Abstract
Dynamic contrast enhanced MRI is often used to assess response to therapy in small animal models of cancer. Rigorous quantification of DCE-MRI data using common pharmacokinetic models requires dynamic determination of the concentration of contrast in tumor tissue and in blood. Measurement of the blood concentration, or vascular input function, requires high temporal resolution and is prone to distortion due to flow and partial volume artifacts when measured in local blood vessels. We have developed a strategy for robust measurements of the VIF in mice that uses a constrained reconstruction algorithm to enable sampling from the left ventricle of the heart. The feasibility of the algorithm and its resistance to cardiac motion are demonstrated in vivo and through numerical simulations. VIF sampling is interleaved with slices dedicated to tumor coverage to yield a fast VIF sampling period (81ms) that is decoupled from the temporal resolution of tumor data (3.9s). The algorithm provides results that agree with fully encoded measurements in the slowly varying component of the VIF to within a 4% rms signal difference. Analysis of a parametric representation of VIFs measured in a population of mice showed a significant reduction in variations observed within subjects (from 5% to 585 over four parameters; p < 0.05) and a reduction in variations between subjects (from 19% to 62%) when using this technique. Preliminary dynamic measurements in an orthotopic xenograft model of anaplastic thyroid cancer reveal a decrease in the variation of pharmacokinetic parameters between mice by a factor of two.
Keywords: DCE-MRI, constrained reconstruction, small animal MRI, VIF reproducibility, VIF measurement
Introduction
Non-invasive quantification of microvascular function using dynamic, contrast-enhanced (DCE-)MRI allows insight into the tumor microenvironment and provides a powerful tool for monitoring response to novel therapeutic interventions. The dynamic measurement is typically performed by rapid acquisition of T1-weighted MR images before, during, and after injection of an exogeneous T1-reducing contrast agent. Changes in signal intensity are used to derive estimates of the concentration of the contrast agent in blood and tissue as a function of time. Physiological models are used to relate the exchange of contrast between blood and tissue to arrive at descriptors of the local microvasculature in the form of pharmacokinetic parameters. Successful performance of these measurements in small animal models is challenging due to demanding requirements in spatial and temporal sampling (1,2). Rapid sampling is required to resolve the fast dynamics of contrast enhancement and exchange, but the sampling rate must be balanced against the need for good spatial resolution and a sufficient number of slices to resolve the heterogeneity of diseased tissue over the whole tumor volume.
While powerful insights may be gained from pharmacokinetic analysis of DCE-MRI data, the pharmacokinetic parameters are subject to large uncertainties from many sources. One particular source of uncertainty is that of the vascular input function (VIF), the measurement of contrast agent concentration in blood plasma (3). Although the most reproducible measurements of pharmacokinetic parameters are obtained with measurement of the VIF on an individual basis (4), this is challenging, particularly in small animal MRI (5). Severe distortion and scaling of the VIF can result from partial volume effects and inflow enhancement because of the small size of blood vessels and the flow of blood (6). Additionally, the low blood volumes and rapid heart rates of mice cause the kinetics of the contrast agent in blood to be particularly fast (7). These effects decrease the reproducibility of VIF measurements and thus the reproducibility of the resulting DCE-MRI parameter estimates.
In small animals, many attempts have been made to avoid the artifacts that arise from sampling the VIF in local blood vessels and improve the precision of DCE-MRI. Reference region techniques permit implicit consideration of the blood dynamics through measurements in normal tissues (5), although these techniques have demonstrated a modest penalty in the reproducibility of the pharmacokinetic parameters compared to a successful blood vessel measurement (8). Other approaches have resulted in improved VIF measurement by imaging relatively large structures, such as the tail vein or heart, to avoid partial volume effects (9–13), but these approaches have been unable to simultaneously achieve high temporal and spatial coverage has been limited to only 1–3 slices.
Beyond the need for artifact avoidance, VIF sampling requires high temporal resolution for accurate measurement of pharmacokinetic parameters. Henderson found that sampling the VIF at least once per second was required in humans (14). Although no analogous calculation has been performed for the VIFs expected in small animals, the rapid heart rates (9) and the small blood volumes of mice (5) can introduce very rapid changes in the VIF, strongly suggesting that faster temporal sampling requirements may be needed to capture the initial passage the contrast agent bolus in mice.
We have developed an approach to VIF sampling that is insensitive to partial volume and flow artifacts, while also providing very high temporal resolution. Sampling is performed in a slice prescribed for this purpose to traverse the heart and exploit the relatively large size of the left ventricle (~5 mm2 in cross section) to avoid partial volume artifacts. This slice is interleaved with other slices that are prescribed in the usual way to provide necessary coverage of tumor tissue. Dedicating a single slice to VIF sampling permits optimization of the acquisition of that slice for the measurement of vascular dynamics, with only minor costs to the measurements of the slower tissue dynamics. Here, flow enhancement suppression is applied to the cardiac slice using an RF pulse which performs spatial presaturation of spins before their arrival in the ventricle to avoid inflow enhancement (15). The use of a novel combination of signal encoding and reconstruction algorithms achieves cardiac-anatomy constrained, temporally unrestricted sampling (CACTUS) of the VIF with high temporal resolution and relative insensitivity to blood flow and cardiac motion. This algorithm uses prior information from fully-encoded images and segmentation to directly measure the mean signal in regions of interest without reconstruction of a full image, which allows higher temporal resolution sampling than would be possible otherwise.
In addition to demonstrating high temporal resolution, we show that VIF measurement with this strategy is more reproducible within subjects and has lower variation between subjects than conventional sampling in a local vessel. This improvement in the precision of VIF measurement will lead to more reproducible measurements of pharmacokinetic parameters. Preliminary in vivo measurements in a pilot tumor study reveal a reduction in the variations of pharmacokinetic parameters that is consistent with the results of the VIF measurement.
Theory
Ultimately, measurement of the VIF depends on changes in the steady-state signal intensity of blood due to the presence of contrast agent, and time spent encoding the details of the image represents inefficiency. The use of a constrained reconstruction technique reduces the data required for VIF sampling and improves the temporal resolution of the measurement. Very high acceleration factors are achieved by representing contrast enhancement as a function of anatomic structures, rather than of the individual voxels. In this paradigm, the minimum number of data points required for image reconstruction decreases from the size of the entire imaging matrix to only a number equal to the number of identified regions. A similar structure-based assumption has been used to reduce the data required for spectroscopic imaging (16) although our approach differs in the inclusion of a reference image in the reconstruction and in the spatial encoding strategy. This approach achieves similar temporal resolution to a method for VIF sampling in humans using fixed-angle projections, but also allows separation of vascular signal from tissue signal (17).
