Finally, it should be noted that the orange oil samples used here were considered to be free of simazine and atrazine according to the LC-MS/MS analysis (LOQ is 20 µg/L). In addition, synthetic mimics of 1- and 10-fold oil showed very similar matrix effects on the ELISA. Therefore, we believe that the observed matrix effect is due to nonspecific oil interferences.
Figure 2.
Matrix effect of 1-fold (A) and 10-fold (B) oil on the simazine ELISA. Orange oil is extracted with 50% MeOH/PBS. ELISA calibration curves were run in 10% MeOH/PBS buffer (squares) and in methanolic extracts of (A) 1-fold orange oil (1:20, 1:50, and 1:250 total oil dilution) and (B) 10-fold orange oil (1:250, 1:500, and 1:1000 total oil/simazine dilution).
Accuracy and Precision
With the aim of obtaining the highest simazine recoveries with a minimum of sample dilution in a reproducible manner, we tested MeOH/PBS extraction of 1-fold orange oil samples, varying the amount of MeOH in the aqueous phase (50% MeOH/PBS and 70% MeOH/PBS) and the oil/methanolic PBS phase ratio (1:1 and 1:2). A summary of the data are presented in Table 1. Highest recoveries (73–108%) were obtained using the 70% MeOH/PBS extraction (procedure C), but the higher dilution needed to reduce ELISA matrix effects resulted in an increase in the LOQ of the method. Thus, we chose procedure B (50% MeOH/PBS) as the optimum procedure to achieve the highest sensitivity and quantitative recovery. Procedure B was used in the analysis of spiked 1-fold orange oil samples provided by Givaudan for the instrumental validation study. The practical LOQ for simazine analysis in 1-fold orange oil following procedure B was determined by triplicate analysis of fortified samples with concentrations in the range from 20 to 100 µg/L. The LOQ was 40 µg/L (CV of 26%). We were able to detect levels of 30 µg/L with a CV of 35%.
Table 1.
Simazine Analysis by ELISA in Methanolic Extracts of 1-Fold Orange Oil
| extraction procedure: % MeOH: (oil–hexane):MeOH–PBS: total simazine dilutiona: |
A 50% MeOH–PBS 1:1 1/250 |
B 50% MeOH–PBS 1:2 1/500 |
C 70% MeOH–PBS 1:2 1/700 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| spike concn, µg/L |
measured concn, µg/L |
recovery, % | CV%b | measured concn, µg/L |
recovery, % | CV% | measured concn, µg/L |
recovery, % | CV% |
| 20 | 12.8 | 63.8 | 17 | <LODc | <LOD | ||||
| 30 | ntd | nt | nt | 33.5 | 111.6 | 35 | nt | nt | nt |
| 40 | nt | nt | nt | 35 | 87.5 | 26 | nt | nt | nt |
| 50 | 32.5 | 65 | 12.8 | 34.2 | 68.3 | 13 | 52.9 | 105.8 | 6.7 |
| 80 | 41.6 | 52 | 11.7 | 74.2 | 92.7 | 17 | 58.4 | 73 | 5.1 |
| 200 | 100 | 50 | 22 | 141.2 | 70.6 | 19 | 157.6 | 78.8 | 13.4 |
| 500 | 337.5 | 67.5 | 10.6 | 482.5 | 96.5 | 6 | 540 | 108 | 17.6 |
Total simazine dilution corresponds to the dilution factor of the simazine from the original oil sample to the final extract analyzed by ELISA
Samples were spiked in triplicate, and each sample was analyzed in triplicate by ELISA
LOD is the limit of detection of the ELISA defined as 80% of the maximal signal.
nt, not tested.
Similarly, we tested the accuracy and precision of the simazine analysis in spiked 10-fold oil (Table 2). Poor recoveries were obtained in this case. We believe that the main reason for the unsatisfactory accuracy of the immunoanalysis of 10-fold orange oil is due to the formation of unstable emulsions in the methanolic PBS extracts.
Table 2.
