Abstract
Purpose
Cetuximab, an antibody targeting the epidermal growth factor receptor (EGFR), is active in colorectal cancer (CRC). However, response rates range from only 10% to 20%. Here, we investigate hepatocyte growth factor (HGF)-dependent mesenchymal-epithelial transition factor (MET) activation as a mediator of cetuximab resistance through signal diversification in CRC cell lines.
Experimental Design
DiFi, GEO, and LIM1215 cells were treated with varying concentrations and combinations of EGF, HGF, cetuximab, and PHA-665752 (a highly specific MET kinase inhibitor). Biological end points included proliferation, cell-cycle arrest, and apoptosis. Proliferation was measured using WST-1 assays and synergy investigated via isobolograms. Expression and signaling were examined using immunoblotting.
Results
EGFR and MET are co-expressed in these CRC cell lines, and dual receptor activation synergistically increased proliferation. Cetuximab inhibited cell growth by 60% to 80%, with an associated dephosphorylation of EGFR, MAPK and/or AKT. Addition of HGF to cetuximab-treated cells phosphorylated MET, but not EGFR or ErbB3, re-stimulated the MAPK and AKT pathways, restored cell proliferation, and rescued cells from G1 arrest and apoptosis. Importantly, this effect could be abrogated by inhibiting MET activation with PHA-665752 or by downregulating MET expression with RNAi.
Conclusions
HGF-induced MET activation is a novel mechanism of cetuximab resistance in CRC. Inhibition of the HGF-MET pathway may improve response to EGFR inhibitors in CRC, and combination therapy should be further investigated.
Keywords: Colorectal neoplasms, MET oncogene, tyrosine kinase inhibition, resistance, EGFR
INTRODUCTION
Colorectal Cancer (CRC) is the third most common malignant disease in the U.S., with more than 150,000 estimated new cases in 2007, and one of the leading causes of cancer-related death in the western world (1). Recent therapeutic strategies for CRC have focused on developing molecularly targeted therapies. The epidermal growth factor receptor (EGFR), a member of the ErbB family of receptors, is a potential target in colorectal cancer as it is expressed in 60% to 80% of colorectal cancers (2).
Cetuximab is a human:murine chimeric anti-EGFR IgG1 antibody that binds to the extracellular domain of inactive EGFR, thereby competitively inhibiting binding of the natural ligand and subsequent receptor activation. Cetuximab demonstrated in vivo and in vitro antitumor activity in tumors, leading to its approval in the United States in 2004 for use in combination with irinotecan, or as monotherapy in irinotecan-refractory colorectal cancer (3). However, cetuximab, when used as a single agent or in combination therapy, has an objective response rate of only 9% and 23%, respectively (3, 4). Furthermore, anti-EGFR is not curative, and all responding patients subsequently progress (3-5). Understanding the mechanisms of resistance is necessary in order to fully realize the benefit of EGFR-directed therapy.
It was initially hypothesized that EGFR targeted therapy would be most effective in tumors overexpressing the protein, however it was quickly documented that the levels of EGFR expression were not correlated with response to cetuximab(3, 4, 6). On the other hand, increased EGFR gene copy number, overexpression of EGFR ligands, and more recently TP53 mutations have been shown to be associated with response to EGFR inhibitors in CRC (7-11). Intrinsic resistance to EGFR-targeted therapy can be the result of downstream effector molecule activation such as KRAS which is seen in 35-40% of CRCs. Multiple studies have now shown that KRAS mutations in CRC confer resistance to cetuximab and have led the American Society of Clinical Oncology to put forward a provisional recommendation limiting cetuximab therapy to patients with wild-type KRAS tumors (5, 7, 12-16). Recent studies have demonstrated that oncogenic activation of effector molecules downstream of EGFR, other than KRAS, can also lead to cetuximab resistance (17). Mutations in BRAF, the serine protein recruited by KRAS, which occur in approximately 3%-10% of KRAS wild-type CRC cancer patients are associated with resistance to monoclonal antibodies targeting EGFR (18-20). Similarly, activating mutations in the PIK3CA p110 subunit and inactivation of the PTEN phosphatase (which can occur parallel to KRAS or BRAF mutations) have also been shown to be associated with cetuximab resistance (21-25). However, approximately 25% of CRC patients not responding to EGFR inhibitors are wild-type at KRAS, BRAF, PIK3CA, and PTEN and the mechanism of resistance in these “quadruple negative” patients is still unknown (17).