This structure-oriented reconstruction is developed in the context of a radial data acquisition scheme. Through the projection-slice theorem, a spoke in k-space is related to the full image by
| (1) |
where Sk is the spoke in k-space at angle θ at time t, F is the one-dimensional Fourier transform along the spatial dimension, Pt is the projection at angle θ, the position along the projection is given by r, 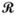 is the Radon transform, and S(x, y, t) is the transverse magnetization, modified by the system gain and coil sensitivity. Solving this system of equations for a full set of projections reconstructs the time-dependent image I(x,y,t=t0) α S(x, y, t=t0); this approach is used in conventional algebraic projection-reconstruction techniques (18).
is the Radon transform, and S(x, y, t) is the transverse magnetization, modified by the system gain and coil sensitivity. Solving this system of equations for a full set of projections reconstructs the time-dependent image I(x,y,t=t0) α S(x, y, t=t0); this approach is used in conventional algebraic projection-reconstruction techniques (18).
To simplify the system of equations, reduce the number of unknowns and decrease the number of projections required, we partition the image into N ROIs, each of which corresponds to a distinct structure. We define the indicator function for the ith ROI as:
| (2) |
We assume that the ROIs enhance smoothly, in the sense that, for a voxel at position x,y located within the ith partition:
| (3) |
| (4) |
where Ii(x,y,t=0) is the reference image and wi(x,y,t) is a smooth contrast modulation function for the partition that relates the dynamic images to the reference. Because spatial details within individual ROI are unimportant to measurement of the average changes in the signal level in blood, all but the 0th order term of the Taylor expansion of wi(x,y,t) in space can be ignored, allowing the use of a single coefficient per region to describe average signal change in a region:
| (5) |
This physically corresponds to assuming homogeneous enhancement within the ROI. From the above equations, it follows that
| (6) |
where Ii(t) is the mean intensity in the ith ROI at time t.
Combining the above equations leads to
| (7) |
The linearity of the Radon transform allows the spatially constant, temporally varying value of wi to be brought outside of the transformation. This produces a set of linear equations with the number of unknowns (wi) equal to the number of partitions(χi). Because there is one equation for each point along the readout direction, the set of equations will be overdetermined. The system of equations may be solved in the least-squares sense (19) after each measurement of P(r,θ,t) to produce coefficients describing the dynamic signal changes in the ROIs. A schematic display of the algorithm is given in Figure 1.
Figure 1.
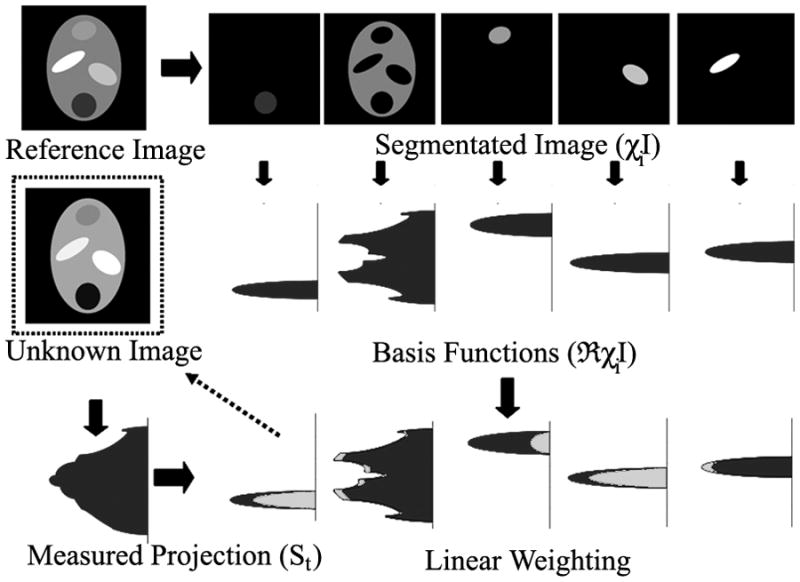
Diagram of CACTUS algorithm. A reference image is segmented into several distinct structures, which are transformed into basis vectors Radon transform. Single projections of dynamically changing data is fit to decomposed into basis vectors from the reference image, yielding a set of scaling coefficients that describe changes in the average signal level in each ROI.
Methods
All experiments, procedures and animal care were approved by our Institutional Animal Care and Use Committee, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. All data was acquired on a Biospec 4.7T small animal MR scanner (Bruker Biospin MRI, Billerica, MA) with 60-mm inner diameter imaging gradients and a linear volume coil (35-mm inner diameter). . All simulations and reconstructions were implemented in Matlab (The MathWorks, Natick, MA).
Numerical simulations
The feasibility of the CACTUS reconstruction was tested in a dynamic numerical phantom, shown in Figure 2a, consisting of several regions of varying size and shape that represent thoracic features. Heterogeneous dynamic enhancement in these areas was simulated according to the two-parameter Toft’s model in response to a predetermined biexponential VIF:
| (8) |
Figure 2.
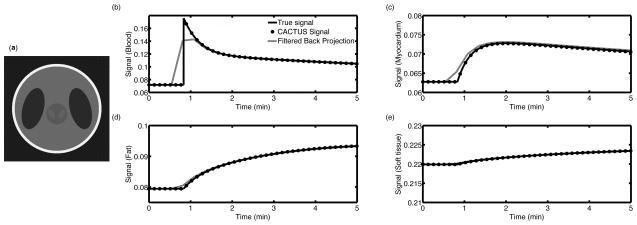
Numeric simulation of enhancement in four regions. Within the numerical phantom structure, (a), the vascular region enhances more rapidly than the update rate for a fully encoded image, which distorts both the magnitude and position of the initial peak (b). More slowly enhancing tissues (c–e) are well-described at normal image update rates: the signal of the FBP curves agree to within 1%, except during the initial rise of the myocardial signal.
Here, CT(t) represents the concentration of contrast agent in tissue, with the biexponential VIF described via CP(t), the concentration of agent present in blood plasma. Ktrans is the forward transfer rate at which contrast extravasates from blood to tissue, and ve represents the extravascular, extracellular volume fraction. Each region of the numerical phantom was assigned distinct enhancing characteristics; one region represented the VIF, while the other regions approximated slower enhancing tissues. Kinetic parameter values were taken from Padhani, et al.(20), and adjusted to produce greater differences between tissues. To provide heterogenous enhancement, each voxel of the phantom was randomly assigned independent values for T1, KTrans, and ve, chosen from a Gaussian distribution with a standard deviation of 25% of the mean of that parameter for that tissue type. Mean tissue characteristics for this numerical phantom are given in Table 1. A radial acquisition was simulated with TR = 40 ms, flip angle = 30°, number of spokes = 384, readout points = 729, reconstruction matrix size = 512 × 512 over a time of 250 s. Both filtered backprojection (FBP), which is used to reconstruct fully encoded radial images, and the constrained reconstruction algorithm were used to measure the mean signal within each ROI. CACTUS analysis of individual projections was performed using a fully encoded pre-contrast image as its reference to provide measurements of the change in the mean signal in each ROI. The signal change in the vascular ROI was used as the measured VIF. The mean signals from both FBP and CACTUS were compared to the true value of the mean signal in each ROI.
