Abstract
Considerable evidence suggests that receptor-mediated excitation and inhibition of brainstem pedunculopontine tegmental (PPT) neurons are critically involved in the regulation of sleep-wake states. However, the molecular mechanisms operating within the PPT controlling sleep-wake states remain relatively unknown. This study was designed to examine sleep-wake state-associated extracellular-signal-regulated kinase 1 and 2 (ERK1/2) transduction changes in the PPT of freely moving rats. The results of this study demonstrate that the levels of ERK1/2 expression, phosphorylation, and activity in the PPT increased with increased amount of time spent in sleep. The sleep-associated increases in ERK1/2 expression, phosphorylation, and activity were not observed in the cortex, or in the immediately adjacent medial pontine reticular formation. The results of regression analyses revealed significant positive relationships between the levels of ERK1/2 expression, phosphorylation, and activity in the PPT and amounts of time spent in slow-wave sleep, rapid eye movement sleep, and total sleep. Additionally, these regression analyses revealed significant negative relationships between the levels of ERK1/2 expression, phosphorylation, and activity in the PPT and amounts of time spent in wakefulness. Collectively, these results, for the first time, suggest that the increased ERK1/2 signaling in the PPT is associated with maintenance of sleep via suppression of wakefulness.
Keywords: ERK1/2 signaling, pedunculopontine tegmental nucleus, sleep, REM sleep, wakefulness
Introduction
The pedunculopontine tegmental nucleus (PPT) is situated in the dorsolateral tegmentum and contains a prominent group of cholinergic neurons as well as some noncholinergic neurons, which project widely throughout the brainstem and forebrain (Mesulam et al. 1983; Garcia-Rill, 1991; Thakkar et al. 1998; Jones, 2004; Wang and Morales, 2009). In a growing body of literature, it is evident that the activation of PPT cells plays a pivotal role in the regulation of wakefulness (W) and rapid eye movement (REM) sleep in both animals and humans (Pace-Schott and Hobson, 2002; Jones, 2004; Datta and MacLean, 2007; Lim et al. 2007; Lydic and Baghdoyan, 2008; Garcia-Rill et al. 2008). The regulation of REM sleep and W both involve important processes of neurotransmitter-mediated excitation and inhibition of PPT cells (Datta, 2010). For example, neuropharmacological studies have demonstrated that glutamate activation of kainate receptors on PPT cholinergic cells induces REM sleep, whereas glutamate activation of N-methyl-D-aspartate (NMDA) receptors induces W (Datta and Siwek, 1997; Datta et al. 2001, 2002; Datta, 2002). On the other hand, the inhibitory neurotransmitter γ-aminobutyric acid (GABA) activates GABA-B receptors which inhibit PPT cholinergic cells and suppress REM sleep and W (Ulloor et al. 2004, Datta, 2007). Recently, it has also been shown that PPT cholinergic neurons exhibit REM sleep-associated immunoreactivity of phosphorylated cyclic adenosine monophosphate (cAMP) response element-binding protein (pCREB), indicating that the PPT intracellular transcription process is involved in the regulation of W and REM sleep (Datta et al. 2009).
Coordinated regulation of intracellular signaling pathways is essential for the receptor activation mediated transcription and translation processes in neuronal function that appear to underlie complex behavioral responses, including the regulation of sleep/wake states (Datta, 2010). Several signaling pathways are thought to play important roles in neuronal receptor activation/inhibition-mediated regulation of the sleep/wake cycle, including cAMP-protein kinase A (cAMP-PKA), Ca2+/calmodulin-dependent protein kinase II (CaMKII) and the mitogen-activated protein kinases (MAPK). Recent studies using a combination of pharmacological and molecular techniques have demonstrated that the activation of cAMP-PKA within the PPT is involved in the induction of REM sleep (Datta and Prutzman, 2005; Bandyopadhya et al. 2006; Datta and Desarnaud, 2010). Similarly, the activation of CaMKII within the PPT has been shown to be involved in promoting W (Stack et al. 2010). Unlike the cAMP-PKA and CaMKII pathways, the role of the MAPK signaling pathway within the PPT in sleep/wake regulation remains unknown.
MAPK represents a highly conserved family of enzymes comprising the extracellular signal-regulated kinases that contain many isoforms (ERK1/2/3/4/5/7), the c-Jun N-terminal kinases/stress-activated protein kinases (JNK/SAPK) and p38 MAPK (Raman et al. 2007). Among the MAPK family, ERK1/2, which contains the subtypes 1 and 2, represents the most studied and thoroughly characterized signaling pathway. ERK1/2 are serine-threonine kinases that are highly expressed in the central nervous system and have emerged as important elements in neuronal signal transduction (Thomas and Huganir, 2004; Gerits et al. 2008). The activation of ERK1/2 is a critical component of the neuronal response (Kornhauser and Greenberg, 1997; Atkins et al. 1998; Coogan et al. 1999; Dolmetsch et al. 2001; Wu et al. 2001). ERK1/2 participates in diverse important neuronal processes, for instance neuronal maturation and survival, synaptic plasticity and learning and memory (Paul et al. 2003; Sweatt, 2004; Braithwaite et al. 2006; Paul and Connor, 2010). A variety of neurotransmitters including glutamate, GABA, acetylcholine, dopamine, and nitric oxide regulate ERK1/2 activity (Yan et al. 1999; Tu et al. 2007; Wang et al. 2007; Socodato et al. 2009). The expression and activity of the ERK1/2 signaling pathway are also known to be modulated by the activation and inactivation of both the cAMP-PKA and the CaMKII signaling pathways (Wang et al. 2004; Gerits et al. 2008). It is also known that the activity of ERK1/2 signaling can be modulated by the activation and inactivation of NMDA, kainate, and GABA-B receptors (Tu et al. 2007; Wang et al. 2007). Therefore, it is reasonable to suggest that the ERK1/2 signaling pathway within the PPT might play a role in neuronal receptor activation/inhibition-mediated regulation of the sleep/wake cycle.
The aim of the present study was to examine the hypothesis that the ERK1/2 signaling pathway within the PPT is involved in the regulation of sleep/wake behavior. To achieve this goal, the levels of ERK1/2 expression and ERK1/2 phosphorylation (pERK1/2) and ERK1/2 activity were quantified within the PPT of freely-moving rats as a function of high and low sleep. The results of this study demonstrate that the expression of ERK1/2, pERK1/2, and the activity of ERK1/2 increased proportionately with increased sleep. These findings suggest that the ERK1/2 signaling pathway within the PPT is involved in the maintenance of sleep by suppressing W.
Materials and Methods
Subjects
Experiments were performed on 25 adult male Wistar rats (Charles River Labs, Wilmington, MA) weighing between 300–350 g. The rats were housed individually at 24°C in a 12/12 hour light/dark cycle (lights on 7:00 a.m.) and allowed ad libitum access to food and water. Experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Boston University Animal Care Committee (AN-14084). Every effort was made to minimize animal suffering and to reduce the number of animals used. To reduce additional stress that might be imposed by the experimental handling, animals were gently handled daily for 15–20 min between 09:00 a.m. and 10:00 a.m. This habituation handling began one week prior to surgery and continued up to the experimental recording sessions.
Surgical procedures for electrode implantation
Stereotaxic surgeries were carried out as previously described (Datta et al. 2009), under pentobarbital anesthesia (40 mg/kg, i.p; Ovation Pharmaceuticals, Deerfield, IL, USA). To record the behavioral states of vigilance, stereotaxically positioned electrodes were chronically implanted to record cortical electroencephalogram (EEG), dorsal neck muscle electromyogram (EMG), and hippocampal EEG (HPC-EEG) activity. Post-operative pain was controlled with buprenorphine (0.05 mg/kg, s.c; Ben Venue Laboratories, Bedford, OH, USA). After a post-surgical recovery of 5–7 days, rats were habituated to the recording cage and free-moving polygraphic recording apparatus (Grass Model 15 Neurodata Amplifier System, Astro-Med, Inc., West Warwick, RI, USA) for about 7 days as described earlier (Bandyopadhya et al. 2006). All adaptation recording sessions were performed between 9:00 a.m. and 11:00 a.m., when rats are normally sleeping.
