Abstract
Brain-specific angiogenesis inhibitor-1 (BAI1) is a transmembrane protein highly expressed in normal brain that has been ascribed two apparently distinct functions: inhibition of angiogenesis and recognition and engulfment of apoptotic cells by phagocytes. A previous localization study reported BAI1 expression only in neurons. Because a phagocytic function of BAI1 could be important for neuroglial antigen processing and presentation, we performed immunolocalization studies in adult mouse brain and cultured neural cells, using a pair of antibodies directed against N- and C-terminal epitopes. BAI1 immunoreactivity is enriched in gray matter structures and largely excluded from myelinated axon tracts. Neuronal BAI1 expression was readily detectable in the cerebellar molecular layer as well as in primary hippocampal cultures. In some brain regions, especially olfactory bulb glomeruli, BAI1 was expressed by GFAP-positive astrocytes. Cultured cortical astrocytes show small (∼0.4μm2) BAI1 immunoreactive membrane puncta as well as prominent focal adhesion localization in a subset of cells. In mixed neuronal-glial cultures, BAI1-expressing astrocytes frequently contained engulfed apoptotic debris. Cultured astrocytes engulfed apoptotic targets, and BAI1 showed accumulation within the phagocytic cup. We hypothesize that glial BAI1 may subserve an engulfment function in adult brain regions such as olfactory bulb with ongoing apoptotic turnover, whereas neuronal-derived BAI1 may serve primarily as an anti-angiogenic factor in the mature neuropil.
Introduction
The recognition and phagocytic clearance of apoptotic cells is a critical process in all multicellular organisms (Elliott and Ravichandran, 2010; Kinchen and Ravichandran, 2008), necessary for normal morphogenesis and potentially important for the prevention of autoimmunity. One mechanism for immunological tolerization could be the presentation of self antigens acquired by engulfment of apoptotic cells (Albert et al., 1998; Russo et al., 2000). It is unclear whether such processing and presentation of self antigens from apoptotic brain cells occurs and whether it plays any role in CNS autoimmunity. Brain-specific angiogenesis inhibitor-1 (BAI1) is one of several recently identified phosphatidylserine receptors that functions in apoptotic cell engulfment (Bratton and Henson, 2008). BAI1 serves as a phosphatidylserine receptor that binds apoptotic cell membranes and triggers activation of the best-studied apoptotic engulfment pathway, via its interaction with ELMO1 and Dock180, leading to the activation of the small GTPase Rac1 (Park et al., 2007). Rac1 activity is essential for the extensive actin remodelling and membrane trafficking during engulfment (Tosello-Trampont et al., 2001). Despite its high expression in the central nervous system, studies addressing its regional and cellular expression have been minimal (Mori et al., 2002; Kaur et al., 2003) with no reports on its subcellular localization. Because phagocytosis of apoptotic neurons and other brain cells is a necessary step in CNS antigen processing and presentation, we sought to characterize the regional, cellular and subcellular expression of BAI1 in the mature mouse brain and culture systems.
Materials and methods
Cell culture
Neonatal primary astrocyte cultures were prepared as previously described (Heffron and Mandell, 2005). Briefly, the forebrain was dissected from newborn pups, meninges were removed, and cells were dissociated in 0.05% trypsin EDTA for 5 min at 37°C. Following trituration, cells were pelleted and resuspended in DMEM supplemented with 10% fetal bovine serum, penicillin (50 U/ml), and streptomycin (50 ug/ml), all from Gibco. Media were replaced twice per week for two weeks to obtain astrocyte monolayers. Mixed glial/neuronal cultures were prepared from neonatal rat hippocampus as previously described (Goodkin et al., 2008). LR73 fibroblasts were stably transfected with a full length BAI1 construct to produce LR73-BAI1 cells, as previously described (Park et al., 2007).
In vitro phagocytosis assay
Mouse astrocytes were incubated with fluorescently labelled 2 μm carboxylate-modified latex beads exactly as previously described (Park et al., 2007). After 2 h, the cells were extensively washed with cold PBS and fixed in 4% paraformaldehyde, prior to immunofluorescence staining for BAI1 (h1570).
Tissue processing
Mice were anesthetized with a lethal dose of pentobarbital and transcardially perfused at room temperature with 10 ml PBS followed by 10 ml of PBS/4% paraformaldehyde over a period of 3-5 minutes. For some studies using antibody h103, mice were perfusion fixed with a high pH fixative (Berod et al., 1981). Brains were further fixed in PBS/4% paraformaldehyde for 24 hours at 4°C and processed into paraffin by standard methods. All animal procedures were approved by the University of Virginia Animal Care and Use Committee.