Table 1.
Pharmacokinetic parameter values used in the simulation
| T1 | KTrans | ve | |
|---|---|---|---|
| Blood | 1800 ms | - | - |
| Myocardium | 2100 ms | 0.25 min−1 | 0.2 |
| Muscle | 1600 ms | 0.125 min−1 | 0.5 |
| Fat | 400 ms | 0.05 min−1 | 0.4 |
Motion sensitivity simulations
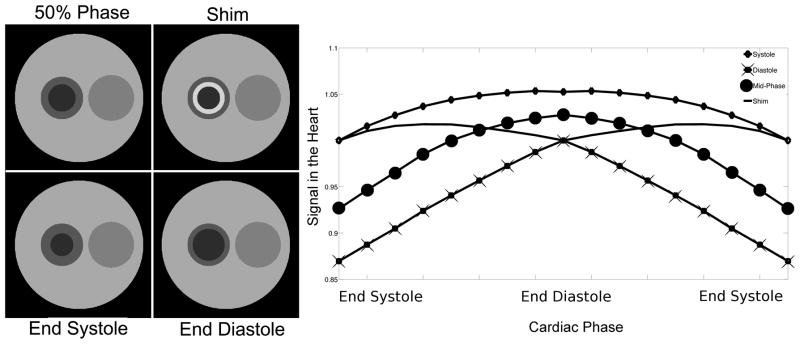
An additional simulation was performed to investigate the sensitivity of the algorithm to the cardiac motion-related mismatches between the segmentation and the anatomy. A dynamic digital phantom was created, and the structures representing the ventricle and myocardium were constricted to simulate cardiac motion at 17 cardiac phases. Four segmentation schemes were considered:
Segmentation representing the anatomy at the end of systole
Segmentation representing the anatomy at the end of diastole
Segmentation representing the anatomy at the 50% point of the cardiac cycle
Segmentation with a ring-shaped transition ROI between the myocardium and ventricle encompassing the spatial difference between end systole and end diastole
The four segmentation schemes are shown in Figure 3. Synthetic data was generated by calculating projections during each of the cardiac phases at 1° increments. Single projection CACTUS analysis was performed of individual projection with each of the four segmentation schemes to generate signal curves across the cardiac cycle for each structure in the phantom at each orientation. No actual changes in the mean signal of each structure were introduced; signal fluctuations in this measurement are only due to motion. The mean error and root-mean-square (RMS) error of the signal in the ventricle were calculated to compare the effects of motion on the signal measured with each of the four segmentation schemes.
Figure 3.
Impact of four approaches to segmentation on variations due to cardiac motion. Left: The four segmentation schemes are shown. Right: Signal fluctuations due to cardiac motion for each segmentation scheme are shown. The shim segmentation produced the lowest overall variations across the cardiac cycle, while the diastole segmentation produced the largest.
Software implementation
Postprocessing software was developed to use CACTUS for in vivo VIF measurement, which requires that I(x,y,t) and χi(x,y) be identified prior to pharmacokinetic analysis. Projections that are measured during the dynamic acquisition directly provide I(x,y,t) with reference anatomic images obtained from filtered backprojection (FBP) of the data acquired prior to injection of contrast. To generate χi, the operator is prompted to manually segment structures found in the cardiac slice reconstructed with FBP: left ventricle, myocardium, right ventricle, lungs, great vessels, liver/diaphragm, portal vein, chest cavity, soft tissue, and the fat around the skin. A two-voxel ring is automatically generated at the boundary of the ventricle and the myocardium to approximate the shim ROI used in the previous simulation. Intersections are removed, and the resulting segmentations are used in the constrained reconstruction algorithm. Consecutively acquired pairs of projections are decomposed by least-squares fit to the basis vectors generated by I(x,y,t=0) and χi to produce coefficients that describe changes in the mean signal within each ROI with high temporal resolution. The use of projection pairs leads to the temporal resolution of CACTUS being twice TR.
In vivo motion sensitivity
A series of measurements were made in vivo to test the accuracy of the CACTUS algorithm and assess signal stability in the presence of noise and cardiac motion. Short-axis cardiac projections were acquired at multiple excitation angles to modulate contrast between blood and tissue without administration of contrast. Mice were anesthetized using 0.5%–2% isoflurane in oxygen and placed head-first and supine on an imaging sled. Respiratory rates were monitored using a small animal physiological monitoring system (Model 1025 Monitoring & Gating System, Small Animal Intruments, Inc., Stony Brook, NY) and anesthesia levels were adjusted as necessary to maintain a rate of 20–30 breaths per minute. A warm water circulating system was used to maintain a constant body temperature.
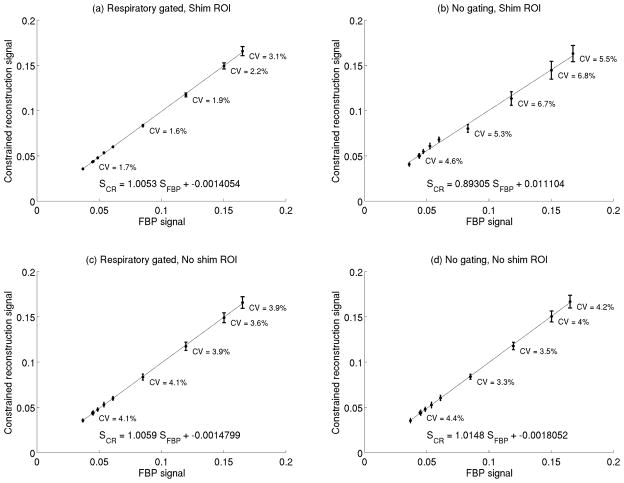
After pilot scans to confirm animal positioning, a radial cardiac acquisitions were performed using a fast spoiled gradient echo (FSPGR) sequence (TE = 1.5 ms, TR = 41 ms, flip angle = 10°, 20°, 30°, and 40°, frequency resolution = 256, frequency FOV = 4 cm, number of spokes = 384, angular increment = 120.5°, receiver bandwidth = 85 kHz, number of repetitions = 8). Constrained reconstruction using the fully encoded data set to produce a reference image was performed with and without retrospective respiratory gating (21) according to fluctuations in the signal in the liver ROI, and with and without the use of the shim ROI. For each series of CACTUS reconstructions at a constant excitation angle, the temporal coefficient of variation of the mean signal level of blood in the left ventricle was calculated. A linear regression between signal levels in blood measured from FBP images or CACTUS was performed to assess the accuracy of the constrained reconstruction algorithm.