Assessment of sleep/wake states
After adaptation, rats underwent an experimental recording session, during which polygraphic signals of all rats were recorded between 9:00 a.m. and 11:00 a.m. To determine wake-sleep states, polygraphic data was captured on-line using Gamma software (Grass product group, Astro-Med, Inc., West Warwick, RI, USA). From this captured data, using previously described criteria (Bandyopadhya et al. 2006; Datta et al. 2009), three behavioral states [wakefulness (W), slow-wave sleep (SWS), and REM sleep] were distinguished and scored visually in 5-second epochs using Rodent Sleep Stager software (Grass product group, Astro-Med, Inc., West Warwick, RI, USA). These scored data of the two-hour recording period were then expressed as percentages of total time spent in W, SWS, REM sleep, and total sleep (SWS + REM sleep). These rats were then divided into two experimental groups based on total percentages of time spent in sleep: 1) High sleep (total sleep > 50%) and 2) Low sleep (total sleep < 50%).
Tissue collection
Immediately after the end of the experimental recording session (at 11 a.m.), rats were sacrificed with carbon dioxide, and the brains were quickly removed and frozen using dry ice. In order to minimize possible variations due to differences in the sleep-wake state, at the time of death all animals were awakened by shaking their cages and kept awake for 1 min before they were sacrificed with carbon dioxide. To rule out any diurnal factors contributing to the different levels of ERK1/2 expression, phosphorylation, and activity in the different groups, all rats were sacrificed at a fixed time of day. The frozen brains were stored in 50 ml falcon tubes at −80°C until dissection. From the frozen brain, the pedunculopontine tegmentum (PPT), medial pontine reticular formation (mPRF) and frontal cortex (CTX) were then dissected on an ice-chilled petri dish as described earlier (Bandyopadhya et al. 2006; Stack et al. 2010). The dissected brain parts were processed immediately for the assessment of ERK1/2 expression, ERK1/2 phosphorylation, and ERK1/2 activity.
Assessment of ERK1/2 expression and phosphorylation by Western blotting
The brain regions were homogenized on ice using a tissue dounce in 500 µl of an ice-cold tissue lysate buffer containing 50 mM Tris-Hcl pH 7.4, 1.5% Triton X-100, 150 mM sodium chloride, 5 mM EDTA, 1 mM sodium orthovanadate, 25 mM sodium fluoride, 5 mM phenylmethylsulfonyl fluoride, 2 µg/ml pepstatin A and one Mini tablet (Roche, Indianapolis, IN, USA). The lysate was then centrifuged at 20,000 g for 10 minutes at 4°C. The crude protein extract contained in the supernatant was removed from each sample and assayed for total protein concentration using a bicinchoninic acid assay kit (Thermo Fisher, Rockford, IL, USA), measuring the spectrophotometric signal at 562 nm with a Benchmark Plus spectrophotometer (Bio-rad, Hercules, CA, USA). An appropriate volume of 4× sample buffer was added to the homogenate, and the sample was incubated in a 95°C water bath for 5 minutes. Equivalent amounts of proteins were loaded onto 10% SDS-polyacrilamide gels and resolved by standard electrophoresis (Bio-Rad minigel apparatus, Hercules, CA, USA). For ERK1/2 expression analysis, 10 µg of PPT protein extract, 2 µg of mPRF protein extract or 5 µg of CTX protein extract were loaded per lane. For phospho-ERK1/2 expression analysis, 30 µg of PPT, mPRF or CTX protein extracts were loaded per lane. Then the gels were blotted electrophoretically to polyvinylidene difluoride (PVDF) membranes (Millipore, San Jose, CA, USA) using a transfer tank maintained at 4°C with typical parameters being a one hour transfer at a constant current of 350 mA. PVDF membranes were blocked for one hour at room temperature in PBST buffer containing 10 mM phosphate buffered saline pH 7.4, 0.1% Tween 20 and supplemented with 5% non-fat dry milk (Bio-Rad, Hercules, CA, USA). All antibody applications were carried out in PBST.
To assess total ERK1/2 expression, PVDF membranes were incubated overnight at 4°C in PBST containing 5% bovine serum albumin (BSA) and an anti-ERK1/2 rabbit monoclonal antibody (Clone D13.14.4E, Cell Signaling Technology, Danvers, MA) at a 1:1000 dilution. Then PVDF membranes were incubated for two hours at room temperature in PBST containing 5% non-fat dry milk and a horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (GE Healthcare, Piscataway, NJ, USA) at a 1:3000 dilution. In this and all the other Western blot protocols described below, the blots were washed extensively in PBST after incubations with the primary or secondary antibodies (typically 3 washes, each for 5 to 10 minutes).
To assess ERK1/2 phosphorylation, PVDF membranes were incubated overnight at 4°C in PBST containing 5% bovine serum albumin (BSA) and an anti-phosphothreonine 202-phosphotyrosine 204 – ERK1/2 rabbit monoclonal antibody (Clone 137F5, Cell Signaling Technology, Danvers, MA, USA) at a 1:1000 dilution. Then PVDF membranes were incubated for 2 hours at room temperature in PBST containing 5% non-fat dry milk and an HRP-conjugated anti-rabbit secondary antibody (GE Healthcare, Piscataway, NJ, USA) at a 1:2000 dilution.
Variations of ERK1/2 and pERK1/2 amounts due to sample preparation and protein loading onto the electrophoresis gels were controlled by subsequent assessment of alpha-tubulin immunoreactivity on the same PVDF membrane. To prepare reprobing with an anti alpha-tubulin mouse monoclonal antibody (clone DM1A, Millipore, Billerica, MA, USA), blots were incubated at 70°C in three changes of stripping buffer (150 mM Tris-Hcl, pH 6.8, 100 mM β–mercaptoethanol and 2% SDS) with constant agitation for a total of one hour. The blots were then extensively washed with PBST and blocked for one hour at room temperature in PBST containing 5% non-fat dry milk. The blots were incubated sequentially overnight at 4°C with the anti alpha-tubulin antibody at a 1:1000 dilution and two hours at room temperature with an HRP-conjugated anti-mouse secondary antibody (GE Healthcare, Piscataway, NJ, USA) at a 1:5000 dilution. The resulting immunocomplexes were detected with a chemiluminescent substrate (SuperSignal, WestDura, Pierce, Rockford, IL, USA). The quantification of the immunoreactive bands was carried out using a Kodak Imaging Station (PerkinElmer, Shelton, Connecticut, USA). The net intensity of each ERK1/2 and pERK1/2 chemiluminescent signal was normalized with the net intensity of the corresponding alpha-tubulin signal.
Assessment of ERK1/2 activity
The brain regions were homogenized on ice using a tissue dounce in 500 µl of an ice-cold tissue lysate buffer (buffer A) containing 50 mM Tris-Hcl pH 7.4, 1 % Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.5 mM sodium orthovanadate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.1 mM phenylmethylsulfonyl fluoride, 0.1% 2-mercaptoethanol, 10 mM sodium β-glycerol phosphate, 1 µg/ml pepstatin A, aprotinin, leupeptin and 1 µM microcystin. The lysate was centrifuged at 20,000 g for ten minutes at 4°C. The crude protein extract contained in the supernatant was removed from each sample and assayed for total protein concentration using a bicinchoninic acid assay kit as described above.
ERK1/2 activity was assayed using a MAP Kinase/ERK immunoprecipitation kinase assay kit following the manufacturer’s instructions (Millipore, Temecula, CA, USA). The assay kit is designed to measure the phosphotransferase activity in an immunocomplex formed between the MAP kinase R2 antibody and ERK1/2. The precipitated enzyme is used to phosphorylate a specific substrate, myelin basic protein (MBP). The phosphorylated substrate is then analyzed by Western immunoblot using an antibody specific for phosphorylated MBP.
