Abstract
Little is known about brain function in the oldest old, although this is the fastest growing segment of the population in developed countries and is of paramount importance in public health considerations. In this study, we investigated the cerebral response to a memory task in healthy subjects over age 90 compared with healthy younger elderly.
We studied 29 healthy elderly subjects, 12 over age 90 and 17 between age 70 and 80. All subjects were cognitively intact, as verified by a neuropsychological battery, and performed a nonverbal memory task while undergoing a functional MRI (fMRI). Activation results were analyzed by a random-effects ANCOVA using SPM5. The task resulted in activation of similar areas of the posterior temporal, parietal, and posterior frontal cortexes, but the activation was more robust in the younger subjects, especially in the right hippocampus, and parietal and temporal cortices. This finding remained after controlling for education, cognition, task performance or cerebral atrophy.
The phenomenon of relatively maintained performance, despite significant brain atrophy and lower activation is consistent with the cognitive reserve theory and may be specific to subjects with extremely successful aging. Further investigation of brain activation patterns in the oldest old is warranted.
Keywords: functional MRI, nonagenarians, brain activation, cognitive reserve
Introduction
In the United States, while the population under the age of 65 has tripled since the beginning of the 20th century, the number of those over age 65 has increased 11-fold. Among the elderly, the "oldest old" - those aged 85 and over - are the most rapidly growing age group. Between 1960 and 1994, their numbers rose by 274 percent in the USA. In contrast, the elderly population in general rose by 100 percent and the entire U.S. population grew only by 45 percent. The oldest old numbered 4.4 million in 2001, making them 10 percent of the elderly and just over 1 percent of the total population. By 2050, it is expected the oldest old will number 19.3 million, constituting one quarter of all elderly Americans and 5 percent of all Americans (1998).
The incidence rate of AD is substantially higher in the ninth decade (>6.5% per year) than at earlier ages (between 1% at 60–65 and 2.2% at 75–80)(Kawas et al., 2000). The expansion of the oldest old population will increase its significance as a major public health burden, emphasizing the importance of identifying characteristics distinguishing the cognitively intact oldest old. Yet this group has rarely been studied in epidemiological investigations (Corrada et al., 2008) or in neuroimaging studies(Rosano et al.,, 2005).
Epidemiological, neuropathological and clinical studies have indicated that the characteristics of elderly AD patients differ significantly from those observed in younger(Prohovnik et al., 2006;van den Biessels et al., 2007), and have suggested that the “oldest old” population may represent a genetically and pathophysiologically distinct group (Sobel et al., 1995;Rebeck et al., 1994;Silverman et al., 2008). For example, the cumulative survival from AD was significantly greater in the relatives of non-demented probands above 90 years old compared with relatives of non-demented probands younger than 90(Silverman et al., 2008). This suggests that genetic factors conferring an extended reduction of risk for AD may be more highly concentrated among non-demented probands aged above 90 (Silverman et al., 1999).
Longitudinal studies (Brayne et al., 1995) have found evidence of decline in cognition associated with age among normal elderly populations who do not necessarily go on to develop dementia (Brayne et al., 1999). AD may not be an inevitable concomitant of the aging process (Hickman et al., 2000). Identifying modifiable protective factors, associated with decreased risk of AD, may suggest preventive strategies. It is therefore important to elucidate the possible mechanisms by which some individuals maintain high cognitive abilities at advanced ages. The aim of this study was to compare the patterns of brain activation by fMRI, during a recognition memory task, of cognitively intact subjects aged 90 years and above (OO- old old) to those 70 to 80 (YO-young old). Based on previous studies suggesting a compensatory mechanism used by especially high functioning elderly compared to normal functioning elderly (Cabeza et al., 2002;Sole-Padulles et al., 2007), we hypothesized that while the YO would activate the hippocampus during a task targeted to activate this area(Stark and Squire 2000b), the OO would also activate other areas, as a compensatory mechanism for age-associated hippocampal functional decline.
Methods
Subjects
Subjects were recruited through talks on memory at senior centers in the tri-state area (New York, New Jersey, and Connecticut), and through newspaper ads. In addition, subjects were asked to invite acquaintances to participate in the study. Individuals who reported having no memory problems were visited at their residences. After informed consent, a Clinical Dementia Rating (CDR(Fillenbaum et al., 1996)) was obtained based on information from both the subject and an informant. Only subjects with CDR=0 (not demented) and a MMSE within the norms for age and education(Crum et al., 1993) were included in the study. Those with CDR ≥ .5 (questionable dementia or dementia) were invited to participate in other ADRC projects. Subjects were excluded if they had a stroke, or a significant psychiatric or neurological disease, based on medical charts or self reported. All subjects were living independently in the community. Forty one subjects were approached for this fMRI study. Twelve subjects were excluded due to contraindications to MRI. Therefore, we studied 29 subjects, 12 OO (9 females) and 17 YO (9 females). The mean ages were 92±1 and 76±3. All subjects maintained a CDR=0 and a MMSE above 25 at their 12 month follow-up, suggesting lack of incipient cognitive decline at baseline.