Reproducibility of VIF measurement
The reproducibility of VIF measurements using CACTUS and traditional methods were measured and compared to assess the suitability of the constrained reconstruction algorithm for experiments involving DCE-MRI. Healthy nude mice were anesthetized as previously described. A catheter loaded with 40 μL of heparinized saline followed by 110 μL of 0.25 M Magnevist was inserted into the tail vein prior to placement on the imaging sled. Acquisition was performed using a multiple-slice FSPGR sequence (TE = 1.55 ms, TR = 41 ms, flip angle = 30°, slice thickness = 1 mm). A radially-encoded trans-cardiac slice for VIF measurement was interleaved with seven Cartesian-encoded slices of the head and neck for tumor coverage. The acquisition matrix for the axial slices through the head and neck was 256 × 96 over a 4 cm × 3.84 cm FOV, providing one sample every 3.9 s. The acquisition matrix for the radial encoded cardiac slice was 256 points over 4 cm with 384 spokes and an angular increment of 120.5° over 30 repetitions, equivalent to 120 repetitions of the Cartesian sequence. A 5 mm presaturation band was applied on both sides of the trans-cardiac slice to suppress inflow enhancement during excitation (15). 10 μL of contrast was injected at a rate of 0.5 mL/min approximately 75 seconds after the start of the acquisition using an automated MR-compatible injection system (Harvard Apparatus, Holliston, MA). This low-dose protocol reduced the amount of time required for the contrast agent to clear, in turn reducing the total amount of time the mice were under anesthesia and promoting physiological stability of the mice. After the dynamic acquisition was complete, single repetitions of the radial images were acquired at 15 minute intervals to monitor the clearance of contrast agent by observing the signal in the left ventricle. Once the contrast agent had cleared from the blood, the dynamic acquisition was repeated. This measurement was performed three times per mouse.
Comparison of the VIF from the left ventricle analyzed with CACTUS against those taken from the FBP reconstruction was performed to assess the soundness of the constrained reconstruction approach. Filtered backprojection was used as the reference because it is the best standard against which constrained reconstruction of radial projection data can be compared. Its relative robustness to motion (22) reduces the effect of artifacts in the presence of cardiac motion. VIFs were measured in Cartesian-encoded dynamic image data by averaging the signal among manually selected voxels. The same vessel, generally the internal carotid artery (ICA) when available or another artery if not, was used for measurements for all injections in a single mouse. The shape and amplitude of signal enhancement was observed for all voxels corresponding to the chosen vessel, and those with the greatest enhancement and most similar shape were included. The use of a single vessel minimizes the dispersion of signal enhancement among voxels but also limits the number of voxels available for use. . To test the agreement between the two approaches at low temporal resolution, the values of the VIF measured using CACTUS were averaged over a window corresponding to the sampling period of the FBP data. The RMS difference between the mean downsampled signal and the FBP signal was calculated for all mice and all injections. To qualitatively evaluate differences at high temporal resolution, the FBP VIF was linearly interpolated to match the resolution of the CACTUS VIF and the resulting signal curves were subtracted.
Dynamic signal measurements were converted into concentration estimates by inverting the signal equation from a spoiled gradient echo sequence (23), assuming a baseline T1 of 1800 ms for blood and a relaxivity of 4.8 mM−1s−1 for Gd-DTPA. Concentration as a function of time was fit to a biexponential model to facilitate evaluation of reproducibility:
Here, C0 is the amplitude of the slow component of the VIF, α is the relative amplitude of the fast component, and kslow and kfast are respective decay constants. The amplitude of the fast component was defined relative to the amplitude of the slow component to segregate artifacts which scale the VIF and to ease interpretation. The model was fit to the decaying portion of the model after the initial signal rise, using a Levenberg-Marquardt algorithm(24) in Matlab.
The coefficients of variation across the injections of the four biexponential parameters were calculated for each mouse. To evaluate changes in reproducibility, the mean coefficients of variation were compared using the paired Hotelling T2 test, the multivariate analogue of the paired t-test (25) to assess the within-subject reproducibility. In addition, the inter-subject coefficients of variation were calculated from the variance of the intra-animal parameter means and the means of the parameters across mice were compared with the Hotelling T2 test.
Constrained VIF reconstruction for DCE-MRI
The effects of the proposed VIF measurement strategy on pharmacokinetic parameters was measured by comparing parameters resulting from CACTUS and local vessel VIF measurements in three tumor-bearing mice. We employed an orthotopic xenograft model of anaplastic thyroid cancer as previously described (26). The cell line (Hth83) was grown in RPMI 1640 media with 10% fetal bovine serum, sodium pyruvate, L-glutamine, non-essential amino acids, penicillin and streptomycin. Hth83 cells were grown to 70% confluence in 10-cm tissue culture dishes. Cells were gently trypsinised, washed with serum-free RPMI 1640 and re-suspended at 5 × 104 cells/μL. Female athymic nude mice (4–6 wks old) were anesthetized and injected with 2.5 × 105 cells orthotopically into the right thyroid gland. Twenty days after injection, DCE-MRI imaging was performed on the mice with the same pulse sequence and protocols as were used for the VIF reproducibility measurements. Imaging was performed with a transmit/receive birdcage coil. VIFs were simultaneously measured from the heart with CACTUS and from jugular veins near the tumor and orthogonal to the fully encoded axial slices. Concentration was calculated from the signal as was done previously. Voxel-by-voxel pharmacokinetic parameter maps were calculated by fitting the extended Toft’s model (27) to the concentration time courses using a Levenberg-Marquardt optimization algorithm. The mean and standard deviation of KTrans, kep, and vb were calculated for each mouse as well as the inter-subject variance of the parameters.
Results
Numerical simulations
The numerical simulations of dynamic enhancement showed very close agreement between the true signal means and those calculated through CACTUS; the worst case error for any ROI and any time point was 0.8%. The fluctuations in T1 and kinetic parameters resulted in a spatial coefficient of variation in ‘true’ pixel intensities ranging from 14% to 25% for each structure, indicating that even in the presence of large variations in signal across each ROI, the constrained reconstruction approach can provide an accurate measurement of the signal mean. The fully encoded images, while suitable for measuring enhancement in more slowly enhancing solid tissues, did not accurately capture the peak of the simulated VIF and significantly distorted the initial rise of the signal, as shown in Figure 2(b).
Motion sensitivity simulations
Simulations reveal that the use of a static anatomic model does not severely compromise the constrained reconstruction measurement in the presence of cardiac motion, as seen in Figure 3. Both the error in the mean and the RMS error were less than 1% when using the additional ring ROI as a dynamic shim. The other segmentation schemes fared slightly worse with RMS errors of up to 8% and errors in the mean of up to 7%.