Simazine Analysis by ELISA in Methanolic Extracts of 10-Fold Orange Oil
| 50% MeOH–PBS | 70% MeOH–PBS | |||
|---|---|---|---|---|
| spike concn, µg/L | recovery, % | CV% | recovery, % | CV% |
| 100 | <LOD | 65.6 | 38 | |
| 200 | 36.3 | 9.72 | 46.9 | 35 |
| 500 | 35.2 | 16.2 | 39.8 | 18.9 |
Validation of the Immunochemical Method for Simazine with LC-MS/MS Analysis
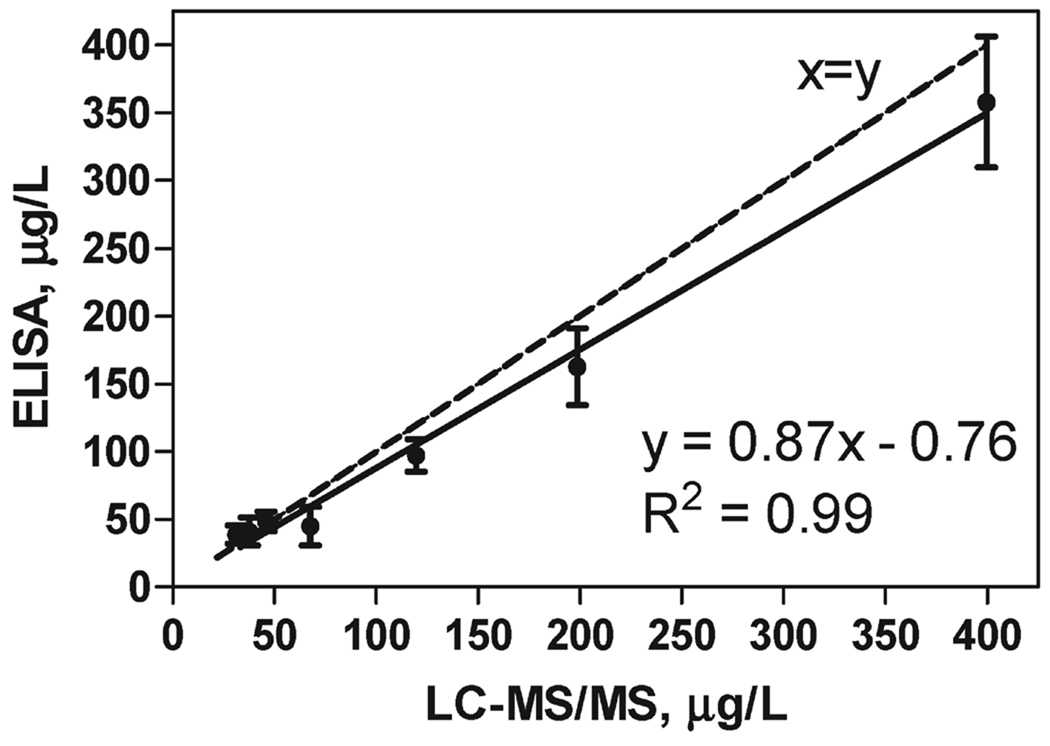
Ten fortified 1-fold orange oil samples (in the range from 20 to 400 µg/L) were provided by Givaudan for the validation study. The samples were split, and one part was directly analyzed by LC-MS/MS. The other part was analyzed by ELISA after extraction with 50% MeOH–PBS in triplicate on three different days (N = 9). In parallel, a blank 1-fold oil sample was processed and the standard ELISA curve was run in the obtained extract. This strategy “normalizes” for some of the interferences found in the extracts. The parameters of the standard curve in the methanolic extract were as follows: Amax = 0.99 ± 0.26; Amin = 0.15 ± 0.05; slope = 0.92 ± 0.06; R2 = 0.994; IC50 = 0.53 ± 0.17 µg/L; and dynamic range from 0.12 ± 0.06 to 2.19 ± 0.8 µg/L simazine. (The data correspond to eight standard curves run on different days and reflect the assay interday variability.) Samples were quantified from the linear (dynamic) range of the ELISA calibration curve. Samples with values lower than the lower limit of that range were assigned as “<LOQ”. Mean percentage recoveries of 93.7 and 101% by ELISA and LC-MS, respectively, were reached with CV values lower than 32%. The correlation between simazine concentration in 1-fold orange oil samples determined by ELISA and LC-MS/MS is presented in Figure 3. The dashed line indicates an ideal correlation (x = y) between the methods. The proposed procedure correlated well with the reference method (y = 0.87x − 0.76; R2 = 0.99), indicating that there is no significant bias between the techniques. The observed “under-estimation” for the ELISA analysis can be explained by the lower recovery (68–111%) in the extraction procedure prior to immunoanalysis. In contrast, instrumental analysis was performed directly on the spiked oil samples (no extraction step).