Two hundred µg of lysate proteins were immunoprecipitated for 2 hours at 4°C with 10 µl of anti-MAP Kinase/ERK1/2 agarose conjugate in a final volume of 500 µl of buffer A. The agarose/ERK1/2 immunocomplex was washed twice with 500 µl of buffer A and then incubated for 20 minutes at 30°C in a total volume of 40 µl of buffers provided by the manufacturer (manufacturer details). For the PPT and mPRF, 2.5 µl and for the CTX, 0.8 µl of the reaction mixture was analyzed on a 15% SDS-polyacrilamide gel and electrotransferred on a PVDF membrane as described above. PVDF membrane was blocked in PBS buffer containing 3% nonfat dry milk for 20 minutes at room temperature and then incubated overnight at 4°C with an anti-phospho MBP antibody (clone P12, Millipore) at a 1:2000 dilution in freshly prepared PBS containing 3% nonfat dry milk. The PVDF membrane was washed twice with water and incubated for 2 hours at room temperature with an HRP-conjugated anti-mouse secondary antibody (GE Healthcare, Piscataway, NJ, USA) at a 1:5000 dilution. After 2 washes with water and one wash with PBS containing 0.05% Tween, the immunocomplexes were detected and quantified as described above. Controls for endogenous phosphorylation of proteins in the sample extract were carried out by omitting the manufacturer’s buffer containing MBP substrate. No phosphorylation of endogenous proteins was detected.
Statistical analyses
Analysis of interval scale data involving only two independent groups was accomplished through utilization of the standard unpaired t-test (two-tailed). To assess relative correlations between ERK1/2 expression, ERK1/2 phosphorylation or ERK1/2 activity in the PPT, mPRF, or CTX, and the percentages of time spent in W, SWS, REM sleep, or total sleep during the two hours of recording period, Pearson correlations, which assumes a Gaussian distribution, were calculated. As a standard rule of statistical analysis, before individual statistical tests, group data were subjected to normality testing, which confirmed that the data meets normality assumptions. All analyses were performed using Graphpad Prism statistical software (v5.0; GraphPad Software, La Jolla, CA).
Results
Sleep-wake associated changes in the expression and phosphorylation of ERK1/2
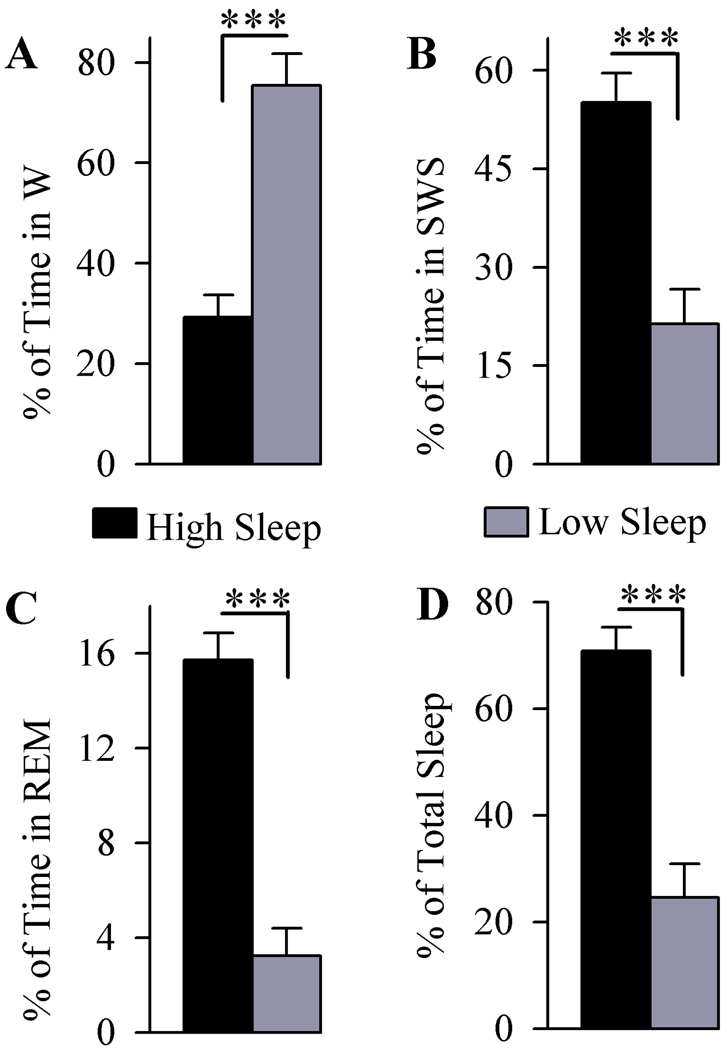
To determine the sleep-wake associated changes in the expression and phosphorylation of ERK1/2, a total of 12 rats were used (6 with high sleep and 6 with low sleep). Figure 1 shows these animals’ total percentages of time in W, SWS, REM sleep and total sleep during the two hours of recordings with free sleep-wake cycles. Immediately at the end of these two hours of free sleep-wake recordings, animals were sacrificed to analyze the ERK1/2 expression and phosphorylation in the PPT, mPRF, and CTX. During the two hours of free sleep-wake recording, a comparison of the total percentage of time spent in W between high and low sleep groups revealed significantly less W time in the high sleep group (62.25% less; df = 10, t = 5.98, p<0.001). Compared with the low sleep group, the high sleep group spent significantly more time in SWS (157.96% more; df = 10, t = 4.84, p<0.001) and REM sleep (386.38% more; df = 10, t = 7.57, p<0.001). These results revealed that the animals in the high sleep group spent 188.08% more time in sleep than the low sleep group (df = 10, t = 5.99, p<0.001). Since these two groups of rats were subjected to identical experimental conditions and brain tissues were collected at an identical time of day, any variations in the expression and phosphorylation of ERK1/2 in the PPT, mPRF, and CTX between these two groups of rats would be mainly due to their differences in the time spent in sleep and/or wake.
Figure 1.
Analysis of the total percentage (mean and SEM) of time spent in wakefulness (W; A), slow-wave sleep (SWS; B), rapid eye movement sleep (REM sleep; C) and total sleep (D) in animals used for expression and phosphorylation of ERK 1/2. Examination of the total time spent in W (A) revealed that the high sleep group spent less time in W compared to low sleep group. Analysis of the total time spent in SWS (B) and REM sleep (C) revealed that the high sleep group spent more time than low sleep group did in SWS and REM sleep. Overall, the high sleep group spent more time in sleep than the low sleep group (D). *** p<0.001.