In vivo motion sensitivity
The in vivo cardiac measurements made at varying excitation angles show that motion-related signal fluctuations can be reduced when both a shim-ROI and respiratory gating are used. With both techniques in use, the line of best fit relating the FBP signal to the CACTUS signal had a slope of 1.01, an intercept of −0.001, and an r2 of 0.9951, indicating a very strong agreement between the two (Figure 4a). Additionally, the signal CV, which includes variations due to both motion and acquisition noise, was low at all flip angles(28). Omitting the corrections for motion increased the signal variation by a factor of up to 3, indicating that the use of the retrospective gating and a shim ROI does provide an improvement in resistance to motion.
Figure 4.
The relationship between signal levels measured in blood measured using constrained reconstruction signal and filtered backprojection A combination of retrospective respiratory gating and shim-segmentation approaches (a) reduce variation compared to other combinations of their use (c–e). Across a wide range of signal levels, the constrained reconstruction algorithm matches well to the signal from filtered backprojection.
Comparison to fully encoded images
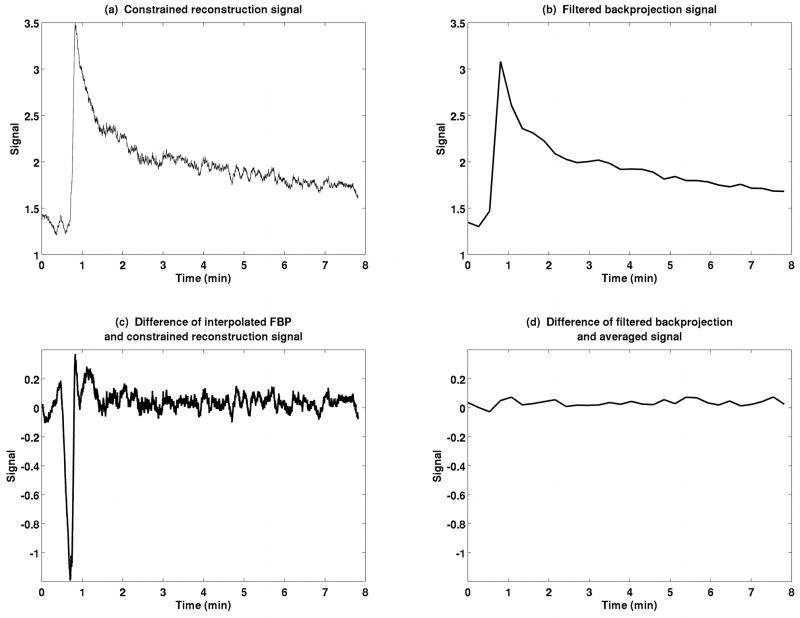
VIF measurements show close agreement between the filtered backprojection signal and the CACTUS method at low temporal resolution, as shown for a typical mouse in Figure 5. Averaging the CACTUS measurement to decrease the temporal resolution effectively recovers the original FBP signal. The RMS difference between FBP and downsampled curves was 4.0% of the mean FBP signal for this representative data (Figure 5d) and 4.0±2.1% across all measurements. When high-resolution data from constrained reconstruction is compared to the interpolated FBP signal (Figure 5c), the two curves agree with a root-mean-square error of less than 3% except at the initial rise of the curve, indicating both good agreement and the ability to capture rapid enhancement more clearly using CACTUS.
Figure 5.
Comparison of constrained reconstruction (a) with filtered backprojection (b). The difference between the CACTUS VIF and a linearly interpolated FBP VIF, at high temporal resolution, (c), emphasizes the heightened temporal resolution achieved with constrained reconstruction. The difference between a time-averaged constrained reconstruction VIF and the FBP signal, (d), illustrates the consistency of the low-frequency information of the two techniques. Note that the peak of the VIF is higher and the rise steeper when measured using constrained reconstruction than conventional sampling.
Reproducibility comparison
Comparison of the biexponential parameter values reveals greater reproducibility of the VIFs measured in the heart using CACTUS compared to those made in local vessels using traditional anatomic image encoding. Example VIFs from several animals are shown in Figure 6. Parameters for all mice are summarized in Table 2. The within-mouse reproducibility of VIF measurement was considerably improved when using CACTUS (p<0.05), with the CV of kfast and α decreasing the most, from 86% to 28% and 62% to 23% in constrained and local vessels, respectively, while the changes in both kslow and C0 were more modest. The inter-subject variation also decreased for all four parameters, although this was not statistically significant. Uncertainties in these parameters include both errors due to measurements and also true alterations in the blood dynamics due to variation in the injections and drift in the physiological state of the mouse, however, the paired nature of the experiments allows meaningful comparison of the two techniques because true fluctuations are present in both measurements.
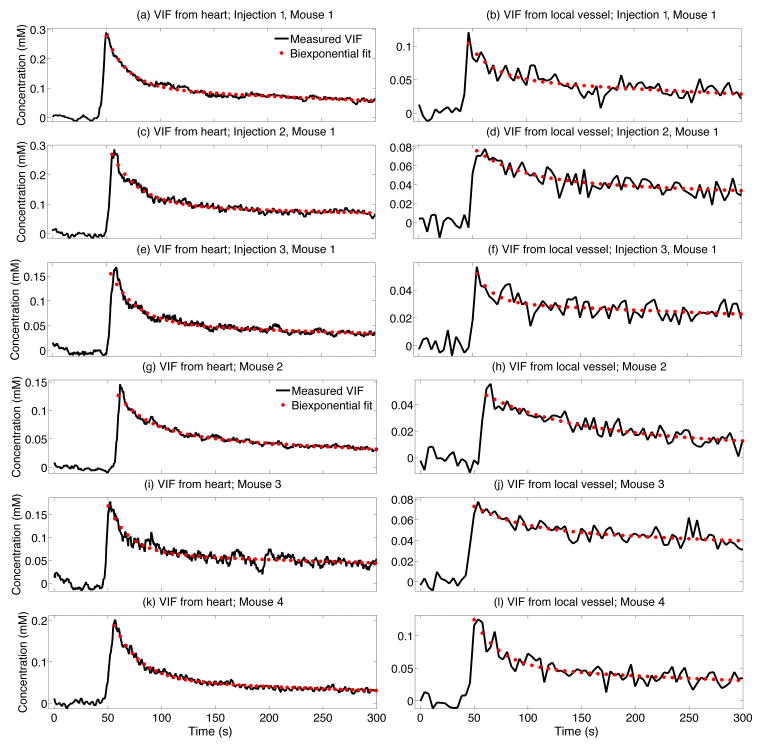
Figure 6.
Paired measurements of the VIF using the constrained reconstruction algorithm (a,c,e,g,i,k) and manually in the internal carotid artery using conventional sampling (b,d,f,h,j,l). All VIF measurements from one mouse (a–f) and representative curves from three additional mice (g–l) demonstrate the consistency of acquisitions within and between animals.