Figure 3.
Correlation between simazine concentrations in 1-fold orange oil samples determined by ELISA and LC-MS/MS. The dashed line indicates an ideal correlation (x = y) between both methods.
Cypermethrin Immunoanalysis
The ELISA for detection of cypermethrin is a competitive (inhibition) immunoassay in indirect format (21). Because pyrethroids are lipophilic, a water-miscible organic solvent is needed to ensure their solubility. We explored MeOH and DMSO as cosolvents to solubilize the cypermethrin and Tween 20 and Tween 80 as commonly used detergents in immunoassays to reduce nonspecific binding. The cypermethrin ELISA can tolerate up to 40% MeOH in PBS, up to 20% DMSO in PBS, and up to 0.2% Tween 20 or Tween 80. Higher organic solvent (or detergent) concentrations reduced the maximum signal (Amax) and increased the IC50 value. The parameters of the sigmoidal standard curve in 40% MeOH–PBS were as follows: Amax = 0.88 ± 0.22; Amin = 0.11 ± 0.06; slope = 0.79±0.29; R2 = 0.995; IC50 = 33.77±12.56 µg/L; and dynamic (working) range from 6.37± 2.44 to 258.8 ± 78.35 µg/L cypermethrin. The values reported here correspond to 10 standard curves run on different days over 3 months.
Initially, we evaluated the cypermethrin ELISA performance in oil-in-organic solvent/buffer emulsions stabilized by Tween 20, Tween 80, and casein as emulsifiers. We tested both 40% MeOH–PBS and 20%DMSO–PBS emulsions. Emulsions were prepared by homogenization in a blender. Matrix effect studies demonstrated that both 1- and 10-fold orange oils have a strong inhibitory effect on the immunoassay (data not shown) and that the assay can tolerate up to 0.1–0.2% oil content, thus limiting significantly the assay sensitivity. Furthermore, we explored liquid–liquid “extraction” with methanol and DMSO. Extraction efficiency of 14C-cypermethrin from spiked 1-fold oil was 8% for MeOH extraction and 80% for the DMSO extraction. Due to the presence of oil residue in the DMSO extract, significant dilution of the extract was needed to minimize inhibition of the immunoassay. This yielded an estimated LOQ of >1 ppm. As none of the methods tested led to the targeted sensitivity, we focused on the development of a more detailed sample preparation method. As the orange oil has an inhibitory effect on the immunoassay, our aim was to separate the target analyte cypermethrin from the oil and thus reduce the oil residue in the extract prior to immunoanalysis.
Development of Sample Preparation Method (LLE followed by SPE) for Cypermethrin
Sample preparation is the most difficult step in the determination of pyrethroids in fatty materials (oils, animal fat, dietary products, etc.), due to their difficult separation from the fatty matrix (11). As stated above, cypermethrin and limonene (the major orange oil component) have similar physicochemical properties. The most commonly reported extraction procedures for pyrethroids in liquid fatty samples are those based on a liquid–liquid partitioning with acetonitrile–hexane followed by SPE with different phases, such as mixed Florisil-C18 used for multiresidue extraction from lemon oil (7) and graphitized carbon black for vegetable oils (26). As our goal was to introduce minimal sample preparation steps prior to immunoanalysis, we explored the effectiveness of each of the steps, acetonitrile–hexane partitioning and silica gel C18-SPE of orange oil samples, in reducing interferences in the cypermethrin ELISA.