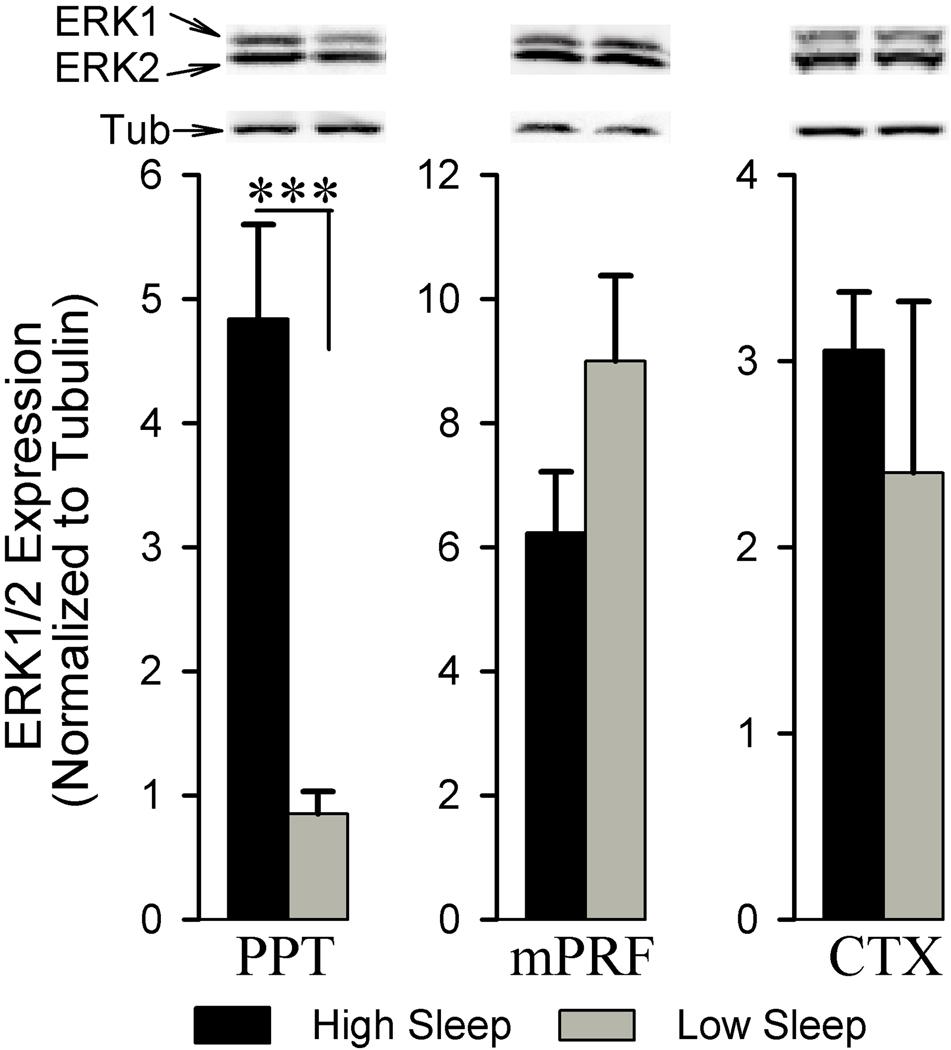
Western blot analyses were performed to probe the ERK1/2 expression within the PPT, mPRF, and CTX associated with high and low sleep. Figure 2 shows sleep-wake associated ERK1/2 expression changes in the PPT, mPRF, and CTX. The results show that the ERK1/2 expression within the PPT was significantly higher in the high sleep group compared to the low sleep group (466.53% higher; df = 10, t = 5.13, p<0.001). ERK1/2 expression in the mPRF was relatively less in the high sleep group than in the low sleep group, but this difference was not statistically significant(df = 10, t = 1.64, p = 0.133). Similar comparisons between high and low sleep groups did not reveal any significant difference in the ERK1/2 expression within the CTX (df = 10, t = 0.68, p = 0.509). These data suggest that alterations in the ERK1/2 expression within the PPT, but not in the mPRF and CTX, are coincident with the amounts of time spent in W and sleep.
Figure 2.
Analysis of ERK1/2 expression in the pedunculopontine tegmentum (PPT), medial pontine reticular formation (mPRF), and cortex (CTX) of high and low sleep subjects. Densitometric measurements from western blot of ERK1/2 expression in the PPT revealed a significant increase (466.53%) in the high sleep group compared to the low sleep group. In contrast, western blot analysis of ERK1/2 expression in the adjacent mPRF and the CTX revealed no significant difference between the high and low sleep groups. All analyses of ERK1/2 expression are normalized against α-tubulin. *** p<0.001.
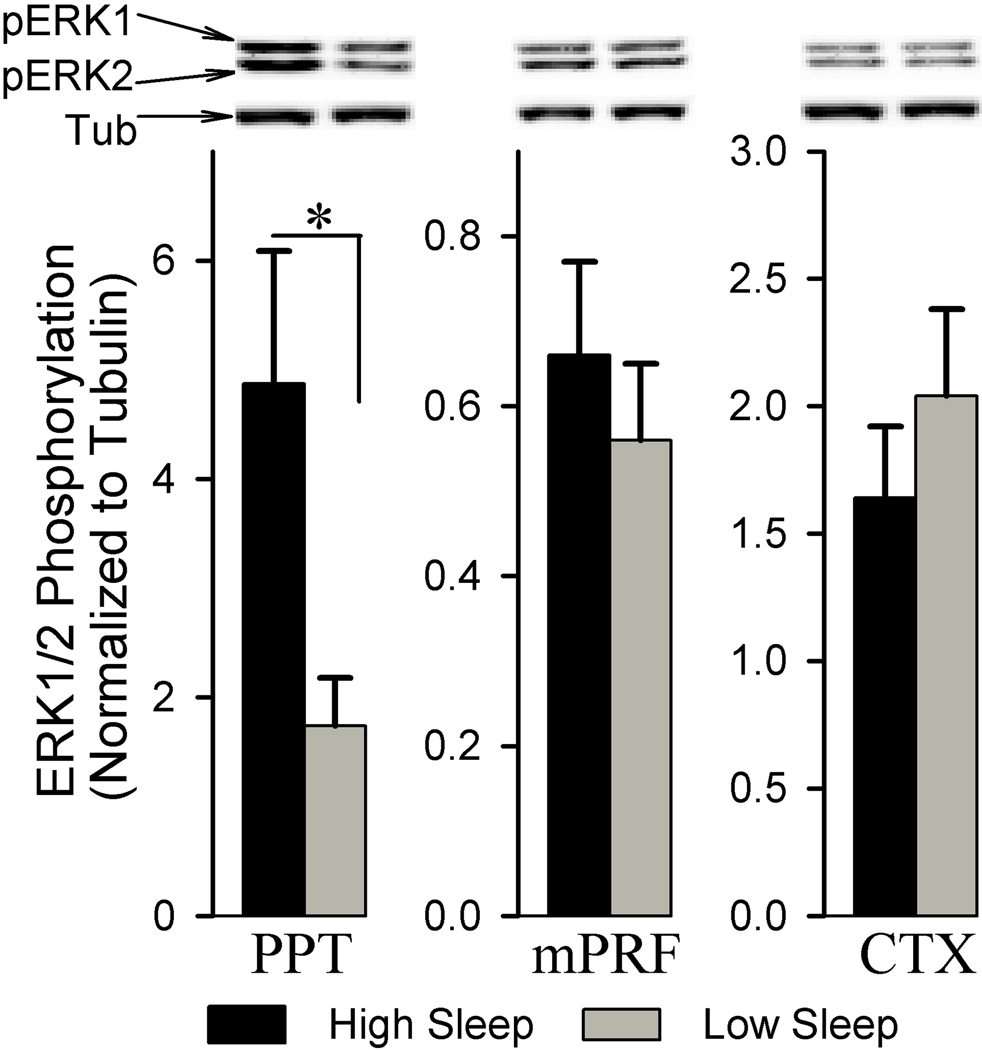
We also examined the phosphorylation of ERK1/2 in the PPT, mPRF, and CTX associated with the amount of time spent in W and sleep. Figure 3 shows sleep-wake associated changes in ERK1/2 phosphorylation within the PPT, mPRF, and CTX. Analysis of ERK1/2 phosphorylation reveals that the phosphorylation of ERK1/2 within the PPT was significantly higher in the high sleep group than in the low sleep group (179.90% higher; df = 10, t = 2.41, p<0.05). This sleep-wake associated change in the ERK1/2 phosphorylation was anatomical region-specific, as the similar analysis of ERK1/2 phosphorylation within the mPRF (df = 10, t = 0.70, p = 0.498) and CTX (df = 10, t = 0.90, p = 0.387) reveals no significant difference between the high and low sleep groups.
Figure 3.
Analysis of ERK1/2 phosphorylation in the pedunculopontine tegmentum (PPT), medial pontine reticular formation (mPRF), and cortex (CTX) of high and low sleep subjects. Densitometric measurements from western blot of pERK1/2 expression in the PPT revealed a significant increase (179.90%) in the high sleep group compared to the low sleep group. In contrast, western blot analysis of pERK1/2 expression in the adjacent mPRF and the CTX revealed no significant difference between the high and low sleep groups. All analyses of pERK1/2 expression are normalized against α-tubulin. *p<0.05.