Another possible mechanism of resistance to EGFR targeted therapy may include activation of parallel pathways such as the MET receptor tyrosine kinase (26-31). MET amplification has been shown to be responsible for acquired resistance to the EGFR tyrosine kinase inhibitor (TKI) gefitinib in non-small-cell lung cancer (NSCLC) harboring activating mutations (27, 31). Resistance there was mediated by MET-ErbB3 transactivation, leading to restored signaling via the PI3K/AKT pathway (27). HGF-dependent MET activation also proved to be the mechanism of intrinsic resistance to gefitinib in NSCLC cells with EGFR-activating mutations that are not MET-amplified (29). Similarly, in ErbB2 (HER2)-overexpressing breast cancer cells, MET contributes to trastuzumab resistance (28). Conversely, MET-amplified gastric cancer cells were shown to be resistant to a TKI specific for MET when co-cultured with EGF or heregulin-β1 (26). In all these cases, treatment of cells with inhibitors targeting both MET and EGFR overcame resistance to a single inhibitor.
MET and HGF are often co-expressed in the CRC microenvironment, and increased expression is associated with advanced stage disease and poor prognosis (32). Ligand-independent MET activation, by mutation or overexpression, has been demonstrated in a minority of cancers (33, 34). More commonly solid tumors, including CRC, are ligand-responsive and require either autocrine or paracrine HGF for malignant transformation (33, 35). We therefore investigated whether HGF-mediated MET activation could rescue CRC cells from cetuximab inhibition. We observed that EGF and HGF have a synergistic effect on cellular proliferation. We then noted that HGF induces resistance to cetuximab by restoring signaling through the AKT and MAPK pathways independent of ErbB3. Importantly, EGFR sensitivity could be restored by treating cells with a combination of cetuximab and PHA-665752 (a highly specific MET TKI (36)) in the presence of HGF, providing a rationale for combined inhibition of EGFR and MET in CRC.
MATERIALS AND METHODS
Cell lines
The DiFi human colorectal cancer cell line was provided by Dr. José Baselga (Vall d’Hebron University Hospital, Barcelona, Spain). The SW620 human colorectal cancer cell line was purchased from American Type Culture Collection (Manassas, VA). The GEO and LIM1215 human colorectal cancer cell lines were a gift from Dr. John Mariadason (Ludwig Institute for Cancer Research, Melbourne, Australia). The cell lines DiFi, GEO, and LIM1215 were checked for mislabeling, contamination, and misidentification using a multiplexed PCR/MS-based genetic fingerprinting assay as described by Janakiraman et al.(37). DiFi cells were grown in Dulbecco’s Modified Eagle’s medium, high glucose, supplemented with Ham’s F-12 (DME HG F-12). GEO and LIM1215 were grown in Minimal Essential Medium (MEM), and SW620 was grown in Leibovitz’s L-15 Medium. All media were supplemented with 10% FCS and maintained at 37°C in a humidified atmosphere containing 5% CO2 (except for L-15, which was cultured without CO2).
Chemicals
Cetuximab was purchased from ImClone Systems, Inc. (Branchburg, NJ). PHA-665752 was provided by Pfizer Global Research and Development (La Jolla, CA). Human EGF and HGF were purchased from R&D systems (Minneapolis, MN).
Cell Proliferation Assays
Cell proliferation was measured by a modified methylthiazoletetrazolium (MTT) assay, using the WST-1 cell proliferation assay (Roche) according to the manufacturer’s instructions. Briefly, the cells were seeded in triplicate or sextuples in flat-bottomed 96-well plates at 1,000-10,000 cells per well and allowed to adhere for 24 hours in serum supplemented (10% fetal calf serum) media. Thereafter, the cells were treated for 24 to 72 hours as indicated in serum-reduced (2% fetal calf serum) media. After incubation with 10 ul of WST-1 reagent for 4 hours, the absorption of the samples (with a background control as a blank) was measured at 440 nm and 650 nm using a microplate reader.
Isobologram Analysis
The concentrations of EGF and HGF required to produce a defined single-agent effect (the concentration required to cause a 30% increase in proliferation), when used as single agents, were placed on the x and y axes in a two-coordinate plot, corresponding to (CEGF, 0) and (0, CHGF), respectively. The line connecting these two points is the line of additivity. Second, the concentrations of the two drugs used in combination to provide the same effect—isoeffect points—(expressed relative to the single-agent EGF and HGF concentrations), are placed in the same plot. Synergy, additivity, or antagonism are indicated when the isoeffect point is located below, on, or above the line, respectively. Combination index (CI) analysis, provides a quantitative measure of the extent of drug interaction and was calculated as described by Zhao et al. (38). A CI of less than, equal to, and more than 1 indicates synergy, additivity, and antagonism, respectively.
siRNA
For siRNA experiments, cells were seeded in sextuples in 96-well plates at 7,500 cells/well in antibiotic-free complete medium, and allowed to adhere for 24h at 37°C. Thereafter, the cells were transfected with Dharmacon (Chicago, IL) siGenome ON-TARGET plus human MET (sense 5′-GAACUGGUGUCCCGGAUAUUU-3′, antisense 5′-AUAUCCGGGACACCAGUUCUU-3′) siRNA or Non-Targeting siRNA (#3) according to the manufacturer’s instructions. After 4-6h the transfection medium was removed and cells were treated as indicated. After 72h of incubation, cell proliferation was determined using a WST-1 cell proliferation assay.