Extraction recoveries were tested with 1-fold oil spiked with 14C-cypermethrin. The orange oil was mixed with hexane and washed with basic aqueous phase. Three consecutive extractions with acetonitrile were performed by shaking in an orbital shaker for 15 min. Then the acetonitrile phases were evaporated under a nitrogen blanket, and radioactivity was measured in each of the three extracts. Ninety-seven percent of the cypermethrin was recovered in two extractions. Therefore, we recommend two extractions with acetonitrile to be performed. After the acetonitrile had been evaporated to dryness, an oily residue was obtained. It was redissolved in MeOH and further diluted with PBS. The extracts obtained (40% MeOH–PBS) were tested for matrix effects on the cypermethrin immunoassay. Due to the presence of oil residue, significant dilution of the extract was needed and the sensitivity of the immunoanalysis was again greater than the targeted 1 ppm.
Furthermore, we tested normal phase SPE using a Sep-Pak Plus silica cartridge as a direct cleanup (without prior acetonitrile–hexane partitioning). The column was prewashed with 5 mL of hexane. An oil sample (0.3 mL of oil + 1.5 mL of hexane) was loaded onto the column. The column was washed with 5 mL of hexane, and cypermethrin was eluted with 4 mL of ethyl acetate–hexane. Increasing polarity (5, 10, 50, 100% of ethyl acetate) in the elution solvent led to increased oil coelution. Only 5% ethyl acetate–hexane was suitable to elute the maximum amount of cypermethrin (94% recovery based on radio-activity tests) while keeping the oil coelution at a minimum. The evaluation of the matrix effect of the 40% MeOH–PBS extract obtained after SPE demonstrated that at least 200-fold dilution was needed to eliminate interferences from the oil residue for 1-fold oil. Therefore, LLE or SPE alone does not provide enough cleanup of the oil to reach the desired LOQ.
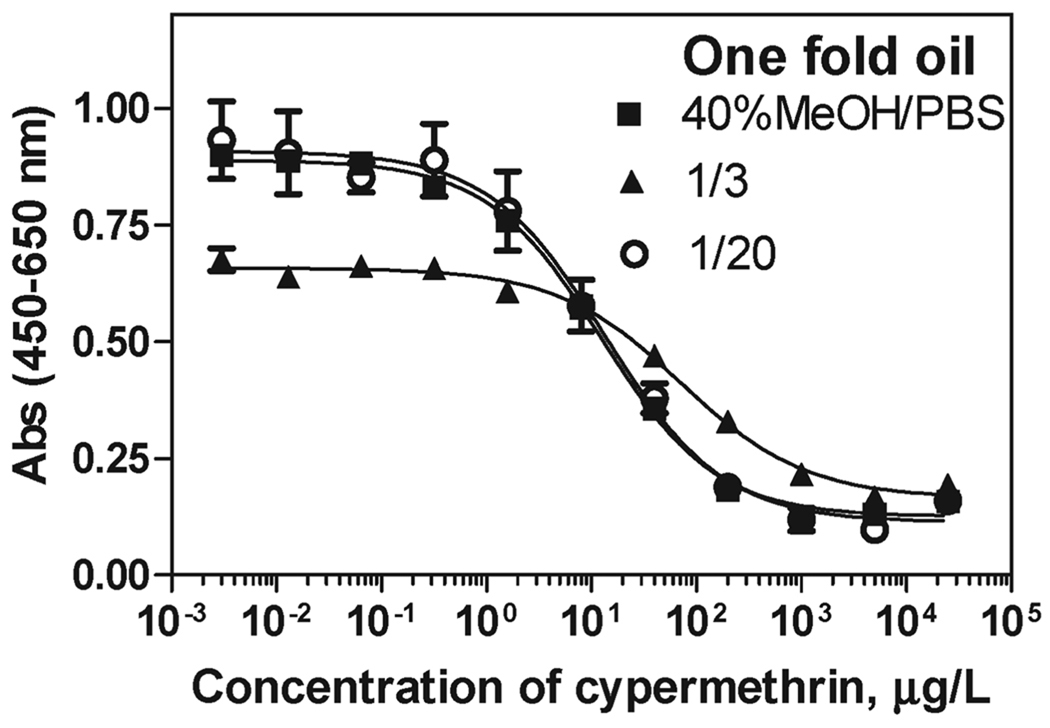
Combined LLE and SPE are required to minimize the oil residue sufficiently for the analysis of cypermethrin by immunoassay at the targeted levels of detection. Recoveries for the entire procedure (LLE + SPE) determined with radioactive cypermethrin were 77.2±3.1% (N = 3) for 1-fold orange oil and 91.4 ± 7.1% (N = 3) for 10-fold oil. Matrix effects studies demonstrated that the 40% MeOH–PBS extracts obtained after the LLE silica SPE cleanup needed to be further diluted 20 times for 1-fold oil (Figure 4) and at least 40 times for 10-fold oil to obtain ELISA standard curves similar to those run in buffer. (For comparison, a 1/200 dilution was needed when SPE was applied directly to 1-fold orange oil without prior acetonitrile–hexane partitioning.)
