Abstract
The reactions of two bacterial TIM barrel prenyltransferases (PTs), MoeO5 and PcrB, were explored. MoeO5, the enzyme responsible for the first step in moenomycin biosynthesis, catalyzes the transfer of farnesyl to 3-phosphoglyceric acid (3PG) to give a product containing a cis-allylic double bond. We show that this reaction involves isomerization to a nerolidyl pyrophosphate intermediate followed by bond rotation prior to attack by the nucleophile. This mechanism is unprecedented for a prenyltransferase that catalyzes an intermolecular coupling. We also show that PcrB transfers geranyl and geranylgeranyl groups to glycerol-1-phosphate (G1P), making it the first known bacterial enzyme to use G1P as a substrate. Unlike MoeO5, PcrB catalyzes prenyl transfer without isomerization to give products that retain the trans-allylic bond of the prenyl donors. The TIM barrel family of PTs is unique in including enzymes that catalyze prenyl transfer by distinctly different reaction mechanisms.
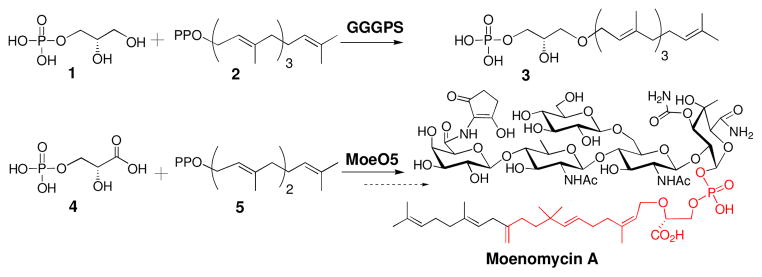
Nature uses prenyltransferases (PTs) to make a wide variety of primary and secondary metabolites containing prenyl groups, and there is enormous interest in understanding the scope and utility of these enzymes.1 The best studied classes of PTs, the polyprenyl synthases and the cyclizing terpene synthases, contain an alpha helical fold, as do the protein farnesyl and geranylgeranyl transferases.2 Recently, the first PT having a triosephosphate isomerase (TIM) barrel fold was identified in archaea.3–5 This PT, geranylgeranylglyceryl phosphate synthase (GGGPS), catalyzes the transfer of a geranylgeranyl (C20) group to glycerol-1-phosphate (G1P) during the first step in the biosynthesis of the major phospholipid component of archaeal membranes (Figure 1).6,7 We recently discovered a bacterial GGGPS homolog, MoeO5, in the gene cluster for the antibiotic moenomycin. MoeO5 couples 3-phosphoglyceric acid (3PG) to a farnesyl group to form the starter unit for moenomycin biosynthesis (Figure 1).8,9 Here, we study the mechanism of MoeO5, describe key residues that can be used to predict the acceptor substrate in TIM barrel PTs, apply these criteria to a bacterial GGGPS homolog of unknown function, PcrB, and establish its substrates biochemically. We conclude that TIM barrel PTs are dedicated to the transfer of prenyl groups to oxygen on phosphorylated triose acceptors, but the reactions they catalyze can proceed by two very different mechanisms.4
Figure 1.
GGGPS converts glycerol-1-phosphate (G1P, 1) and GGPP (2) to compound 3. MoeO5 farnesylates 3-phosphoglycerate (3PG, 4) using farnesyl pyrophosphate (FPP, 5). The portion of MmA synthesized by MoeO5 is highlighted in red.
The TIM barrel PTs, GGGPS and MoeO5, both make ether-linked products but with different allylic bond geometries (Figure 1). Unlike the GGGPS product, which retains the trans-geometry of the starting material and likely proceeds by a straightforward displacement mechanism, the MoeO5 product (6) contains a cis-allylic double bond.8 MoeO5 is the only known prenyltransferase that forms an intermolecular cis-allylic bond using a trans-allylic precursor.10 This linkage suggests that bond isomerization occurs prior to coupling during the catalytic reaction, and a possible reaction mechanism involving the intermediacy of nerolidyl pyrophosphate (NPP, 7) is shown in Figure 2A.11
Figure 2.
