Abstract
Systemic injection of high doses of 11-deoxycortisol succinate had been reported to induce status epilepticus in rats and cats that was associated with paroxysmal epileptiform activity refractory to first generation antiepileptic drugs (AEDs). Using patch-clamp recordings we have investigated the mechanisms of 11-deoxycortisol-induced excitability and we have discovered that this molecule accelerates the decay time of the inhibitory postsynaptic currents (IPSCs) mediated by GABAA receptors, both in neuronal cultures and in hippocampal slices. In addition, it reduces the amplitude and frequency of IPSCs. Thus, 11-deoxycortisol action on GABAergic neurotransmission may be one of the underlying causes of convulsive seizures that had been observed in rats. In the present study, we have reproduced the ability of 11-deoxycortisol to induce convulsive seizures after intravenous infusion in mice. The threshold dose of 11-deoxycortisol necessary for seizure induction was also determined (0.95 mmol/kg). Furthermore, we have established that these seizures are completely refractory to several AEDs such as phenytoin (up to 100 mg/kg), carbamazepine (up to 56 mg/kg), and valproate (up to 300 mg/kg). Levetiracetam and diazepam afforded only limited protection at high doses, 540 and 3–10 mg/kg, respectively. Interestingly, long-lasting seizures induced by 11-deoxycortisol in mice were not associated with typical neuropathological changes observed in other models of status epilepticus. We propose that 11-deoxycortisol-induced seizures may be an advantageous experimental model of drug-resistant epilepsy. Finally, better understanding of the pro-epileptic properties of 11-deoxycortisol is very important, because this endogenous steroid precursor may play a role in the pathophysiology of epilepsy.
Keywords: Seizures, hippocampus, 4-aminopyridine, neurosteroids, cortisol, GABA
1. Introduction
Steroid hormones are well known to modulate neuronal excitability through various mechanisms. Fast actions of steroids on firing patterns of neurons are mediated by altering the properties of neuronal membranes and receptors, while delayed actions are mediated by binding to nuclear receptors and ensuing transcriptional changes (Jöels, 1997). The steroids synthesized in the brain, called neurosteroids, act not through classic steroid hormone nuclear receptors, but mostly through ion-gated neurotransmitter receptors (Baulieu, 1998).
A major target of neurosteroids is GABA (γ-aminobutyric acid), the most abundant inhibitory neurotransmitter of mammalian brain that hyperpolarizes neuronal membranes by opening GABA-gated Cl− channels of GABAA receptors. Brain specific neurosteroids are synthesized in glial cells (Baulieu, 1998) and allosterically modulate as well as directly activate GABA gated Cl− channels (Majewska et al., 1992; Maguire and Mody, 2009). The action on GABAA receptors by neurosteroids is strongly dependent on specific structural determinants (Harrison et al., 1987). Most neurosteroids such as allopregnanolone, allotetrahydrodeoxycorticosterone and adrosterone are positive modulators at GABAA receptors (Majewska et al., 1986; Puia et al., 1990) and exert potent anticonvulsant activity (Gasior et al., 1999; Kaminski et al., 2004, 2005a). In contrast, few other neurosteroids, such as pregnenolone sulfate, negatively modulate GABA-gated Cl− currents (Majewska and Schwartz, 1987; Shen et al., 2000) and exert convulsant actions (Kokate et al., 1999). Direct injection of pregnenolone sulfate to rodent brain elicits seizures which increase in severity and frequency with time and eventually progress to status epilepticus, tonic hindlimb extension and death of the animals (Kokate et al., 1999; Williamson et al 2004). However, systemic administration of pregnenolone sulfate does not induce overt convulsant activity (Kokate et al., 1999). The pharmacology of pregnenolone sulfate seizures is not well described, but clonazepam, allopregnanolone and dizocilpine effectively protected against its convulsive actions (Kokate et al 1999).
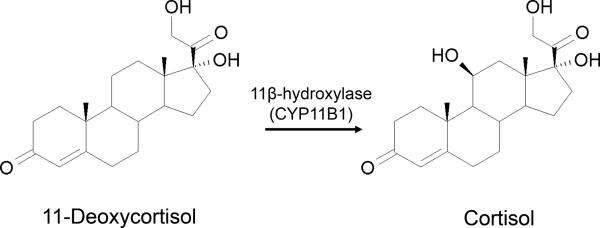
11-Deoxycortisol (pregn-4-ene-17,21-diol-3,20-dione) is an immediate precursor of cortisol, the main corticosteroid produced by the zona fasciculata of the adrenal cortex. Enzymatic conversion of 11-deoxycortisol to cortisol is mediated by 11β-hydroxylase (CYP11B1) (Fig. 1). 11-Deoxycortisol acts as a competitive antagonist of glucocorticoid receptor in vitro, but is ineffective as a glucocorticoid antagonist in vivo due to adrenal 11-hydroxylation (Cutler et al 1979). Nearly 50 years ago, Heuser and Eidelberg (1961) have observed that systemic administration of large doses of 11-deoxycortisol succinate (300–400 mg/kg) induce long-lasting seizure activity (status epilepticus) associated with paroxysmal electroencephalographic (EEG) epileptiform activity in rats and cats. Interestingly, 11-deoxycortisol-induced status epilepticus was refractory to antiepileptic drugs (AEDs) available at the time (Heuser and Eidelberg, 1961).
Fig. 1. Enzymatic conversion and chemical structures of 11-deoxycortisol and cortisol.
The mechanism underlying 11-deoxycortisol seizures remained unexplored ever since the initial finding of Heuser and Eidelberg (1961). We believe that understanding of the pro-epileptic properties of 11-deoxycortisol is very important, because it is an endogenous steroid precursor which may play a role in the pathophysiology of epilepsy. Therefore, we aimed to reproduce the above mentioned findings and scrutinize the mechanisms of 11-deoxycortisol-induced excitability.
First, we confirm that 11-deoxycortisol is capable of inducing long-lasting status epilepticus in rodents. Furthermore, we also find that seizures induced by 11-deoxycortisol are refractory to several currently used AEDs with diverse mechanisms of action. Finally, we unravel the most probable mechanism of induction of seizures by 11-deoxycortisol. Using a range of electrophysiological approaches we show that 11-deoxycortisol has a unique property to accelerate the decay time of the inhibitory postsynaptic currents (IPSCs) mediated by GABAA receptors and recorded in both neuronal cultures and brain slices. Together with reduction of the amplitude and frequency IPSCs 11-dexocortisol significantly impedes GABAergic neurotransmission which may lead to paroxysmal epileptiform network activity and convulsive seizures. This quite significant reduction of GABAergic neurotransmission may also contribute to the fact that 11-deoxycortisol seizures are refractory to treatment.
2. Materials and Methods
2.1 Cell culture & Electrophysiology
2.1.1 Cell Culture
Hippocampal neuronal cultures were prepared from 1-day-old C57Bl6 mice. Mouse pups were sacrificed by decapitation in agreement with the guidelines of the Georgetown University Animal Care and Use Committee. Briefly, after careful dissection from diencephalic structures, the hippocampi were chopped and digested in 0.25% trypsin (Sigma, St Louis, MO) for 15 min at 37°C with gentle shaking. Dissociated cells were plated at a density of 0.2 × 106 in a 35-mm dish on poly-L-lysine coated coverslips (Fisher Scientific, Pittsburgh, PA) in Basal Medium Eagle (BME, Invitrogen, Carlsbad, CA) containing 10% FBS, 2 mM glutamine, 100 μg/ml gentamycin (all from Invitrogen Corporation Carlsbad, CA), and 25mM KCl, and maintained at 37°C in 5% CO2. After 24 hours in vitro, the medium was replaced with a half-half mixture of BME and Neurobasal medium containing 2% B27 supplement, 20 μg/ml gentamycin, and 0.25% glutamine (Invitrogen). At 5 days in vitro (DIV5), cytosine arabinofuranoside was added at a final concentration of 10 μM. Thereafter, half of the medium was replaced twice a week with Neurobasal medium containing 2% B27 supplement, 20 μg/ml gentamycin, and 0.25% glutamine.