Western Blotting
LR73 parental or LR73-BAI1 cells were lysed directly in Laemmli sample buffer and separated by electrophoresis using standard procedures. Gels were transferred to nitrocellulose for 90 min with a semidry transfer apparatus and treated with blocking reagent (LI-COR block; LI-COR, Lincoln NE) overnight at 4°C and then probed with primary antibodies (BAI1 h1570 1:10,000; alpha-tubulin, Sigma, 1:4000) for one hour at room temp. Secondary antibodies were goat anti-mouse InfraRed800 and goat anti-rabbit Cy5.5 (Rockland) at 1:2000 for infrared imaging. Blots were imaged on an Odyssey LI-COR Odyssey infrared scanner (LI-COR, Lincoln NE).
Primary Antibodies and Immunohistochemistry/Immunofluorescence
Custom polyclonal rabbit antisera against C-terminal (intracellular epitope, Ab h1570) and N-terminal (extracellular epitope, Ab h103) regions of BAI1 were generated by injecting rabbits with corresponding KLH-linked peptides derived from human amino acid sequences (Novus Biologicals, Littleton, CO). Antibodies were used after one round of affinity purification on peptide columns. Peptide competition experiments were used to test antibody specificity in immunohistochemistry (Figure 1). As an irrelevant control peptide, a 15 amino acid peptide derived from the human AMP-activated kinase-alpha-1 with no sequence similarity was used at the same concentration as the h1570 peptide. Other primary antibodies: rabbit polyclonal anti-GFAP (DAKO #Z0334; 1:4000); mouse monoclonal anti-GFAP conjugated to Cy3 (Sigma, 1:800); rabbit polyclonal S-100beta (DAKO #Z0311; 1:500); rabbit polyclonal anti-IBA1 (Wako, 019-19741; 1:500); mouse monoclonal anti-SV2 (Developmental Studies Hybridoma Bank; 1:10). Microglia were labeled with biotinylated Lycopersicon Esculentum (tomato) lectin (Vector, 20μg/ml) and visualized with streptavidin-conjugated TRITC (Jackson, 1:2000). Free floating frozen 30 μm sections or 5 μm paraffin sections were prepared and processed for immunohistochemistry using standard techniques. Immunoperoxidase detection was performed using the ImPress polymeric peroxidase reagents (Vector, Burlingame, CA), according to the supplier's instructions. The chromogen used was diaminobenzidine (Dako S3000) 1 mg/ml in PBS plus 0.02% hydrogen peroxide applied for 3-5 min. Immunofluorescence detection was performed using Alexa-488 and Alexa-546 dyes (Invitrogen, Carlsbad, CA). Brightfield and immunofluorescence images were acquired with an Olympus BX40 upright microscope and a Scion Firewire CCD camera (Scion, Frederick, MD). Whole section imaging (Figure 2) was performed using an Aperio ScanScope (Aperio, Vista, CA). Confocal microscopy was performed using a Zeiss 510 Meta microscope with a 60x or 63x oil immersion objective lens (Keck Biological Imaging Center, Dept. Biology, Univ. of Virginia).
Figure 1.
