Abstract
Improved non-invasive localization of the epileptogenic foci prior to epilepsy surgery would improve surgical outcome in patients with partial seizure disorders. A critical component for the identification of the epileptogenic brain is the analysis of electrophysiological data obtained during ictal activity from prolonged intracranial recordings. The development of a noninvasive means to identify the seizure onset zone (SOZ) would thus play an important role in treating patients with intractable epilepsy. In the present study, we have investigated non-invasive imaging of epileptiform activity in patients with medically intractable epilepsy by means of a cortical potential imaging (CPI) technique. Eight pediatric patients (1M/7F, ages 4–14 year) with intractable partial epilepsy were studied. Each patient had multiple (6 to 14) interictal spikes (IIS) subjected to the CPI analysis. Realistic geometry boundary element head models were built using each individual’s MRI in order to maximize the imaging precision. CPI analysis was performed on the IISs, and extrema in the estimated CPI images were compared with SOZs as determined from the ictal electrocorticogram (ECoG) recordings, as well as the resected areas in the patients and surgical outcomes. The distances between the maximum cortical activities of the IISs reflected by the estimated cortical potential distributions and the SOZs were determined to quantitatively evaluate the performance of the CPI in localizing the epileptogenic zone. Ictal ECoG recordings revealed that six patients exhibited a single epileptogenic focus while two patients had multiple foci. In each patient, the CPI results revealed an area of activity overlapping with the SOZs as identified by ictal ECoG. The distance from the extreme of the CPI images at the peak of IIS to the nearest intracranial electrode associated with the onset of the ictal activity was evaluated for each patient and the averaged distance was 4.6 mm. In the group of patients studied, the CPI imaged epileptogenic foci were within the resected areas. According to the follow-up of the eight patients included, two were seizure free and six had substantial reduction in seizure frequency. These promising results demonstrate the potential for noninvasive localization of the epileptogenic focus from interictal scalp EEG recordings. Confirmation of our results may have a significant impact on the process of presurgical planning in pediatric patients with intractable epilepsy by dramatically reducing or potentially eliminating the use of intracranial recording.
Keywords: Cortical imaging, localization, epileptogenic focus, interictal spike, pediatric
Introduction
In the presurgical evaluation and diagnosis of the medically intractable epilepsy patients, it is of importance to accurately locate the epileptogenic brain areas to guide the neurosurgical operations. In most clinical cases, the electrocorticogram (ECoG) collected by subdural electrodes as well as stereotactic EEG (SEEG) by depth electrodes have been a gold standard to find the epileptogenic brain areas (Engel et al., 1981). Although there are high concordances between subdural and depth electrodes, the two techniques are applied differently in clinical diagnosis of epilepsy. Depth electrodes are necessary in detecting seizures originating in deep brain areas such as hippocampus. Subdural electrodes can be accurate when seizures are focal and lateralized (Spencer et al., 1990). They are often used in localizing seizures for pediatric patients who have epileptiform activities originating from neocortex, which are close to the recording surface.
However, the use of ECoG has been limited in clinical routine due to its invasiveness, morbidity risk, and high cost (Huppertz et al., 2001a). Meanwhile, the scalp EEG provides noninvasive means to monitor the brain electrical activity, which offers much of the information for the localization of epileptogenic foci but with less accuracy. Many methods have been applied to localize the epileptiform activities based on the EEG source analysis, such as the dipole localization (He et al., 1987; Ebersole 1994), the distributed source analysis (Michel et al., 1999; Huppertz 2001b; Sidman et al., 1992; Ding et al, 2007a,b; Brodbeck et al., 2010) and the localization from EEG patterns (Ebersole et al., 1996; Ebersole 1997) in both research and clinical studies.
This study aims to apply cortical potential imaging (CPI), a high resolution EEG technique (He et al., 1999), to determine the epileptogenic foci from the noninvasive EEG recordings of epileptiform interictal spikes (IIS). The feasibility of this technique has been demonstrated in a previous study (He et al., 2002) by analyzing the clinically collected somatosensory evoked potentials. It was also used to localize and image cortical regions displaying interictal epileptiform activities in pediatric epilepsy patients, where the epileptogenic cortical zones at different lobes (frontal, temporal and parietal) were successfully revealed in the study, and confirmed by the neurosurgical resections and subsequent favorable outcomes (Zhang et al., 2003). However, in this previous study, a spherical head model with standard electrode locations were used to test feasibility of the CPI approach in clinical routines where a whole-head MRI and measured electrode positions are not available (as is frequently the case in a clinical setting). In addition, due to the lack of structural information from MRI in some patients, only qualitative comparison of the CPI results with SOZs were presented, and the localization accuracy could not be quantitatively established.
Accurate determination of the epileptogenic zone(s) is especially important in guiding neurosurgical resection. Studies have shown that, assuming a single dipole source model, the average error reflected by the difference between the locations of the calculated dipole and the intracranial source was 10mm for a four-shell spherical head model (Cohen et al., 1990), and 8.5 mm for a three-shell realistic model (Homma et al., 1994). Previous work has shown that the use of realistic geometry (RG) head models is able to increase the dipole localization accuracy for epileptiform spikes compared with the spherical head model (Silva et al., 1999). And another study reported that use of the RG head model increased source localization accuracy as compared with that using the 3-sphere head model (Roth et al., 1997).
In the present study, the CPI approach is applied to localize and image the interictal epileptiform spikes to reveal the epileptogenic cortical zones from scalp EEG recordings for pediatric epilepsy patients. The realistic geometry boundary element head volume conductor models have been employed in the analysis, in order to obtain more accurate location and extent of the epileptogenic zones to guide the presurgical planning. The localization accuracy is quantified by the distances between the CPI extrema and associated SOZs determined by intracranial measurements and surgical outcomes.
Methods
Figure 1 illustrates the study protocol. For each patient, EEG was acquired using scalp electrodes. MR images were obtained to build the multi-layer (scalp, skull and cortex) realistic geometry boundary element head model (He et al., 1987; Hamalainen & Sarvas, 1989). With these measurements in place, the CPI technique was then applied to estimate the electrical potential distribution over the whole cortical surface. The long term monitoring ECoG was also recorded by the implanted subdural electrodes to identify the seizure onset zone for each included pediatric epilepsy patient. The CPI results were then compared with the ECoG findings quantitatively by measuring the physical distance between CPI maximum and SOZs, as well as with the surgical resection and outcome in the patients.
Figure 1.