Primary cultures of cerebellar granule neurons were prepared from postnatal day 5–7 C57Bl6 mice. The cerebella were removed, dissociated with 0.25 mg/ml trypsin (Sigma) and plated in 35 mm Nunc dishes at a density of 1.1×106 cells/ml, on glass coverslips (Fisher Scientific) coated with 10 μg/ml poly-D-lysine (Sigma). The cells were cultured in basal Eagle's medium supplemented with 10% bovine calf serum, 2 mM glutamine, and 100 μg/ml gentamycin (all from Invitrogen Corporation Carlsbad, CA), and maintained at 37°C in 5% CO2. The final concentration of KCl in the culture medium was adjusted to 25 mM. At 5 days in vitro (DIV5) the medium was replaced with low (5 mM) K+ medium (MEM supplemented with 5 mg/ml glucose, 0.1 mg/ml transferrin, 0.025 mg/ml insulin, 2 mM glutamine, 20 μg/ml gentamicin (Invitrogen) and cytosine arabinofuranoside 10 μM, Sigma) as previously described (Chen et al., 1987; Losi et al., 2002) to facilitate the formation of a synaptic network between cerebellar granule cells and GABAergic interneurons in culture.
2.1.2 Electrophysiology from cultures
Coverslips with hippocampal pyramidal neurons (HPNs) or cerebellar granule cell (CGCs) were placed on the stage of an inverted microscope (TM2000, Nikon) equipped with fluorescent and phase-contrast optics. All recordings were performed at room temperature (24–26°C) from neurons maintained for 7 to 14 days in vitro.
Osmolarity was adjusted to 315 mOsms with sucrose. Electrodes were pulled in two stages on a vertical pipette puller to a resistance of 4–6 MΩ, from borosilicate glass capillaries (Wiretrol II, Drummond, Broomall, PA), and filled with a KCl-based internal solution containing (in mM): KCl (145), HEPES (10), ATP.Mg (5), GTP.Na (0.2), and EGTA (10), adjusted to pH 7.2 with KOH. In some experiments, KCl was substituted with equimolar K-gluconate or Csmethanesulfonate. Whole-cell voltage-clamp and current-clamp recordings were performed at room temperature using an Axopatch-1D amplifier (Molecular Device Co., Sunnyvale CA, USA). A transient current response to a hyperpolarizing 10 mV pulse was used to assess access resistance and capacitance throughout the recordings, and recordings with >20% over time changes were discarded. Currents were filtered at 2 kHz with an 8-pole low-pass Bessel filter (Frequency Devices, Haverhill, MA), digitized at 5–10 kHz using an IBM-compatible microcomputer equipped with Digidata 1322A data acquisition board and pCLAMP9 software (both from Molecular Devices Co). All drugs were applied with a Y-tube (Murase et al., 1989) unless otherwise stated. 11-Deoxycortisol and pregnenolone sulfate (both from Sigma) were first diluted in DMSO at a concentration of 100 mM. All control application contained DMSO at 0.1% concentration. Cortisol and 4-aminopyridine (4-AP, Sigma) were diluted directly in the extracellular medium.
Miniature spontaneous inhibitory postsynaptic currents (mIPSCs) were isolated by application of TTX (0.5 μM), NBQX (5 μM), and strychnine (0.5 μM) and verified to be GABAergic by subsequent application of bicuculline methobromide (25 μM). All detected events were used for event frequency analysis, but overlapping mIPSCs were eliminated from averages obtained to determine amplitude, rise time, and decay kinetic analysis. GABA-mIPSCs were identified using a semi-automated threshold based mini detection software Mini Analysis (Synaptosoft, Fort Lee, NJ) and were visually confirmed. The decay of GABA-mIPSCs was fitted using Clampfit 9.2 (Axon Instruments) from averages of more than 50 events selected with Mini Analysis in each cell studied. The decay phase of currents was fitted using a simplex algorithm for least squares exponential fitting routine with a double exponential equation of the form I(t)=I1×exp(−t/τ1)+I2×exp(−t/τ2), where I1 and I2 are the peak current amplitude of each decay component and τ1 and τ2 are the corresponding decay time constants. To allow for easier comparison of decay times between experimental conditions, the two decay time components were combined into a weighted time constant τw = [I1/(I1+I2)]×τ1 + [I2/(I1+I2)] ×τ2. All data are expressed as mean ± s.e.m. P values represent the results of Analysis of variance (ANOVA) for multiple comparisons or two-tailed unpaired Student's t tests.
2.2 Hippocampal slices and Electrophysiology
2.2.1 Hippocampal slices
Balb/c mice (main animal facility, University of Liege, Belgium), aged 12–18 days, were decapitated. The local ethics committee for the use of animals in research of the University of Liège approved the experimental protocol on hippocampal slices (protocol 1018). Brains were quickly removed and 300-μm thick transverse hippocampal slices were cut with a DTK-1000 microslicer (DTK-1000, Dosaka Co. Ltd, Kyoto, Japan) in ice-cold artificial cerebrospinal fluid (aCSF) containing (mM): 120 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, and 10 D-glucose, 300–305 milliosm/l, pH 7.3–7.4 when bubbled with 95% O2-5% CO2. Slices were immediately placed in a holding chamber containing oxygenated aCSF and were incubated at 36°C for 30–40 min before being transferred to the recording chamber.
2.2.2 Electrophysiology from slices
Recording pipettes were filled with an internal solution of the following composition (mM): 130 CsCl, 1 EGTA, 0.5 CaCl2, 2 MgCl2, 2 ATP-Mg, 10 Hepes, 1.5 QX-314, 300–305 milliosm/l, pH adjusted to 7.2–7.4 with CsOH.
Patch pipettes were pulled from thick-walled borosilicate glass capillaries (2.0 mm outer diameter, 0.5 mm wall thickness; Hilgenberg, Malsfeld, Germany) using a Sutter P-87 puller (Sutter Instrument Company, Novato, CA, USA). Pipette resistances were 3–6 MΩ when filled with the above solution.
Whole-cell recordings were made at 30 – 32°C in a chamber where slices were completely submerged. Viable pyramidal neurons in the CA1 region were visualized with Infrared-aided Nomarski differential interference contrast video microscopy using a Zeiss Axioskop FS I microscope (Zeiss, Heidelberg, Germany), fitted with a 40 × 0.90 numerical aperture water-immersion objective. Whole-cell voltage-clamp recordings were obtained from CA1 pyramidal neurons. Data was acquired using a Patch clamp EPC-9 amplifier (HEKA Elektronik, Lambrecht, Germany). The membrane potential of CA1 pyramidal neurons was held at −70 mV. Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in the presence of the glutamate receptor antagonists D-AP5 (25 μM, Tocris, Bristol, UK) and CNQX (10 μM, Tocris). mIPSCs were recorded in the additional presence of 500 nM TTX (Tocris), in order to block presynaptic action potentials. The GABAergic nature of both mIPSCs and sIPSCs was verified by using the GABAA receptor antagonist gabazine (SR 95531, 10 μM, Sigma, St Louis, MO). 11-deoxycortisol (Sigma) was stored as a stock solution in DMSO (100 mM) and diluted to a 100 μM concentration in aCSF (0.1% DMSO). Control solutions also contained DMSO at 0.1%. In all experiments, 100 μM 11-deoxycortisol was superfused after recording sIPSCs or mIPSCs in the control solution. Parallel experiments showed that there was no significant change over time in the parameters of IPSCs in the control solution.
Recordings were filtered at 1 kHz (4-pole low-pass Bessel filter) and digitally sampled at 6 kHz. Series resistance was monitored repetitively throughout the recording by applying a hyperpolarizing voltage step of 5 mV. Only recordings with a stable access resistance (less than 25 % changes over the course of recording) were used for data analysis. Recordings with series resistance >30 MΩ were discarded. Events were analyzed by using the Mini Analysis (Synaptosoft, Fort Lee, NJ), which collects events using a manually controlled threshold detector and is capable of detecting events as small as two to three times the baseline noise. The sIPSC and mIPSC parameters, i.e frequency, amplitude, rise and decay times, as well as total charge transfer (over a period of 2 minutes), were measured. The sIPSCs and mIPSCs parameters were measured during 2-min epochs. All data were expressed as mean ± s.e.m. and statistical analysis was performed with a paired or unpaired Student's t test (GraphPad InStat 3.0, GraphPad Software Inc, San Diego, CA).