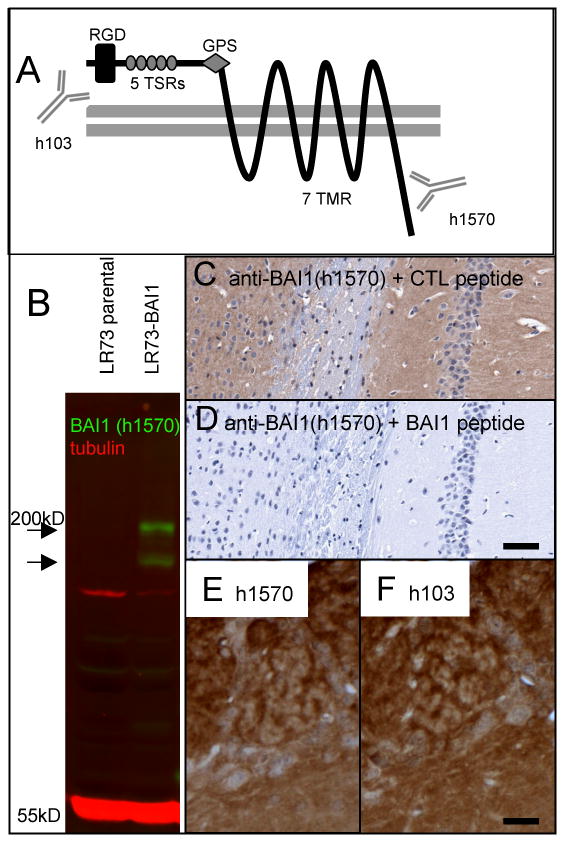
Demonstration of anti-BAI1 (h1570) antibody specificity. A) BAI1 is a 7-transmembrane (7 TMR) protein of the GPCR superfamily. The extracellular domain contains an RGD sequence, 5 thrombospondin type I repeats (TSRs) which are necessary for both the anti-angiogenic properties and apoptotic cell engulfment, and a G-protein coupled receptor (GPCR) proteolytic cleavage site (GPS). Antibody h103 is directed at an extracellular (N-terminal) sequence. The protein is cleaved at the GPS to release the anti-angiogenic fragment known as vasculostatin. The polyclonal h1570 antibody generated for the present study recognizes an amino acid sequence near the intracellular C-terminus. B) Western blotting of untransfected LR73 fibroblasts (parental) shows no detectable endogenous BAI1 signal (green) at the expected molecular weight of ∼180 kD. A stably transfected line (LR73-BAI1) shows strong h1570 blotting signal at the expected molecular weight as well as in a presumed proteolytic fragment. (C, D) Preincubation of diluted h1570 antibody with an irrelevant peptide (AMP-activated kinase, alpha-1, C-terminal peptide, Santa Cruz Biotechnology) had no effect on immunostaining of mouse hippocampus (C), whereas preincubation with the immunizing peptide completely abolished immunostaining (D). Immunohistochemistry on adjacent paraffin sections using the h1570 (C-terminal) and the h103 (N-terminal-directed) antibodies gave nearly identical staining patterns, with olfactory bulb shown as an example (E, F). Scale bars: D=100 μm; F=25 μm.
Figure 2.
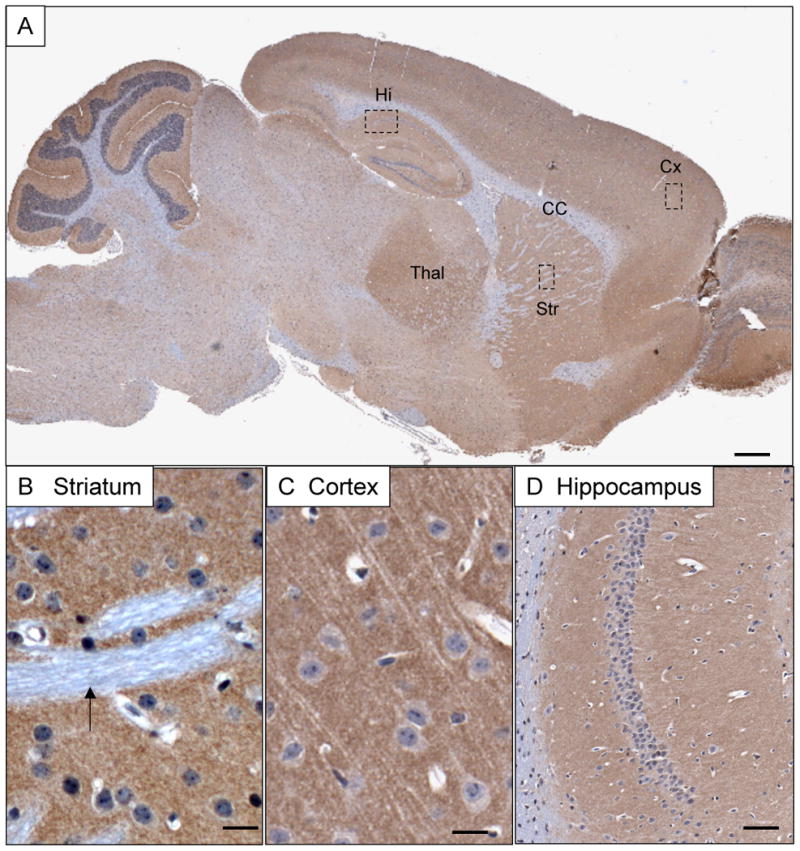
Overview of BAI1 immunoreactivity in the adult mouse brain. A) BAI1 expression detected by antibody h1570 is enriched in neuropil structures such as cortex (Cx), thalamus (Th), striatum (Str) and hippocampus (Hi), and largely absent from white matter structures such as the corpus callosum (CC). B) Striatal pencil fibers (small white matter tracts-arrow) lack BAI1 expression whereas surrounding neuropil shows finely punctate staining. C) Cortical pyramidal neurons show little somatodendritic cytoplasmic staining, but are surrounded by intense granular neuropil immunoreactivity. D) Hippocampal neurons show a neuropil pattern confined to the exent of the apical (left) and basal (right) dendritic fields of CA1. Scale bars: A=500 μm; B, C=20 μm; D=100 μm.