Sleep-wake associated changes in the ERK1/2 activity
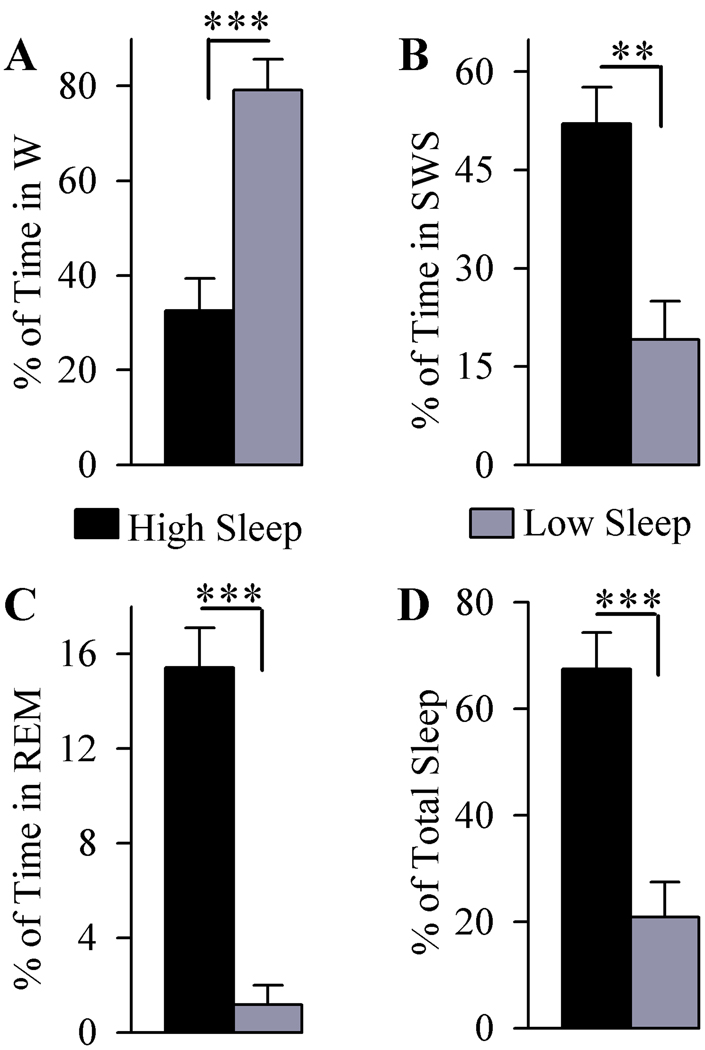
To determine the sleep-wake associated changes in the activation of ERK1/2, a total of 13 rats were used (6 with high sleep and 7 with low sleep). Figure 4 shows the total percentages of time spent in W, SWS, REM sleep and total sleep during the two hours of recordings with free sleep-wake cycles. Compared to the low sleep group, the total percentage of time spent in W was significantly less in the high sleep group (58.85% less; df = 11, t = 4.91, p<0.001). Conversely, comparisons between high and low sleep groups revealed that the high sleep group spent significantly more time in SWS (172.14% more; df = 11, t = 4.03, p<0.01), REM sleep (768.58% more; df = 11, t = 7.68, p<0.001), and total sleep (222.87% more; df = 11, t = 4.92, p<0.001).
Figure 4.
Analysis of the total percentage (mean and SEM) of time spent in wakefulness (W; A), slow-wave sleep (SWS; B), rapid eye movement sleep (REM sleep; C) and total sleep (D) in animals used for ERK1/2 activity. Examination of the total time spent in W (A) revealed, compared to the low sleep group, high sleep group spent less time in W (58.85% less) and more time in SWS (172.14% more) and REM sleep (768.58% more). The high sleep group spent more time (222.87% more) in total sleep (D) than the low sleep group. *** p<0.001, ** p<0.01.
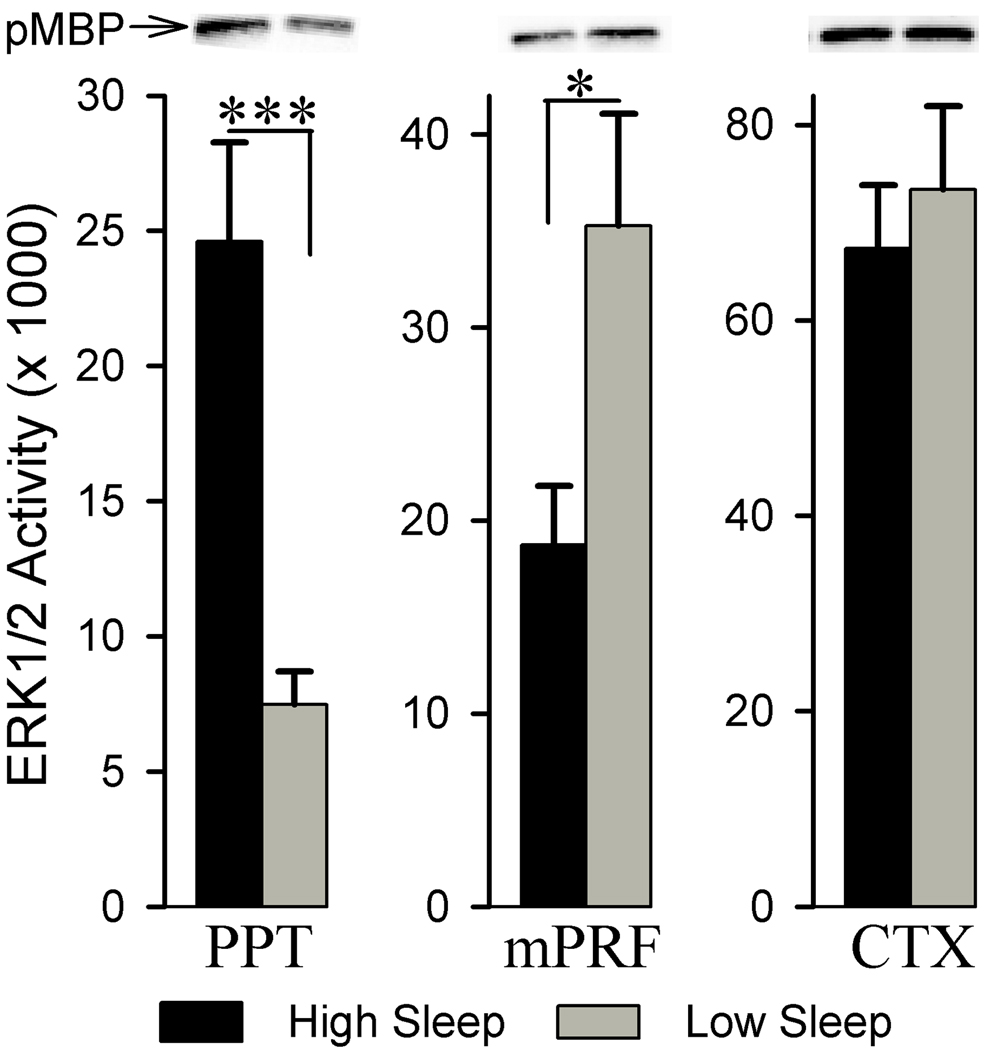
Comparison between these high and low sleep groups reveals that the ERK1/2 activity within the PPT was significantly greater in the high sleep group compared to the low sleep group (229.24% more; df = 11, t = 4.74, p<0.001, Fig. 5). On the contrary, ERK1/2 activity within the mPRF was significantly less in the high sleep group compared to the low sleep group (46.87% less; df = 11, t = 2.40, p<0.05). Unlike in the PPT and mPRF, ERK1/2 activities within the CTX are not significantly different between the high sleep and low sleep groups (df = 11, t = 0.55, p = 0.593).
Figure 5.