2.3 In vivo experiments
2.3.1 Induction of seizures by 11-deoxycortisol and testing of AEDs in mice
Male NMRI mice (Charles River, France) were used as the experimental subjects in all pharmacological studies, while male C57Bl6 mice (Charles River, France) were used for EEG recordings. All animal experiments were done according to the Helsinki declaration and conducted in accordance with the guidelines of the European Community Council directive 86/609/EEC. 11-Deoxycortisol and cortisol (both from Sigma) were dissolved in 20% solution of β-cyclodextrin and infused i.v. in freely moving animals through the tail vein catheter as previously described for other convulsants (Kaminski et al. 2005b). Before infusion, mice were briefly restrained and a 30-gauge needle attached to a 0.3-m long polyethylene tubing (PE-10) was inserted into the lateral tail vein. Proper localization of the needle was confirmed by the appearance of blood in the tubing. The tubing was connected to a syringe mounted on infusion pump (Harvard Apparatus, Holliston, MA, USA). The needle was gently secured to the tail with plastic tape, the mouse was removed from the restrainer and immediately transferred to an observation cage. The infusion and observation of seizures were performed on freely moving mice. Infusion of 11-deoxycortisol (0.038 mmol/ml, 0.2 ml/min) was used for seizure threshold determination. A bolus dose (1.0 mmol/kg) producing seizures in 100% of animals was used for assessment of the protective efficacy of the anticonvulsant drugs (AEDs). The following AEDs were used as pretreatment: phenytoin (10–100 mg/kg, ip, −120 min., Fluka), carbamazepine (10–56 mg/kg, ip, −15 min., Sigma), valproate (30–300 mg/kg, ip, −15 min., Sigma), levetiracetam (17–540 mg/kg, ip, −60 min., UCB) and diazepam (0.3–10 mg/kg, ip, −30 min., Apin). Phenytoin, carbamazepine and diazepam were dissolved in 0.1% solution of Tween 80 (Merck), while levetiracetam and valproate were dissolved in saline. Each experiment consisted of several groups of mice (n = 5–14) receiving different doses of tested compounds or vehicle. A control group (n = 11–18), pretreated with vehicle and infused with 11-deoxycortisol was used in each experiment.
2.3.2 EEG recordings
The EEG recordings in mice were performed according to previously described protocol (Kaminski et al., 2009). The mice were anesthetized with medetomidine hydrochloride (0.5 mg/kg ip, Domitor, Pfizer) and ketamine (50 mg/kg ip, Imalgene, Rhône-Mérieux) for implantation of EEG electrodes. A monopolar depth electrode was implanted into the CA1 region of the hippocampus (coordinates vs. bregma: −1.94 mm anteroposterior, −1.0 mm lateral, −1.25 mm depth). Three monopolar cortical electrodes, frontal left (+1.0 mm anteroposterior, −2.0 mm lateral) and occipital left/right: (−4.0 mm anteroposterior, +/−4 mm lateral) were also positioned on the dura mater. A ground electrode was placed in the left prefrontal bone. EEG activity from both hippocampal and cortical electrodes was recorded with a Model 15 Neurodata Amplifier System (Grass Technologies; West Warwick, RI, USA).
2.4 Histology and immunohistochemistry
NMRI male mice were sacrificed 48 hours after status epilepticus induced by infusion of 11-deoxycortisol. Their brains were rapidly removed, immersed in 10% formalin, and then paraffin embedded. Coronal sections (6 μm-thick) were cut at the level of the dorsal hippocampus (Paxinos and Franklin, 2001), and mounted onto polarized slides (Superfrost slides, Thermo Scientific).
Hematoxylin-Eosin staining sections were de-waxed through two washes in xylol for 10 min, one in ethanol 100% for 5 min, one in ethanol 95% for 5 min, and one in ethanol 80% for 5 min; incubated in Mayer' hematoxylin solution (Klinipath) for 6 min; washed in water for 5 min; incubated in alcohol eosin (Labonord) solution for 2 min; dehydrated in increasing ethanol baths and finally immersed twice in xylol. Coverslips were mounted using DPX Mountant for histology (Labonord). Hematoxylin-Eosin staining was evaluated after acquiring images through an AxioCam ICc1 1× camera (Zeiss) mounted on an Axioplan microscope (Zeiss). Images were captured with a 2.5× objective.
Fluoro-Jade C staining (FJC) was performed as described by Schmued et al., (2005). Sections were dewaxed as described above and incubated in a solution containing 1% NaOH in 80% ethanol for 5 min, then in 70% ethanol for 2 min, then in distilled water for 2 min. They were then incubated in 0.06% potassium permanganate and placed on an oscillating plate for 10 min in the dark, washed for 2 min in distilled water and transferred to a 0.001% FJC staining solution for 20 min, during which slices were continuously stirred in the darkness. After staining, sections were washed three times in distilled water and dried for 30 min at 50°C. Coverslips were mounted using DPX Mountant for histology.
For immunofluorescence sections were de-waxed and rehydrated as described for Hematoxylin-Eosin staining. All antigens were unmasked in a bath of citrate buffer using a Microwave Processing Labstation for Histology (Milstone T/T Mega). After washing in PBS, sections were incubated with Triton x-100 (0.3% in PBS 1×) at room temperature for 10 min, washed twice in PBS 1×, and incubated with 5% BSA and 5% of Normal Goat Serum (serum of the species in which the secondary antibody was produced) for 3 min. Slices were then incubated with the primary antibodies in humid atmosphere at 4°C overnight as follows: MAP2abc (mouse monoclonal, Immunological Sciences) 1:25 dilution and GFAP (rabbit polyclonal, Invitrogen) 1:50 dilution. The day after, after 5 min rinses in PBS, sections were immersed again in triton x-100 0.3% for 30 min, washed in PBS and incubated with a solution containing the goat anti-mouse Alexa Fluor 488-conjugated and the goat anti-rabbit Alexa Fluor 594-conjugated, secondary antibodies (both diluted 1:500, Invitrogen) at room temperature in a humid atmosphere for 3 h. After staining, sections were washed in PBS, counterstained with 0.0001% DAPI for 10 min (Invitrogen), and washed again three times. Coverslips were mounted using anti fading Shur/Mount Mounting Media water based (proLab). Fluorescence images were captured using an AxioCam MRm 0.63× camera (Zeiss) mounted on an Axio Observer microscope (Zeiss). Illumination was kept constant throughout all experiments. Images were acquired with a 5× objective.
3. Results
3.1. Electrophysiology
3.1.1 Cell cultures
3.1.1.1. 11-Deoxycortisol prolongs spontaneous bursts and increases the number of action potentials per burst in HPN cultures
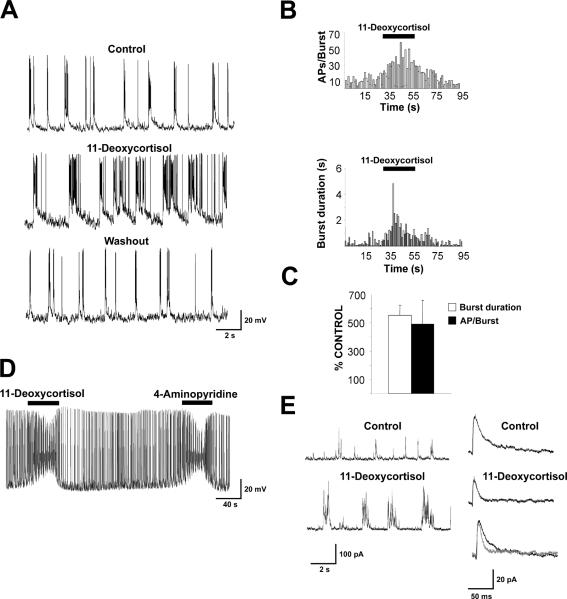
We first performed electrophysiological recording from neurons in primary cultures to investigate the action of 11-deoxycortisol. As shown in Fig. 2A, current clamp recordings from hippocampal neurons in culture for at least 3 weeks revealed spontaneous activity consistent in random action potentials or short bursts. Upon local application of 11-deoxycortisol, the duration of these bursts increased dramatically with a parallel increase of the number of spikes per burst (Fig. 2A,B). Fig. 2C summarizes the percent increase in burst duration and action potential/burst in the neurons tested. In additional experiments, we sought to compare the action of a known convulsant 4-AP (50 μM) (Avoli and Perreault, 1987) to those of 11-deoxycortisol using a slower bath perfusion. As shown in Fig. 2D, action potential firing reached a paroxysmal status resulting in a steady state depolarization and spike size reduction in the presence of both drugs. These effects were readily reversible and were reproducible. Spontaneous synaptic activity was evident in hippocampal cultures. When neurons were transiently clamped at 0 mV (close to the reversal potential of excitatory synaptic currents), outward currents due to GABAergic (GABAA) IPSCs were readily observed. As seen in Fig. 2E (left panels) 11-deoxycortisol increased the duration of the IPSC bursts, but failed to strongly reduce their amplitude, contrary to what would be expected for a potential GABAergic antagonist. However, upon inspection of the average (Fig. 2E, right panel) of isolated IPSCs occurring in between bursts in these experimental conditions, we observed that 11-deoxycortisol decreased IPSC decay times (see normalized average IPSCs in the absence and the presence of 11-deoxycortisol)
Fig. 2. Spontaneous synaptic activity is enhanced by 11-deoxycortisol application.