Recognition memory task
Since hippocampal atrophy is strongly related to cognitive function in the elderly population (Devanand et al., 2007;Li et al., 2006), a well documented recognition memory task that activates the hippocampus was used (Stark and Squire 2000b), with minor modifications.
Subjects were presented with 50 line drawings with common nameable objects (Snodgrass and Vanderwart 1980) on a laptop computer before entering the scanner and were asked to name them. Each picture was presented to the subject for 2.5 seconds with an inter-trial interval of 0.5 seconds. They were instructed to learn the pictures, to be able to recognize them in a memory task while in the scanner. After entering the scanner, each subject viewed 100 pictures (the 50 that they had learned and 50 new pictures) while BOLD fMRI images were acquired. Subjects rested supine in the scanner and viewed the images via a set of fiber optic goggles (SV2000; Avotec, Inc.) positioned in the head coil above their eyes.
Subjects were asked to press a button with the right hand if they recognized the picture (old) and with the left hand if they did not recognize the picture (new). Two sets of 50 pictures were counterbalanced as old and new between subjects. The two sets of pictures were ordered in a random fashion, and were presented in five blocks of 20 images. Each image was presented for two seconds with an inter-trial interval of 1 second. Subjects were instructed to rest for thirty seconds between each block (while looking at a slide indicating “rest”).
Image acquisition and analysis
MRI scanning was performed on a Siemens 1.5T Symphony with enhanced (Quantum) gradients using the standard quadrature head coil. Following a localizer, anatomical images (T1W) were acquired with a spin-echo sequence (TR/TE/FA 524/14/90, 20 axial 5 mm slices with 1.5mm gap, FOV 220 mm, matrix 256x256). BOLD images were acquired at the same slices with single-shot EPI (TR/TE/FA 3000/60/90, matrix 64x64 with fat saturation); 220 BOLD images were acquired, but the first 10 were discarded to ensure magnetization steady-state and the last 10 were also discarded. Each block consisted of 10 images of 3 sec duration.
All BOLD data were visually examined for any slice dropout or other severe signal abnormalities (timediff, http://www.mrc-cbu.cam.ac.uk/Imaging/Common/diagnostics.shtml) and if necessary, the slice was replaced with a mean image created from adjacent images of the same activation. Image analysis was carried out using SPM5 (Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/). Motion correction was applied by realigning all images to the first image using 6 parameter rigid body transformation and re-slicing with 4th degree B-spline interpolation. The mean BOLD image was then spatially normalized with SPM5’s EPI template using both affine and non-linear normalization followed by 8 mm Gaussian kernel smoothing. Non-linear frequency cutoff, iterations, and regularization were set to 25mm, 16, and 1, respectively; high-pass filtering was done with 128 sec cutoff. The first-level fixed-effects analysis included motion realignment parameters as regressors.
The volumetric fraction of cerebral spinal fluid (CSF) for each brain was also computed and defined by CSF/(GM+WM), where CSF, GM (grey matter) and WM (white matter) represent the total intracranial volume of each compartment. Segmenting the anatomical T1W image into each compartment was based on the SPM5 segmentation routine, using MNI tissue probability maps with warping regularization and frequency cutoff at 1 and 25mm, respectively. Bias regularization was set to 0.0001.
The activation response (activation BOLD signal minus resting BOLD signal) was analyzed by a random-effects ANCOVA design, with age (OO vs. YO) as a grouping factor and education, performance accuracy, MMSE, and CSF fraction as covariates in separate analyses. The cluster threshold was k=20 with a search range of implicitly masked whole brain, and all results below are reported at p=.001 uncorrected. Final anatomical labeling of findings in Talairach space was based on the MRU matlab toolbox (http://www.ihb.spb.ru/~pet_lab/MSU/MSUMain.html).
Covariates
Education was recorded as years of formal schooling. Performance accuracy was the percentage of correct response (recognition of old or rejection of new pictures) a subject made and reflected the overall difficulty of the task. CSF fraction was measured as detailed above and reflected degree of brain atrophy.