Real-time-PCR
TaqMan® Gene Expression Assays for MET and 18S rRNA were purchased from Applied Biosystems (Foster City, CA). Gene expression was measured using the ABI Prism 7900HT Sequence Detection System from Applied Biosystems. Real-time PCR of cDNA specimens was conducted as described previously (32).
Cell Cycle Analysis
Cells were seeded in 100 mm dishes at a density of 5 × 105 per dish. Twenty-four hours later, the cells were treated with drug or media for 24h. Both adherent and floating cells were harvested and stained with ethidium bromide. Quantification of the cell cycle distribution was done by flow cytometric analysis.
Western Blot
Cells were treated with inhibitors or growth factors in serum supplemented (10% fetal calf serum) medium. The medium was then aspirated, and tissue culture flasks were placed on ice and washed twice with ice-cold Tris-buffered saline (TBS). Cells were scraped off the culture flasks, centrifuged and placed in ice-cold RIPA lysis buffer (Upstate, Temecula, CA) containing protease and phosphatase inhibitors (Halt™ Protease and Phosphatase, [Thermo Scientific, Waltham, MA]). After shaking for 15 minutes at 4°C, the lysates were centrifuged at 20,000g for 15 minutes and stored at −80°C until further use. For Western Blotting, equal amounts of protein (50 ug) were boiled in Laemmli buffer for 5 minutes, resolved by 10% SDS-polyacrylamide gel electrophoresis (Invitrogen Life Technologies, Carlsbad, CA) and electrophoretically transferred onto a polyvinylidene difluoride membrane (BioRad, Hercules, CA). After blocking nonspecific binding sites with 5% nonfat dry milk in TBS + 0.05% Tween 20 (TBS-T), the membrane was incubated with the respective antibodies overnight at 4°C. After 3 washes with TBS-T, the membrane was incubated for 1h at room temperature with a horseradish peroxidase-linked secondary antibody, followed by several washes with TBS-T. The immunocomplexes were visualized using the ECL Plus detection system (GE Healthcare, Uppsala, Sweden).
Antibodies
Antibodies against MET (C-12), EGFR (1005), as well as secondary goat anti-mouse IgG HRP and secondary goat anti-mouse IgG2b HRP antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phospho-MET (Tyr1234/1235), phospho-EGFR (Tyr1068), phospho-HER3 (Tyr1289), AKT, phospho-AKT (Ser473), p44/42 MAPK, and phospho-p44/42 MAPK (Thr202,Tyr204) were from Cell Signaling Technology (Danvers, MA). Anti-α-Tubulin was from Calbiochem (San Diego, CA). Anti-HER3 was from Lab Vision (Fremont, CA). Secondary donkey anti-rabbit HRP antibody was from GE Healthcare (Uppsala, Sweden).
Cleaved caspase-3 assays
Lysates were prepared in the same buffer used for Western blotting. One hundred micrograms were used for the PathScan cleaved caspase-3 sandwich ELISA (Cell Signaling), following the manufacturer’s instructions. In brief, extracts were mixed with sample diluent and incubated in antibody-coated microwell strips. One hundred microliters of cleaved caspase-3 detection antibodies were added to each well. Binding was detected with 100 μL of horseradish peroxidase-linked streptavidin antibody and 100 μL of TMB substrate solution. The colored reaction product was measured in a microplate reader at 450 nm.
Statistical Analysis
The statistical significance of differences was analyzed by one-way ANOVA. In cases in which the P values for the overall comparisons were <0.05, post hoc pairwise comparisons were done with the Neuman-Keuls Multiple comparison test. One sample Wilcoxon test was used to test whether the combination index is significantly different than 1. Statistical analyses were done using GraphPad Prism ver. 5.00 (GraphPad Software, Inc., San Diego, CA).