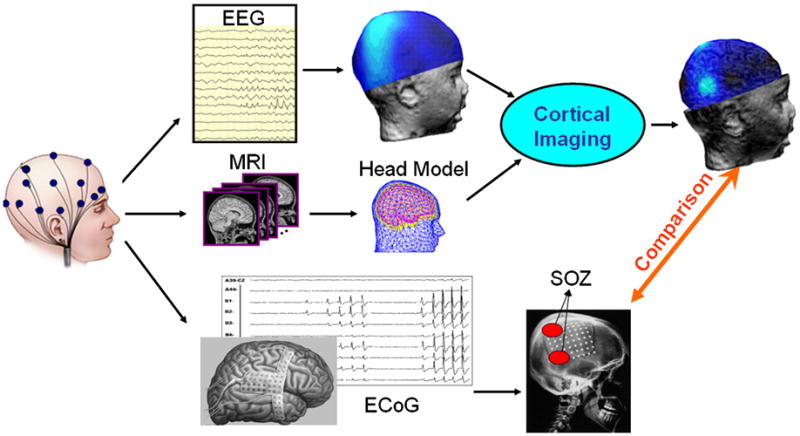
Schematic illustration of the Cortical Imaging Technique and study design.
Patients
Table I summarizes the patients’ information, diagnoses, operation, surgical outcome, and follow-up length. Eight pediatric patients (1 male, 7 female, 4 to 14 years old) with medically intractable partial epilepsy were studied using a protocol approved by the Institute Review Boards of the University of Minnesota and the University of Chicago. All patients had similar presurgical evaluation which included preoperative structure MRI, interictal and ictal long term video/EEG monitoring with both scalp electrodes and subsequent subdural electrodes (Radionics Medical Products Inc., Burlington, Massachusetts) at the Pediatric Epilepsy Center at the University of Chicago Children’s Hospital. The follow-up (1 to 8 years) shows that all patients either are seizure free or have reduction of seizure frequency after the surgical resections (Table I).
Table I.
Clinical information of the patients included in the present study.
| Pt. | Sex | Age | No. foci by ECoG | Operation | #Resection | Pre-op Sz Freq | Post-op Sz Freq | Engel Class | Follow up years |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 10 | 1 | Right frontal topectomy | 1 | 20/day | Sz Free | I | 4 |
| 2 | F | 14 | 1 | Left temporal lobectomy, removal of hippocampus | 1 | 6/week | 2/week | II | 3 |
| 3 | F | 7 | 1 | Right medial temporal lobectomy, right posterior temporal-parietal lobectomy | 2 | 10/week | 3/week | II | 8 |
| 4 | F | 4 | 1 | Right inferior-posterior frontal topectomy; posterior superior temporal topectomy; temporal tip lobectomy with removal of amygdala and anterior hippocampus, back approximately 5.5 to 6 cm from the temporal tip | 3 | Status epilepticus | >90% Reduction | II | 4 |
| 5 | F | 9 | 1 | Left frontal, then temporal then parietal topectomies; Posterior temporal subpial transactions | 3 | Numerous daily szs | >90% Reduction | II | 4 |
| 6 | M | 12 | 2 | Right post frontal and parietal lobectomy | 2 | 7/week | Sz Free | I | 1 |
| 7 | F | 10 | 2 | Left sided frontal and parietal occipital lobectomy | 2 | 4/week | 3/week | III | 1 |
| 8 | F | 12 | 1 | Left frontal and occipital topectomy | 2 | 7/week | 2/week | II | 2 |
Data Acquisition
During the presurgical monitoring, the EEGs were obtained using 24 scalp electrodes placed according to 10-10 system (American Electroencephalographic Society, 1994) with 400Hz sampling rate and band-pass filtering at 1–100Hz (BMSI 6000, Nicolet Biomedical Inc., Madison, Wisconsin). The positions of the surface electrodes as well as three fiducial points (nasion, left and right preauricular points) were digitized using a magnetic digitizer (Polhemus Inc., Colchester, VT). The interictal records from each patient were reviewed for occurrence of spikes. For each patient, multiple (6 to 14) interictal spikes were visually identified according to the International Federation of Societies for Electroencephalography and Clinical Neurophysiology (IFSECN) criteria (Chatrian et al., 1974). Spikes were selected sequentially as they occurred in the long term EEG monitoring records. Epochs with artifact (eye movement and blinks, muscle activity etc) were excluded from analysis. The highest peak of the interictal spike was determined by a peak detection algorithm. The artifact-free preoperative scalp EEG epochs of 2 second duration centered at the peak of the spikes were isolated for further analysis. Baseline correction was based on the scalp EEG data from 300 to 100ms before the peak of the spikes.
Three-dimensional (3-D) MRI were obtained for each patient with a Siemens 1.5T scanner using T1-weighted images composed of 120 continuous coronal slices with 1.5mm slice thickness. These MR images have been employed to build the 3-layer boundary element model for each individual using the software of Curry (Neuroscan Labs, TX). The normalized conductivity of the scalp and the brain was taken as 1.0 and that of the skull as 1/20 (Oostendorp et al., 2000; Lai et al., 2005; Zhang et al., 2006). In order to compare the noninvasive estimation and invasive recording, the postoperative CT images were also coregistered with the preoperative MRI to obtain the intracranial electrode positions in the same coordinate system as the boundary element model. The coregistration was based on a surface-fitting technique by matching the two skull surfaces reconstructed from both MR and CT images completed in Curry software (Neuroscan Labs, TX). It was a manual approach which interactively adjusted the transformation until a satisfactory visual result was achieved. Once the coregistration was done, the locations of corner electrodes and the dimension of the grid (e.g., 4X8 or 8X8) were then provided to the software, where the position of each surface electrode was automatically calculated.
Cortical Potential Imaging
The CPI technique used in the present study has been previously developed (He, 1999; He et al., 1999), and validated experimentally using the Somatosensory Evoked Potential (SEP) data collected from human patients (He et al., 2002). In brief, the head was approximately represented by a volume conductor with three shells representing the scalp, the skull, and the brain. Each shell is homogeneous but has different electrical conductivity. Since brain electrical sources exist only inside the brain, Green’s second identity can be applied to the volume between the scalp and the skull, and the volume between the skull and the brain, respectively. After mathematical manipulations, a linear relationship has been shown to exist between the scalp potential and cortical potential. By solving the inverse problem, the cortical potential distribution can be estimated from the scalp EEG measurement. Due to the illposedness of the system, zero-order Tikhonov regularization (Tikhonov and Arsenin, 1977), was used in the present study to suppress the noise effect.
In order to capture both early onset and the peak of the interictal epileptiform activity, CPI was applied to the scalp EEG measurements, at each time instant independently of other time instants, within a time window (typically from 20ms before to 10ms after the peak of spike). The regularization parameters corresponding to each time point were determined separately by means of the L-curve approach (Hansen, 1990, 1992).