Figure 4.
Matrix effect of 1-fold orange oil extracts on the cypermethrin ELISA after LLE-SPE cleanup. ELISA calibration curves were run in 40% MeOH–PBS buffer (■) and in methanolic extracts further diluted 1/3 (▲) and 1/20 (○) in 40% MeOH–PBS.
Accuracy and Precision of the LLE-SPE-ELISA Analysis of Cypermethrin in Orange Oil
One-fold orange oil samples were fortified with cypermethrin (in the range from 400 to 2000 µg /L) and analyzed in triplicate by the LLE-SPE-ELISA procedure described above. In parallel, a blank (unspiked) 1-fold orange oil sample was processed and the standard curve was run in the obtained extract. The parameters of the standard curve in the methanolic extract are as follows: Amax = 1.03 ± 0.26; Amin = 0.04 ± 0.02; slope = 0.57 ± 0.15; R2 = 0.994; IC50 = 37.91 ± 14.11 µg/L. The dynamic range was from 4.2 ± 1.8 to 431.9 ± 180.3 µg/L cypermethrin. (The data correspond to eight standard curves run on different days and reflect the assay interday variability.) Samples were quantified from the linear (dynamic) range of the ELISA calibration curve. The results are presented in Table 3. Recoveries in the range of 65–71% were obtained for levels >500 µg/L cypermethrin. As the accuracy (46.2%) and precision (CV = 37%) for the 400 µg/L spiked level were not satisfactory, we estimated that the LOQ for a reliable cypermethrin analysis of 1-fold orange oil by LLE-SPE-ELISA is ~0.5 ppm (mg/L). This LOQ is acceptable as it is below the U.S. EPA tolerance level of 0.9 ppm for cypermethrin in citrus oil.
Table 3.
Analysis of Cypermethrin in 1-Fold Orange Oil by LLE-SPE-ELISA
| measured concn, µg/L | ||||
|---|---|---|---|---|
| spike concn, µg/L | av, µg/L | SD | CV% | recovery, % |
| 400 | 185 | 55.5 | 37 | 46.2 |
| 500 | 350.5 | 57.5 | 16.4 | 70.1 |
| 1000 | 650 | 117.3 | 18.1 | 65 |
| 1500 | 1040 | 332.8 | 32 | 69.3 |
| 2000 | 1428 | 485.5 | 34 | 71.4 |
Similarly, we have tested the accuracy of the LLE-SPE-ELISA analysis of 10-fold orange oil with spiked samples. Significant data variability was observed, probably due to the presence of an oily residue in the methanolic extracts resulting in nonstable emulsions during ELISA analysis. It has been demonstrated that different solid phases and their combined use result in different amounts of oil residue after each treatment of olive oil (11). Sample preparation methods based on other SPE phases (Florisil, carbon, etc.) could be further explored to improve sample cleanup of 10-fold orange oil and obtain more acceptable sensitivity and reproducibility in the cypermethrin analysis.