RESULTS
EGFR and MET are co-expressed in a genotypically diverse panel of cetuximab-sensitive CRC cell lines
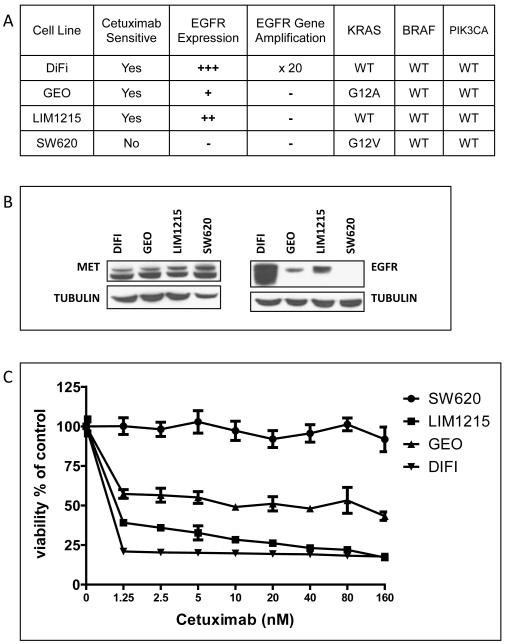
The CRC cell lines selected for our study constitute a genotypically diverse panel of cell lines in having (1) an EGFR-amplified cell line in DiFi, (2) a KRAS mutant cell line in GEO, and (3) a non-EGFR-amplified, KRAS wild-type cell line in LIM1215 (Fig. 1A)(22, 37). EGFR and MET are frequently overexpressed in CRC, and the intensity of their respective expressions has been linked to worse prognosis (39-42). To establish the possibility of MET-mediated rescue from EGFR inhibition in our cell lines we examined them for the co-expression of the two RTKs. As shown in Fig. 1B, analyses of three cetuximab-sensitive lines (DiFi, GEO, LIM1215) shows that MET and EGFR are co-expressed to varying degrees in all three lines, with DiFi demonstrating marked overexpression of EGFR due to gene amplification, and none of the cell lines showing MET amplification. The three cell lines are sensitive to cetuximab at nanomolar concentrations (60% to 80% growth inhibition), with DiFi showing the strongest response and SW620 (control cell line) being resistant to cetuximab at the highest concentration tested (Fig. 1C). Like others (22), we found that GEO cells are sensitive to cetuximab despite harboring a KRAS mutation. Cetuximab’s inhibitory effect in this cell line is probably due to the cetuximab-dependent interruption of an autocrine growth-stimulatory loop created by the cells’ secretion of the EGFR ligand amphiregulin (22, 43). Clinically, expression levels of the EGFR ligands amphiregulin and epiregulin have also been found to be associated with response to cetuximab (7, 11).
Figure 1. MET and EGFR are co-expressed in a panel of cetuximab sensitive, genotypically diverse CRC cell lines.
(A) Summary of genotypical data of cell lines used in experiments. (B) Whole cell lysates from the cell lines DiFi, GEO, LIM1215, and SW620 were analyzed for MET, EGFR, and Tubulin by SDS-PAGE followed by Western blot. The three cetuximab-sensitive cell lines co-express MET and EGFR to varying degrees. (C) CRC cell lines were treated for 72 h in serum-reduced media with increasing concentrations of cetuximab followed by determination of cell proliferation as described in Materials and Methods. Data points average of replicates of six; bars SD. DiFi, LIM1215, and GEO are sensitive to Cetuximab at nanomolar concentrations.
Combined activation of EGFR and MET enhances CRC cell proliferation by augmented activation of AKT and MAPK
EGFR and its ligands are frequently overexpressed in CRC, and receptor activation leads to uninhibited tumor proliferation (2, 44). Similarly, HGF and MET are co-overexpressed in the majority of CRCs, and the level of overexpression is associated with outcome (32). To investigate the effects of ligand-dependent activation of MET in CRC cell lines already driven by EGFR, we treated our cell lines with EGF and HGF and analyzed the effects of dual receptor activation on cell proliferation and signaling. As shown in Figure 2A, treatment of LIM1215 cells with either EGF or HGF results in a modest growth response, whereas treatment with both growth factors leads to an enhanced proliferative response. DiFi cells exhibit a similar response to dual receptor activation, whereas GEO do not have a significant proliferative response to EGF stimulation (data not shown). Isobologram analyses (38) show a synergistic interaction (Combination Index30 (CI30) = 0.78 ± 0.11, P < 0.05 vs. CI30 = 1) between the two growth factors, indicating that HGF-dependent MET activation can play an important role in the proliferation of CRC cells already driven by EGFR activation (Figure 2B). To examine the mechanism by which MET activation contributes to proliferation of EGF-treated cells, the activation status of downstream signaling molecules was evaluated. LIM1215 and DiFi cells were treated with EGF, HGF, or both, and the activation of PI3K/AKT and RAS/MAPK pathways were examined by Western blotting with phosphorylated specific antibodies. As shown in Figure 2C, activating EGFR and MET by treatment of cells with a combination of EGF and HGF augments the activation of AKT and MAPK when compared with treatment by either growth factor alone.
Figure 2. Combined activation of EGFR and MET enhances cell proliferation by augmented activation of AKT and MAPK.
(A) LIM1215 cells were treated in serum-reduced media with EGF (0.1 ng/ml), HGF (75 ng/ml), or both EGF and HGF. Columns average of replicates of six; bars SD; *, p < 0.0001. Combining EGF and HGF significantly enhances the proliferation of LIM1215 cells. (B) Isobologram analyses of LIM1215 cells. The isobologram is a geometric method of determining drug interactions. The concentration of EGF producing a desired (30% increase in proliferation) effect was plotted on the horizontal axis, and the concentration of HGF producing the same proliferative effect was plotted on the vertical axis. The diagonal line represents a theoretical additive interaction between EGF and HGF. An experimental isoeffect point is the concentration (expressed relative to EGF and HGF concentrations) of the two drugs which, when combined, yielded a 30% increase in proliferation. Points below the line represent a synergistic interaction, whereas points above represent an antagonistic interaction. EGF and HGF synergize to increase proliferation of LIM1215 cells. (C) LIM1215 and DiFi cells were treated with EGF and/or HGF for 15 minutes, and whole-cell lysates were analyzed by SDS-PAGE followed by Western blot. Combined activation of EGFR and MET augments phosphorylation of AKT and/or MAPK in LIM1215 and DiFi cells.