Table 2.
Summary of the measured VIF parameter values and their uncertainties
| Mean Value (Vessel) | Mean Value (Heart) | Intrasubject CV (Vessel) | Intrasubject CV (Heart) | Intersubject CV (Vessel) | Intersubject CV (Heart) | |
|---|---|---|---|---|---|---|
| kfast min−1 | 6.01 min−1 | 2.14 | 86% | 28% | 120% | 58% |
| α | 0.6042 | 1.82 | 62% | 23% | 51% | 24% |
| kslow min−1 | 0.17 min−1 | 0.09 | 75% | 65% | 97% | 38% |
| C0 | 57 μmol | 81 | 45% | 40% | 55% | 36% |
Quantitative differences in the VIFs measured with the two techniques were observed, indicated by the mean biexponential parameters differing between conventional vessel sampling and cardiac sampling (p<0.05). The mean concentration measured in the heart was larger, which is consistent with a decrease in the presence of partial volume and flow artifacts. The mean relative amplitude of the fast component was larger when measured using constrained reconstruction in the heart, which is consistent with better sampling of the peak.
Constrained VIF reconstruction for DCE-MRI
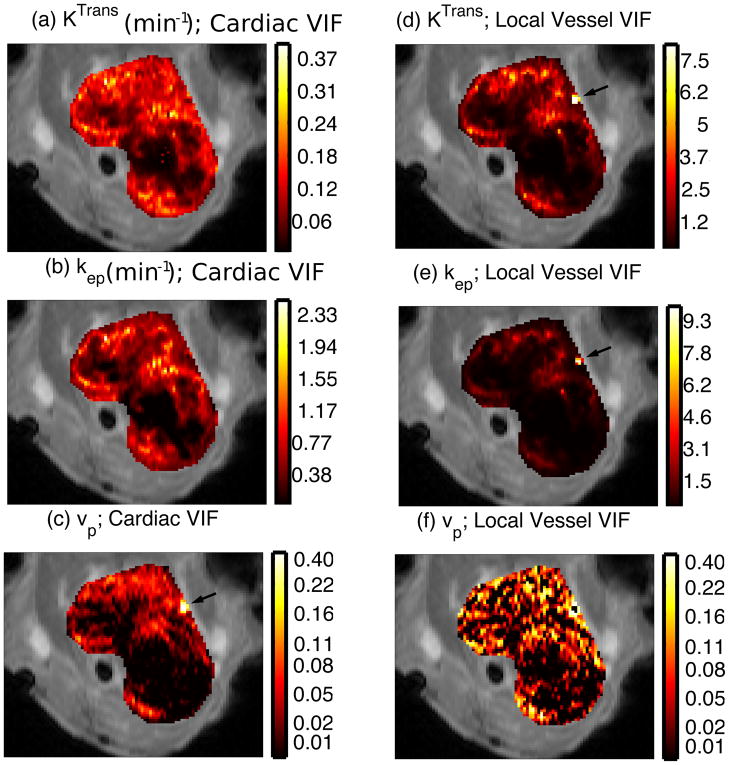
Parameter maps were calculated for the tumor models using VIFs measured with both techniques, in Figure 7 and summarized in Table 3. Although the number of animals imaged is insufficient for rigorous statistical comparison of the inter-subject coefficients of variation, this quantity is reduced for all three pharmacokinetic parameters: the CVs, between mice, of KTrans, kep, and vb from the cardiac measurement are 36%, 25%, and 28%, respectively, while for measurements derived from the local vessel, these quantities are 71%, 79%, and 61%. The values of vb derived from the cardiac measurements displayed structure more similar to the other parameters than the noise-like measurements of vb derived from the local vessel.
Figure 7.
Pharmacokinetic parameter maps calculated using VIFs from CACTUS and a local vessel. Comparison of KTrans (a vs. d) and kep (b vs. e) reveal similar patterns of regional perfusion and clearance. However, pseudopermeability artifacts due to vessels are visible in the maps calculated using the local vessel VIF (arrows), but correctly attributed to high vascular volume fraction when the VIF is sampled with high temporal resolution. Noise is visibly reduced in vp (c vs. f)
Table 3.
Summary of measured pharmacokinetic parameters and coefficient of variation (percent).
| Vessel Mouse 1 | Heart Mouse 1 | Vessel Mouse 2 | Heart Mouse 2 | Vessel Mouse 3 | Heart Mouse 3 | |
|---|---|---|---|---|---|---|
| KTrans min−1 | 0.286 ± 94% | 0.052 ± 34% | 1.099 ± 94% | 0.112 ± 49% | 0.438 ± 37% | 0.086 ± 47% |
| kep min−1 | 2.251 ± 91% | 0.775 ± 43% | 1.332 ± 98% | 0.509 ± 76% | 0.247 ± 35% | 0.526 ± 46% |
| vb | 0.041 ± 86% | 0.017 ± 72% | 0.091 ± 122% | 0.030 ± 102% | 0.158 ± 89% | 0.026 ± 99% |
A blood vessel at the edge of the tumor produced a large pseudopermeability artifact that is visible in the maps of KTrans and kep that were calculated based on VIF measurement from local vessels. This artifact does not appear in the KTrans and kep maps created from the rapidly sampled cardiac VIF, but rather it manifests in the measurement of vb, as is physiologically appropriate.
Discussion
We have developed a strategy for rapid sampling the vascular input function that addresses prominent challenges in DCE-MRI involving small animal models of human disease. Changes in blood signal levels are measured from within the left ventricle of the heart to reduce partial volume effects, and the use of a constrained reconstruction technique provides very high temporal resolution to more accurately capture rapid vascular kinetics. VIF estimates with fewer confounding artifacts that more clearly depict rapid signal enhancement improve the reproducibility of VIF and may lead to a reduction of variabilities in parameter maps that depend on its measurement(5).
The vascular input function is determined by the average signal enhancement that is observed in blood, due to injection of contrast, as a function of time. Identification of vessels for traditional VIF measurement could lead to averaging of venous and arterial enhancement, leading to temporal blurring. The proposed method enforces consistency in the VIF measurement while still sampling from a large volume by observing signal changes only in the left ventricle, the last chamber of the heart through which oxygenated blood circulates before being supplied to the body. Spatial details within the left ventricle are not necessary for measurement of the VIF, but the ventricle must still be separated from the surrounding tissues. The constrained reconstruction algorithm relies upon the simple approximation that mean signal enhancement can be separated among manually segmented regions of anatomically consistent structures, allowing very rapid temporal measurement of course signal enhancement. The assumption of homogenously enhancing ROIs is reasonable for the slowly enhancing soft tissues and muscle found in the chest. Simulations and the comparison of the CACTUS signal to the FBP measurement indicate that the uncertainty in the signal is on the order of 5%, which is much less than the large signal changes of up to 400% that can be observed in vivo due to the presence of contrast agent. In vivo application of the algorithm demonstrates that it provides a high temporal resolution measurement of the VIF that is able to capture the rapid initial enhancement due to arrival of the bolus of contrast.