Evaluation of Imaging Results
The results from the CPI analysis of IIS have been compared quantitatively with the ECoG ictal recordings, and compared qualitatively with resected areas. At first the long term monitoring ECoG recordings (Figure 1) were visually inspected for the ictal events according to the IFSECN criteria (Chatrian et al., 1974). On the ictal ECoG recordings, the channels where the repetitive activity (typically shown as fast oscillation) started first were considered as representation of SOZs. In cases of doubt or vague repetitive activities, the electrodes with largest amplitude were used instead. SOZs were defined as the cortical areas enclosed by these “active” electrodes. In addition, from the CPI-estimated cortical potential distribution, it is assumed that the cortical sites with maximal potential amplitude represent the generators of epileptiform activities. The physical distances of these sites to the SOZs were measured for each patient to evaluate the performance of the CPI analysis of interictal spikes.
Results
Eight pediatric epilepsy patients with medically intractable seizures were studied in the present work following the procedures as described above. All patients were enrolled in the University of Chicago Children’s Hospital. After the neurosurgical resections, all patients either are seizure free or have substantial seizure reduction (see Table I). Table II summarizes the results revealed by the CPI analysis. The mean distance between CPI and SOZ localization is 4.6 mm.
Table II.
Summary of results of the CPI analysis of interictal spikes.
| Pt. | No. IIS | CPI results | Epileptogenic zones determined by CPI | Distance of CPI peak to SOZ (mm) |
|---|---|---|---|---|
| 1 | 8 | 6/8 show right frontal activity; 2/8 show one right frontal activity and one activity on right temporal lobe. | Two on Right Frontal lobe | 0 |
| 2 | 14 | 13/14 show one activity on left temporal tip; 1/14 shows one activity on left temporal tip and one extra activity on left median temporal lobe. | Left inferior temporal lobe | 8.7 |
| 3 | 7 | 7/7 show both activities on right parietal lobe and right posterior temporal lobe | One on right parietal lobe, one on right posterior temporal lobe | 0 |
| 4 | 6 | 2/6 show 3 activities on right anterior temporal lobe, posterior temporal lobe, and inferior frontal lobe; 3/6 show right frontal and posterior temporal activities; 1/6 shows right frontal activity. | One on right anterior temporal lobe, one on right posterior temporal lobe, one on right inferior frontal lobe | 4.7 |
| 5 | 9 | 1/9 shows left parietal activity; 5/9 show left parietal and anterior temporal activities;3/9 show left parietal and posterior temporal activities | Two on left temporal lobe, one on left parietal lobe | 7.2 |
| 6 | 9 | 4/9 show right frontal and parietal-occipital activities; 1/9 shows right parietal activity; 4/9 show right frontal activity | One on right posterior frontal lobe, one on right parietal lobe | (5.7, 0)* |
| 7 | 7 | 5/7 show left occipital activity; 2/7 show left occipital and frontal activities. | One on left frontal lobe, one on parietal-occipital lobe | (5.3, 0)** |
| 8 | 13 | 13/13 show both left frontal and left parietal activities. | One on left frontal lobe, one on left occipital lobe | 5.0 |
Patient 6: the distance was 5.7mm to the frontal SOZ, 0 to the parietal SOZ.
Patient 7: the distance was 5.3mm to the frontal SOZ, 0 to the parietal-occipital SOZ.
Results from three patients are shown because of their representative and challenging aspects in terms of the localization of their epileptogenic zones. The first example (patient #1) had two spatially adjacent foci, and both the second and the third examples (patients #4 and #5) each had three foci located in different lobes.
Identification of spatially adjacent foci
Patient #1 was a 10-year-old female with intractable seizures. The pre-operative long-term scalp EEG monitoring showed that she had a seizure focus on the right side of her brain. Subsequently, her right temporal and parietal lobe were monitored intracranially. This revealed that the seizure focus was located in the right frontal region. Surgical resection (right frontal lobectomy) made the patient seizure free.
Eight pre-operative interictal spikes were selected and subjected to the CPI analysis. Figure 2 shows the results of the CPI source analysis of one typical interictal spike. Figure 2d shows the waveform of the interictal spike used for the analysis. Figure 2a shows the scalp potential distributions between 15ms before and 10ms after the peak interictal activity. The estimated cortical potential distributions at the corresponding time points are shown in Figure 2b. Compared with the smeared scalp potential maps, the cortical potential estimation clearly show the epileptiform activities originated from two foci located at right frontal region. To precisely illustrate the exact location of the epileptogenic foci and provide a comparable indication of epileptogenic zone, the cortical potential distributions above a 50% amplitude level were superimposed onto the realistic cortical surface reconstructed from the patient’s MRI, as displayed in Figure 2c. Figure 2e displays the right and top view of the two epileptogenic foci (N1 and N2) revealed by the estimated cortical potential distributions. The resected area of this patient is illustrated in Figure 2f, where there is only one large resection area (diameter about 3.5cm) which includes both foci shown in Figure 2e.
Figure 2.
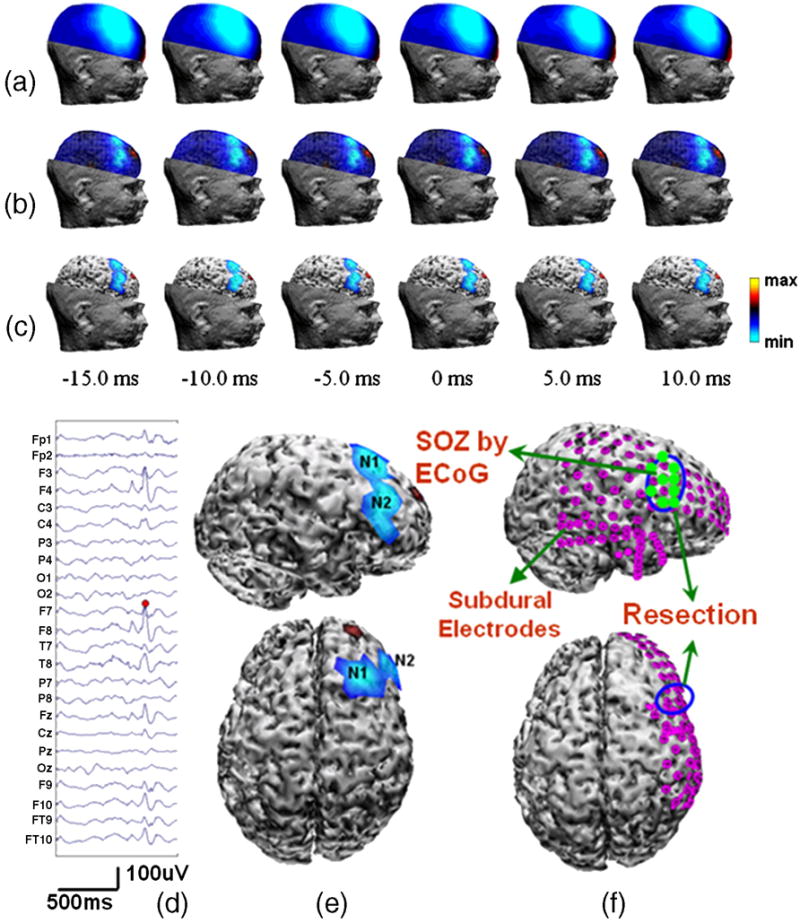
Patient #1 (a) Right-view scalp potential maps during an interictal discharge; (b) Corresponding estimated cortical potential distributions at each time point; (c) Top 50% cortical potential distributions superimposed onto the realistic cortical surface reconstructed from the patient’s MRI; (d) Scalp EEG waveform of the interictal spike, red dot representing the highest negative peak; (e) Epileptogenic foci revealed by the CPI analysis of the peak interictal spike activity in right and top views; (f) Illustration of surgical resections in right and top views.