HGF rescues CRC cells from cetuximab inhibition
HGF induces resistance to cetuximab (5 nM and 50 nM) in DiFi, GEO, and LIM1215 in a dose-dependent fashion (Fig 3A). The rescue effect is more pronounced in GEO and LIM1215 than in DiFi, probably due to DiFi cells’ “addiction” to the EGFR pathway secondary to EGFR amplification. When examining the effects of cetuximab and HGF over a 72-hour time course we found that HGF can overcome the complete growth inhibition induced by cetuximab and restore proliferation in all three cell lines (Fig. 3B).
Figure 3. HGF-dependent MET activation rescues LIM1215, DiFi, and GEO cells from cetuximab inhibition.
(A) DiFi, LIM1215, and GEO cells were treated with serum-reduced media and cetuximab (left panel 5nM, right panel 50nM) and increasing concentrations of HGF for 72 hours followed by determination of cell proliferation, as described in Materials and Methods. Data points average of triplicates; bars SD. HGF reverses inhibitory effects of cetuximab in tested cell lines. (B) Cell lines were treated with serum-reduced media, cetuximab (5 nM), cetuximab + HGF (75 ng/ml), and cetuximab + HGF + PHA-665752 (0.4 uM), followed by determination of cell proliferation at 24, 48, and 72 hours. Data points average of triplicates; bars SD. All three cell lines showed cetuximab-dependent inhibition of proliferation, which was reversed with HGF. Inhibition of MET with PHA-665752 abrogated the HGF rescue effect. (C) Left panel: LIM1215 cells were treated with serum-reduced media, cetuximab (5nM), cetuximab + HGF (75 ng/ml), cetuximab + HGF + non-silencing siRNA (5 ul/well), and cetuximab + HGF + MET siRNA (5 ul/well). Proliferation was determined after 72 hours. Columns average of replicates of six; bars SD; *, p < 0.0001. Right Panel: LIM1215 cells were treated with non-silencing siRNA and MET siRNA for 72 hours followed by determination of relative MET mRNA expression using RT-PCR. Columns average of triplicates; bars SD; *, p < 0.0001. Knocking down MET expression abrogates the HGF rescue effect and restores cetuximab’s inhibitory effects on cell proliferation.
MET inhibition or down-regulation completely abrogates the HGF rescue effect
The selective tyrosine kinase inhibitor PHA-665752 has no effect on the proliferation of DiFi, GEO, or LIM1215. When treating CRC cells with a combination of cetuximab, HGF, and PHA-665752 we found that MET inhibition completely abrogates the HGF rescue effect and essentially restores the growth-inhibitory effects of cetuximab in all three cell lines (Fig. 3B). We used RNAi technique to confirm these results. Down-regulation of MET expression by a MET-specific siRNA canceled HGF-induced resistance to cetuximab in LIM1215 (Fig. 3C), indicating that HGF induces cetuximab resistance via MET activation.
HGF rescues CRC cells from cetuximab induced G1 arrest
As shown in Fig. 4, DiFi, GEO, and LIM1215 cells treated with cetuximab for 24 hours accumulate in G1 phase. Cetuximab reduces the percentage of cells in S phase from 33% to 17%, 27% to 13%, and 18% to 3% for LIM1215, GEO, and DiFi respectively. HGF is able to counteract the effects of cetuximab, increasing the proportion of cells in S phase from 18% to 27%, 13% to 20%, and 3% to 10%. MET inhibition with PHA-665752 abrogates the effects of HGF on cetuximab-treated cells and restores the cetuximab-induced G1 arrest in all three cell lines by reducing the percentage of cells in S to 17%, 15%, and 4%.
Figure 4. HGF rescues CRC cells from cetuximab-induced G1 arrest, and PHA-665752 abrogates HGF rescue effect.
(A) DiFi, GEO, and LIM1215 cells were treated for 24 hours with complete media, cetuximab (5 nM), cetuximab +HGF (75 ng/ml), and cetuximab + HGF + PHA-665752 (0.4 uM) followed by flow cytometric determination of cell cycle distribution, as described in Materials and Methods. Cetuximab induced G1 arrest in all cell lines, which could be overcome by HGF. Addition of PHA-665752 abrogated the effect of HGF in cetuximab-treated cells. Representative results of three independent experiments.