Suppression of flow-related enhancement improves sensitivity to the presence of contrast agent and is a crucial component of VIF measurement. The CACTUS algorithm can be used in conjunction with any technique that prepares spins for imaging. In this work, we have employed a partially refocused excitation pulse that simultaneously excites the imaging slice and spoils spins within adjacent regions(15)to minimize the effect of flow preparation on sequence timing.
No tissues are perfectly homogeneous, and all tissues are comprised of some level of small-scale detail or structure, such as vasculature, that would be tedious to manually identify and segment. The coarse segmentation used in this work will necessarily represent an imperfect representation of the dynamic changes in the image. Nevertheless, fluctuations of signal in small structures contribute to and are accounted for by variations of the mean signal within the surrounding ROI. Fluctuations due to larger, more prominent structures may be segmented into additional regions to more closely approximate the assumption of uniform enhancement.
Numerical simulations indicate that the algorithm can accurately reconstruct highly undersampled data, even in the presence of spatially incoherent signal fluctuations. Mismatches between the segmentation and the actual structures of the image present larger challenges, but these may be minimized by careful segmentation and the use of a shim ROI to account for changes in the size of the left ventricle through the cardiac cycle. Comparison of the CACTUS measurements to those from FBP demonstrates that the constrained approach produces a measurement that is consistent with the slowly changing component of the VIF, yet which reveals a sharper and more repeatable initial rise of the contrast agent. The temporal resolution achieved is faster than the cardiac cycle of a mouse, enabling a reasonable sacrifice in temporal resolution for the sake of motion and noise suppression.
Dedication of a single slice of VIF measurement that is interleaved with dynamic anatomic imaging permits separate optimization of both acquisitions. With this approach, the VIF sampling rate is decoupled from the anatomic image update rate. This separation also allows the use of an alternative k-space trajectory for the dedicated slice. Additionally, because the optimal flip angle for VIF measurement is higher than the optimal flip angle for tissue (23), different excitation strategies can be used for each quantity. Time that would otherwise be dedicated to dynamic anatomic imaging is only reduced by the time required to acquire the single slice for CACTUS and thus has only a minimal effect on tumor coverage.
These results indicate a significant improvement in the within-mouse reproducibility when the VIF is measured by constrained reconstruction of vascular enhancement in the left ventricle. The experimental setup was designed to maintain stable animal physiology, ensuring that the variation in VIFs between injections is dominated by measurement effects and not by physiological drift. We observed large variations in the fast component for all mice with both techniques, although the fluctuations were lower when high-temporal resolution cardiac sampling was used generate the VIF. In addition, we observed both a reduction in the inter-animal variation and a significant change in the shapes of the VIFs compared to conventional vessel sampling.
Inter-subject variation in the VIF from the cardiac measurements is comparable to that reported by Pickup, et al. (9), who reported CVs of 61% for kfast, 34% for α, 77% for the kslow, and 56% for C0. Because of differences in mouse strain, animal preparation, injection, and imaging conditions these numbers should not be used to compare one measurement technique against another, but they do provide a broad indication of the inherent variability of VIF measurements in mice.
Measurements in tumors revealed a substantial reduction in the variation of pharmacokinetic parameters across subjects; this change in relative variation would decrease the number of mice needed for an experiment by a factor of four, assuming similarly proportional effects. Additionally, the parameter maps calculated using the constrained VIF show resistance to pseudopermeability artifacts and improved measurements of vp, compared to maps calculated from the VIF measured using conventional encoding in a local vessel. Further studies are needed to confirm these preliminary results.
Our technique relies on a coil-dependent proximity between the heart and tumor tissue. In this work, distance between the heart and thyroid were easily within the homogeneous region of our volume coil. Careful animal placement would enable interleaved acquisition of cardiac and tumor data from any structure anterior to the heart or posterior to the heart until approximately the thigh. The homogeneity of the RF coil should be verified for larger separation between heart and tumor. Specialized coil configurations may be necessary to address this limitation.
The improvements in VIF measurement afforded by the CACTUS technique will provide substantial benefits to the measurement of pharmacokinetic parameters in DCE-MRI experiments involving small animal models of cancer. High temporal resolution will reduce sampling distortions, and provide a reduction in partial volume artifacts, decreasing errors in the VIF. This will in turn lead to greater reproducibility and greater accuracy of measured pharmacokinetic parameters, which will improve the sensitivity and power of DCE-MRI in preclinical research.
Acknowledgments
Support provided by NIH Grants P30 CA16672 and P50 CA83639
The authors would like to express their thanks to Valen E. Johnson, Ph. D., for helpful enlightenment on statistical matters. They further thank Charles Kingsley, Jorge DeLacerda and Yunyun Chen, Ph. D., for their assistance in animal handling and preparation. Financial assistance was provided by NIH Grants P30 CA16672 and P50 CA83639, as well as University of Texas M. D. Anderson Cancer Center start-up funds.