Results
Subject characteristics are presented in Table 1. The two groups were matched in education (YO=15.6±3.0 and OO=15.4±2.5), and all subjects performed well (>70% accuracy) on the activation task. Due to mechanical malfunction, performance accuracy measures in the scanner were only available for 21 subjects; among those, the YO performed significantly better than the OO on the activation memory task (87±5 vs. 80±6%, t(19)=2.54, p<.05). On the MMSE, the YO group had significantly higher values (28.9±1.3 Vs 27.6±1. 7, t(27)=2.50, p<.02), but these scores were consistent with the difference in norms for age and education for the respective age groups (Beeri et al., 2006;Welsh et al., 1994).
Table 1.
Characteristics of the sample- Mean (SD) or %
| Young-Old (n=17) | Old-Old (n=12) | p-value* | |
|---|---|---|---|
| Age (years) | 75.3 (1.9) | 91.0 (1.0) | <.005 |
| Sex (%F) | 52.9 | 75.0 | N.S. |
| Education | 15.6 (2.97) | 15.4 (2.48) | N.S. |
| MMSE | 28.9 (1.3) | 27.5 (1.7) | .03 |
| Performance accuracy % | 86.5 (5.3) | 80.1 (6.1) | .02 |
| CSF fraction | .62 (.08) | .53 (.05) | p<.001 |
p-value for t-test.
Detailed regional findings by SPM are provided in Table 2. The basic analysis contrasted the activation response of the two age groups. They showed similar patterns of posterior temporal, parietal, and posterior frontal activation, but the activation was more robust in the YO. The OO did not activate more than the YO in any location. The YO had significantly greater activation (Figure 1), especially in the right hippocampus (Figure 2), parietal and temporal cortex. We then proceeded to evaluate the effects of possible covariates, each evaluated separately. Education and performance, when added to the model, did not change the group differences, nor did they exhibit significant correlations with activation in any area. The inclusion of MMSE as a covariate left the group differences largely unchanged, but the difference between age groups for the left supramarginal gyrus and the right middle frontal gyrus (BA 8) also became significant. A significant positive correlation of MMSE with activation response was only noted in one cluster in the left middle temporal gyrus, and this was accompanied by two clusters with negative correlations, in left superior parietal lobule and right middle frontal gyrus.
Table 2.
Detailed regional findings by SPM
| Contrast | Hemis phere | Area | # Voxels | x | y | z |
|---|---|---|---|---|---|---|
| OO < YO | Rt | Sup. Temp. g/BA 38 | 42 | 46 | 12 | −26 |
| Lingual g/BA 18 | 40 | 2 | −80 | −4 | ||
| Middle Occ g/BA 18/19 | 21 | 34 | −86 | −2 | ||
| Hippocampus/BA 13 | 271 | 26 | −34 | 20 | ||