A. An example of intracellular current clamp recording with K+ gluconate of action potential bursts occurring in a hippocampal neuron in culture at DIV 21. Local application of 50 μM 11-deoxycortisol prolongs burst duration and increases the number of action potentials per burst.
B. Time course of the 11-deoxycortisol-induced increase in number of action potential per burst (AP/Burst) (top) and burst duration in the neuron illustrated in A (bottom).
C. Summary of the % increase of burst duration and action potential number per burst with 50 μM 11-deoxycortisol. Data derived from 8 cells in 2 culture preparations.
D. A comparison of the effect of 50 μM 11-deoxycortisol with 50 μM 4-AP in another HPC neuron. Compounds were applied by bath perfusion.
E. IPSCs recorded from a HPC neuron at DIV 23 in voltage clamp with Cs-methanesulfonate at 0 mV holding potential illustrating the effect of 11-deoxycortisol on their occurrence (left). In the panels on the right averages of isolated IPSCs recorded from this cell in the presence and absence of 100 μM 11-deoxycortisol are shown individually and superimposed to illustrate the decrease in decay time with 11-deoxycortisol. IPSC decay time constants were 15.3 ms in control condition and 8.9 ms in the presence of 11-deoxycortisol.
3.1.1.2. 11-Deoxycortisol decreases peak amplitude and decay time of mIPSCs in CGCs
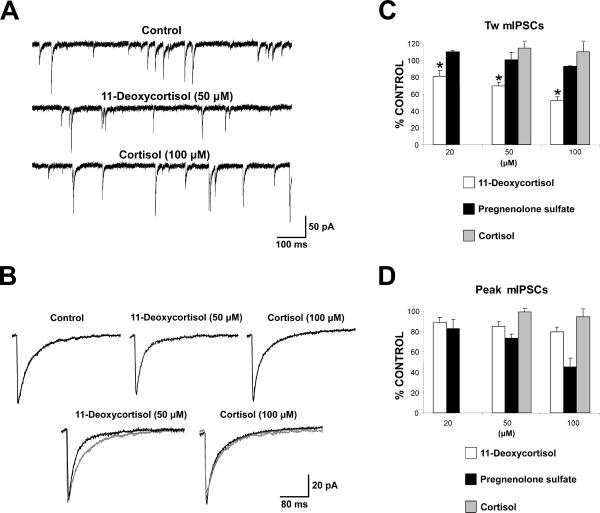
To further investigate the mechanism of action of 11-deoxycortisol and to perform concentration-response curves together with comparison to analogous compounds, we next investigated mIPSCs recorded in cultured CGCs because HPNs do not offer the possibility to achieve high quality voltage clamp. These cultures contain a homogeneous cell population, in which neurons have a small body size (only 3–4 μm diameter) and simple dendritic arborization, thus allowing good voltage clamping, when compared to other types of cultured neurons (Silver et al., 1992; Ortinski et al., 2004; Fiszman et al., 2005; Kaminski et al., 2006). GABAA mIPSCs were recorded at DIV7-14 in the presence of TTX, AMPA receptor and glycine receptor antagonists as described previously (Ortinski et al., 2004). Sample traces illustrating these mIPSCs recorded are shown in Fig. 3A. In the example shown, local application of 50 μM 11-deoxycortisol only slightly decreased the amplitude and the frequency of occurrence of mIPSCs, but substantially decreased the decay time as shown by the comparison of the normalized and superimposed average traces (Fig. 3B). These effects were not shared by 100 μM cortisol. Fig. 3C and 3D illustrate the dose dependency of the effects of 11-deoxycortisol on weighted time constant of decay and amplitude respectively by three concentrations of 11-deoxycortisol in comparison to cortisol and to pregnenolone sulfate, another steroid that cause alterations of GABAergic synaptic transmission (Shen et al. 2000). Decay of mIPSCs was significantly faster even with the lowest 11-deoxycortisol concentration tested, while this effect was not shared by cortisol or pregnenolone sulfate (Fig. 3C). On the other hand, as seen in Fig. 3D, pregnenolone sulfate decreased mIPSCs amplitude more robustly than 11-deoxycortisol. In contrast to 11-deoxycortisol and cortisol, pregnenolone sulfate significantly increased mIPSCs occurrence in most cells tested (not illustrated), perhaps because of the reported potentiating effects of this steroid on NMDA receptors and the role of NMDA receptor in regulating GABA release in GABAergic interneurons (Fiszman et al., 2005).
Fig. 3. 1-Deoxycortisol, but not pregnenolone sulfate or cortisol, shortens mIPSC decay in cerebellar granule neurons (CGCs) cultures.
A. An example of voltage clamp recordings with KCl of mIPSCs occurring in a cerebellar granule neuron in culture at DIV 8. Local application of 50 μM 11-deoxycortisol decreased the duration and frequency of the occurrence of these currents. In contrast 100 μM cortisol failed to alter mIPSCs occurrence and their biophysical properties.
B. Average mIPSCs in the 3 experimental condition illustrated for the cell in panel A. Traces shown individually (top) and normalized and superimposed (bottom). Decay values were 28 ms for control, 19 ms for 11-deoxycortisol and 29 ms for cortisol.
C,D. Summary of the % changes from control for decay (C) with mIPSC amplitude (D) after application of 50 μM 11-deoxycortisol, 50 μM pregnenolone sulfate and 100 μM cortisol in all CGCs studied (at least 7 in each condition). * p<0.05
3.1.1.3 11-Deoxycortisol equally accelerate mIPSC decay in cultured CGCs from α1 subunit (−/−) mice
We and others have shown that the α1 subunit of GABAA receptors is highly expressed in adult CGCs and it is also the predominant α subunit in the mature rodent cerebellum. The switch of α subunits of GABAA receptors from α2/3 to α1 is the main cause underlying developmental speeding of the IPSC decay (Vicini et al., 2001, Ortinski et al., 2004). To assess whether the reduced decay time of mIPSCs by 11-deoxycortisol was due to a selective antagonism of non α1 containing GABAA receptors at the synapses characterized by slower decay, we examined the effects on mIPSCs in CGSs from α1 subunit (−/−) mice. 11-Deoxycortisol did not affect synaptic currents decay (62 ± 3 % of control τw with 50 μM 11-deoxycortisol) suggesting that the steroid does not preferentially inhibit GABAA receptors at postsynaptic sites other then those α1 subunit-containing. This is further supported by lack of differential effect of 11-deoxycortisol on the decay of mIPSCs in cells at DIV7–9 versus cells at DIV13–15 (66 ±5 % versus 69 ±3 % of control τw with 50 μM 11-deoxycortisol), when α1-containing GABAA receptors (α1β2γ2) become predominant (Ortinski et al., 2004).