Abbreviations
- CACTUS
Cardiac Anatomy Constrained, Temporally Unrestricted Sampling
- CV
Coefficient of variation
- DCE-MRI
Dynamic contrast enhanced MRI
- FBP
Filtered backprojection
- ICA
Internal carotid artery
- VIF
Vascular input function
Bibliography
- 1.Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst. 2005;97(3):172–187. doi: 10.1093/jnci/dji023. [DOI] [PubMed] [Google Scholar]
- 2.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. Journal of magnetic resonance imaging: JMRI. 1999;10(3):223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Cheng HL. T1 measurement of flowing blood and arterial input function determination for quantitative 3D T1-weighted DCE-MRI. J Magn Reson Imaging. 2007;25(5):1073–1078. doi: 10.1002/jmri.20898. [DOI] [PubMed] [Google Scholar]
- 4.Parker GJ, Roberts C, Macdonald A, Buonaccorsi GA, Cheung S, Buckley DL, Jackson A, Watson Y, Davies K, Jayson GC. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med. 2006;56(5):993–1000. doi: 10.1002/mrm.21066. [DOI] [PubMed] [Google Scholar]
- 5.Yankeelov TE, Luci JJ, Lepage M, Li R, Debusk L, Lin PC, Price RR, Gore JC. Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magnetic Resonance Imaging. 2005;23(4):519–529. doi: 10.1016/j.mri.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Hansen AE, Pedersen H, Rostrup E, Larsson HB. Partial volume effect (PVE) on the arterial input function (AIF) in T1-weighted perfusion imaging and limitations of the multiplicative rescaling approach. Magn Reson Med. 2009;62(4):1055–1059. doi: 10.1002/mrm.22098. [DOI] [PubMed] [Google Scholar]
- 7.Zhou R, Pickup S, Yankeelov TE, Springer CS, Jr, Glickson JD. Simultaneous measurement of arterial input function and tumor pharmacokinetics in mice by dynamic contrast enhanced imaging: effects of transcytolemmal water exchange. Magnetic Resonance in Medicine. 2004;52(2):248–257. doi: 10.1002/mrm.20143. [DOI] [PubMed] [Google Scholar]
- 8.Yankeelov TE, Cron GO, Addison CL, Wallace JC, Wilkins RC, Pappas BA, Santyr GE, Gore JC. Comparison of a reference region model with direct measurement of an AIF in the analysis of DCE-MRI data. Magnetic Resonance in Medicine. 2007;57(2):353–361. doi: 10.1002/mrm.21131. [DOI] [PubMed] [Google Scholar]
- 9.Pickup S, Zhou R, Glickson J. MRI estimation of the arterial input function in mice. Academic Radiology. 2003;10(9):963–968. doi: 10.1016/s1076-6332(03)00291-5. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre DJ, Ludwig C, Pasan A, Griffiths JR. A method for interleaved acquisition of a vascular input function for dynamic contrast-enhanced MRI in experimental rat tumours. NMR Biomed. 2004;17(3):132–143. doi: 10.1002/nbm.868. [DOI] [PubMed] [Google Scholar]
- 11.Pradel C, Siauve N, Bruneteau G, Clement O, de Bazelaire C, Frouin F, Wedge SR, Tessier JL, Robert PH, Frija G, Cuenod CA. Reduced capillary perfusion and permeability in human tumour xenografts treated with the VEGF signalling inhibitor ZD4190: an in vivo assessment using dynamic MR imaging and macromolecular contrast media. Magn Reson Imaging. 2003;21(8):845–851. doi: 10.1016/s0730-725x(03)00186-3. [DOI] [PubMed] [Google Scholar]
- 12.McGrath DM, Bradley DP, Tessier JL, Lacey T, Taylor CJ, Parker GJ. Comparison of model-based arterial input functions for dynamic contrast-enhanced MRI in tumor bearing rats. Magn Reson Med. 2009;61(5):1173–1184. doi: 10.1002/mrm.21959. [DOI] [PubMed] [Google Scholar]
- 13.Bradley DP, Tessier JL, Checkley D, Kuribayashi H, Waterton JC, Kendrew J, Wedge SR. Effects of AZD2171 and vandetanib (ZD6474, Zactima) on haemodynamic variables in an SW620 human colon tumour model: an investigation using dynamic contrast-enhanced MRI and the rapid clearance blood pool contrast agent, P792 (gadomelitol) NMR Biomed. 2008;21(1):42–52. doi: 10.1002/nbm.1161. [DOI] [PubMed] [Google Scholar]
- 14.Henderson E, Rutt BK, Lee TY. Temporal sampling requirements for the tracer kinetics modeling of breast disease. Magn Reson Imaging. 1998;16(9):1057–1073. doi: 10.1016/s0730-725x(98)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Ragan DK, Esparza-Coss E, Bankson J. A Method for Updating the Aterial Input Function Each Cardiac Cycle with Flow Compensation. Proceedings of the 16th Annual Meeting of the ISMRM; 2008. p. 3837. [Google Scholar]
- 16.Hu X, Levin DN, Lauterbur PC, Spraggins T. SLIM: spectral localization by imaging. Magn Reson Med. 1988;8(3):314–322. doi: 10.1002/mrm.1910080308. [DOI] [PubMed] [Google Scholar]
- 17.Taylor NJ, Rowland IJ, Tanner SF, Leach MO. A rapid interleaved method for measuring signal intensity curves in both blood and tissue during contrast agent administration. Magn Reson Med. 1993;30(6):744–749. doi: 10.1002/mrm.1910300613. [DOI] [PubMed] [Google Scholar]
- 18.Kak AC, Slaney M. Principles of Computerized Tomographic Imaging. 1988. 7 Algebraic Reconstruction Algorithms. [Google Scholar]
- 19.Foster M. An Application of the Wiener-Kolmogorov Smoothing Theory to Matrix Inversion. Journal of the Society for Industrial and Applied Mathematics. 1961;9(3):387–392. [Google Scholar]
- 20.Padhani AR, Hayes C, Landau S, Leach MO. Reproducibility of quantitative dynamic MRI of normal human tissues. NMR in Biomedicine. 2002;15(2):143–153. doi: 10.1002/nbm.732. [DOI] [PubMed] [Google Scholar]
- 21.Bishop J, Feintuch A, Bock NA, Nieman B, Dazai J, Davidson L, Henkelman RM. Retrospective gating for mouse cardiac MRI. Magnetic Resonance in Medicine. 2006;55(3):472–477. doi: 10.1002/mrm.20794. [DOI] [PubMed] [Google Scholar]
- 22.Haacke EM. Magnetic Resonance Imaging: Physical Principles and Sequence Design. New York: John Wiley & Sons; 1999. Projection Reconstruction of Images; pp. 303–330. [Google Scholar]
- 23.Schabel MC, Parker DL. Uncertainty and bias in contrast concentration measurements using spoiled gradient echo pulse sequences. Phys Med Biol. 2008;53(9):2345–2373. doi: 10.1088/0031-9155/53/9/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill PE, Murray W. Algorithms for Solution of Non-Linear Least-Squares Problem. Siam Journal on Numerical Analysis. 1978;15(5):977–992. [Google Scholar]
- 25.Hotelling H. The Generalization of Student’s Ratio. The Annals of Mathematical Statistics. 1931;2(3):360–378. [Google Scholar]
- 26.Kim S, Park YW, Schiff BA, Doan DD, Yazici Y, Jasser SA, Younes M, Mandal M, Bekele BN, Myers JN. An orthotopic model of anaplastic thyroid carcinoma in athymic nude mice. Clin Cancer Res. 2005;11(5):1713–1721. doi: 10.1158/1078-0432.CCR-04-1908. [DOI] [PubMed] [Google Scholar]
- 27.Buckley DL. Uncertainty in the analysis of tracer kinetics using dynamic contrast-enhanced T1-weighted MRI. Magnetic Resonance in Medicine. 2002;47(3):601–606. doi: 10.1002/mrm.10080. [DOI] [PubMed] [Google Scholar]
- 28.Aerts HJ, van Riel NA, Backes WH. System identification theory in pharmacokinetic modeling of dynamic contrast-enhanced MRI: influence of contrast injection. Magn Reson Med. 2008;59(5):1111–1119. doi: 10.1002/mrm.21575. [DOI] [PubMed] [Google Scholar]