Results
Validation of C-terminal (intracellular) and N-terminal (extracellular) anti-BAI1 antibodies
BAI1 is a 7-pass transmembrane protein belonging to the type II adhesion type G-protein coupled receptor family. The N-terminal extracellular domain contains 5 thrombospondin type-I repeats and an RGD (putative integrin-binding) sequence (Figure 1A). We tested the specificity of our C-terminal (intracellular epitope, Ab h1570) antibody using two approaches. First, we showed that western blotting using h1570 detected strong bands near the expected weight (∼180 kD) in LR73 fibroblasts stably transfected with human BAI1, but not in untransfected cells (Figure 1B). An additional immunoreactive band of lower molecular mass was present, which could be the result of proteolytic cleavage, as previously described (Kaur et al., 2005). Second, we showed that preincubation of antibody h1570 with excess immunizing peptide, but not an irrelevant peptide, completely blocked immunohistochemical staining (Figures 1 C, D). The N-terminal anti-BAI1 antibody h103, although useful for immunohistochemistry and immunofluorescence, where it produced qualitatively very similar staining patterns on adjacent sections as h1570 (Figures 1E, F), did not recognize denatured BAI1 on western blot. We found that h103 staining of tissue fixed with standard pH 7.4 4% paraformaldehyde was weaker than that obtained with antibody h1570. However, the intensity of h103 immunostaining could be increased by using a high pH (11.0) fixation buffer as previously described (Berod et al., 1981). The similarity in immunostaining profiles with the two antibodies suggests that in the normal adult brain there is not a large pool of cleaved and translocated extracellular fragment (referred to in the literature as vasculostatin (Kaur et al., 2005), since this pool of released protein would be detected with the h103, but not the h1570 antibody. However, we cannot exclude the possibility that vasculostatin is lost from the extracellular space due to perfusion and fixation procedures.
BAI1 expression in the normal adult mouse brain: enrichment in neuropil and exclusion from myelinated white matter tracts
BAI1 immunoreactivity detected with antibody h1570 was widespread in all neuropil-rich zones, including spinal cord gray matter, cerebellar molecular layer, cerebral cortex, thalamic nuclei and basal ganglia (Figure 2). Close examination of hippocampus and cortex revealed that large pyramidal neurons lacked significant somatodendritic cytoplasmic BAI1, but that the intervening neuropil was rich in BAI1. White matter, both large tracts such as corpus callosum and anterior commissure, as well as microscopic tracts such as striatal pencil fibers, were devoid of BAI1 immunoreactivity.
Complex cellular patterns of expression in specialized neuropil zones: cerebellum, olfactory bulb and retina
Examination of several brain regions with architecturally distinct neuropil zones revealed several interesting aspects of BAI1 localization. In the cerebellar molecular layer, strongly labeled fine branching processes were seen to emanate from interneurons, whereas Purkinje cells and their dendrites showed weaker expression (Figure 3A). GFAP-immunoreactive Bergmann glial processes were negative for BAI1, but it was difficult to determine whether distal Bergmann glial membranous processes might account for some BAI1 immunostaining (Figure 3B). In the olfactory bulb, glomeruli showed the highest intensity of BAI1 staining (Figure 3C). Unlike other neuropil-rich zones, the pattern of staining in olfactory glomeruli showed stellate forms, consistent with the distal membranous processes of astrocytes. This was confirmed by co-staining with GFAP, which showed GFAP-positive process cores surrounded by BAI1- reactive distal processes (Figure 3D). Extraglomerular astrocytes, which occupy the neuropil zones just above the glomeruli in (C) show much lower BAI1 immunoreactivity. In the retina BAI1 was highly concentrated in the two neuropil layers, the outer and inner plexiform layers (Figure 3E). Double immunofluorescence labeling with the universal presynaptic marker SV2 showed that not all presynaptic terminals co-localized with BAI1: in the outer plexiform layer (OPL), brightly SV2-immunoreactive photoreceptor terminals were devoid of BAI1 immunostaining, but synaptic zones in the more inner portion of the OPL, where horizontal cell and interplexiform cells synapse upon bipolar cell dendrites (Dowling, 1987), did show significant BAI1 immunoreactivity (Figure 3F).