| Lingual g/cuneus/post cg | 71 | 10 | −76 | 4 | ||
| Precuneus/BA 7/19 | 23 | 24 | −74 | 42 | ||
| Precuneus/post cg/BA 7/31 | 91 | 6 | −50 | 42 | ||
| Precuneus/BA 7/19 | 40 | 8 | −78 | 40 | ||
| Inferior Parietal/BA 40 | 23 | 40 | −42 | 46 | ||
| Lt | Temp WM | 40 | −26 | −42 | 20 | |
| OO < YO, MMS Covariance | Rt | Sup Temp g/BA 38 | 32 | 44 | 14 | −24 |
| Hippocampus | 27 | 26 | −44 | −2 | ||
| Cuneus/post cg/BA 17/23 | 22 | 12 | −76 | 4 | ||
| Cuneus/post cg/BA 23/30 | 22 | −2 | −64 | 2 | ||
| Insula/BA 13 | 40 | 28 | −34 | 20 | ||
| Precuneus/BA 7/19 | 28 | 10 | −78 | 42 | ||
| Precuneus/post cg/BA 7/31 | 84 | 6 | −52 | 42 | ||
| MFG/BA 8 | 22 | 38 | 22 | 50 | ||
| Inf Parietal/BA 40 | 32 | −46 | −48 | 58 | ||
| Lt | Supramarginal g | 23 | −46 | −56 | 30 | |
| + MMS | Lt | MTG/BA 19 | 56 | −34 | −80 | 22 |
| − MMS | Rt | MFG/BA 8 | 37 | 40 | 22 | 46 |
| Lt | Precuneus/BA 7 | 34 | −24 | −62 | 44 | |
| OO < YO, CSF Covariance | Rt | Culmen/cerebellar Lingual | 57 | 8 | −42 | −16 |
| Hippocampus/parahypocampal g | 37 | 26 | −44 | 0 | ||
| STG | 25 | 36 | −56 | 10 | ||
| Insula/cg/CC/BA 13 | 162 | 28 | −36 | 20 | ||
| Posterior cg/BA 23/30/31 | 31 | 2 | −48 | 22 | ||
| Precuneus/Cuneus/BA 7/19 | 175 | 10 | −80 | 42 | ||
| Precuneus/BA 7 | 58 | 22 | −74 | 42 | ||
| Precuneus/post cg/BA 7/31 | 35 | 4 | −52 | 40 | ||
| Postcentral g/inf. Parietal/BA 3/40 | 138 | 38 | −34 | 54 | ||
| MFG/BA 6/31 | 27 | 6 | −22 | 52 | ||
| Precuneus/BA 7 | 29 | 12 | −54 | 60 | ||
| Lt | Insula/STG/BA 13/29/41 | 33 | −34 | −34 | 10 | |
| Post cg/BA 31 | 67 | −10 | −58 | 24 | ||
| Precuneus/Cuneus/BA 7/31 | 88 | −16 | −66 | 24 | ||
| Supramarginal g/STG | 28 | −42 | −56 | 28 | ||
| Cg | 56 | −16 | −34 | 32 | ||
| Precuneus/BA 7 | 32 | −24 | −48 | 52 | ||
| + CSF | Rt | Ant cg/BA 32 | 26 | 20 | 10 | 42 |
| Lt | STG/Insula/BA 13 | 51 | −52 | −42 | 14 | |
| Post cg/Precuneus/BA 31 | 100 | −10 | −58 | 24 | ||
| Inf Parietal/BA 40 | 22 | −56 | −36 | 32 | ||
| Precuneus | 31 | −26 | −48 | 52 |
Figure 1.
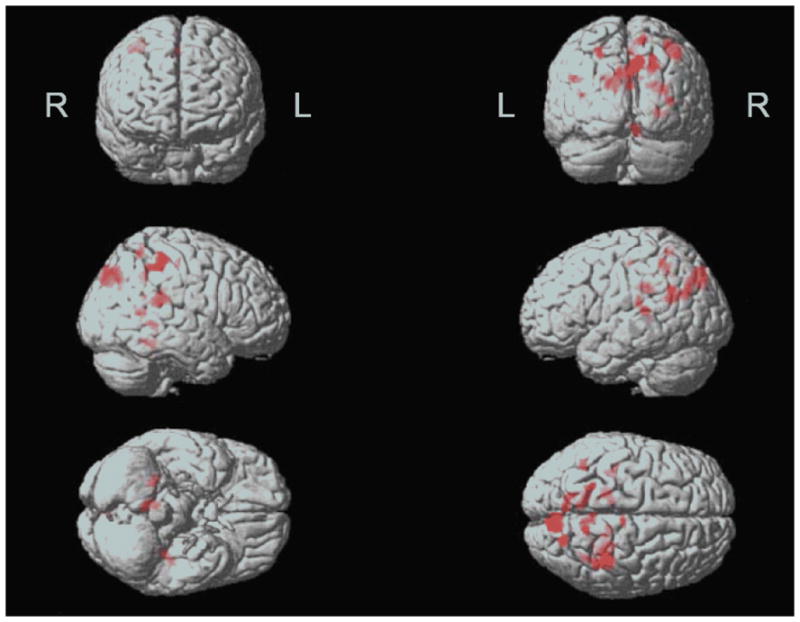
Areas where the OO group demonstrated significantly lower activation than the YO group in CSF-controlled ANCOVA (thresholded at p<.001, cluster size 20 voxels) are indicated by the red color. L/R denote the left and right hemisphere, respectively. In this display, the brain is viewed (from top left) from the front, rear, right side, left side, bottom and top.
Figure 2.
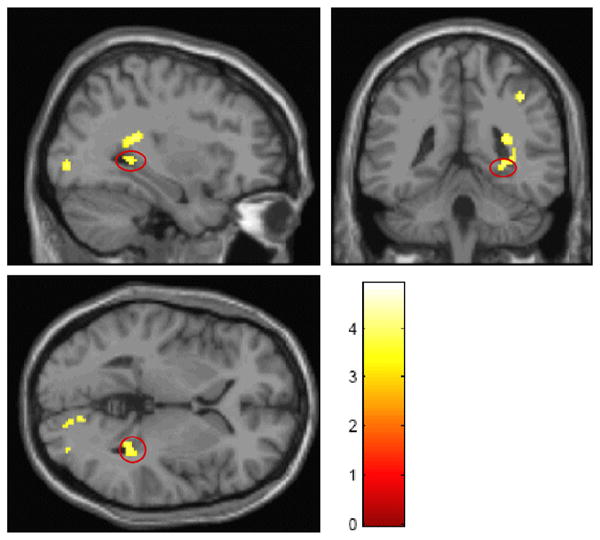
Orthogonal display of hippocampal activation (color scale depicts magnitude of t statistic). Significant voxels are shown in parasaggital, coronal and axial slices that include the hippocampus, with activation noted in its posterior tip.