3.1.2 Hippocampal slices
3.1.2.1 Effect of 11-deoxycortisol on sIPSCs in hippocampal CA1 pyramidal neurons
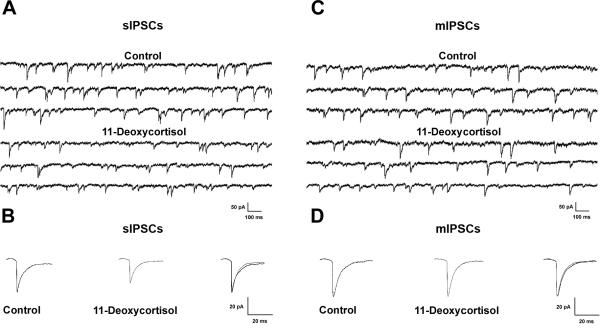
In order to test whether the effect of 11-deoxycortisol on GABAA receptor-mediated synaptic transmission also occurs in more physiological conditions, we performed whole-cell recordings from hippocampal CA1 pyramidal neurons in slices from 12–18 days old mice. We initially examined the effects of 11-deoxycortisol (100 μM) on sIPSCs recorded in the presence of CNQX (10 μM) and D-APV (25 μM) to block AMPA and NMDA responses, respectively. Cells were held at −70 mV and sIPSCs appeared as inward currents because ECl was close to 0 mV with the solutions that were used in these experiments (see Methods) (Fig 4A). It has previously been shown that these sIPSCs originate from Cl− currents mediated by presynaptic release of GABA (Haage et al., 1998). They were completely and reversibly blocked by 10 μM SR 95531, indicating that they were mediated by GABAA receptors (Bai et al., 2001). As shown in Fig. 4A, application of 11-deoxycortisol (100 μM) for 10 min significantly decreased the frequency and the amplitude of sIPSCs recorded from CA1 pyramidal neurons as compared to the control condition (frequency: 74% of control; amplitude: 81% of control, Table 1, P < 0.05 for both parameters). 11-deoxycortisol also significantly decreased the mean decay time of the averaged sIPSCs recorded from CA1 pyramidal neurons as compared to control (84% of control, Table 1, P < 0.05). However, the mean rise time of the averaged sIPSCs was unaffected (98% of control, P = 0.76, Table 1). Not surprisingly, the charge transfer of sIPSCs (represented by the area of the currents) was also significantly decreased by 11-deoxycortisol (67% of control, Table 1, P < 0.05). A complete reversal of the effects of 11-deoxycortisol could not be achieved. However, its effect on sIPSC frequency was partially reversed (91.6% of control) 30 minutes after its washout in three neurons in which a stable recording was achieved for that duration (not illustrated). The slow and partial reversal of the 11-deoxycortisol effect was probably caused by the lipophilic nature of the steroid.
Fig. 4. Effects of 11-deoxycortisol (100 μM) on sIPSCs and mIPSCs parameters recorded in hippocampal slices.
A. Representative traces of sIPSCs recorded in mice CA1 pyramidal neurons. Application of 100 μM 11-deoxycortisol decreased both the frequency and amplitude of the sIPSCs (see also Table 1).
B. Averages of isolated sIPSCs recorded in the absence (left) and presence (middle) of 100 μM 11-deoxycortisol are shown individually and superimposed (right) to illustrate the decrease in amplitude and decay time with 11-deoxycortisol. Synaptic currents decay time constants were 18.5 ms in control condition and 15.2 ms in the presence of 11-deoxycortisol.
C. Representative traces of mIPSCs recorded in mice CA1 pyramidal neurons. Application of 100 μM 11-deoxycortisol decreased the frequency, but not amplitude of the sIPSCs (see also Table 1).
D. Averages of isolated mIPSCs recorded in the absence (left) and presence (middle) of 100 μM 11-deoxycortisol are shown individually and superimposed (right). 11-Deoxycortisol remained without effect on the amplitude and decay time constants. Synaptic currents decay time constants were 16.3 ms in control condition and 14.9 ms in the presence of 11-deoxycortisol.
Table 1.
Effects of 11-deoxycortisol on the parameters of spontaneous inhibitory postsynaptic currents (sIPSC) and miniature inhibitory postsynaptic currents (mIPSC) recorded in mouse hippocampal CA1 pyramidal neurons.
| Group | N | Frequency (Hz) | Amplitude (PA) | Rise time (ms) | Decay time (ms) | Charge transfer (pA ms) |
|---|---|---|---|---|---|---|
| sIPSC | ||||||
| Control | 10 (10) | 15.8 ± 1.7 | − 41.2 ± 2.7 | 2.4 ± 0.2 | 18.1 ± 0.7 | 381.2 ± 24.5 |
| 11-Deoxycortisol | 10 (10) | 11.2 ± 1.2* | − 34.5 ± 2.5* | 2.4 ± 0.5 | 15.1 ± 0.5* | 248.9 ± 12.5* |
| mIPSC | ||||||
| Control | 12 (10) | 10.6 ± 1.0 | − 41.9 ± 1.5 | 2.0 ± 0.1 | 16.1 ± 0.8 | 286.2 ± 18.6 |
| 11-Deoxycortisol | 12 (10) | 8.7 ± 0.9* | − 37.6 ± 1.5 | 2.1 ± 0.2 | 14.8 ± 0.9 | 229.9 ± 12.7* |
Values are means ± s.e.m. N - number of cells, number of animals is in brackets. sIPSCs were recorded in the presence of CNQX (10 μM) and D-AP5 (25 μM). mIPSCs were recorded in the presence of TTX (500 nM), CNQX (10 μM) and D-AP5 (25 μM).
P <0.05 compared to control (paired Student's t-test)
3.1.2.2 Effect of 11-deoxycortisol on mIPSCs in hippocampal CA1 pyramidal neurons
To investigate the effect of 11-deoxycortisol on action potential-independent GABAergic transmission, we further studied mIPSCs by applying 0.5 μM TTX. These mIPSCs are resistant to blocking Ca2+ entry into the terminal (Fatt and Katz, 1952). We exposed hippocampal slices to 11-deoxycortisol (100 μM) for 10 min and mIPSCs were recorded over 2 min epochs (Fig. 4C,D). Bath application of 11-deoxycortisol induced a significant decrease in the frequency of mIPSCs (81% of control, P < 0.05, Table 1). In these conditions, application of 11-deoxycortisol significantly decreased the charge transfer of mIPSCs recorded from CA1 pyramidal neurons as compared to control (82% of control, P < 0.05, Table 1). There was however only a tendency toward a decrease in the amplitude (90% of control, Table 1, P = 0.26) and decay time (92% of control, Table 1, P = 0.21) of mIPSC. Finally, the rise time of mIPSCs was unaffected (106% of control, Table 1, P = 0.69). The effect of 11-deoxycortisol on the charge transfer of mIPSCs was smaller than the one which was observed for sIPSCs (82% of control vs 67% of control, Table 1, P <0.01, unpaired Student's t-test).
3.2 In vivo
3.2.1 Seizure activity induced by 11-deoxycortisol in mice
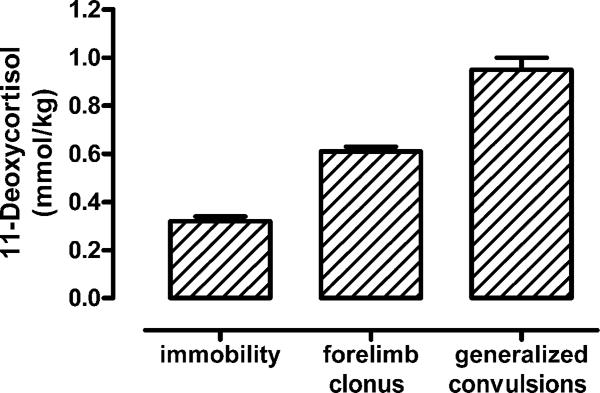
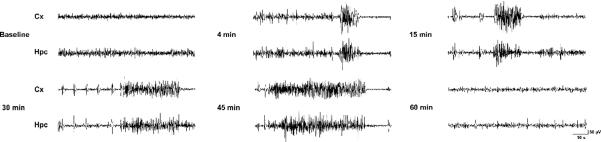
Continuous infusion of 11-deoxycortisol through the later tail vein produced an evolution of seizure activity which started with behavioral arrest (immobility) followed by forelimb clonus and generalized clonic convulsions with loss of balance. The mean threshold doses for induction of these seizure endpoints were 0.32, 0.61 and 0.95 mmol/kg, respectively (Fig 5). In contrast, continuous i.v. infusion of cortisol at the same rate and concentration did not produce any seizure activity (not illustrated) confirming that 11-deoxycortisol does have specific pro-convulsant activity and arguing that it is not just due to administration of large pharmacological doses of a steroid. In further experiments, a fixed dose of 11-deoxycortisol (1 mmol/kg) was infused i.v. to induce generalized convulsive activity. As evidenced from the EEG recordings quickly after injection of 11-deoxycortisol, generalized ictal epileptiform activity appeared, which was associated with convulsive seizures (forelimb clonus) (Fig. 6). This epileptic activity then progressed into longer duration ictal events associated with generalized convulsive seizures and loss of balance (Fig. 6). At this point the mice exhibited nearly continuous seizure activity, which could be described as status epilepticus. Convulsive and electrographic seizures continued up to 45 min after infusion, but single interictal spikes could be observed even after 60 min post-infusion (Fig. 6).