Figure 3.
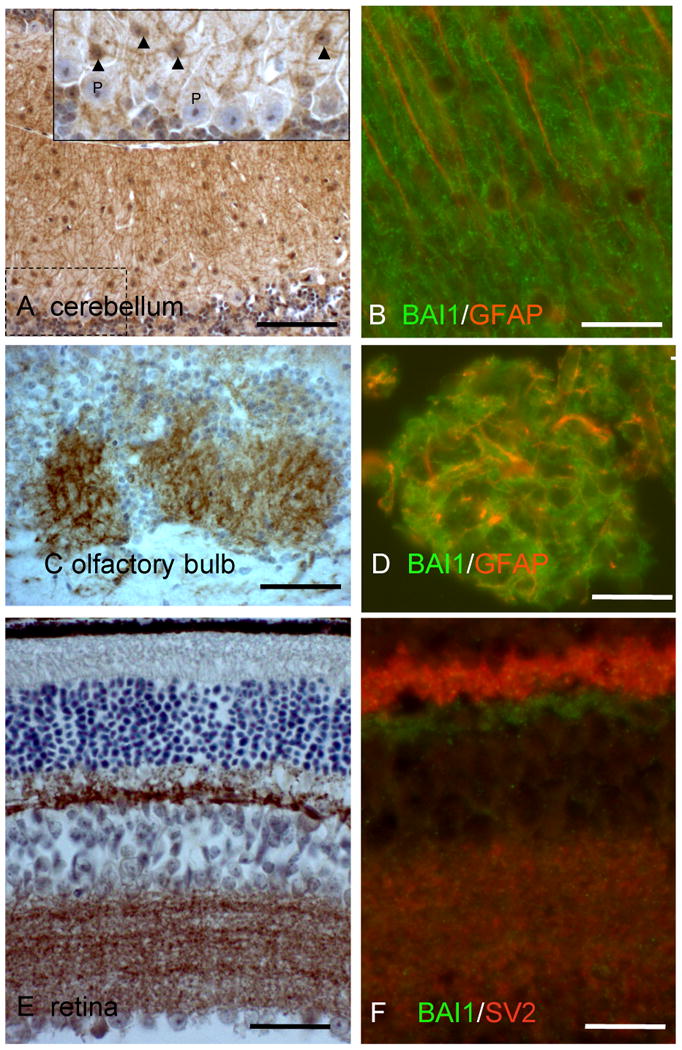
Fine patterns of neuropil BAI1 immunoreactivity in cerebellum, olfactory bulb, and retina. (A) Cerebellular staining reveals fine interlacing processes emanating primarily from small molecular layer interneurons (inset, arrowheads), not from Purkinje cells (P) or Bergmann glia. (B) BAI1 (green) fine radial processes do not colocalize with Bergmann glial fibers (GFAP, red). (C) Olfactory bulb glomeruli show intense staining in a reticular pattern coincident with distal membranes of GFAP+ astrocytes (D). Extraglomerular astrocytes, which occupy the neuropil zones just above the glomeruli in (C) show much lower BAI1 immunoreactivity. (E) Retinal BAI1 is confined to the outer (top) and inner (bottom) plexiform layers. (F) Double labeling for BAI1 and presynaptic marker SV2 reveals lack of BAI1 in large photoreceptor synaptic terminals (bright red upper portion of IPL), but strong expression in structures located beneath these terminals. In the inner plexiform layer, BAI1 is intermingled with, but does not colocalize with SV2. Scale bars: A, C=125 μm, B, D, F=25 μm, E=50 μm.