Localization of multiple foci at different lobes
Patient #4 was a 4-year-old female with intractable epilepsy. The preoperative long-term EEG monitoring revealed a right temporal focus. She had subdural electrode array placed on the right temporal lobe and right frontal lobe. The long-term subdural EEG monitoring revealed three cortical areas arousing seizure discharges, which were over the right sided anterior temporal lobe, right posterior temporal lobe and right inferior frontal lobe.
Six preoperative interictal spikes were subjected to the CPI analysis to find the epileptogenic foci. Figures 3a and 3b show waveforms of two typical EEGs of interictal discharges. Figures 3c and 3d show the scalp potential maps, the estimated cortical potential distributions, and the top 50% cortical activity at 6 selected time points surrounding the peak activity of spike #1 and #2, respectively. Figure 3e shows the right-view cortical potential distributions estimated from peak activity of interictal spike #1, where three localized areas of negativity N1 (right posterior-superior temporal), N2 (right inferior-posterior frontal) and N3 (anterior temporal) are revealed. Figure 3f shows the right-view cortical potential distribution of peak activity of interictal spike #2, where only N1 activity is revealed. But from Figure 3d, it can be seen that both N2 and N3 activities appeared in spike #2, but diminished and terminated before the peak activity. Figure 3g shows the location of SOZ identified by ECoG, which was well predicted by the corresponding CPI maximum. Of all 6 interictal spikes analyzed for this patient, two showed all three activities, three showed one frontal (N2) and one posterior temporal (N1) activities, and one showed only frontal activity (N2). All these three activities (N1, N2 and N3) are confirmed by the neurosurgical resections. The seizure frequency was reduced by more than 90% after the inferior-posterior frontal topectomy, posterior-superior temporal topectomy and temporal tip lobectomy.
Figure 3.
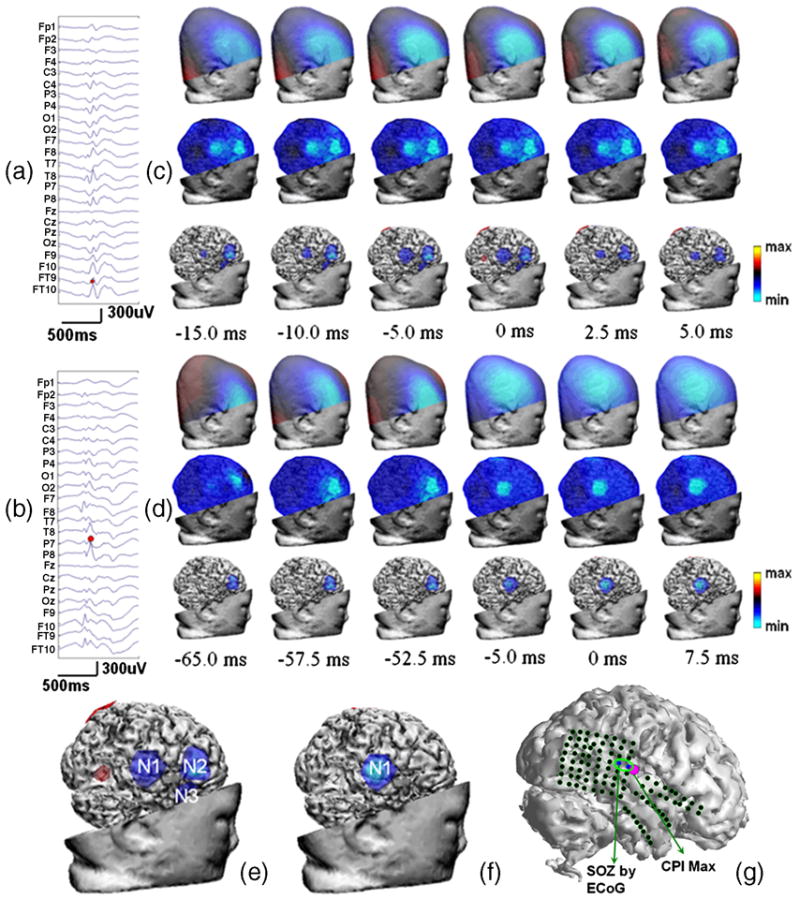
Patient #4. (a,b) Scalp EEG waveforms of interictal spike #1 and #2; (c,d) Right-view scalp potential maps, estimated cortical potential distributions, and top 50% cortical activities superimposed onto the cortical surface at 6 time points of interictal spike #1 and #2; (e,f). The epileptogenic foci revealed by the CPI analysis of the peak activity of interictal spikes #1 and #2 in right view; (g). Illustration of subdural electrodes, SOZ determined by ECoG, and CPI maximum.
Qualitative comparison with ECoG and surgical resections
Patient #5 was a 9-year-old female with intractable seizures. The preoperative long-term scalp EEG monitoring suggested presence of interictal discharges over the left hemisphere. Four electrode grids were placed subdurally to cover the posterior part of the left sided frontal lobe, the parietal lobe, the posterior parietal temporal lobe, and the superior and anterior temporal lobe. Preoperative long term subdural EEG monitoring revealed 3 areas of seizure discharges, one of which was over the left parietal lobe and two were over the left temporal lobe. The neurosurgical resections of these areas (left temporal lobectomy and left parietal topectomy) the seizure frequency was reduced by >90%.
Nine preoperative scalp EEG-recorded interictal spikes were analyzed using CPI, which were categorized into two types as shown in Figure 4 according to their spatial potential patterns. Figures 4a and 4b show the waveforms of two typical surface EEGs of interictal spikes #1 and #2, separately. Figures 4c and 4d show the scalp potential maps, the estimated cortical potential distributions and the top 50% cortical activities at 6 time points surrounding the peak activity for spike #1 and #2, respectively. Figure 4e shows the left-view and top-view estimated cortical potential distributions of the peak activity of interictal spike #1, where two localized areas of negativity N1 (left parietal) and N2 (left temporal) are revealed. Figure 4f shows the left-view and top-view cortical potential estimations of the peak activity of interictal spike #2. The localized area of N1 activity is the same as that of spike #1 shown in Figure 4e while N2 activity disappears, and an additional focus N3 is also revealed to reside in the left temporal lobe. Of nine interictal spikes analyzed using CPI, the N1 activity is revealed in all 9 spikes, N2 in five, and N3 in three.