Fig. 5. Threshold for convulsions induced by continuous i.v. infusion of 11-deoxycortisol in mice.
Infusion of 11-deoxycortisol quickly induced a range of convulsive behaviors in mice. Initially, the mice displayed immobility followed by forelimb clonus (1–2 min.), which progressed into generalized clonic seizures which continued for more than 30 min. in most animals. Tonic seizures (hindlimb extension) were never observed and all animals fully recovered following spontaneous termination of convulsions.
Fig. 6. Electroencephalogram (EEG) recording of convulsions induced by i.v. bolus infusion of 11-deoxycortisol in mice.
Cortical (Cx) and hippocampal (Hpc) EEG recordings before and after 11-deoxycortisol (1 mmol/kg) infusion revealed generalized ictal activity that appeared very quickly (few minutes) and was congruent with seizure behaviors lasting for more than 30 min. Interictal spikes and sharp waves were still noticeable 60 min. after the infusion.
3.2.2 Effects of AEDs on seizures induced by 11-deoxycortisol in mice
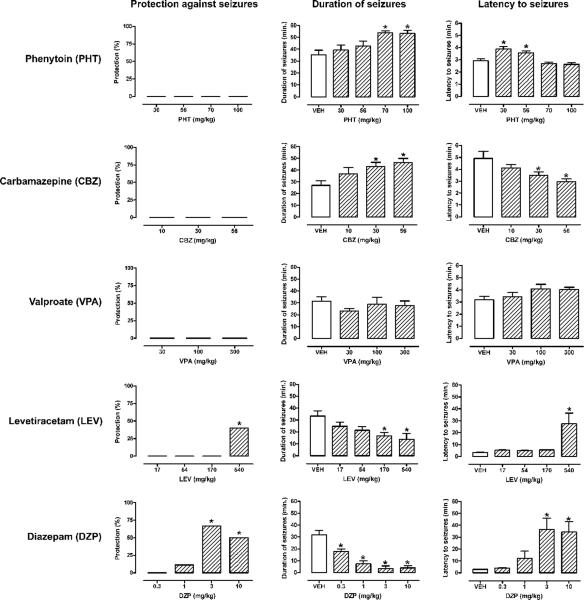
Pretreatment with several different AEDs before i.v. infusion of 11-deoxycortisol remained largely ineffective against seizures induced by this steroid (Fig. 7). Phenytoin tested in a wide dose-range (30–100 mg/kg) was completely ineffective in protection against 11-deoxycortisol seizures (Fig. 7; first row). Lower doses of phenytoin (30 and 56 mg/kg) only slightly increased the latency to the seizure onset, while higher doses (70 and 100 mg/kg) prolonged the duration of 11-deoxycortisol seizures (Fig. 7; first row). Carbamazepine (17–56 mg/kg) was not only completely ineffective in protection against 11-deoxycortisol seizures, but significantly accelerated the onset and prolonged the duration of seizures (Fig. 7; second row). Valproate (30–300 mg/kg) remained without any significant effect on 11-deoxycortisol seizures (Fig. 7; third row). Levetiracetam afforded a 40% protection against seizures and significantly delayed their onset only at the highest dose tested (540 mg/kg) (Fig. 7; fourth row). Levetiracetam also induced a dose-dependent reduction of the duration of seizures, which reached statistical significance only at the two highest doses (170 and 540 mg/kg) (Fig. 7; fourth row). Diazepam appeared to be the most effective AEDs against 11-deoxycortisol seizures (Fig. 7; fifth row). Even at very high doses (3 and 10 mg/kg) complete protection against seizures could not be achieved, but delayed seizure onset and shorter duration of seizures was clearly observed (Fig. 7; last row).
Fig. 7. Effects of antiepileptic drugs (AEDs) on generalized seizures, their latency and duration after bolus i.v. infusion (1 mmol/kg) of 11-deoxycortisol.
The following AEDs were used: phenytoin (PHT, 10–100 mg/kg, ip, −120 min.), levetiracetam (LEV, 17–540 mg/kg, ip, −60 min.), valproate (VPA, 30–300 mg/kg, ip, −15 min.) and diazepam (DZP, 0.3–10 mg/kg, ip, −30 min.). *p < 0.05 vs. vehicle-treated group.
3.3 Histology and immunohistochemistry
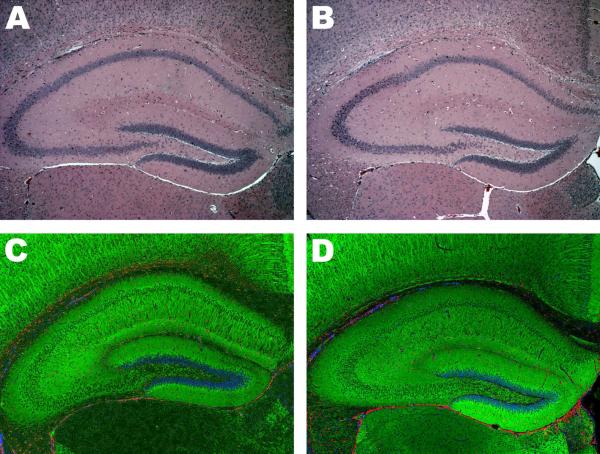
Histological assessment performed 48 hrs after status epilepticus induced by 11-deoxycortisol failed to reveal any significant change in morphology or cellular organization of hippocampus (Fig. 8). Hematoxylin-Eosin staining yielded very comparable results between vehicle- and 11-deoxycortisol-infused mice (Fig. 8A,B). Immunofluorescence labeling directed towards neuronal (MAP2), astrocytic (GFAP) and nuclear (DAPI) markers also produced nearly identical results for both control and 11-deoxycortisol groups (Fig. 8C,D). FJC staining also failed to detect degenerating neurons in the hippocampus 48 hrs following status epileptics induced by 11-deoxycortisol (not illustrated). In contrast, very strong FJC signal was detected in CA3 and CA1 hippocampal regions of mice (not illustrated) that sustained pilocarpine-induced status epilepticus which is consistent with available literature (Poirier et al., 2000).
Fig. 8. Hippocampal histology assessed 48 h after induction of seizures by i.v. bolus infusion (1 mmol/kg) of 11-deoxycortisol in mice.
Sections show dorsal hippocampus of control animals (panels A,C) of 11-deoxicortisone infused animals (panels B,D). Hematoxylin-Eosin staining did not reveal any noticeable changes in the overall morphology of hippocampus (panels A,B). No observable effect of 11-deoxycortisol seizures was detected by immunofluorescence assay (panels C,D); green color marks the neuronal component (MAP2-positivie), red color depicts astrocytic component (GFAP-positive) and nuclei are labeled in blue (DAPI-positive).
4. Discussion
In the present study we have demonstrated that 11-deoxycortisol, the immediate precursor of cortisol, inhibits GABAergic neurotransmission and exerts specific effects on the kinetics of GABAA mediated currents. This effect of 11-deoxycortisol was correlated with paroxysmal epileptiform activity observed in vitro and in vivo. Furthermore, seizures induced by administration of 11-deoxycortisol to mice resembled status epilepticus and were highly refractory to a range of current AEDs, but did not lead to an overt neuronal degeneration. Our data may suggest potential contribution of 11-deoxycortisol to the pathophysiology of seizures in patients with epilepsy.