BAI1 is expressed in cultured neurons, astrocytes, and microglia
In order to more definitively determine the cellular origin and subcellular localization of BAI1 in the brain, we performed immunofluorescence on mixed neuronal/glial cell cultures from rat hippocampus (Figure 4). Neurons, astrocytes, and microglia, identified both by characteristic morphologies and specific markers, showed definitive punctate BAI1 immunostaining. In general, neurons and astrocytes showed the most intense labeling, with microglia showing variable and patchy staining. In older cultures, where many cells had undergone apoptosis, we observed numerous GFAP-positive astrocytes with punctate BAI1 labeling that had engulfed apoptotic nuclear debris (identified by condensed DAPI staining). Confocal microscopy with z-sectioning showed the debris to be within the confines of GFAP-positive astrocyte cytoplasm and surrounded by BAI1-positive membrane (Figure 4C). In order to determine whether astrocytic BAI1 associates with apoptotic targets (2-micron red fluorescent carboxylate-modified beads), we performed confocal microscopy on astrocytes 2 h after addition of beads (Figure 4D). A single confocal slice shows that some beads are surrounded by a higher concentration of BAI1 staining (h1570) than surrounding plasma membrane (see bead at convergence of cross hairs). In addition, XZ and YZ orthogonal slices reveal BAI1 immunoreactivity surrounding a bead, indicating engulfment and not merely superficial membrane adhesion.
Figure 4.
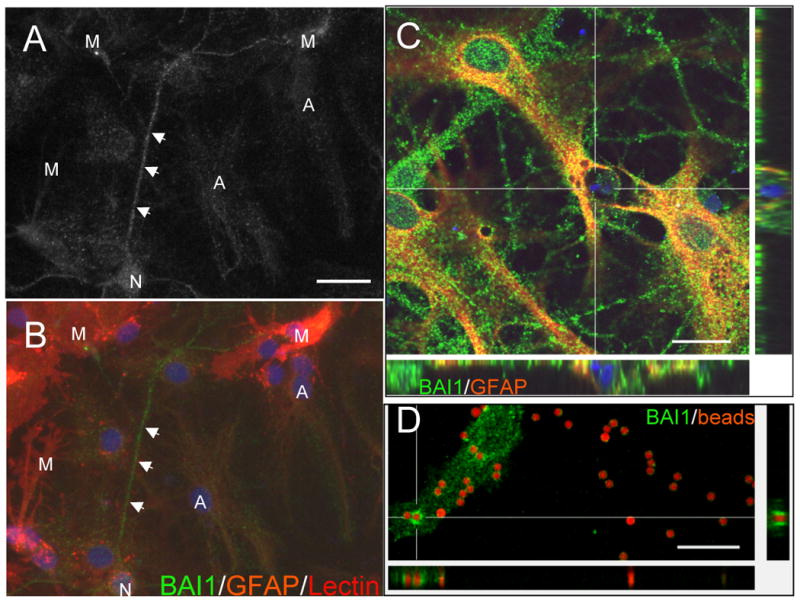
(A,B) BAI1 is expressed by cultured neurons, astrocytes, and microglia. In general, neurons (N), recognized by their extensive neuritic networks, showed the strongest BAI1 immunoreactivity. Astrocytes (A), labeled lightly with GFAP-Cy3, also revealed significant punctate labeling. Microglia (M), labeled with the tomato lectin, showed variable and patchy BAI1 staining. (C) In older culture preparations in which numerous cells had undergone apoptosis, we frequently observed GFAP-positive astrocytes with punctate BAI1 labeling that had engulfed apoptotic nuclear and cytoplasmic debris. Confocal microscopy with z-sectioning showed the DAPI-positive nuclear debris to be within astrocyte cytoplasm. (D) BAI1-immunoreactive astrocyte membrane surrounds apoptotic targets (2 μm carboxylate-modified red fluorescent beads) added to cultured astrocytes for 2 hours. A single confocal slice shows that some beads are surrounded by a higher concentration of BAI1 staining (h1570) than surrounding plasma membrane (see bead at convergence of cross hairs). In addition, XZ and YZ orthogonal slices reveal BAI1 immunoreactivity surrounding a bead, consistent with its engulfment. Scale bars: A=25 μm, C=20 μm, D=15 μm.
Punctate membrane and focal adhesion localization of BAI1 in purified cultured astrocytes
An additional pattern of astrocyte BAI1 labeling was observed in purified astrocytes, grown in serum-containing medium in the absence of neurons. In a subset (20-30% of GFAP-positive cells), BAI1 localized to focal adhesions, confirmed by double-labeling with paxillin (Figures 5A-C). Interestingly, BAI1 was not uniformly distributed through the length of the focal adhesions, but showed a speckled distribution which was sometimes polarized towards one end of the focal adhesion. All cells, whether or not they formed prominent focal adhesions, also exhibited punctate membrane BAI1 staining (Figures 5D-F). The area of these puncta determined from single confocal sections of 4 different fields of astrocytes was 0.422 +/- 0.387 (S.D.) μm2.