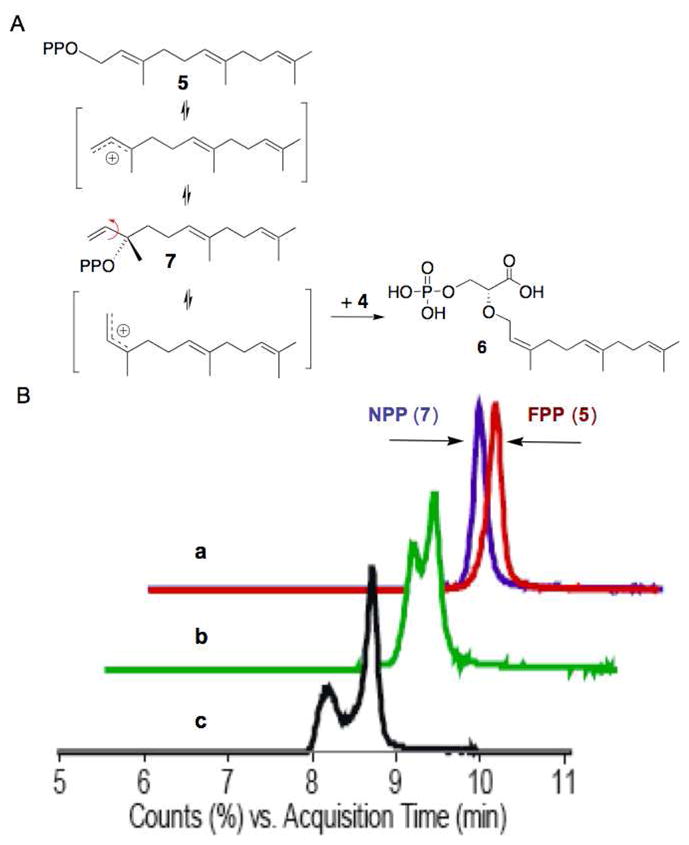
A. MoeO5 catalyzes the formation of 6 from 3PG (4) and FPP (5) via a mechanism proposed to involve isomerization to NPP (7). B. NPP forms during the MoeO5 reaction. NPP and FPP have the same [M-H]−1 (381.1237). LC/MS extracted ion chromatograms of a: an overlay of NPP and FPP standards (blue and red respectively) demonstrating small but reproducible differences in retention times; b: MoeO5 reaction of FPP spiked with NPP following quenching of the enzymatic reaction (green); c: MoeO5 reaction with FPP showing the formation of a new peak with a RT consistent with NPP (black).14
To test this hypothesis, we compared the reaction rates for FPP (5) and (3S)-NPP (7) using LC/MS. Under initial rate conditions, the turnover numbers for FPP and NPP were indistinguishable (7.8±0.1 min−1 for FPP and 7.4±0.7 min−1 for NPP).12 In addition, we detected a compound with a retention time and mass consistent with NPP (7) in reactions of MoeO5 incubated with FPP in the absence of 4 (Figure 2B).13 These results support a mechanism in which MoeO5 isomerizes FPP (5) to NPP (7), which undergoes rotation about the C-2,3-bond to the cisoid conformer prior to prenyltransfer to phosphoglyceric acid (4, Figure 2).
To gain insight into how MoeO5 and GGGPS are capable of catalyzing prenyltransfer to acceptors in different oxidation states via fundamentally different mechanisms, we aligned the sequences of fifty archaeal TIM barrel PTs and MoeO5, and compared them to the GGGPS crystal structure, which contains a bound acceptor substrate.3 Amino acids in the β-6-loop-6 region of GGGPS make direct contacts to the acceptor substrate, and there are several striking changes in this region of MoeO5 (Figure 3).3 For example, E167, which contacts both the C-2 and C-3 hydroxyl groups of G1P in the GGGPS crystal structure, is replaced by tyrosine. This change likely prevents repulsive interactions with the 3PG carboxylate. In addition, S169 is replaced by arginine, which may favor attractive interactions with the substrate carboxylate.