HGF rescues DiFi cells from cetuximab-induced apoptosis
In most CRC cells, the effect of cetuximab is cytostatic, not cytocidal (22); however, the EGFR-amplified cell line DiFi does undergo apoptosis when treated with EGFR inhibitors. As seen in Fig. 5A, we found that DiFi cells treated with either HGF or PHA-665752 had insignificant effects on cellular apoptosis. However, HGF was able to rescue DiFi cells from cetuximab-induced apoptosis. Treating the cells for 24 hours with a combination of HGF and PHA-665752 restored the pro-apoptotic effects of cetuximab.
Figure 5. HGF rescues DiFi cells from cetuximab-induced apoptosis. HGF-dependent phosphorylation of MET reactivates AKT and MAPK in cetuximab-treated cells.
(A) DiFi cells were treated for 24 hours with serum-reduced media cetuximab (50 nM), cetuximab + HGF (75 ng/ml), and cetuximab + HGF + PHA-665752 (0.4 uM) followed by determination of cleaved caspase-3 levels, as described in Materials and Methods. Columns average of triplicates; bars SD; *, p < 0.0001. HGF rescues DiFi cells from cetuximab-induced apoptosis. MET inhibition with PHA-665752 restores pro-apoptotic effects of cetuximab in HGF-stimulated DiFi cells. (B) and (C) DiFi and LIM1215 cells were treated overnight with cetuximab (5 nM) and/or PHA-665752 (0.4 uM) with or without the addition of HGF (75 ng/ml) for the final 15 minutes. Whole-cell lysates were analyzed by SDS-PAGE, followed by Western blot. HGF-dependent phosphorylation of MET restored signaling through the AKT and MAPK pathways in cetuximab-treated cells. HER3 is not cross-activated by MET phosphorylation. A combination of cetuximab and PHA-665752 blocks the activation of AKT and MAPK by inhibiting MET and EGFR phosphorylation in HGF-stimulated cells.
HGF rescue occurs via MET-dependent activation of AKT and MAPK
To evaluate the mechanism by which HGF rescues CRC cancer cells from cetuximab-induced inhibition of proliferation, cell cycle arrest, and apoptosis, we examined the activation status of downstream signaling molecules. DiFi, GEO, and LIM1215 were treated with cetuximab, HGF, and PHA-665752 as indicated, and the activation of PI3K/AKT and RAS/MAPK pathways were examined by Western blotting with phosphorylated specific antibodies. As shown in fig. 5B and 5C, cetuximab inhibits EGFR phosphorylation and activation of AKT and MAPK in DiFi and LIM1215 cells. Treating the cells with a combination of cetuximab and HGF leads to MET phosphorylation and reactivation of AKT and MAPK. The HGF rescue effect is not mediated by activation of HER-3. However, when treating the cells with a combination of cetuximab and PHA-665752, inhibition of EGFR and MET phosphorylation leads to sustained inhibition of the AKT and MAPK pathways in the presence of HGF. In GEO cells the HGF rescue effect was also by p-MET-mediated activation of AKT and MAPK (data not shown).
DISCUSSION
To fully realize the benefits of EGFR-targeted therapy in CRC, mechanisms of escape and resistance to therapy must be elucidated. KRAS has been identified as an important factor in selecting patients who will derive benefit from cetuximab (12-15). However, little is known about the mechanism for cetuximab resistance in KRAS wild-type patients. There are now many examples illustrating the potential weakness inherent in targeted therapy; cancer cells develop complex signaling networks, often resulting in redundancy and overlap of cell survival pathways and thereby potentially allowing cancer cells to escape the therapeutic effects of targeting one single pathway (45).
Engelman and colleagues’ study was the first in a series of studies to show that cancer cells can overcome EGFR inhibition by signaling through the MET receptor, thereby activating shared downstream pathways (26-31). Similar to EGFR, the MET receptor is overexpressed in CRC (39). Furthermore, HGF is co-expressed with MET in the CRC tumor microenvironment, and the levels of co-expression have been found to correlate with outcomes (32, 39). We hypothesized that HGF-dependent activation of MET would enable CRC cells to escape the inhibitory effects of cetuximab by activating shared downstream survival and proliferation pathways.
In the present study we selected three genotypically diverse CRC cell lines and established the possibility for interaction between MET and EGFR by documenting their co-expression in all three cell lines. We then found that EGF and HGF act in synergy to increase the proliferation of CRC cells by augmenting signaling through the AKT and MAPK pathways. These results indicate that the MET-HGF axis plays an important role even in cells already driven by EGFR activation. We observed that, while the CRC cells DiFi, GEO, and LIM1215 do not show MET amplification, stimulation with HGF rescues these cetuximab-sensitive cell lines from EGFR inhibition. We found that HGF rescues CRC cells from cetuximab-induced cell cycle arrest and restores proliferation in all three cell lines. The rescue effect is less pronounced in the EGFR-amplified cell line DiFi, given its dependence on the EGFR pathway; however, HGF is able to rescue those cells from cetuximab-induced apoptosis. The mechanism underlying the rescue effect is phospho-MET-dependent activation of the AKT and MAPK pathways. Unlike the case of acquired resistance in NSCLC where amplified MET cross-activates ErbB3 to overcome EGFR inhibition (27), our study showed that HGF rescues non-MET-amplified cells from the effects of cetuximab, independent of ErbB3 activity.