Analysis of ERK1/2 activity in the pedunculopontine tegmentum (PPT), medial pontine reticular formation (mPRF), and cortex (CTX) of high and low sleep subjects. Densitometric measurements from western blots of phosphorylated myelin basic protein (pMBP) revealed that, compared to the low sleep group, in the high sleep group, ERK1/2 activity is higher (229.24% higher) within the PPT and lower within the mPRF (46.87% less). *** p<0.001, * p<0.05.
Relationship between the expression, phosphorylation, and activity of ERK1/2 and amount of time spent in W, SWS, REM sleep, and total sleep
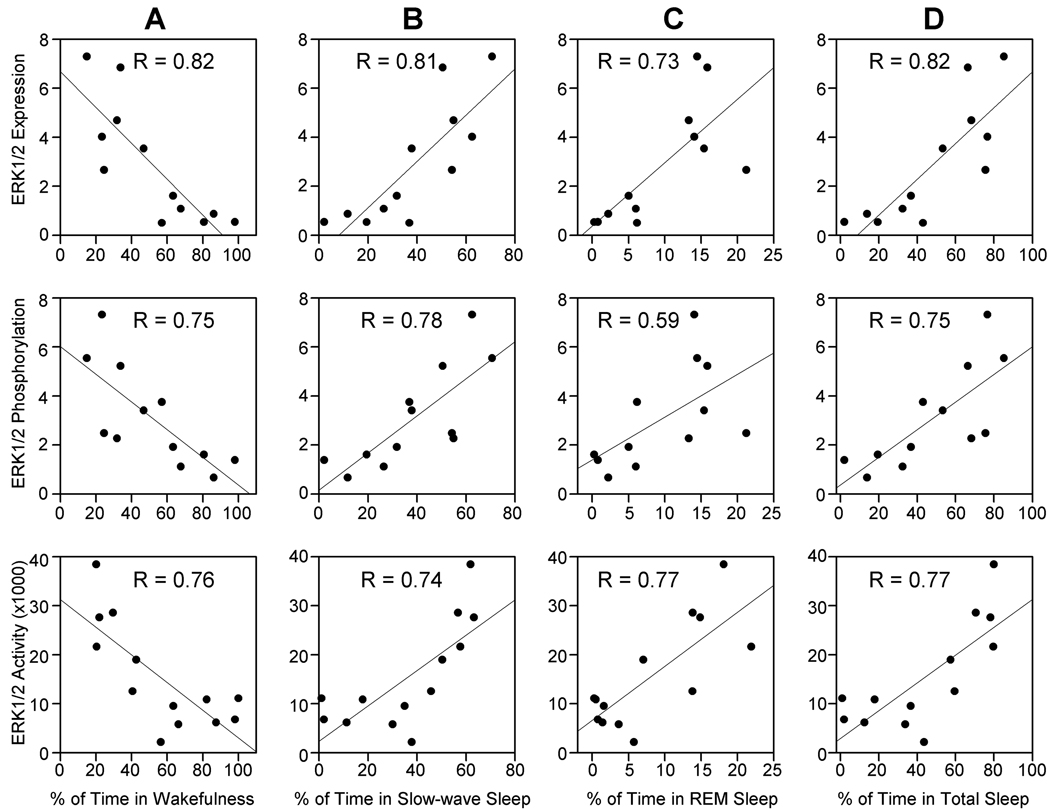
The results of this study document that the expression, phosphorylation, and activity of ERK1/2 within the PPT were higher in the high sleep group of rats. Based on these results, we expected to see some relationships (linear regression analysis) between individual animals’ total percentages of time spent in W, SWS, REM sleep, or total sleep and the levels of ERK1/2 expression, phosphorylation, or activity within the PPT. The results of these regression analyses reveal significant negative relationships between (Fig. 6): total percentages of time spent in W and PPT levels of ERK1/2 expression (R = 0.82, df = 12, F = 20.17, p<0.01); total percentages of time spent in W and PPT levels of ERK1/2 phosphorylation (R = 0.75, df = 12, F = 13.04, p<0.01); total percentages of time spent in W and PPT levels of ERK1/2 activity (R = 0.76, df = 12, F = 15.42, p<0.01). Significant positive relationships were found between: total percentages of SWS and PPT levels of ERK1/2 expression (R = 0.81, df = 12, F = 19.58, p<0.01); total percentages of SWS and PPT levels of ERK1/2 phosphorylation (R = 0.78, df = 12, F = 15.17, p<0.01); total percentages of SWS and PPT levels of ERK1/2 activity (R = 0.74, df = 12, F = 13.35, p<0.01). To our surprise, the regression analyses also reveals significant positive relationships between: total percentages of REM sleep and PPT levels of ERK1/2 expression (R = 0.73, df = 12, F = 11.73, p<0.01); total percentages of REM sleep and PPT levels of ERK1/2 phosphorylation (R = 0.59, df = 12, F = 5.40, p<0.05); total percentages of REM sleep and PPT levels of ERK1/2 activity (R = 0.77, df = 12, F = 16.07, p<0.01). The results of these regression analyses suggest that the increased ERK1/2 expression, phosphorylation, and activation may not be responsible for the regulation of any specific stages of sleep, but rather suggest that ERK1/2 is involved in the maintenance of sleep in general by suppressing W state. This suggestion is also supported by the results of regression analyses that demonstrate significant positive relationships between: total percentages of total sleep and PPT levels of ERK1/2 expression (R = 0.82, df = 12, F = 20.14, p<0.01); total percentages of total sleep and PPT levels of ERK1/2 phosphorylation (R = 0.75, df = 12, F = 13.00, p<0.01); total percentages of total sleep and PPT levels of ERK1/2 activity (R = 0.77, df = 12, F = 15.62, p<0.01).
Figure 6.
The relationships between the levels of ERK1/2 expression, phosphorylation, and activation in the PPT and percentages of time spent in wakefulness (A), slow-wave sleep (B), REM sleep (C), and total sleep (D). Linear regression analyses showed negative correlation between ERK1/2 expression, phosphorylation, and activity within the PPT and individual animals’ total percentage of time spent in W (A), whereas ERK1/2 expression, phosphorylation, and activity within the PPT were positively correlated with individual animals’ total percentage of time spent in SWS (B) REM sleep (C) and total sleep (D). For each linear regression analysis, the correlation coefficient (R) is indicated in the respective graph.
Discussion
The present study, for the first time, highlights the ERK signaling system in the PPT and its role in the regulation of sleep-wake behavior. The principal findings of this study are as follows: (1) levels of ERK1/2 expression and phosphorylation in the PPT, but not in the mPRF or CTX, increased with increased amount of time spent in sleep; (2) with increased amount of time spent in sleep, levels of ERK1/2 activity increased in the PPT and decreased in the mPRF; (3) levels of ERK1/2 expression, phosphorylation, and activity in the PPT of individual animals were positively correlated with their total percentages of time spent in SWS, REM sleep and total sleep; (4) levels of ERK1/2 expression, phosphorylation, and activity in the PPT of individual animals were negatively correlated with their total percentages of time spent in W. These results suggest that the ERK1/2 signaling system in the PPT is involved in the maintenance of sleep.
The ERK1/2 signaling pathway is an important component of numerous signaling cascades within neurons (Grewal et al. 1999). It is known that once activated, ERK1/2 translocate from the cytoplasm to the nucleus and activate a specific set of transcription factors, including pCREB (Vanhoutte et al. 1999), which facilitates the subsequent expression of target genes. ERK1/2 activity is tightly regulated by the opposing actions of MAPK kinases and several protein phosphatases (Keyse, 2000; Saxena and Mustelin, 2000). These regulating actions tune the duration and magnitude of activation of ERK1/2, which are critical components of the neuronal response (Marshall, 1995; Vincent at al. 1998; Yan et al. 1999). Activation of ERK1/2 by MAPK kinases requires phosphorylation of a threonine and a tyrosine residue in the activation domain (Robinson and Cobb, 1997). Three different classes of protein phosphatases reverse this process by dephosphorylation of the phosphothreonine and/or phosphotyrosine residues. These include the dual-specificity phosphatases (Camps et al. 2000), serine/threonine phosphatases (Alessi et al. 1995), and tyrosine phosphatases (Pulido et al. 1998; Saxena et al. 1999).