Validation of the Immunochemical Method for Cypermethrin with LC-MS Analysis
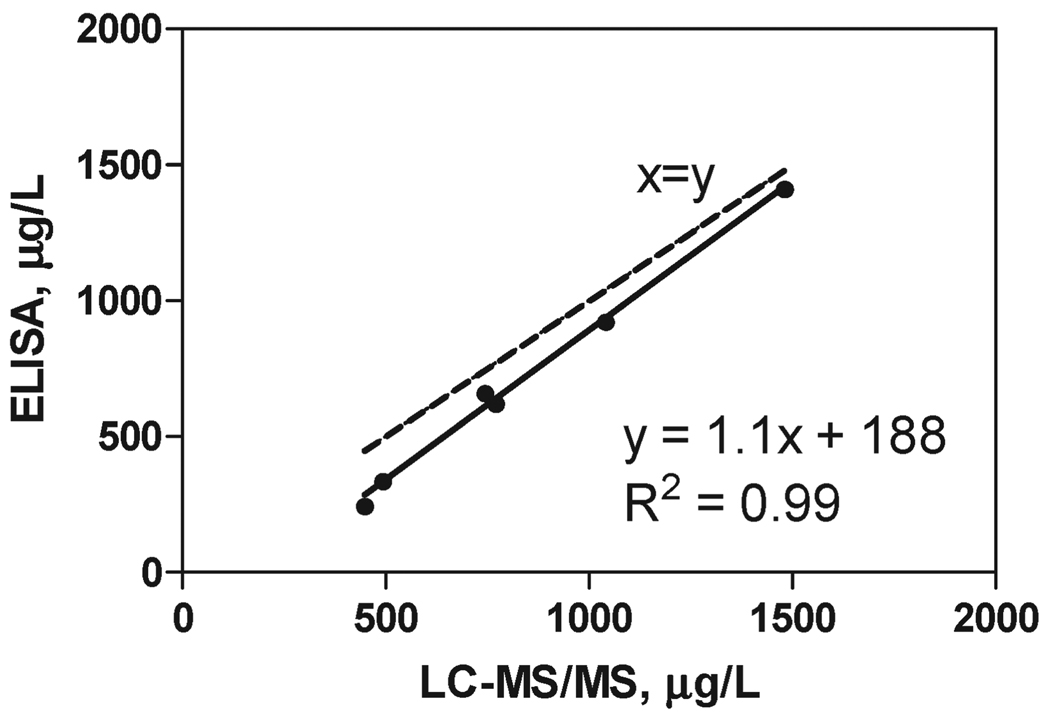
Six fortified 1-fold orange oil samples (in the range from 400 to 1500 µg /L) were provided by Givaudan for the validation study. The samples were split, and one part was subjected to the LLE-SPE cleanup method described above and analyzed by ELISA in triplicate on two different days (N = 6). The other part was cleaned up and analyzed in duplicate by LC-MS/MS. Recoveries in the range of 98–112% were observed for the LC-MS/MS analysis. The correlation (R2 = 0.99) between the immunochemical and instrumental analyses is presented in Figure 5. The observed underestimation for the ELISA analysis is due to the lower recoveries (65–71%) for this method related to the additional step of solvent exchange and the presence of oil traces in the final extract. Nevertheless, the proposed LLE-SPE-ELISA can be used as a reliable screening method for cypermethrin in 1-fold orange oil at levels lower than the regulatory limits.
Figure 5.
Correlation between cypermethrin concentrations in 1-fold orange oil samples determined by ELISA and LC-MS/MS. The dashed line indicates an ideal correlation (x = y) between both methods.
In conclusion, the pesticides (simazine and cypermethrin) studied cannot be analyzed by ELISA by direct addition of orange oil. The significant inhibitory effect of the oil on the immunoassays is probably due to the disruption of antibody–antigen interaction and/or a result of the limited solubility of the target analyte (especially for cypermethrin) in the aqueous phase. Because the triazine is relatively more water-soluble and less lipophilic than the pyrethroid, it was efficiently extracted (recovery of 65–112%; LOQ of 40 µg/L) from 1-fold orange oil using a simple partitioning with 50% MeOH–PBS. However, analysis of the lipophilic pyrethroid, cypermethrin, required additional cleanup to reduce the oil residue prior to the immunoassay. LLE of 1-fold oil with acetonitrile followed by silica SPE allowed the detection of cypermethrin levels of >0.5 ppm with accuracy of 65–71%. According to U.S. EPA regulation, the recommended tolerance for cypermethrin is 4 ppm (proposed tolerance is 0.9 ppm) in citrus oils. Therefore, the LLE-SPE-ELISA method is suitable for cypermethrin screening of 1-fold orange samples for regulatory purposes. The immunoanalysis of both pesticides in 10-fold orange oil had unsatisfactory accuracy and precision due to the formation of unstable emulsions in the methanolic PBS extracts. The sample preparation for 1-fold oil can be performed simultaneously for many samples in a high-throughput manner. Pesticide ELISA can be applied for prescreening purposes as a complementary tool to instrumental analysis.