Figure 3.
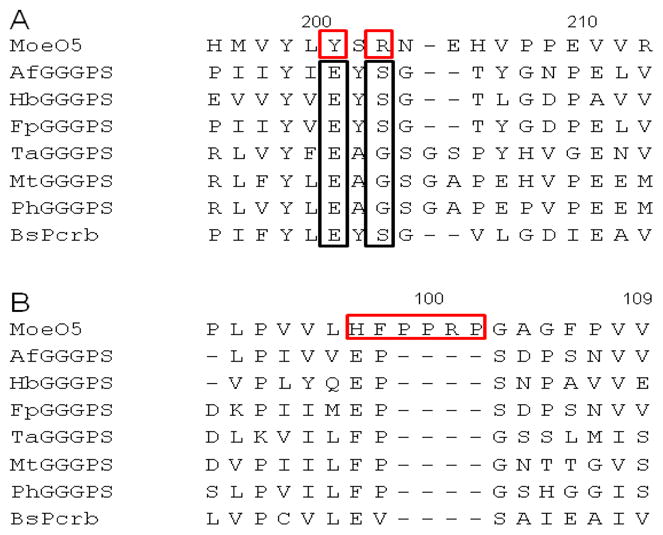
Selections of the sequence alignment of MoeO5 (S. ghanaensis), representative archaeal GGGPSs, and PcrB from B. subtilis (BsPcrB). A. Alignments of the acceptor binding loop. The tyrosine and arginine changes in MoeO5 are outlined in red B. Alignments of the “swinging door” region of AfGGGPS described in the text.
Changes in the acceptor binding loop help explain the changes in acceptor substrate preferences, but other features are likely responsible for the ability of MoeO5 to catalyze isomerization of the allylic diphosphate bond of FPP. Notably, MoeO5 contains a proline rich insertion (Figure 3B) between β-3 and α-3*, a region in GGGPS that is suggested to form a “swinging door” that closes over the prenyl chain.3 In addition, while the GGGPS binding tunnel is largely hydrophobic and acidic, the MoeO5 homology model shows a nest of basic and π-electron containing residues in the vicinity of the “swinging door” that could help stabilize a partially ionized nerolidyl intermediate. Using the absence of glutamate and the presence of arginine in the acceptor binding loop, combined with the presence of a proline insertion in the α-3* strand, we have identified several putative MoeO5 homologues in other bacteria, always clustered with genes putatively involved in secondary metabolite biosynthesis.15
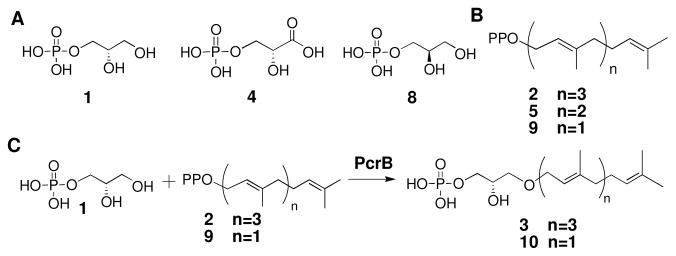
We also investigated the enzymatic function of PcrB, a putative prenyltransferase with a TIM barrel fold.16 PcrB is conserved in several Gram-positive genera, including bacilli, staphylococci, and listeriae. Based on sequence analysis, it has been speculated that PcrB is either an aromatic prenyltransferase or has a function similar to GGGPS.3,7,17 Since PcrB contains an acceptor binding loop and “swinging door” similar to those in GGGPS, it seemed likely that PcrB is a eubacterial prenyltransferase that uses G1P as a substrate.