In the present study, we observed that the expression, phosphorylation, and activation of ERK1/2 in the PPT increased with increased sleep. The results presented here also provide evidence that this sleep-associated up-regulation of ERK1/2 signaling in the PPT is anatomically site-specific. However, this anatomical site specificity requires future testing and validation by a microinjection study that will examine the effects of pharmacologically active agonists and antagonists of ERK1/2 in the PPT and additional sleep-wake state regulating sites. Since this is the first study that examined sleep-wake associated changes in ERK1/2 signaling in the PPT, the results could not be compared directly with any other published investigations. However, a recent study on Drosophila melanogaster has shown that the activation of ERK1/2 signaling increases sleep in a dose-dependent manner, and that blockade of its expression in the nervous system decreases sleep (Foltenyi et al. 2007). Collectively, these results suggest that the up-regulation of ERK1/2 signaling in the brain is a critical step in the regulation of sleep.
Neurotransmitter receptor activation-mediated excitation and inhibition of PPT cells are important processes for the regulation of sleep-wake behavior (Datta and MacLean, 2007; Datta, 2010). The initiation of W requires NMDA receptor activation-mediated postsynaptic depolarization and Ca2+ influx into the PPT cells (Datta and Siwek, 1997; Datta et al. 2001, 2002) and increased activation of CaMKII signaling in the PPT (Stack et al. 2010). It is also known that the activation of CaMKII increases ERK1/2 phosphorylation and activity (Wang et al. 2007). Therefore, it would be expected that the phosphorylation and activation of ERK1/2 in the PPT would increase with increased W. On the contrary, however, the results of the present study demonstrate that the phosphorylation and activation of ERK1/2 in the PPT decreased with increased W. This seemingly surprising observation can be reconciled by the fact that the activation of NMDA receptors also activates a negative–feedback pathway that involves dephosphorylation and subsequent activation of Striatal Enriched Tyrosine Phosphatase (STEP) by calcineurin (Paul et al. 2003). This activated STEP then quickly dephosphorylates and inactivates ERK1/2 activity (Paul et al. 2003; Venkitaramani et al. 2009). Since STEP is involved in the inactivation of ERK1/2, STEP activation by the NMDA receptor may have prevented the sustained activation of ERK1/2 in the PPT during W. An alternative explanation is that during W, when PPT neurons are firing robustly, metabolic byproducts, including adenosine diphosphate (ADP), accumulate (Datta and Siwek, 2002; Thakkar et al. 2003; Datta and MacLean, 2007). It has been established that dephosphorylation increases with increasing ADP concentrations, and this process could, in turn, cause activation of STEP (Kim et al. 2001). Activated STEP dephosphorylates and inactivates ERK1/2 (Paul et al. 2003; Venkitaramani et al. 2009); therefore, the neuronal activation-dependent increase of ADP concentrations is another potential mechanism by which the phosphorylation and activation of ERK1/2 in the PPT decreased with increased W.
Earlier physiological and pharmacological studies have shown that the activation of GABA-B receptors on PPT cells increases SWS by suppressing W and REM sleep (Gauthier et al. 1997; Ulloor et al. 2004; Datta, 2007). It is known that the activation of GABA-B receptors on many different cell types, including brain cells, increases phosphorylation and activation of the ERK1/2 signaling pathway (Vanhoose et al. 2002; Wetzker and Bohmer, 2003; Tu et al. 2007; Richer et al. 2009) and enhances down-regulation of CaMKII and PKA signaling pathways (Datta, 2007; Stack et al. 2010). Thus, it is reasonable to suggest that, in the present study, during SWS, the increased expression, phosphorylation, and activation of ERK1/2 signaling in the PPT may have been caused by the activation of GABA-B receptors on the PPT cells. To our surprise, the results of this study also demonstrate that the activity of ERK1/2 signaling increased with increased REM sleep. Since the activation of GABA-B receptors on the PPT suppresses REM sleep, it is unlikely that this increased activation of ERK1/2 signaling in the PPT during REM sleep was caused by the activation of GABA-B receptors. Earlier pharmacological studies have shown that the activation of kainate receptors in the PPT increases REM sleep by suppressing W (Datta, 2002; Datta et al., 2002). It has also been shown that the activation of kainate receptors activates ERK1/2 signaling (Cruise et al. 2000; Fuller et al. 2001; Mao et al. 2004; Grabauskas et al. 2007). Since the activation of PPT kainate receptors increases REM sleep without suppressing SWS, it is logical to suggest that the increased activation of ERK1/2 signaling in the PPT during REM sleep may have been caused by the activation of kainate receptors (Datta and Desarnaud, 2010). Since the increased PPT ERK1/2 expression and activity are maintained during both SWS and REM sleep, the up-regulation of the PPT ERK1/2 signaling might be involved in the consolidation of sleep by suppressing W rather than in the regulation of a specific sleep stage. Therefore, the sustained ERK1/2 activity during sleep could enable the expression of specific down stream PPT target genes, which might be responsible for the consolidation of sleep.
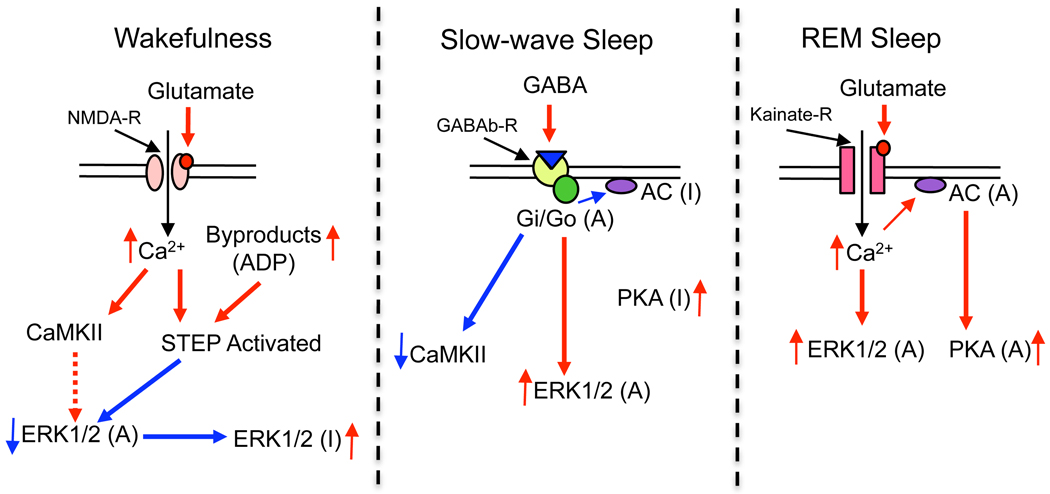
Mammalian cells possess numerous signal transduction pathways and their interactions with each other are different in different cell types (Impey et al. 1998; Saxena et al. 1999; Soderling, 1999; Cammarota et al. 2008; Gerits et al. 2008; Ji et al. 2009; Alter et al. 2010). These differential interactions of numerous signaling pathways regulate the distribution, duration, intensity and specificity of the cellular response (Marshall, 1995; Enslen et al. 1996; Vincent et al. 1998; Yan et al. 1999; Fujisawa, 2001; Praskova et al. 2002; Paul et al. 2003). Recent studies have shown that the activation of the PKA signaling pathway in PPT cells promotes REM sleep (Datta and Prutzman, 2005; Bandyopadhya et al. 2006; Datta and Desarnaud, 2010), whereas activation of the CaMKII pathway in PPT cells promotes W by suppressing REM sleep (Stack et al. 2010). Additionally, the results of the present study show that activation of the ERK1/2 signaling pathway in PPT cells promotes both SWS and REM sleep by suppressing W. Thus, it appears that like in other cell types, in the PPT cells, PKA, CaMKII, and ERK1/2 signaling systems interact to regulate sleep-wake stages. On the basis of present findings and our previous studies as well as the studies of others (Datta et al. 2001, 2002; Datta and Siwek, 2002; Vanhoose et al. 2002; Paul et al. 2003; Wetzker and Bohmer, 2003; Ulloor et al. 2004; Bandyopadhya et al. 2006; Datta, 2007; Tu et al. 2007; Wang et al. 2007; Richer et al. 2009; Stack et al. 2010; Venkitaramani et al. 2009; Wang et al. 2007; Datta and Desarnaud, 2010), we propose a working model for the signal transduction interactions in the PPT that lead to the regulation of sleep-wake stages (Fig. 7). W is the behavioral and physiological manifestation of NMDA receptor activation-mediated up-regulation of CaMKII and ERK1/2 signaling and activation of STEP in the PPT. This transient up-regulation of the ERK1/2 signal is then quickly down-regulated by the activated STEP. However, the up-regulation of CaMKII remains throughout the W period. SWS is the behavioral and physiological representation of GABA-B receptor activation-mediated up-regulation of ERK1/2 and down-regulation of CaMKII and PKA signaling in the PPT. REM sleep is the physiological and behavioral result of kainate-receptor activation-mediated up-regulation of PKA and ERK1/2 and down-regulation of CaMKII signaling in the PPT.