ACKNOWLEDGMENT
We thank Chris Courter and Dr. Thorsten Koenig of Givaudan S.A. for consultancy and useful discussion.
This work has been supported by Givaudan S. A. and Synthia-LLC. Previously developed ELISA analyses were made possible by Grant 5 P42 ES004699 from the National Institute of Environmental Health Sciences (NIEHS).
ABBREVIATIONS USED
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- IC50
concentration of analyte giving 50% inhibition
- LLE
liquid–liquid extraction
- LOD
limit of detection
- LOQ
limit of quantitation
- PBS
phosphate-buffered saline
LITERATURE CITED
- 1.U.S. EPA. Summary of analytical chemistry and residue data, ζ-cypermethrin. [accessed April 25, 2009];2007 www.regulations.gov/fdmspublic/ContentViewer?-objectId=090000648036e2f2&disposition=attachment&content-Type=pdf.
- 2.Verzera A, Trozzi A, Dugo G, Di Bella G, Cotroneo A. Biological lemon and sweet orange essential oil composition. Flavour Fragrance J. 2004;19:544–548. [Google Scholar]
- 3.Florida Citrus Pest Management Guide. [accessed April 25, 2009];2008 http://edis.ifas.ufl.edu/ CG026. [Google Scholar]
- 4.U.S. EPA. Amitraz, atrazine, ethephon, ferbam, lindane, propachlor, simazine; proposed tolerance actions. Fed. Regist. 2007;72:32570–32582. [Google Scholar]
- 5.U.S. EPA. ζ-cypermethrin; pesticide tolerance. Fed. Regist. 2008;73:1517–1525. [Google Scholar]
- 6.Robbat A, Hoffmann A, MacNamara K, Huang Y. Quantitative identification of pesticides as target compounds and unknowns by spectral deconvolution of gas chromatographic/mass spectrometric data. J. AOAC Int. 2008;91:1467–1477. [PubMed] [Google Scholar]
- 7.Barrek S, Paisse O, Grenier-Loustalot MF. Analysis of pesticide residues in essential oils of citrus fruit by GC-MS and HPLC-MS after solid-phase extraction. Anal. Bioanal. Chem. 2003;376:157–161. doi: 10.1007/s00216-003-1899-9. [DOI] [PubMed] [Google Scholar]
- 8.Di Bella G, Serrao L, Salvo F, Lo Turco V, Croce M, Dugo G. Pesticide and plasticizer residues in biological citrus essential oils from 2003–2004. Flavour Fragrance J. 2006;21:497–501. [Google Scholar]
- 9.Hernando MD, Ferrer C, Ulaszewska M, Garcia-Reyes JF, Molina-Diaz A, Fernandez-Alba AR. Application of high-performance liquid chromatography–tandem mass spectrometry with a quadrupole/linear ion trap instrument for the analysis of pesticide residues in olive oil. Anal. Bioanal. Chem. 2007;389:1815–1831. doi: 10.1007/s00216-007-1464-z. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Reyes JF, Ferrer C, Gomez-Ramos MJ, Molina-Diaz A, Fernandez-Alba AR. Determination of pesticide residues in olive oil and olives. Trends Anal. Chem. 2007;26:239–251. [Google Scholar]
- 11.Esteve-Turrillas FA, Pastor A, de la Guardia M. Determination of pyrethroid insecticide residues in vegetable oils by using combined solid-phases extraction and tandem mass spectrometry detection. Anal. Chim. Acta. 2005;553:50–57. [Google Scholar]
- 12.Bushway RJ, Perkins LB, Hurst HL, Ferguson BS. Determination of atrazine in milk by immunoassay. Food Chem. 1992;43:283–287. [Google Scholar]
- 13.Nunes GS, Toscano IA, Barcelo D. Analysis of pesticides in food and environmental samples by enzyme-linked immunosorbent assays. Trends Anal. Chem. 1998;17:79–87. [Google Scholar]