Figure 4.
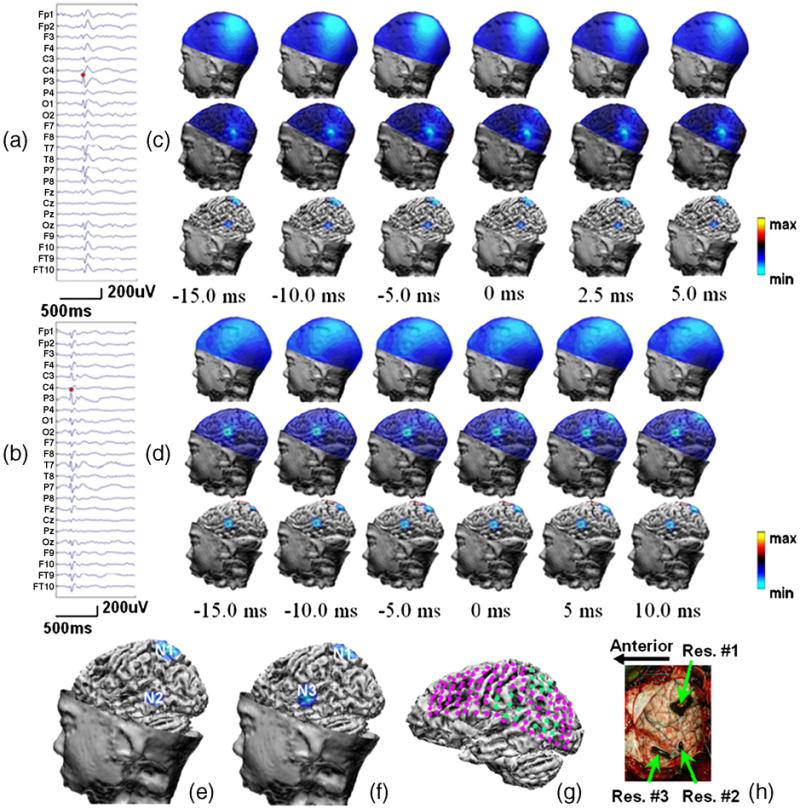
Patient #5. (a,b) Waveform of interictal spike #1 and #2; (c,d) Left-view scalp potential maps, estimated cortical potential distributions, and top 50% cortical activities superimposed onto the cortical surface at 6 time points of interictal spike #1 and #2; (e,f). The epileptogenic foci revealed by the CPI analysis of the peak activity of interictal spike #1 and #2 in left view; (g) Clinical diagnosis by neurologist, where pink dots are intracranial electrodes and green dots are SOZs; (h) Lateral view of the surgical resections.
Figure 4g shows the ECoG findings of the SOZs, where the pink dots display subdural electrodes and the green dots represent the cortical area with seizure activities. Figure 4h illustrates the resected area indicated by the green arrows. Visual comparison of figures 4g and 4h with figures 4e and 4f indicates that the three epileptogenic areas N1, N2 and N3 are successfully identified by the CPI results.
Quantitative Comparison with Seizure Onset Zone
On the CPI-estimated cortical potential distribution, it is assumed that the cortical sites with maximal potential amplitude reveal the locations of the generators of epileptiform activities. The physical distances of these sites to the SOZs defined by ictal ECoG recordings were measured for each patient to evaluate the performance of the CPI analysis of interictal spikes. The distances are included in Table II, where 0 means that the cortical sites with maximal amplitude were within the SOZs. For the patients with multiple seizure foci (two in patients #3, #6, #7 and #8; three in patients #4 and #5), averaged distance is calculated and included in the table. In Figures 5, the relative position of the CPI maximum and SOZs are displayed for patients #1. In patient #1 (Figure 5), the cortical site with maximum estimated potential is located within the SOZ on the right frontal lobe. The averaged distance for all of the eight patients included in this study is 4.6 mm.
Figure 5.
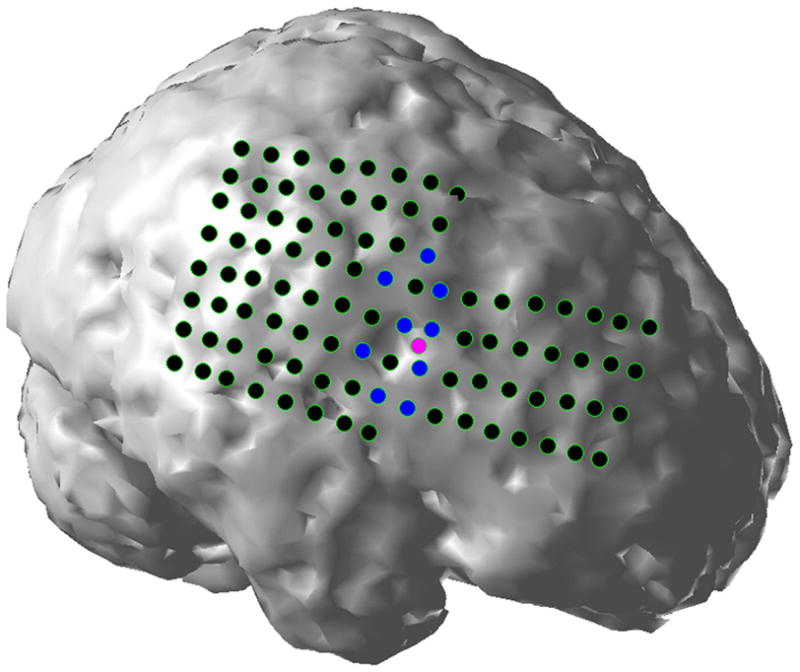
Quantitative evaluation of the CPI analysis of interictal spikes in patient #1. Black dots represent intracranial subdural electrodes, blue dots ictal onset zones identified by ECoG ictal recordings, and pink dots cortical sites with maximum estimated potential amplitude.
Discussion
In the present study, eight pediatric epilepsy patients’ pre-operative interictal spikes were subjected to the cortical potential imaging analysis to locate the epileptogenic foci using the patients’ realistic geometry boundary element head model. The most active cortical areas revealed by the estimated cortical potential distributions were confirmed by the neurosurgical resections, which made the patients seizure free or have substantial seizure reduction. Compared with the adult patients, the pediatric epilepsy patients may frequently have epileptic discharges involving multiple neocortical areas at different lobes with a greater variety in the EEG patterns (Gilliam et al., 1997; Otsubo et al., 1997; Ochi et al., 2000). However, this study shows that the epileptogenic foci located at the frontal, parietal, occipital as well as the temporal lobe can be located within 10 mm precision using the CPI analysis.