To test this possibility, we cloned, expressed, and purified Bacillus subtilis PcrB and incubated it with three potential acceptors, glycerol-1-phosphate (G1P, 1), glycerol-3-phosphate (G3P, 8), and 3-phosphoglycerate (3PG, 4), and three potential prenyl donors, GGPP (2), FPP (5), or GPP (9) (Figure 4A and B).14 The reactions, analyzed by mass spectrometry, showed that PcrB uses G1P (1) exclusively as an acceptor, but is able to transfer both the C10 and C20 carbon donors (GPP, 9; GGPP, 2) at similar rates (Figure 3C, Table S1, turnover: 1.8±0.1 min−1 and 0.7±0.1 min−1, respectively); furthermore, upon extended incubation both reactions went to completion.18 In contrast, FPP (5), which contains 15 carbons, is a poor substrate for PcrB and reactions using it as a donor proceeded to less than 10% conversion. NMR analysis showed that the PcrB product formed using GPP as a donor has structure 10 (Figure 3, Figures S1-S3) in which the allylic bond is trans and the ether linkage is to the C3 hydroxyl of G1P.14 Hence the reaction is identical to that catalyzed by GGGPS, except that both short (C10) and long (C20) prenyl chains are substrates.19 Although G1P (1) is a common building block for membrane lipids in archaea, PcrB is the first known bacterial enzyme to use it as a substrate.17,20 The pcrB gene is always found in the same operon as pcrA,21 which encodes an essential helicase involved in the replication and transfer of DNA elements between bacteria.22 This genetic linkage suggests a possible functional connection. Knowledge of PcrB’s enzymatic function may facilitate efforts to elucidate its cellular role.
Figure 4.
A. Phosphosugar acceptors tested as substrates for PcrB: G1P (1), 3PG (4), and G3P (8). B. Prenylpyrophosphate donors tested as substrates for PcrB: GGPP (2), FPP (5), and GPP (9) C. The reactions shown to be catalyzed by PcrB.
The reactions catalyzed by GGGPS, MoeO5, and PcrB suggest that the TIM barrel family of PTs is dedicated to the transfer of prenyl groups to oxygen acceptors of triose phosphates. We have shown that the oxidation state of the acceptor and the regiochemistry of reaction can vary depending on the PT. More remarkably, this family of enzymes can catalyze the formation of products containing either cis- or trans-allylic double bonds from trans-prenyl donors.14,23 Thus, relatively small changes in three dimensional architecture lead to prenyltransfer by two distinct mechanisms, one involving direct displacement and the other requiring bond isomerization and rotation. This is unprecedented for a single PT family. Structural information on MoeO5 or its homologs should provide clues to the specific sequence changes that have led to this dramatic switch in mechanism.
Supplementary Material
Acknowledgments
We thank Charles Sheahan for training and assistance at the HMS East Quad NMR Facility. This work was partially supported by NERCE (NIAID U54AI057159), NIH grants GM076710 and AI083214 (S.W.) and GM30301 (D.E.C.). E.H.D. was supported by an NSF graduate research fellowship and D.L.P. was supported by NIH post-doctoral fellowship. All LC/MS data were acquired on an Agilent 6520 Q-TOF spectrophotometer supported by the Taplin Funds for Discovery Program (P.I., S. Walker). We thank the Biomolecule Production Core of NERCE for reagents (NIAID U54AI057159).