We investigated the mechanism of action of 11-deoxycortisol on primary neuronal cultures. Using HPNs we observed that 11-deoxycortisol increased epileptiform action potential bursts and shared with 4-AP the capability to induce strong paroxysmal excitation. Recording of mIPSCs from CGCs reveal that the potential mechanism for the effect of 11-deoxycortisol is an acceleration of the decay of the inhibitory synaptic currents resulting in decreased charge transfer at the GABAergic synapse and increased network synchronization. Our experiments performed in α1 subunit (−/−) mice clearly demonstrated that the reduced decay time of GABAergic mIPSCs induced by 11-deoxycortisol was not due to a preferential effect on slower decaying synaptic GABAA receptors that contains α subunits distinct from α1. Interestingly, the in vitro effects of 11-deoxycortisol on GABAergic neurotransmission were not reproduced by cortisol, which is structurally very similar (Fig. 1). These effects were also not shared by the steroid pregnenolone sulfate, which had been shown to decrease inhibitory synaptic activity (Shen et al., 2000). Although both pregnenolone sulfate and 11-deoxycortisol decrease synaptic charge, in contrast to 11-deoxycortisol, pregnenolone sulfate was more effective in decreasing amplitude then decay of mIPSCs. It remains to be seen what distinct GABAA receptor conformational state is preferentially affected by each of these compounds and what are the consequences of the distinct mode of action on the network synchronization and epileptiform activity. Our data clearly show that 11-deoxycortisol decreases the GABAA inhibitory tone not only in neuronal cultures but also in acute hippocampal slices, since it similarly affected sIPSCs in CA1 hippocampal neurons. This inhibitory effect of 11-deoxycortisol persisted in the presence of TTX, but was significantly less robust. Thus, 11-deoxycortisol actions may have a presynaptic component, as also suggested by the significant reduction of mIPSCs frequency in hippocampal slices. Alternatively, endogenous modulation of an active site, or a difficult access to this site in the slice preparation may reduce the effectiveness of 11-deoxycortisol on synaptic transmission in hippocampal slices.
11-Deoxycortisol infused i.v. to mice produced robust seizure activity. Shortly after infusion, the seizures quickly progressed from an initial behavioral arrest to forelimb clonus and finally long-lasting convulsive status epilepticus. Importantly, this evolution of convulsive behavior in mice had very clear EEG correlates with periods of bursting ictal activity ending with a prolonged period of interictal spikes. Our observations were consistent with those initially reported by Heuser and Eidelberg (1961), who used high doses of 11-deoxycortisol succinate to induce convulsions in rats and cats. We have also demonstrated that cortisol infused at the same rate and concentration as 11-deoxycortisol is not capable of inducing seizures in mice. This, together with the fact that 11-deoxycortisol seizures appear very quickly after infusion, strongly suggests that metabolic conversion of cortisol is not responsible for seizure induction. Furthermore, cortisol failed to reproduce the effects of 11-deoxycortisol on GABAergic neurotransmission in vitro. However, it remains to be determined whether the free brain concentration achieved after i.v. infusion of 11-deoxycortisol correlates with the concentration required for inhibition of GABAergic neurotransmission, which was observed in vitro.
Interestingly, seizures induced by 11-deoxycortisol succinate were refractory to AEDs available at the time (Heuser and Eidelberg, 1961). In the present study we have tested a range of clinically used AEDs with diverse mechanisms of action. To our surprise, even very high doses of phenytoin, carbamazepine and valproate were completely devoid of any protective activity against seizures induced by 11-deoxycortisol. Furthermore, sodium channel blockers such as phenytoin and carbamazepine (Rogawski and Löscher, 2004) actually exacerbated these seizures by either accelerating their onset or prolonging duration. Levetiracetam, a synaptic vesicle 2A protein (SV2A) ligand (Lynch et al., 2004) was able to protect up to 40% of mice, but only at the highest dose tested. Even diazepam, a benzodiazepine compound which positively modulates GABAA receptors and is characterized by powerful and broad-spectrum anticonvulsant activity (Rogawski and Löscher, 2004) was not fully efficacious against 11-deoxycortisol seizures. Why are convulsions induced by 11-deoxycortisol so refractory to AEDs with diverse mechanisms of action? It is tempting to speculate that the unique capability of 11-deoxycortisol to accelerate the decay time of GABAA currents, which results in a profound failure of inhibitory neurotransmission and renders the animals unable to respond to AEDs. However, other than GABAA inhibitory effects of 11-deoxycortisol may have contributed to the observed seizure refractoriness. In fact, diazepam which was only partially efficacious against these seizures had been demonstrated to increase the decay time GABAA-mediated currents (Segal and Barker 1984). Also, levetiracetam had been reported to reverse the inhibitory effects of negative allosteric modulators of GABAA receptors (Rigo et al., 2002). It remains to be determined whether diazepam or levetiracetam effectively modulate the inhibitory effects of 11-deoxycortisol on GABAA currents.
The ability of 11-deoxycortisol to generate seizure activity makes it one of the few endogenous pro-convulsant substances (Schwarcz et al., 1983; Jöels, 1997; Kokate et al., 1999). Since relatively high pharmacological doses of exogenously administered 11-deoxycortisol are required for seizure induction, it is not clear whether elevated concentration of the endogenous 11-deoxycortisol would be sufficient to elicit seizures. In fact, high concentrations of 11-deoxycortisol due to 11β-hydroxylase (CYP11B1) deficiency are not generally associated with seizures or epilepsy (New, 1998). Also, pharmacological inhibition of the enzyme by metyrapone, which is used for diagnosis of hypothalamic-pituitary-adrenal axis disorders, does not induce seizures in patients subjected to this procedure (Fiad et al., 1994) However, it is not clear what other metabolic pathways activated or inhibited in these conditions may compensate for the pro-convulsant action of 11-deoxycortisol. It may be that under conditions associated with lowered seizure threshold, such as epilepsy, even small fluctuations of 11-deoxycortisol concentrations in the brain may actually tip off the fragile balance between inhibition and excitation and induce a seizure. To our knowledge, there are no available reports concerning fluctuations of 11-deoxycortisol levels in patients with epilepsy. Limited data exist with respect to physiological and pathological concentrations of 11-deoxycortisol in the brain, however this precursor of cortisol is not a substrate for efflux transporter P-glycoprotein and may readily cross blood-brain barrier (Karssen et al., 2002). In that context, it is widely appreciated that stress may be one of the major factors triggering seizures in patients with epilepsy (Frucht et al., 2000; Spector et al., 2000). The blockade of neurosteroid synthesis under stressful conditions has been demonstrated to lower the seizure threshold of mice below baseline values (Reddy and Rogawski, 2002). Furthermore, chronic stress associated with elevated levels of adrenal hormones may also lead to higher seizure propensity (Kumar et al., 2007; Reddy, 2006). Thus it may be worth to investigate further what may be the contribution of endogenous 11-deoxycortisol to seizures arising in patients with epilepsy.
Our present data clearly demonstrate that status epilepticus induced by 11-deoxycortisol is not associated with any noticeable neuropathological changes in the brain. The overall brain morphology remained intact and more sensitive phenotypic immunofluorescence staining directed towards neuronal and astrocytic populations failed to detect any significant changes. Furthermore, we were not able to detect a significant number of degenerating neurons by FJC staining. These observations are in sharp contrast to the neurodegenerative changes and profound astrocytosis that typically follow status epilepticus induced by pilocarpine (Turski et al 1984; Tang and Loke, 2010) or kainic acid (Sperk et al., 1983). There could be several possible explanations why prolonged seizures induced by 11-deoxycortisol are devoid of a clear neuropathological correlate. First, 11-deoxycortisol seizures are less severe than those induced by pilocarpine or kainate and generally resolve within 60 min. In contrast, the duration of seizures in animals subjected to pilocarpine- or kainate-induced status epilepticus is much longer and associated with high lethality, while all mice survive 11-deoxycortisol-induced seizures. Second, pilocarpine and kainate may exert some direct or indirect neurotoxic and inflammatory effects in addition to their convulsant properties. Finally, it is possible, that 11-deoxycortisol may have neuroprotective activity, but to our knowledge this has not been reported so far. However, estrogens may be a good example of steroids with pro-convulsant activity that could be associated with paradoxical neuroprotective effect (Velísková, 2006). It remains to be determined whether 11-deoxycortisol shares such properties and what neuroprotective pathways may be activated by this steroid.