In pediatric epilepsy patients, the seizures occur more commonly in the extratemporal regions than in the temporal lobe (Gilliam et al., 1997; Otsubo et al., 1997). The accurate localization of epileptogenic foci for the children is especially critical to distinguish the functional brain areas on the large extratemporal lobes from the pathological structures. The RG head model is reported to be able to increase the accuracy of the dipole localization for the interictal spikes (Silva et al., 1999). In another work (Herrendorf et al., 2000), by using the RG head model, the origin of interictal epileptiform discharges was localized to the primary epileptogenic zone in cases of extratemporal epileptogenesis. In a previous study (Zhang et al., 2003), the spherical head model was used and CPI was applied to interictal spikes in a group of pediatric epilepsy patients. The use of the spherical model made it difficulty to quantitatively compare the reconstruction results with resected brain and SOZ as determined from ictal ECoG recordings. The present work employs the RG head model in the CPI analysis, which substantially improves the accuracy of the localization of the epileptogenic foci. Superimposing the cortical potential distribution onto the patients’ realistic cortical surfaces with gyri and sulci reconstructed from MR scan (Figures 2–4) allows clear identification and interpretation of the locations and extents of the epileptogenic activities.
In the presurgical evaluation and diagnosis for epilepsy patients, the ECoG is still playing an essential role as a “gold standard” to determine the location and extent of the epileptogenic zones. The CPI technique provides a noninvasive way to image the cortical electrical activities, which would provide virtual ECoG distributions should subdural electrodes be placed over the epicortical surface. Although it is a two dimensional surface imaging approach without taking the depth of the source into consideration, it may identify the cortical regions associated with underlying sources that generate or participate in the epileptiform activity. As pediatric epilepsy patients often have epilepsy originating from the neocortex in different lobes close to the epicortical surface, the CPI results shall in particular be suitable for localization and imaging of neocortical epileptiform activity. Compared with the scalp potential distribution, the cortical potential distribution has enhanced spatial resolution. As an example, for patient #5, multiple epileptic foci were well distinguished by CPI, while only smeared activity can be observed at scalp potential distribution. This spatial enhancement provides insight for assessing and imaging the underlying cortical activity. It is especially useful in the cases that the cortical regions to be resected are near the major functional brain areas, so that it could be easily recognized if these areas are overlapping with each other. The present results indicate that the CPI approach may become a promising technique to determine the epileptogenic cortical zones to guide the surgical planning for pediatric patients.
Another advantage of the CPI technique is that, compared to the dipole method, a priori estimate of the number of the epileptogenic foci is not required. This is a key feature of CPI since an important problem in the epilepsy evaluation and treatment is that how many pathological areas are involved in the epileptiform activities. The number of sources, as well as the location and extent, can be recognized from the estimated cortical potential distributions to help the surgical decisions without ad hoc assumption with regard to the number of dipoles.
The CPI technique also gains advantage over the ECoG recordings in that the estimated cortical potential is distributed over the whole epicortical surface. Since ECoGs are obtained using subdural electrode arrays, some parts of the cortex may not be covered so that the source localization may often occur at or even beyond the edges of the recording arrays. This edge effect does not exist for the CPI approach, because all spatial information collected from the surface electrodes is used to be deconvolved into the potential distribution over the whole epicortical surface. This is a unique feature of the CPI approach, as it is always desirable in clinical practice to be able to predict various cortical regions displaying epileptiform activities from a limited number of scalp electrodes.
Due to its wider availability than ictal events, interictal spikes are often used in localizing the cortical epileptogenic regions. However, the physiological relationship between IIS generator and seizure onset zone remains unclear. Numerous studies have been conducted to investigate the mechanism of generation of human IISs (Avoli et al., 2006; Staley and Dudek, 2006), but an agreement has not yet been reached among researchers. In a recent EEG-fMRI study of malformation of cortical development (MCD), Tyvaert et al. showed that different structures of the dysplastic cortex and the heterotopic cortex of band heterotopia were involved in interictal and seizure processes (Tyvaert et al., 2008). However, several studies show that for most pediatric patients with intractable neocortical epilepsy, the IISs are of more predictive value to localize SOZs. Sperli et al. applied EEG source imaging technique in analyzing the interictal epileptiform activities where the epileptogenic region was successfully localized in 27 out of 30 pediatric patients (Sperli et al., 2006). Asano et al. analyzed the intracranial interictal spikes with respect to ictal EEG findings in the pediatric patients, and suggested that interictal EEG may predict ictal-onset zones in children with intractable neocortical epilepsy (Asano et al., 2003). Another animal experimental study also suggested the pivotal role of IIS and neuronal network depolarization in subsequent seizure development and epileptogenesis (Rheims et al., 2008). In a very recent study, Wilke et al. employed an adaptive directed transfer function (ADTF) method to identify the cortical sources of the interictal spike activity in eight patients with medically intractable neocortical-onset epilepsy, where the results showed the majority of the ADTF-calculated source activity well correlated with clinically-defined SOZs (Wilke et al., 2009). The present study only included pediatric patients, most of whom had neocortical-onset epilepsy and all of whom demonstrated focal interictal epileptiform activity. The present results show that the epileptogenic foci revealed by the CPI analysis of interictal spikes coincide with the SOZs in each patient studied. This seems to support the finding that IIS is predictive of SOZs in neocortex-onset focal epilepsy.
One important concern regarding the interictal analysis is whether the single spike or the spike average should be used for the source localization. The spike averaging would increase the signal-to-noise ratio, but it is also well known that the individual interictal spikes very often show great variability in waveform so that the averaging of apparently similar spike can introduce localization error (Michel et al., 1999; Baumgartner et al., 1995; Lantz et al., 1997; Diekmann et al., 1998). As shown in the present results, for each patient, different cortical zones were involved in different interictal spikes. For example, Figures 4e and 4f show the CPI results for two spikes for patient #5. Activities N1 and N2 were revealed in spike #1 (Figure 4e), while in spike #2 N1 and N3 were revealed and N2 disappeared. Similar observation can be obtained in patient #4 (Figure 3) as well. Such phenomenon may be neglected if these instantaneous activities were averaged and analyzed. It is thus important to group the IISs with similar patterns before they are averaged. In the present study, due to the limited number of available IISs with the same pattern for each patient, individual spike is used in the analysis
Another important issue related to the interictal analysis is the propagation of the IISs from their origin to surrounding brain areas by uncertain neural pathways (Alarcon et al., 1994, 1997; Ebersole, 2000; Ulbert et al., 2004). Under this circumstance, significant time latency can be observed among spike peaks of the interictal events at the leading channels and other channels. Quantitative analysis based on the intracranial ECoG recordings (Lai et al., 2007) has shown the initiation and propagation areas of the IISs can be quite distant. This moving source could generate blurred EEG activity especially around the area where less surface electrodes are applied. Also considering the epileptiform activities can propagate very fast especially in neocortical epilepsy (Lee et al., 2000), the increase of temporal resolution of EEG recording is necessary when more accurate estimate of the origin and the sequential activation of the neuronal population is needed.