Figure 5.
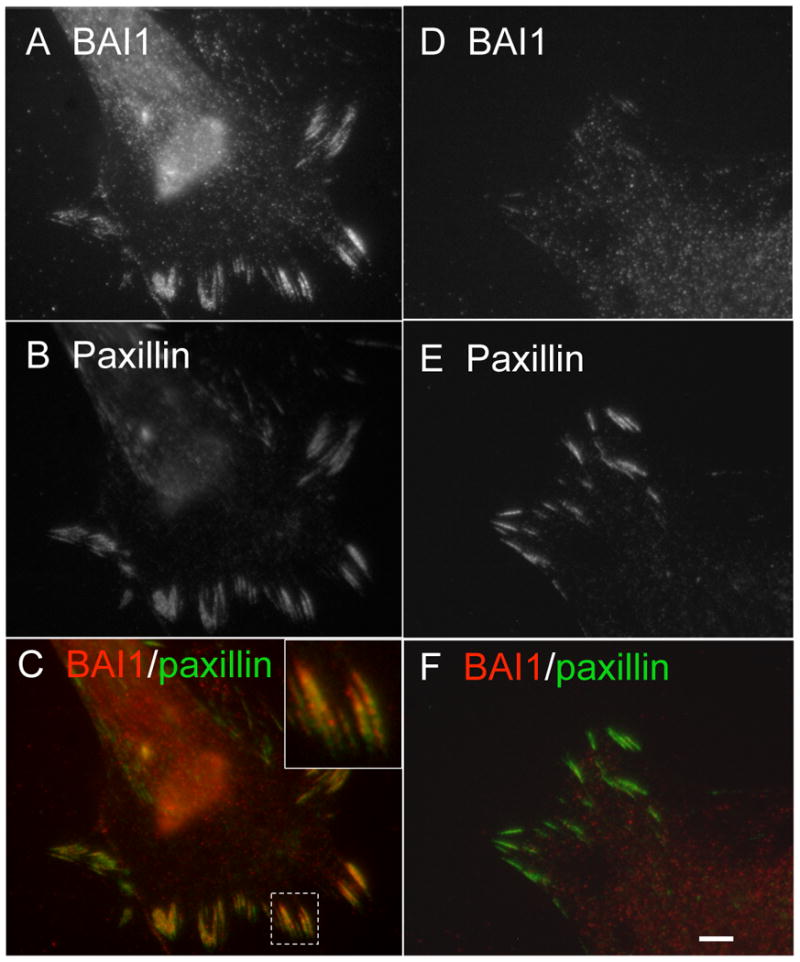
BAI1 is expressed in cultured astrocytes in two distinct patterns, membrane puncta and focal adhesions. (A-C) Some cells show prominent focal adhesion BAI1 localization, demonstrated by colocalization with paxillin, as well as membrane puncta not colocalized with paxillin. (D-F) Other cells show a predominant membrane punctate labeling with little focal adhesion localization. Scale bar: F=5 μm.
Discussion
Our immunohistochemical and immunofluorescence labeling experiments in mouse brain and cultured neural cells reveal new information about the cellular and subcellular localization of BAI1, with implications for its proposed dual functions. A previous study concluded that BAI1 expression was purely neuronal, and completely excluded from GFAP-positive astrocytes (Mori et al., 2002). This study was limited to human postmortem material and examined only the cerebral cortex. A subsequent paper, although focused on the downregulation of BAI1 in malignant gliomas, mentioned as unpublished data that BAI1 immunoreactivity was frequently observed in reactive astrocytes surrounding tumor, in addition to its expression in neurons and neuropil (Kaur et al., 2003). Our previous study showed relatively high levels of BAI1 in primary astrocyte cultues by western blot and demonstrated that siRNA knockdown of BAI1 in astrocytes decreased engulfment of apoptotic targets (Park et al., 2007). Our current demonstration of both neuronal and astrocyte BAI1 patterns of expression is also supported by publically available in situ hybridization data (http://mouse.brainmap.org/brain/Bai1.html).