Importantly, we found that downregulation of MET expression with siRNA or inhibition of MET tyrosine kinase activity using PHA-665752 prevents the HGF-dependent activation of AKT and MAPK and essentially re-sensitizes the cells to the effects of cetuximab. There are a number of MET-targeting therapeutics in clinical development, including TKIs, monoclonal antibodies, and molecular decoys (46-50). In vivo experiments are needed to confirm the efficacy of combined RTK therapy in overcoming resistance to EGFR inhibitors. Such experiments, however, need to overcome the inherent inadequacy of a xenograft model , posed by the fact that murine HGF does not effectively bind human MET (51). Our laboratory is currently working on the development and validation of different mouse models that overcome this problem while maintaining conditions that mirror the nature of CRC encountered in the clinic (52).
In conclusion, we show that HGF-mediated MET activation is a novel mechanism of cetuximab resistance in CRC. The present study adds to growing evidence that a rational combination of tyrosine kinase inhibitors may be capable of overcoming resistance to agents targeting a single growth pathway, and should therefore replace kinase inhibitor monotherapy as the foundation of molecularly targeted therapy strategies in cancer patients.
TRANSLATIONAL RELEVANCE.
Cetuximab, an antibody targeting the human epidermal growth factor receptor (EGFR), is active in colorectal cancer (CRC). However, objective response rates range from only 10% to 20%. Intact KRAS is necessary but not sufficient to derive benefit from EGFR inhibition. Elucidating the resistance mechanisms to this targeted therapy is necessary before it can be utilized to its full potential in the clinic. In this study we found that hepatocyte growth factor (HGF) rescues CRC cells from the effects of EGFR inhibition on proliferation, cell cycle arrest, and apoptosis. PHA-665752, a highly selective inhibitor of the HGF receptor MET, abrogates the effects of HGF and re-sensitizes CRC cancer cells to cetuximab. Given the presence of HGF in the CRC microenvironment, our findings indicate a novel mechanism for resistance to EGFR inhibitors in CRC. Our findings provide a rationale for combined inhibition of EGFR and MET in the treatment of CRC.
Acknowledgments
Grant Support: This work was supported by a grant from the American Society of Colon and Rectal Surgeons. David Liska was supported by a National Cancer Institute T32 Surgical Oncology training grant.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37:285–9. doi: 10.1080/028418698429595. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Meropol NJ, Loehrer PJ, Sr., Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Kohne C-H, Hitre E, et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 6.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–10. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of Epiregulin and Amphiregulin and K-ras Mutation Status Predict Disease Control in Metastatic Colorectal Cancer Patients Treated With Cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 8.Moroni M, Veronese S, Benvenuti S, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–86. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 9.Oden-Gangloff A, Di Fiore F, Bibeau F, et al. TP53 mutations predict disease control in metastatic colorectal cancer treated with cetuximab-based chemotherapy. Br J Cancer. 2009;100:1330–5. doi: 10.1038/sj.bjc.6605008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartore-Bianchi A, Moroni M, Veronese S, et al. Epidermal Growth Factor Receptor Gene Copy Number and Clinical Outcome of Metastatic Colorectal Cancer Treated With Panitumumab. J Clin Oncol. 2007;25:3238–45. doi: 10.1200/JCO.2007.11.5956. [DOI] [PubMed] [Google Scholar]
- 11.Tabernero J, Cervantes A, Rivera F, et al. Pharmacogenomic and pharmacoproteomic studies of cetuximab in metastatic colorectal cancer: biomarker analysis of a phase I dose-escalation study. J Clin Oncol. 28:1181–9. doi: 10.1200/JCO.2009.22.6043. [DOI] [PubMed] [Google Scholar]
- 12.De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 13.Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras Mutations and Benefit from Cetuximab in Advanced Colorectal Cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 15.Lievre A, Bachet J-B, Boige V, et al. KRAS Mutations As an Independent Prognostic Factor in Patients With Advanced Colorectal Cancer Treated With Cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 16.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–6. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 17.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–61. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 18.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–12. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 19.Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR Status in Determining Benefit From Cetuximab Therapy in Wild-Type KRAS Metastatic Colon Cancer. J Clin Oncol. 2009;27:5924–30. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