The present CPI results of IISs have been compared with the SOZs identified from ECoG ictal recordings. Table II shows that the averaged distance between the CPI maximum and SOZs is 4.6 mm for the 8 patients studied, suggesting that the CPI analysis of interictal spikes is predictive of the ictal onset cortical zone.
Conclusion
In summary, we have applied the CPI analysis to localize and image the cortical areas generating epileptiform activities from the EEG recordings during the interictal discharges for 8 pediatric epilepsy patients. Without any a priori knowledge, the estimated cortical potential distributions have been able to reveal the pathological cortical areas of abnormal activity overlapping with the epileptogenic foci which were identified by ictal ECoG recordings and confirmed by surgical resection outcomes. The present study shows that CPI may become an alternative noninvasive means to localize the cortical regions displaying epileptiform activity and potentially be helpful to guide the presurgical planning for pediatric epilepsy patients.
Acknowledgments
This work was supported in part by NIH RO1 EB007920, RO1 EB006433, RO1 EB00178, and a grant from The Dr. Ralph and Marian Falk Medical Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon G, Guy CN, Binnie CD, Walker SR, Elwes RDC, Polkey CE. Intracerebral propagation of interictal activity in partial epilepsy: implications for source localisation. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:435–49. doi: 10.1136/jnnp.57.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Mignel MC, Julen J, Polkey CE, et al. Origin and propagation of interictal discharges in the acute electrocorticogram: implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain. 1997;120:2259–2282. doi: 10.1093/brain/120.12.2259. [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society. American Electroencephalographic Society. Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–3. [PubMed] [Google Scholar]
- Asano E, Muzik O, Shah A, Juhász C, Chugani DC, Sood S, et al. Quantitative Interictal Subdural EEG Analyses in Children with Neocortical Epilepsy. Epilepsia. 2003;44:425 – 34. doi: 10.1046/j.1528-1157.2003.38902.x. [DOI] [PubMed] [Google Scholar]
- Avoli M, Biagini G, de Curtis M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr. 2006;6:203–7. doi: 10.1111/j.1535-7511.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner C, Lidinger G, Ebner A, Aull S, Serles W, Olbrich A, Lurger S, Czech T, Burgess R, Lüders H. Propagation of interictal epileptic activity in temporal lobe epilepsy. Neurology. 1995;45:118–22. doi: 10.1212/wnl.45.1.118. [DOI] [PubMed] [Google Scholar]
- Brodbeck V, Spinelli L, Lascano AM, Pollo C, Schaller K, Vargas MI, Wissmeyer M, Michel CM, Seeck M. Electrical source imaging for presurgical focus localization in epilepsy patients with normal MRI. Epilepsia. 2010;51(4):583–91. doi: 10.1111/j.1528-1167.2010.02521.x. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchtal M, Petersen I. A glossary of term most commonly used by clinical electroencephalographers. Electroenceph clin Neurophysiol. 1974;37:538–48. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, et al. MEG versus EEG localization test using implanted sources in the human brain. Ann Neurol. 1990;28:811–7. doi: 10.1002/ana.410280613. [DOI] [PubMed] [Google Scholar]
- Diekmann V, Becker W, Jürgens R, Grözinger B, Kleiser B, Richter HP, Wollinsky KH. Localisation of epileptic foci with electric, magnetic and combined electromagnetic models. Electroenceph clin Neurophysiol. 1998;106:297–313. doi: 10.1016/s0013-4694(97)00142-9. [DOI] [PubMed] [Google Scholar]
- Ding L, Wilke C, Xu B, Xu X, van Drongelene W, Kohrman M, He B. EEG Source Imaging: Correlate Source Locations and Extents with ECoG and Surgical Resections in Epilepsy Patients. Journal of Clinical Neurophysiology. 2007a;24(2):130–136. doi: 10.1097/WNP.0b013e318038fd52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Worrell GA, Lagerlund TD, He B. IctalSource Analysis: Localization and Imaging of Causal Interactions in Humans. NeuroImage. 2007b;34(2):575–586. doi: 10.1016/j.neuroimage.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole JS, Pacia SV. Localization of Temporal Lobe Foci by Ictal EEG paterns. Epilepsia. 1996;37(4):386–99. doi: 10.1111/j.1528-1157.1996.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Ebersole JS. Defining Epileptogenic Foci: Past, Present, Future. J Clin Neurophysiol. 1997;14(6):470–83. doi: 10.1097/00004691-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Ebersole JS. Non-invasive localization of the epileptogenic focus by EEG dipole modeling. Acta Neurol Scand. 1994;152:20–8. doi: 10.1111/j.1600-0404.1994.tb05179.x. [DOI] [PubMed] [Google Scholar]
- Ebersole JS. Noninvasive localization of epileptogenic foci by EEG source modeling. Epilepsia. 2000;41:24–33. doi: 10.1111/j.1528-1157.2000.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Rausch R, Lieb JP, Kuhl DE, Crandall PH. Correlation of criteria used for localizing epileptic foci in patient considered for surgical therapy of epilepsy. Ann Neurol. 1981;9:215–24. doi: 10.1002/ana.410090303. [DOI] [PubMed] [Google Scholar]
- Gilliam F, Wyllie E, Kashden J, Fraught E, Kotagal P, Bebin M, et al. Epilepsy surgery outcome: comprehensive assessment in children. Neurology. 1997;48:1368–74. doi: 10.1212/wnl.48.5.1368. [DOI] [PubMed] [Google Scholar]
- Hamalainen M, Sarvas J. Realistic conductor geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Hansen PC. Truncated singular value decomposition solutions to discrete ill-posed problems with ill-determined numerical rank. SIAM J Sci Stat Comput. 1990;11:503–18. [Google Scholar]
- He B, Musha T, Okamoto Y, Homma S, Nakajima Y, Sato T. Electric dipole tracing in the brain by means of the boundary element method and its accuracy. IEEE Transactions on Biomedical Engineering. 1987;34:406–414. doi: 10.1109/tbme.1987.326056. [DOI] [PubMed] [Google Scholar]
- He B. Brain electric source imaging: scalp Laplacian mapping and cortical imaging. Crit Rev Biomed Eng. 1999;27:149–188. [PubMed] [Google Scholar]
- He B, Wang Y, Wu D. Estimating Cortical Potentials from Scalp EEG’s in a Realistically Shaped Inhomogeneous Head Model By Means of the Boundary Element Method. IEEE Trans on Biomed Eng. 1999;46:1264–8. doi: 10.1109/10.790505. [DOI] [PubMed] [Google Scholar]