- 14.Park E-K, Kim J-H, Gee SJ, Watanabe T, Ahn KC, Hammock BD. Determination of pyrethroid residues in agricultural products by an enzyme-linked immunosorbent assay. J. Agric. Food Chem. 2004;52:5572–5576. doi: 10.1021/jf049438z. [DOI] [PubMed] [Google Scholar]
- 15.Kaware M, Bronshtein A, Safi J, Van Emon JM, Chuang JC, Hock B, Kramer K, Altstein M. Enzyme-linked immunosorbent assay and sol-gel-based immunoaffinity purification of the pyrethroid bioallethrin in food and environmental samples. J. Agric. Food Chem. 2006;54:6482–6492. doi: 10.1021/jf0607415. [DOI] [PubMed] [Google Scholar]
- 16.Garces-Garcia M, Morais S, Gonzalez-Martinez MA, Puchades R, Maquieira A. Rapid immunoanalytical method for the determination of atrazine residues in olive oil. Anal. Bioanal. Chem. 2004;378:484–489. doi: 10.1007/s00216-003-2242-1. [DOI] [PubMed] [Google Scholar]
- 17.Garces-Garcia M, Brun EA, Puchades R, Maquieira A. Immunochemical determination of four organophosphorus insecticide residues in olive oil using a rapid extraction process. Anal. Chim. Acta. 2006;556:347–354. [Google Scholar]
- 18.Skerritt JH, Rani BEA. Detection and removal of sample matrix effects in agrochemical immunoassays. Immunoassays Residue Anal. 1996;621:29–43. [Google Scholar]
- 19.Skerritt JH, Hill AS, Rao RBS, Beasley HL, Rani BEA, Kumari CGU, Vijayashankar YN, Venugopal NBRK, Karanth NGK. Sample matrix interference in immunoassays for organochlorine residues in plant-derived foods and some strategies for their removal. Food Agric. Immunol. 2003;15:17–34. [Google Scholar]
- 20.Wortberg M, Goodrow MH, Gee SJ, Hammock BD. Immunoassay for simazine and atrazine with low cross-reactivity for propazine. J. Agric. Food Chem. 1996;44:2210–2219. [Google Scholar]
- 21.Lee HJ, Shan GM, Ahn KC, Park EK, Watanabe T, Gee SJ, Hammock BD. Development of an enzyme-linked immunosorbent assay for the pyrethroid cypermethrin. J. Agric. Food Chem. 2004;52:1039–1043. doi: 10.1021/jf030519p. [DOI] [PubMed] [Google Scholar]
- 22.Pino J, Sanchez M, Sanchez R, Roncal E. Chemical-composition of orange oil concentrates. Nahrung–Food. 1992;36:539–542. [Google Scholar]
- 23.Lopes D, Raga AC, Stuart GR, de Oliveira JV. Influence of vacuum distillation parameters on the chemical composition of a five-fold sweet orange oil (Citrus sinensis Osbeck) J. Essent. Oil Res. 2003;15:408–411. [Google Scholar]
- 24.Goh KS, Spurlock F, Lucas AD, Kollman W, Schoenig S, Braun AL, Stoddard P, Biggar JW, Karu AE, Hammock BD. Enzyme-linked immunosorbent assay of simazine for Delhi and Yolo soils in California. Bull. Environ. Contam. Toxicol. 1992;49:348–353. doi: 10.1007/BF01239636. [DOI] [PubMed] [Google Scholar]
- 25.Shan GM, Leeman WR, Stoutamire DW, Gee SJ, Chang DPY, Hammock BD. Enzyme-linked immunosorbent assay for the pyrethroid permethrin. J. Agric. Food Chem. 2000;48:4032–4040. doi: 10.1021/jf000351x. [DOI] [PubMed] [Google Scholar]
- 26.Ramesh A, Balasubramanian M. Rapid preconcentration method for the determination of pyrethroid insecticides in vegetable oils and butter fat and simultaneous determination by gas chromatography–electron capture detection and gas chromatography-mass spectrometry. Analyst. 1998;123:1799–1802. doi: 10.1039/a803097i. [DOI] [PubMed] [Google Scholar]