Footnotes
Supporting Information Available: Experimental Procedures, including cloning and purification of proteins, and raw MS and NMR data, complete refs 16 and 21b. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Dewick PM. Nat Prod Rep. 2002;19:181–222. doi: 10.1039/b002685i. [DOI] [PubMed] [Google Scholar]; Heide L. Curr Opin Chem Biol. 2009;13:171–179. doi: 10.1016/j.cbpa.2009.02.020. [DOI] [PubMed] [Google Scholar]; (b) Liang PH. Biochemistry. 2009;48:6562–6570. doi: 10.1021/bi900371p. [DOI] [PubMed] [Google Scholar]
- 2.(a) Liang PH, Ko TP, Wang AHJ. Eur J Biochem. 2002;269:3339–3354. doi: 10.1046/j.1432-1033.2002.03014.x. [DOI] [PubMed] [Google Scholar]; (b) Christianson DW. Chem Rev. 2006;106:3412–3442. doi: 10.1021/cr050286w. [DOI] [PubMed] [Google Scholar]; (c) Greenhagen BT, O’Maille PE, Noel JP, Chappell J. Proc Natl Acad Sci U S A. 2006;103:9826–9831. doi: 10.1073/pnas.0601605103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lane KT, Beese LS. J Lipid Res. 2006;47:681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]; (e) Thulasiram HV, Erickson HK, Poulter CD. Science. 2007;316:73–76. doi: 10.1126/science.1137786. [DOI] [PubMed] [Google Scholar]; (f) Vedula LS, Zhao YX, Coates RM, Koyama T, Cane DE, Christianson DW. Arch Biochem Biophys. 2007;466:260–266. doi: 10.1016/j.abb.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Shishova EY, Yu FL, Miller DJ, Faraldos JA, Zhao YX, Coates RM, Allemann RK, Cane DE, Christianson DW. J Biol Chem. 2008;283:15431–15439. doi: 10.1074/jbc.M800659200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Aaron JA, Lin X, Cane DE, Christianson DW. Biochemistry. 2010;49:1787–1797. doi: 10.1021/bi902088z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payandeh J, Fujihashi M, Gillon W, Pai EF. J Biol Chem. 2006;281:6070–6078. doi: 10.1074/jbc.M509377200. [DOI] [PubMed] [Google Scholar]
- 4.TIM barrel proteins catalyze a wide range of enzymatic reactions; their structural details, mechanism, and evolution has been the focus of numerous studies. See: Reardon D, Farber GK. FASEB J. 1995;9:497–503. doi: 10.1096/fasebj.9.7.7737457.Copley RR, Bork P. J Mol Biol. 2000;303:627–640. doi: 10.1006/jmbi.2000.4152.Lang D, Thoma R, Henn-Sax M, Sterner R, Wilmanns M. Science. 2000;289:1546–1550. doi: 10.1126/science.289.5484.1546.Wierenga RK. FEBS Lett. 2001;492:193–198. doi: 10.1016/s0014-5793(01)02236-0.Nagano N, Orengo CA, Thornton JM. J Mol Biol. 2002;321:741–765. doi: 10.1016/s0022-2836(02)00649-6.Anantharaman V, Aravind L, Koonin EV. Curr Opin Chem Biol. 2003;7:12–20. doi: 10.1016/s1367-5931(02)00018-2.Gerlt JA, Raushel FM. Curr Opin Chem Biol. 2003;7:252–264. doi: 10.1016/s1367-5931(03)00019-x.
- 5.A distinct PT barrel structure has recently been determined for several aromatic prenyltransferases, including Orf2 and CloQ. See: Tello M, Kuzuyama T, Heide L, Noel JP, Richard SB. Cell Mol Life Sci. 2008;65:1459–1463. doi: 10.1007/s00018-008-7579-3.Kuzuyama T, Noel JP, Richard SB. Nature. 2005;435:983–987. doi: 10.1038/nature03668.
- 6.(a) Chen AJ, Zhang DL, Poulter CD. J Biol Chem. 1993;268:21701–21705. [PubMed] [Google Scholar]; (b) Zhang DL, Poulter CD. J Am Chem Soc. 1993;115:1270–1277. [Google Scholar]; (c) Zhang DL, Poulter CD. J Org Chem. 1993;58:3919–3922. [Google Scholar]; (d) Soderberg T, Chen AJ, Poulter CD. Biochemistry. 2001;40:14847–14854. doi: 10.1021/bi0111799. [DOI] [PubMed] [Google Scholar]
- 7.Nemoto N, Oshima T, Yamagishi A. J Biochem. 2003;133:651–657. doi: 10.1093/jb/mvg083. [DOI] [PubMed] [Google Scholar]
- 8.Ostash B, Doud EH, Lin C, Ostash I, Perlstein DL, Fuse S, Wolpert M, Kahne D, Walker S. Biochemistry. 2009;48:8830–8841. doi: 10.1021/bi901018q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostash B, Saghatelian A, Walker S. Chem Biol. 2007;14:257–267. doi: 10.1016/j.chembiol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The cis-geometry observed in the products of cis-prenyl chain elongating enzymes results from a different type of reaction mechanism. See: Kharel Y, Koyama T. Nat Prod Rep. 2003;20:111–118. doi: 10.1039/b108934j.