The final covariate for analysis consisted of the CSF fraction in brain. There was a significant difference between the groups, with greater brain atrophy in the OO, as expected (CSF fraction .62±.08 vs. .53±.05, t(27)=3.80, p<.001). As in the analysis without a covariate, the YO had more robust activation than the OO, but there were more significant differences between age groups in the left hemisphere—often in the same areas, reducing asymmetry. Consistent with this, the positive correlations of CSF with activation were primarily left-sided (insular and parietal regions). There were no significant negative correlations.
Discussion
In this study we investigated brain function in very old (above 90 years of age—OO), well-functioning subjects. As a comparison group, we used typical, equally well-functioning, septuagenarians (YO). YO and OO subjects activated similar regions when performing a memory recognition task which did not confirm the hypothesis of activation of more regions for the OO group. However, YO subjects had consistently more robust activation in right temporal, parietal and hippocampal regions, even after controlling for education, performance accuracy, MMSE, or brain atrophy. Specifically, we found weaker activation in our OO subjects mainly in the right hemisphere, in the hippocampus, superior temporal cortex, medial frontal gyrus, and numerous parietal areas (Table 2). The regions activated in this study are in good agreement with brain regions previously reported to be involved in similar memory tasks (Miller et al., 2008).
It is possible that incipient Alzheimer’s disease is involved. The elderly are a highly heterogeneous group. Even when currently healthy, they include subjects who are already, or will soon progress, to MCI and AD. Such heterogeneity can be revealed by neuropsychological testing or imaging studies (Pike et al., 2007). Both the YO and OO groups were selected for living independently in the community and being cognitively intact at the time of the study. This may suggest a greater degree of selection of low risk for AD in the OO than the YO group because these characteristics are rarer in the former. This greater selectivity is supported by the fact that no subject in either group declined cognitively a year after the study, which is less likely in the OO than the YO (Hebert et al., 1995). This may suggest that the YO group is more heterogeneous so it contains more subjects at high risk for AD. This potential inclusion of such subjects in the YO group might explain our observations of stronger brain activation compared to the OO group. A recent review(Dickerson and Sperling 2008) noted that in the few fMRI studies on MCI, results have been inconsistent but generally suggest that early in the course of MCI there may be hyperactivation of medial temporal lobe circuits, possibly representing inefficient compensatory activity. In AD patients, medial temporal regions are hypoactivated during memory task performance while other cortical regions are engaged and hyperactivated, also suggesting compensation(Dickerson and Sperling 2008). AD patients had greater recruitment of the same brain regions as age-matched controls (and not of additional regions) suggesting the latter compensatory mechanism, in another study (Gould et al., 2006).
The most straightforward explanation for our findings of lower activation in the OO is that they had lower MMSE and greater brain atrophy than the YO. However, the difference in MMSE between the groups was modest, and smaller than the differences in norms for such age groups with such education levels(Welsh et al., 1994;Beeri et al., 2006). This suggests that, in the context of age norms for cognitive performance, the OO group performed at least as well as the YO group. It should be emphasized that like the YO group, the OO subjects were highly functional and living independently in the community. Although we are not aware of age and education specific norms for the recognition task used in this study, the group differences in CSF atrophy (p<.001) were substantially larger than for cognitive performance (p<.05 for both MMSE and recognition task) with an effect size between 30% and 50% larger. This distinction between relatively unimpaired cognitive performance and relatively abundant atrophy was interpreted as consistent with the cognitive reserve model (Stern 2006). This model posits that—among individuals who perform well cognitively despite brain pathology due to disease or age-related changes—individual differences provide differential reserve in two forms: “neural compensation” and “neural reserve”. In “neural compensation,” alternate networks compensate for pathology’s disruption of previously existing networks. This adjustment was found in studies comparing young subjects to subjects slightly younger than YO in which high performing older adults counteract age-related neural decline through hemispheric asymmetry reduction, i.e. compensation by use of other brain areas (Cabeza et al., 2002;Grady et al., 2006). This is consistent with the HAROLD model, which states that under similar circumstances, prefrontal activity during cognitive performances tends to be less lateralized in older than younger adults(Cabeza 2002). In our study, both the YO and OO groups exhibited relatively symmetrical use of the hemispheres, consistent with the interpretation that this form of compensation has been developed by high functioning subjects by the time they become YO. This study did not find dramatic prefrontal activations but rather temporal and parietal, which may reflect additional applications of a model like HAROLD in later aging. Insofar as the YO group has subjects in the very early stages of cognitive decline, their “neural compensation” concept might explain the relatively strong activation in the YO group(Dickerson and Sperling 2008).