- He B, Zhang X, Lian J, Sasaki H, Wu D, Towle VL. Boundary Element Method-Based Cortical Potential Imaging of Somatosensory Evoked Potentials Using Subject’s Magnetic Resonance Images. Neuroimage. 2002;16:564–76. doi: 10.1006/nimg.2002.1127. [DOI] [PubMed] [Google Scholar]
- Herrendorf G, Steinhoff BJ, Kolle R, Baudewig J, Waberski TD, Buchner H, et al. Dipole-Source Analysis in a Realistic Head Model in Patients with Focal Epilepsy. Epilepsia. 2000;41(1):71–80. doi: 10.1111/j.1528-1157.2000.tb01508.x. [DOI] [PubMed] [Google Scholar]
- Homma S, Musha T, Nakajima Y, Okamoto Y, Blom S, Flink R, et al. Location of electric current sources in the human brain estimated by the dipole tracing method of the scalp–skull–brain (SSB) head model. Electroenceph clin Neurophysiol. 1994;91:374–82. doi: 10.1016/0013-4694(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Hoegg S, Sick C, Lücking CH, Zentner J, Schulze-Bonhage A, et al. Cortical current density reconstruction of interictal epileptiform activity in temporal lobe epilepsy. J Clin Neurophysiol. 2001b;112:1761–72. doi: 10.1016/s1388-2457(01)00588-0. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Hof E, Klisch J, Wagner M, Lücking CH, Kristeva-Feige R. Localization of interictal delta and epileptiform EEG activity associated with focal epileptogenic brain lesions. Neuroimage. 2001a;13:15–28. doi: 10.1006/nimg.2000.0680. [DOI] [PubMed] [Google Scholar]
- Lai Y, van Drongelen W, Hecox KE, Frim DM, Kohrman M, He B. Cortical Activation Mapping of Epileptiform Activity Derived from Interictal ECoG Spikes. Epielepsia. 2007;48(2):305–314. doi: 10.1111/j.1528-1167.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- Lai Y, van Drongelen W, Ding L, Hecox KE, Towle VL, Frim DM, et al. Estimation of in vivo human brain-to-skull conductivity ratio from simultaneous extra- and intra-cranial electrical potential recordings. Clin Neurophysiol. 2005;116:456–65. doi: 10.1016/j.clinph.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Lantz G, Michel CM, Pascual-Marqui RD, Spinelli L, Seeck M, Seri S, et al. Extracranial localization of intracranial interictal epileptiform activity using LORETA (low resolution electromagnetic tomography) Electroenceph clin Neurophysiol. 1997;102:414–22. doi: 10.1016/s0921-884x(96)96551-0. [DOI] [PubMed] [Google Scholar]
- Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Michel CM, de Peralta RG, Lantz G, Andino SG, Spinelli L, Blanke O, et al. Spatiotemporal EEG analysis and distributed source estimation in presurgical epilepsy evaluation. J Clin Neurophysiol. 1999;16:239–66. doi: 10.1097/00004691-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Shirasawa A, Hunjan A, Sharma R, Bettings M, et al. Systematic approach to dipole localization of interictal EEG spikes in children with extratemporal lobe epilepsies. Clin Neurophysiol. 2000;111:161–8. doi: 10.1016/s1388-2457(99)00208-4. [DOI] [PubMed] [Google Scholar]
- Oostendorp TF, Delbeke J, Stegeman DF. The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans Biomed Eng. 2000;47:1487–92. doi: 10.1109/TBME.2000.880100. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Steinlin M, Hwang PA, Sharma R, Jay V, Becker LE, et al. Positive epileptiform discharge in children with neuronal migration disorders. Pediatr Neurol. 1997;16:23–31. doi: 10.1016/s0887-8994(96)00254-8. [DOI] [PubMed] [Google Scholar]
- Rheims S, Represa A, Ben-Ari Y, Zilberter Y. Layer-Specific Generation and Propagation of Seizures in Slices of Developing Neocortex: Role of Excitatory GABAergic Synapses. J Neurophysiol. 2008;100:620–8. doi: 10.1152/jn.90403.2008. [DOI] [PubMed] [Google Scholar]
- Roth BJ, Ko D, von Albertini-Carletti IR, Scaffidi D, Sato S. Dipole localization in patients with epilepsy using the realistically shaped head model. Electroenceph clin Neurophysiol. 1997;102:159–66. doi: 10.1016/s0013-4694(96)95111-5. [DOI] [PubMed] [Google Scholar]
- Sidman R, Vincent DJ, Smith DB, Lee L. Experimental tests of the cortical imaging technique—applications to the response to median nerve stimulation and the localization of epileptiform discharge. IEEE Trans Biomed Eng. 1992;39:437–44. doi: 10.1109/10.135537. [DOI] [PubMed] [Google Scholar]
- Silva C, Almeida R, Oostendorp T, Ducla-Soares E, Foreid JP, Pimentel T. Interictal spike localization using a standard realistic head model: simulations and analysis of clinical data. Clin Neurophysiol. 1999;110:846–55. doi: 10.1016/s1388-2457(99)00025-5. [DOI] [PubMed] [Google Scholar]
- Spencer S, Spencer D, Williamson P, Mattson R. Combined depth and subdural electrode investigation in uncontrolled epilepsy. Neurology. 1990;40(1):74–9. doi: 10.1212/wnl.40.1.74. [DOI] [PubMed] [Google Scholar]
- Sperli F, Spinelli L, Seeck M, Kurian M, Michel CM, Lantz G. EEG Source Imaging in Pediatric Epilepsy Surgery: A New Perspective in Presurgical Workup. Epilepsia. 2006;47:981 – 90. doi: 10.1111/j.1528-1167.2006.00550.x. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek EF. Interictal Spikes and Epileptogenesis. Epilepsy Curr. 2006;6:199–202. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov AN, Arsenin VY. Solutions of Ill-Posed Problems. Wiley; New York: 1977. [Google Scholar]
- Tyvaert L, Hawco C, Kobayashi E, LeVan P, Dubeau F, Gotman J. Different structures involved during ictal and interictal epileptic activity in malformations of cortical development: an EEG-fMRI study. Brain. 2008;131:2042–60. doi: 10.1093/brain/awn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia. 2004;45(suppl 4):48–56. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Wilke C, van Drongelen W, Kohrman M, He B. Identification of epileptogenic foci from causal analysis of ECoG interictal spike activity. Clin Neurophysiol. 2009;120(8):1449–56. doi: 10.1016/j.clinph.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, van Drongelen W, Hecox KE, Towle VL, Frim DM, McGee AB, et al. High resolution EEG: Cortical potential imaging of interictal spikes. Clin Neurophysiol. 2003;114:1963–73. doi: 10.1016/s1388-2457(03)00194-9. [DOI] [PubMed] [Google Scholar]
- Zhang YC, van Drongelen W, He B. Estimation of in vivo human brain-to-skull conductivity ratio with the aid of intracranial electrical simulation. Applied Physics Letters. 2006;89:223903. doi: 10.1063/1.2398883. [DOI] [PMC free article] [PubMed] [Google Scholar]