- 11.Nerolidyl diphosphate is a demonstrated intermediate in the cyclization-isomerization-cyclization of FPP to 6-, 10-, and 11-membered ring sesquiterpenes with cis-double bonds. See: Cane DE, Ha HJ. Pargellis C, Waldmeier F, Swanson S, Murthy PPN. Bioorg Chem. 1985;13:246–265.Cane DE, Ha HJ. J Am Chem Soc. 1988;110:6865–6870.Cane DE, Pawlak JL, Horak RM, Hohn TM. Biochemistry. 1990;29:5476–5490. doi: 10.1021/bi00475a011.Domingo V, Arteaga JF, del Moral JFQ. Barrero AF. Nat Prod Rep. 2009;26:115–134. doi: 10.1039/b801470c.Noel JP, Dellas N, Faraldos JA, Zhao M, Hess BA, Smentek L, Coates RM, O’Maille PE. ACS Chem Biol. 2010;5:377–392. doi: 10.1021/cb900295g.
- 12.Rates were measured in triplicate using 1 mM 3PG, 40 μM lipid pyrophosphate and 120 nM enzyme. Conversion was <10%. See SI for full details.
- 13.We cannot rule out that the intermediate detected could also be cis, trans-farnesylpyrophosphate.
- 14.See SI.
- 15.MoeO5 homologues are present and clustered with moenomycin type biosynthetic clusters in S. clavuligerus, Photohrabdus luminescens subsp. laumondii TTO1, and P. asymbiotica.
- 16.Badger J, et al. Proteins: Struct, Funct, Bioinf. 2005;60:787–796. [Google Scholar]
- 17.Guldan H, Sterner R, Babinger P. Biochemistry. 2008;47:7376–7384. doi: 10.1021/bi8005779. [DOI] [PubMed] [Google Scholar]
- 18.Rates were measured in triplicate using 1 mM G1P, 40 μM lipid pyrophosphate and 3.4 μM enzyme. Conversion was <15%. See SI for full details.
- 19.How FPP is excluded is unknown, although we note that the hydrophobic tunnel in PcrB has a kink that would require substrates longer than ten carbons to bend.
- 20.A bacterial enzyme that interconverts glyceraldehyde-phosphate and G1P was recently identified, which suggests that bacteria produce this metabolite for some purpose. See: Ref (17).
- 21.(a) Ji YD, Zhang B, Van Horn SF, Warren P, Woodnutt G, Burnham MKR, Rosenberg M. Science. 2001;293:2266–2269. doi: 10.1126/science.1063566. [DOI] [PubMed] [Google Scholar]; (b) Kobayashi K, et al. Proc Natl Acad Sci U S A. 2003;100:4678–4683. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Ruiz-Maso JA, Anand SP, Espinosa M, Khan SA, del Solar G. J Bacteriol. 2006;188:7416–7425. doi: 10.1128/JB.01010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Slatter AF, Thomas CD, Webb MR. Biochemistry. 2009;48:6326–6334. doi: 10.1021/bi900101h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee CA, Babic A, Grossman AD. Mol Microbiol. 2010;75:268–279. doi: 10.1111/j.1365-2958.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Using homology searches, we have identified a putative new subfamily of TIM barrel PTs that are highly conserved in Gram-negative bacteroides, and we predict that these enzymes also use G1P as a substrate and produce a trans product (Figure S4).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.