A descriptive study such as this with several novel observations raises many more questions than it answers. Why might microglia, the professional phagocyte of the CNS, express low levels of BAI1, but utilize a variety of other phagocytosis-relevant receptors? It is possible that microglia, the professional phagocytes of the brain, are equipped with several different receptors for multiple types of clearance, including that of infectious organisms, protein aggregates, and large quantities of necrotic or apoptotic cells. Astrocytes, on the other hand, may have a more dedicated role in the delicate clean-up of apoptotic neuronal debris produced during development and normal turnover, which could be carried out via BAI1. Are the two ascribed functions of BAI1, apoptotic engulfment and anti-angiogenesis, carried out by different cell types that express the protein, e.g., astrocytes and neurons? Is the cleavage of BAI1 to generate the secreted 120 kD vasculostatin fragment carried out primarily by neurons or glia? In situations where high rates of phagocytic clearance are required (e.g., following brain trauma or infarct, or during normal developmental apoptotic events) is the cleavage of BAI1 suppressed so that the released phosphatidylserine-binding vasculostatin fragment does not compete with the intact phagocytic receptor function? What is the significance of BAI1 localization to astrocyte focal adhesions, and is an interaction of the extracellular RGD sequence with integrins essential for this localization?
Finally, astrocytes can serve as antigen presenting cells in the CNS, and are known to express MHC class I molecules including H-2D and H-2K (Carpentier et al., 2008). Since phagocytosis is the first step in antigen presentation, further study of mechanisms of astrocyte engulment of apoptotic neurons is needed. Whether BAI1 is essential for the engulfment of neuronal debris, antigen processing and presentation to T-cells remains to be determined.
Acknowledgments
JWM is supported by NS065447. K.S.R. is supported by grants from the NIH/NIGMS and an award from the Goldhirsh Foundation. We thank Kendra Keith (Lab of Howard Goodkin, U.Va. Dept. of Neurology) for mixed neuronal/glial cultures and Hannelore Asmussen (Lab of Rick Horwitz, U.Va. Dept. of Cell Biology) for hippocampal neuron cultures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Berod A, Hartman BK, Pujol JF. Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981;29:844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Bratton DL, Henson PM. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? Curr Biol. 2008;18:R76–79. doi: 10.1016/j.cub.2007.11.024. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Getts MT, Miller SD. Pro-inflammatory functions of astrocytes correlate with viral clearance and strain-dependent protection from TMEV-induced demyelinating disease. Virology. 2008;375:24–36. doi: 10.1016/j.virol.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. The retina: an approachable part of the brain. Vol. 282 Cambridge: Harvard Univ. Press; 1987. [Google Scholar]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron DS, Mandell JW. Opposing roles of ERK and p38 MAP kinases in FGF2-induced astroglial process extension. Mol Cell Neurosci. 2005;28:779–790. doi: 10.1016/j.mcn.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Kaur B, Brat DJ, Calkins CC, Van Meir EG. Brain-Specific Angiogenesis inhibitor 1 is differentially expressed in normal brain and glioblastoma independently of p53 expression. Am J Pathol. 2003;162:19–27. doi: 10.1016/S0002-9440(10)63794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B, Brat DJ, Devi NS, Van Meir EG. Vasculostatin, a proteolytic fragment of Brain-Specific Angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene. 2005;24:3632–3642. doi: 10.1038/sj.onc.1208317. [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS. Phagocytic signaling: you can touch, but you can't eat. Curr Biol. 2008;18:R521–524. doi: 10.1016/j.cub.2008.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Kanemura Y, Fujikawa H, Nakano A, Ikemoto H, Ozaki I, Matsumoto T, Tamura K, Yokota M, Arita N. Brain-specific angiogenesis inhibitor 1 (BAI1) is expressed in human cerebral neuronal cells. Neurosci Res. 2002;43:69–74. doi: 10.1016/s0168-0102(02)00018-4. [DOI] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- Russo V, Tanzarella S, Dalerba P, Rigatti D, Rovere P, Villa A, Bordignon C, Traversari C. Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc Natl Acad Sci U S A. 2000;97:2185–2190. doi: 10.1073/pnas.040540197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosello-Trampont AC, Brugnera E, Ravichandran KS. Evidence for a conserved role for CRKII and Rac in engulfment of apoptotic cells. J Biol Chem. 2001;276:13797–13802. doi: 10.1074/jbc.M011238200. [DOI] [PubMed] [Google Scholar]