In the “neural reserve” component of the cognitive reserve model, pre-existing brain networks that are more efficient or have greater capacity may be less susceptible to disruption. Therefore, despite other brain pathology, such networks continue to operate effectively, and may require relatively low activation if they are more efficient(Stern 2006;Stern et al., 2000). Despite loss of brain tissue in the OO group (higher CSF fraction), good cognitive performance and low activation may, speculatively, reflect efficient use of the spared brain tissue.
The recognition task used in the present study has been used in previous studies specifically triggering hippocampal activation (Stark and Squire 2000b;Stark and Squire 2000a;Gabrieli et al., 1997). Although the hippocampus was activated in both groups, as expected in this study, several other areas were engaged too. One explanation is that both groups used “neural compensation” to overcome the effects of aging on the hippocampus. This is plausible since the studies using the original recognition task had subjects ranging from 18 to 38 years of age. Another possibility is procedural: slightly longer duration of presentation of the stimuli might have allowed for activation of regions that the original task did not activate.
Encoding tasks have been studied more often in fMRI and aging studies than retrieval tasks such as the one used here. Use of such a task would have permitted richer comparisons with other cognitive aging-related fMRI studies, such as studies on the HERA model of prefrontal encoding and retrieval asymmetry(Duzel et al., 1999). However, we were apprehensive that the nonagenarian subjects would be less willing to participate in a task that requires more time and effort in the MRI machine. Encoding studies have shown different brain activation patterns from the recognition task used in this study, but the pattern of decreased prefrontal asymmetry with increasing age (HAROLD model(Cabeza 2002) might extrapolate to the substantial more posterior symmetry observed in even older subjects in the current study.
Although major stroke was an exclusion criterion, there was no assessment of other types of cerebrovascular disease (CVD) such as silent infarcts or white matter hyperintensities. The BOLD response is a complex hemodynamic signal that is susceptible to alterations in cerebral blood volume, cerebral blood flow, and cerebrovascular reactivity. Changes in cerebrovascular integrity with aging(Gillum 2002) are well-known and might have affected BOLD responses in the observed directions independent of neural activity. However, in the absence of direct information on CVD other than stroke, we compared the YO and OO groups on cardiovascular risk factors (diabetes, hypertension, and hyperlipidemia), with no significant differences (data not shown). Our study suffers from another limitation, laterality of findings is confounded by the lack of counterbalanced response – recognition was always indicated by the right hand.
The interpretation of our findings remains tentative, due to limited current knowledge in two critical topics: the changes of brain structure at advanced ages, and the analysis of activation differences. Secular artifact in cross-sectional aging studies is well documented, as is the age-associated increase of variance in brain morphology. Neuropathological studies show brain weight, cortical thickness, and number of large neurons all decrease with age, while total number of neurons and neuronal density may not change (Terry et al., 1987). Brain imaging confirms the prevalence and variability of age associated changes, with some subjects losing very little brain tissue even at advanced ages (Coffey et al., 1998). Nonetheless, there is a substantial amount of redundancy and reserve capacity in the human brain, which would limit damage due to loss or shrinkage of neurons (Creasey and Rapoport 1985). We also recognize that the interpretation of differences in activation magnitude is nontrivial. When comparing activation to different stimuli within an individual, the ‘intensity’ of activation is not easy to quantify, or even to conceptualize, and has been the subject of debate. The situation is even more complex when comparing across individuals or groups. Volume and signal change may be positively or negatively correlated under different circumstances (Ludemann et al., 2006;Jancke et al., 1998). Moreover, cognitive activity is often associated with ‘deactivation’ (reduced activity) in some brain areas, and such deactivation may be related to, perhaps even a prerequisite, for successful task performance under some conditions (Miller et al., 2008).