- 20.Tol J, Nagtegaal ID, Punt CJA. BRAF Mutation in Metastatic Colorectal Cancer. N Engl J Med. 2009;361:98–9. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 21.Frattini M, Saletti P, Romagnani E, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97:1139–45. doi: 10.1038/sj.bjc.6604009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jhawer M, Goel S, Wilson AJ, et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–61. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrone F, Lampis A, Orsenigo M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 24.Prenen H, De Schutter J, Jacobs B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–8. doi: 10.1158/1078-0432.CCR-08-2961. [DOI] [PubMed] [Google Scholar]
- 25.Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–7. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 26.Bachleitner-Hofmann T, Sun MY, Chen CT, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Mol Cancer Ther. 2008;7:3499–508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 27.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 28.Shattuck DL, Miller JK, Carraway KL, 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471–7. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 29.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res. 2008;68:9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 30.Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. doi: 10.4103/1477-3163.44372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bean J, Brennan C, Shih J-Y, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proceedings of the National Academy of Sciences. 2007;104:20932–7. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kammula US, Kuntz EJ, Francone TD, et al. Molecular co-expression of the c-Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett. 2007;248:219–28. doi: 10.1016/j.canlet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 34.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–21. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 36.Sattler M, Pride YB, Ma P, et al. A Novel Small Molecule Met Inhibitor Induces Apoptosis in Cells Transformed by the Oncogenic TPR-MET Tyrosine Kinase. Cancer Res. 2003;63:5462–9. [PubMed] [Google Scholar]
- 37.Janakiraman M, Vakiani E, Zeng Z, et al. Genomic and Biological Characterization of Exon 4 KRAS Mutations in Human Cancer. Cancer Res. 2010;70:5901–11. doi: 10.1158/0008-5472.CAN-10-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao L, Wientjes MG, Au JL-S. Evaluation of Combination Chemotherapy: Integration of Nonlinear Regression, Curve Shift, Isobologram, and Combination Index Analyses. Clin Cancer Res. 2004;10:7994–8004. doi: 10.1158/1078-0432.CCR-04-1087. [DOI] [PubMed] [Google Scholar]
- 39.Di Renzo MF, Olivero M, Giacomini A, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1:147–54. [PubMed] [Google Scholar]
- 40.Fujita S, Sugano K. Expression of c-met proto-oncogene in primary colorectal cancer and liver metastases. Jpn J Clin Oncol. 1997;27:378–83. doi: 10.1093/jjco/27.6.378. [DOI] [PubMed] [Google Scholar]
- 41.McKay JA, Murray LJ, Curran S, et al. Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastases. Eur J Cancer. 2002;38:2258–64. doi: 10.1016/s0959-8049(02)00234-4. [DOI] [PubMed] [Google Scholar]
- 42.Galizia G, Lieto E, Ferraraccio F, et al. Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006;13:823–35. doi: 10.1245/ASO.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 43.Johnson G, Saeki T, Gordon A, Shoyab M, Salomon D, Stromberg K. Autocrine action of amphiregulin in a colon carcinoma cell line and immunocytochemical localization of amphiregulin in human colon. J Cell Biol. 1992;118:741–51. doi: 10.1083/jcb.118.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–72. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 45.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of Receptor Tyrosine Kinases Affects the Response of Tumor Cells to Targeted Therapies. Science. 2007;318:287–90. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 46.A study Of Oral PF-02341066, a c-Met/Hepatocyte Growth Factor Tyrosine Kinase Inhibitor. Patients With Advanced Cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT00585195?cond=%22Lymphoma%2C+Large-Cell%2C+Ki-1%22&&rank=13.
- 47.Burgess T, Coxon A, Meyer S, et al. Fully Human Monoclonal Antibodies to Hepatocyte Growth Factor with Therapeutic Potential against Hepatocyte Growth Factor/c-Met-Dependent Human Tumors. Cancer Res. 2006;66:1721–9. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 48.Martens T, Schmidt N-O, Eckerich C, et al. A Novel One-Armed Anti-c-Met Antibody Inhibits Glioblastoma Growth In vivo. Clin Cancer Res. 2006;12:6144–52. doi: 10.1158/1078-0432.CCR-05-1418. [DOI] [PubMed] [Google Scholar]
- 49.Zou HY, Li Q, Lee JH, et al. An Orally Available Small-Molecule Inhibitor of c-Met, PF-2341066, Exhibits Cytoreductive Antitumor Efficacy through Antiproliferative and Antiangiogenic Mechanisms. Cancer Res. 2007;67:4408–17. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Yao W, Newton RC, Scherle PA. Targeting the c-MET signaling pathway for cancer therapy. Expert Opinion on Investigational Drugs. 2008;17:997–1011. doi: 10.1517/13543784.17.7.997. [DOI] [PubMed] [Google Scholar]
- 51.Jeffers M, Rong S, Woude GF. Hepatocyte growth factor/scatter factor-Met signaling in tumorigenicity and invasion/metastasis. J Mol Med. 1996;74:505–13. doi: 10.1007/BF00204976. [DOI] [PubMed] [Google Scholar]
- 52.Francone TD, Landmann RG, Chen CT, et al. Novel xenograft model expressing human hepatocyte growth factor shows ligand-dependent growth of c-Met-expressing tumors. Mol Cancer Ther. 2007;6:1460–6. doi: 10.1158/1535-7163.MCT-06-0466. [DOI] [PubMed] [Google Scholar]