In the present study we have confirmed that 11-deoxycortisol is capable of inducing long-lasting status epilepticus in rodents. We have also explored possible mechanisms of action of this steroid and demonstrated for the first time that it impedes GABAergic neurotransmission and has a very unique action on the kinetics of GABA transmission. Furthermore, we have also found that seizures induced by 11-deoxycortisol are highly refractory to several AEDs with diverse mechanisms of action. Thus, we would like to propose that seizures induced by 11-deoxycortisol may be an advantageous model of drug-resistant epilepsy that could be used for the discovery of future AEDs with novel mechanisms of action. Finally, since 11-deoxycortisol is an endogenous substance our data could also suggest that this steroid might actually contribute to an increased seizure propensity in some clinical situations.
Acknowledgements
This work was in part supported by National Institutes of Health Grants (MH64797) to Dr. Stefano Vicini. The present study was also supported by the NEUROCOM grant n° 716747 from the Walloon Region (Belgium), UCB Pharma and the University of Liege (Belgium), as well as by another grant from the Walloon Region – DGO6 (Convention 6086). We thank Dr. Gregg Homanics, University of Pittsburgh, for providing α1 subunit (−/−) mice.
Abbreviations
- AEDs
antiepileptic drugs
- aCSF
artificial cerebrospinal fluid
- 4-AP
4-aminopyridine
- D-AP5
d(-)-2-amino-5-phosphonopentanoic acid
- 11-deoxycortisol
(pregn-4-ene-17, 21-diol-3,20-dione)
- NMDA
N-methyl-D-aspartic acid
- CPP
3-(2-Carboxypiperazin-4-yl)propyl-1-phosphonic acid
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- GABA
γ-aminobutyric acid
- SR95531 or gabazine
2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl) pyridazinium bromide
- IPSCs
inhibitory postsynaptic currents
- CGCs
cerebellar granule cells
- HPNs
hippocampal pyramidal neurons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avoli M, Perreault P. A GABAergic depolarizing potential in the hippocampus disclosed by the convulsant 4-aminopyridine. Brain Res. 1987;400:191–195. doi: 10.1016/0006-8993(87)90671-8. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid (A) receptors in hippocampal neurons. Mol. Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler GB, Jr, Barnes KM, Sauer MA, Lynn D. 11-Deoxycortisol: a glucocorticoid antagonist in vivo. Endocrinology. 1979;104:1839–1844. doi: 10.1210/endo-104-6-1839. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Fiad TM, Kirby JM, Cunningham SK, McKenna TJ. The overnight single-dose metyrapone test is a simple and reliable index of the hypothalamic-pituitary-adrenal axis. Clin. Endocrinol. 1994;40:603–609. doi: 10.1111/j.1365-2265.1994.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Fiszman ML, Barberis A, Lu C, Fu Z, Erdélyi F, Szabó G, Vicini S. NMDA receptors increase the size of GABAergic terminals and enhance GABA release. J. Neurosci. 2005;25:2024–2031. doi: 10.1523/JNEUROSCI.4980-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frucht MM, Quigg M, Schwaner C, Fountain NB. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000;41:1534–1539. doi: 10.1111/j.1499-1654.2000.001534.x. [DOI] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol. Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Haage D, Karlsson U, Johansson S. Heterogeneous presynaptic Ca2+ channel types triggering GABA release onto medial preoptic neurons from rat. J. Physiol. 1998;507:77–79. doi: 10.1111/j.1469-7793.1998.077bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J. Pharmacol. Exp. Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- Heuser G, Eidelberg E. Steroid-induced convulsions in experimental animals. Endocrinology. 1961;69:915–924. doi: 10.1210/endo-69-5-915. [DOI] [PubMed] [Google Scholar]
- Jöels M. Steroid hormones and excitability in the mammalian brain. Front. Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Gillard M, Leclercq K, Hanon E, Lorent G, Dassesse D, Matagne A, Klitgaard H. Proepileptic phenotype of SV2A-deficient mice is associated with reduced anticonvulsant efficacy of levetiracetam. Epilepsia. 2009;50:1729–1740. doi: 10.1111/j.1528-1167.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005a;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Shippenberg TS, Witkin JM, Rocha BA. Genetic deletion of the norepinephrine transporter decreases vulnerability to seizures. Neurosci. Lett. 2005b;382:51–55. doi: 10.1016/j.neulet.2005.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Marini H, Ortinski PI, Vicini S, Rogawski MA. The pheromone androstenol (5α-androst-16-en-3α-ol) is a neurosteroid positive modulator of GABAA receptor. J. Pharmacol. Exp. Ther. 2006;317:694–703. doi: 10.1124/jpet.105.098319. [DOI] [PubMed] [Google Scholar]
- Karssen AM, Meijer OC, van der Sandt IC, De Boer AG, De Lange EC, De Kloet ER. The role of the efflux transporter P-glycoprotein in brain penetration of prednisolone. J Endocrinol. 2002;175:251–260. doi: 10.1677/joe.0.1750251. [DOI] [PubMed] [Google Scholar]
- Kumar G, Couper A, O'Brien TJ, Salzberg MR, Jones NC, Rees SM, Morris MJ. The acceleration of amygdala kindling epileptogenesis by chronic low-dose corticosterone involves both mineralocorticoid and glucocorticoid receptors. Psychoneuroendocrinology. 2007;32:834–842. doi: 10.1016/j.psyneuen.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neurosteroid pregnenolone sulfate in mice. Brain Res. 1999;831:119–124. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Losi G, Prybylowski K, Fu Z, Luo JH, Vicini S. Silent synapses in developing cerebellar granule neurons. J. Neurophysiol. 2002;87:1263–1270. doi: 10.1152/jn.00633.2001. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Schwartz RD. Pregnenolone-sulfate: an endogenous antagonist of the gamma-aminobutyric acid receptor complex in brain? Brain Res. 1987;404:355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci. Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- New MI. Diagnosis and management of congenital adrenal hyperplasia. Annu. Rev. Med. 1998;49:311–328. doi: 10.1146/annurev.med.49.1.311. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Lu C, Takagaki K, Fu Z, Vicini S. Expression of distinct α subunits of GABAA regulates inhibitory synaptic strength. J. Neurophysiol. 2004;92:1718–1727. doi: 10.1152/jn.00243.2004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Second Edition Academic Press; 2001. [Google Scholar]
- Poirier JL, Capek R, De Koninck Y. Differential progression of Dark Neuron and Fluoro-Jade labelling in the rat hippocampus following pilocarpine-induced status epilepticus. Neuroscience. 2000;97:59–68. doi: 10.1016/s0306-4522(00)00026-9. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;138:911–920. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J. Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, Leprince P, Moonen G, Selak I, Matagne A, Klitgaard H. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA-and glycine-gated currents. Br. J. Pharmacol. 2002;136:659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABA(A) receptor desensitization. J. Neurosci. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Silver RA, Traynelis SF, Cull-Candy SG. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992;355:163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr., Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Segal M, Barker JL. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J. Neurophysiol. 1984;52:469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Spector S, Cull C, Goldstein LH. Seizure precipitants and perceived self-control of seizures in adults with poorly-controlled epilepsy. Epilepsy Res. 2000;38:207–216. doi: 10.1016/s0920-1211(99)00093-5. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O. Kainic acid induced seizures: Neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Tang FR, Loke WK. Cyto-, axo- and dendro-architectonic changes of neurons in the limbic system in the mouse pilocarpine model of temporal lobe epilepsy. Epilepsy Res. 2010;89:43–51. doi: 10.1016/j.eplepsyres.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321:237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Williamson J, Mtchedlishvili Z, Kapur J. Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology. 2004;46:856–864. doi: 10.1016/j.neuropharm.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velísková J. The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience. 2006;138:837–844. doi: 10.1016/j.neuroscience.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow L, Homanics GE. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J. Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]