Functional activation studies of cognitively intact elderly below the ages investigated here, have demonstrated variable results, reporting both stronger and weaker activation than in young subjects (Mason and Miller 1992;Sperling 2007). There are many possible confounds that complicate the interpretation of these results, including subject characteristics such as age, risk factors for degenerative and vascular dementias, gender, neuropsychological performance level, educational attainment and cohort effects. The studies are also methodologically diverse, with many differences in the nature and difficulty of the task, data analytic techniques, block-design or event related fMRI, the use of covariates such as cerebral atrophy, separation of activation from deactivation, and many more. Most importantly, the advanced ages studied here have rarely been observed, and comparison of our data to previous literature is, thus, tenuous. In the current study, we attempted to control for confounds by extensive use of covariance analyses, inclusion of brain atrophy measures, exclusion of subjects with impaired cognition and cognitive screening based both on the MMSE and a CDR using informant as well as subject’s information. We ensured that all subjects could perform the task at adequate levels, and that they did perform the learning task while in the scanner. We also ensured that our two groups were equally educated, due to the strong associations of education with the onset of Alzheimer’s disease and its relationship with cerebral perfusion and neuropathology (Del et al., 1999;Stern et al., 1992).
Since education was not strongly correlated with activation, using education as a covariate did not affect the results. Performance accuracy levels were slightly better in the YO group but did not affect the differences in activation between the YO and OO groups. Similarly, controlling for MMSE had minor effects on the results. The lack of changes in the results after using those covariates might be a result of a relatively narrow range in each. CSF fraction, in contrast, had a wider range and was, as expected, significantly higher in the OO group, reflecting greater brain atrophy. Moreover, CSF amount was associated with activation in many regions. Therefore, the analysis of covariance controlling for CSF best identifies the differences between the YO and OO groups. The group comparison controlling for CSF was more symmetric because differences in the left hemisphere became significant, i.e., there were cortical regions in both hemispheres that were more activated in the YO group than in the OO group. Thus, despite limited knowledge regarding the interpretation of activation differences and the age-associated cerebral changes, we have attempted to overcome methodological uncertainties and believe that our findings are valid for this age range.
Acknowledgments
We thank Dr. Mathew Brett, Cambridge University, UK, for the development of timediff, and Dr. Sergei Pakhomov, Institute of the Human Brain, Russian Academy of Science, Saint Petersburg, Russia, for the development of MSU. This study was partially supported by a pilot grant from the ADRC (P50 AG05138, Dr. Sano), a K01 AG023515-01A2 (Dr. Beeri), an educational grant from Siemens Corporation (Dr. Prohovnik), and John Hartford Foundation/American Federation for Aging Research (Dr. Wollman).
Footnotes
Disclosure Statement: The authors report no conflict of interest. This study was approved by the Mount Sinai School of Medicine Institutional Review Board and all subjects signed informed consent.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- United States Census Bureau. 1998 [Google Scholar]
- Beeri MS, Schmeidler J, Sano M, Wang J, Lally R, Grossman H, Silverman JM. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006;67:1006–1010. doi: 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayne C, Gill C, Paykel ES, Huppert F, O'Connor DW. Cognitive decline in an elderly population--a two wave study of change. Psychol Med. 1995;25:673–683. doi: 10.1017/s0033291700034930. [DOI] [PubMed] [Google Scholar]
- Brayne C, Spiegelhalter DJ, Dufouil C, Chi LY, Dening TR, Paykel ES, O'Connor DW, Ahmed A, McGee MA, Huppert FA. Estimating the true extent of cognitive decline in the old old. J Am Geriatr Soc. 1999;47:1283–1288. doi: 10.1111/j.1532-5415.1999.tb07426.x. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- Creasey H, Rapoport SI. The aging human brain. Ann Neurol. 1985;17:2–10. doi: 10.1002/ana.410170103. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- Del ST, Hachinski V, Merskey H, Munoz DG. An autopsy-verified study of the effect of education on degenerative dementia. Brain. 1999;122 ( Pt 12):2309–2319. doi: 10.1093/brain/122.12.2309. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De SS, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer's disease: insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Cabeza R, Picton TW, Yonelinas AP, Scheich H, Heinze HJ, Tulving E. Task-related and item-related brain processes of memory retrieval. Proc Natl Acad Sci U S A. 1999;96:1794–1799. doi: 10.1073/pnas.96.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to Establish a Registry for Alzheimer's Disease. Aging (Milano ) 1996;8:379–385. doi: 10.1007/BF03339599. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gillum RF. New considerations in analyzing stroke and heart disease mortality trends: the Year 2000 Age Standard and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Stroke. 2002;33:1717–1721. doi: 10.1161/01.str.0000016925.58848.ea. [DOI] [PubMed] [Google Scholar]
- Gould RL, Arroyo B, Brown RG, Owen AM, Bullmore ET, Howard RJ. Brain mechanisms of successful compensation during learning in Alzheimer disease. Neurology. 2006;67:1011–1017. doi: 10.1212/01.wnl.0000237534.31734.1b. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Beckett LA, Albert MS, Pilgrim DM, Chown MJ, Funkenstein HH, Evans DA. JAMA. 273:1354–1359. [PubMed] [Google Scholar]
- Hickman SE, Howieson DB, Dame A, Sexton G, Kaye J. Longitudinal analysis of the effects of the aging process on neuropsychological test performance in the healthy young-old and oldest-old. Dev Neuropsychol. 2000;17:323–337. doi: 10.1207/S15326942DN1703_3. [DOI] [PubMed] [Google Scholar]
- Izaks GJ, Gussekloo J, Dermout KM, Heeren TJ, Ligthart GJ. Three-year follow-up of Mini-Mental State Examination score in community residents aged 85 and over. Psychol Med. 1995;25:841–848. doi: 10.1017/s0033291700035091. [DOI] [PubMed] [Google Scholar]
- Jancke L, Peters M, Schlaug G, Posse S, Steinmetz H, Muller-Gartner H. Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Brain Res Cogn Brain Res. 1998;6:279–284. doi: 10.1016/s0926-6410(98)00003-2. [DOI] [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Li Y, Li J, Segal S, Wegiel J, De SS, Zhan J, de Leon MJ. Hippocampal cerebrospinal fluid spaces on MR imaging: Relationship to aging and Alzheimer disease. AJNR Am J Neuroradiol. 2006;27:912–918. [PMC free article] [PubMed] [Google Scholar]
- Ludemann L, Forschler A, Grieger W, Zimmer C. BOLD signal in the motor cortex shows a correlation with the blood volume of brain tumors. J Magn Reson Imaging. 2006;23:435–443. doi: 10.1002/jmri.20530. [DOI] [PubMed] [Google Scholar]
- Mason JB, Miller JW. The effects of vitamins B12, B6, and folate on blood homocysteine levels. Ann N Y Acad Sci. 1992;669:197–203. doi: 10.1111/j.1749-6632.1992.tb17100.x. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Prohovnik I, Perl DP, Davis KL, Libow L, Lesser G, Haroutunian V. Dissociation of neuropathology from severity of dementia in late-onset Alzheimer disease. Neurology. 2006;66:49–55. doi: 10.1212/01.wnl.0000191298.68045.50. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Perls TT, West HL, Sodhi P, Lipsitz LA, Hyman BT. Reduced apolipoprotein epsilon 4 allele frequency in the oldest old Alzheimer's patients and cognitively normal individuals. Neurology. 1994;44:1513–1516. doi: 10.1212/wnl.44.8.1513. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein H, Cochran J, Saxton J, De KS, Newman AB, Kuller LH, Lopez OL, Carter CS. Functional neuroimaging indicators of successful executive control in the oldest old. Neuroimage. 2005;28:881–889. doi: 10.1016/j.neuroimage.2005.05.059. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Schnaider-Beeri M, Grossman HT, Schmeidler J, Wang JY, Lally RC. A phenotype for genetic studies of successful cognitive aging. Am J Med Genet B Neuropsychiatr Genet. 2008;147:167–173. doi: 10.1002/ajmg.b.30483. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Smith CJ, Marin DB, Birstein S, Mare M, Mohs RC, Davis KL. Identifying families with likely genetic protective factors against Alzheimer disease. Am J Hum Genet. 1999;64:832–838. doi: 10.1086/302280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn ] 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sobel E, Louhija J, Sulkava R, Davanipour Z, Kontula K, Miettinen H, Tikkanen M, Kainulainen K, Tilvis R. Lack of association of apolipoprotein E allele epsilon 4 with late-onset Alzheimer's disease among Finnish centenarians. Neurology. 1995;45:903–907. doi: 10.1212/wnl.45.5.903. [DOI] [PubMed] [Google Scholar]
- Sole-Padulles C, Bartres-Faz D, Junque C, Vendrell P, Rami L, Clemente IC, Bosch B, Villar A, Bargallo N, Jurado MA, Barrios M, Molinuevo JL. Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. fMRI activity in the medial temporal lobe during recognition memory as a function of study-test interval. Hippocampus. 2000a;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J Neurosci. 2000b;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Stern Y, Moeller JR, Anderson KE, Luber B, Zubin NR, DiMauro AA, Park A, Campbell CE, Marder K, Bell K, Van Heertum R, Sackeim HA. Different brain networks mediate task performance in normal aging and AD: defining compensation. Neurology. 2000;55:1291–1297. doi: 10.1212/wnl.55.9.1291. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- van den BE, Biessels GJ, de Craen AJ, Gussekloo J, Westendorp RG. The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology. 2007;69:979–985. doi: 10.1212/01.wnl.0000271381.30143.75. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]