Figure 7.
Working model for intracellular signal transduction interactions in the pedunculopontine tegmental (PPT) cholinergic neurons involved in the regulation of wakefulness, slow-wave sleep, and REM sleep. Long red arrows with solid lines between molecules: direction of activating effects on the target molecule. Long blue arrows with solid lines between molecules: inactivating effects on the target molecule. Dotted red arrow: a momentary activation before the effect disappears. Short red and blue arrows: increased and decreased levels of molecules, respectively. Symbols: (A) and (I) next to molecules indicate its active and inactive forms.
In conclusion, molecular data reported here indicate that activation of the ERK1/2 signaling system in the PPT is an important cellular and molecular step for the regulation of consolidated sleep. These data are consistent with the view that receptor-mediated activation and interactions of different signaling pathways in the PPT are important mechanisms for the regulation of sleep-wake behavior. Additionally, the PPT studies of the molecular signaling pathway that regulates sleep-wake behavior provide the molecular background necessary for beginning a genetic analysis.
Acknowledgements
This research was supported by National Institutes of Health Research Grants MH59839 and NS34004. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We acknowledge Mr. Matthew O’Malley for technical assistance. We thank Dr. Elissa H. Patterson and Dr. Donald F. Siwek for their valuable discussions on this manuscript.
Abbreviations used
- ADP
adenosine diphosphate
- cAMP
cyclic adenosine monophosphate
- Ca2+
calcium
- CaMKII
calcium/calmodulin kinase II
- CREB
cAMP response element binding protein
- CTX
cortex
- EEG
electroencephalogram
- EMG
muscle electromyogram
- ERK
extracellular signal-regulated kinase
- GABA
γ-aminobutyrate
- HPC-EEG
hippocampal EEG
- mPRF
medial pontine reticular formation
- MAPK
mitogen-activated protein kinases
- MBP
myelin basic protein
- NMDA
N-methyl-D-aspartate
- pCaMKII
phosphorylated Ca2+/calmodulin kinase II
- PKA
protein kinase A
- PPT
pedunculopontine tegmentum
- REM
rapid eye movement
- STEP
Striatal Enriched Tyrosine Phosphatase
- SWS
slow-wave sleep
- W
wakefulness.
Footnotes
Disclosure/conflict of interest
The authors declare there is no conflict of interest.
References
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr. Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- Alter BJ, Zhao C, Karim F, Landreth GE, Gereau RWt. Genetic targeting of ERK1 suggests a predominant role for ERK2 in murine pain models. J. Neurosci. 2010;30:11537–11547. doi: 10.1523/JNEUROSCI.6103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bandyopadhya RS, Datta S, Saha S. Activation of pedunculopontine tegmental protein kinase A: a mechanism for rapid eye movement sleep generation in the freely moving rat. J. Neurosci. 2006;26:8931–8942. doi: 10.1523/JNEUROSCI.2173-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Medina JH, Izquierdo I. ERK1/2 and CaMKII-mediated events in memory formation: is 5HT regulation involved? Behav. Brain. Res. 2008;195:120–128. doi: 10.1016/j.bbr.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- Coogan AN, O'Leary DM, O'Connor JJ. P42/44 MAP kinase inhibitor PD98059 attenuates multiple forms of synaptic plasticity in rat dentate gyrus in vitro. J. Neurophysiol. 1999;81:103–110. doi: 10.1152/jn.1999.81.1.103. [DOI] [PubMed] [Google Scholar]
- Cruise L, Ho LK, Veitch K, Fuller G, Morris BJ. Kainate receptors activate NF-kappaB via MAP kinase in striatal neurones. Neuroreport. 2000;11:395–398. doi: 10.1097/00001756-200002070-00034. [DOI] [PubMed] [Google Scholar]
- Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J. Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- Datta S. Activation of pedunculopontine tegmental PKA prevents GABAB receptor activation-mediated rapid eye movement sleep suppression in the freely moving rat. J. Neurophysiol. 2007;97:3841–3850. doi: 10.1152/jn.00263.2007. [DOI] [PubMed] [Google Scholar]
- Datta S. Cellular and chemical neuroscience of mammalian sleep. Sleep Med. 2010;11:431–440. doi: 10.1016/j.sleep.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Desarnaud F. Protein kinase A in the pedunculopontine tegmental nucleus of rat contributes to regulation of rapid eye movement sleep. J. Neurosci. 2010;30:12263–12273. doi: 10.1523/JNEUROSCI.1563-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci. Biobehav. Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Prutzman SL. Novel role of brain stem pedunculopontine tegmental adenylyl cyclase in the regulation of spontaneous REM sleep in the freely moving rat. J. Neurophysiol. 2005;94:1928–1937. doi: 10.1152/jn.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J. Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J. Neurosci. Res. 2001;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic AMP response element-binding protein as a function of rapid eye movement sleep. Neurosci. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Mavanji VK, Patterson EH. A novel role of pedunculopontine tegmental kainate receptors: a mechanism of rapid eye movement sleep generation in the rat. Neurosci. 2002;114:157–164. doi: 10.1016/s0306-4522(02)00250-6. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Enslen H, Tokumitsu H, Stork PJ, Davis RJ, Soderling TR. Regulation of mitogen-activated protein kinases by a calcium/calmodulin-dependent protein kinase cascade. Proc. Natl. Acad. Sci. USA. 1996;93:10803–10808. doi: 10.1073/pnas.93.20.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat. Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- Fujisawa H. Regulation of the activities of multifunctional Ca2+/calmodulin-dependent protein kinases. J. Biochem. 2001;129:193–199. doi: 10.1093/oxfordjournals.jbchem.a002843. [DOI] [PubMed] [Google Scholar]
- Fuller G, Veitch K, Ho LK, Cruise L, Morris BJ. Activation of p44/p42 MAP kinase in striatal neurons via kainate receptors and PI3 kinase. Brain Res. Mol. Brain Res. 2001;89:126–132. doi: 10.1016/s0169-328x(01)00071-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. The pedunculopontine nucleus. Prog. Neurobiol. 1991;36:363–389. doi: 10.1016/0301-0082(91)90016-t. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:673–690. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier P, Arnaud C, Stutzmann JM, Gottesmann C. Influence of zopiclone, a new generation hypnotic, on the intermediate stage and paradoxical sleep in the rat. Psychopharmacology (Berl.) 1997;130:139–143. doi: 10.1007/s002130050221. [DOI] [PubMed] [Google Scholar]
- Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostility. Cell Signal. 2008;20:1592–1607. doi: 10.1016/j.cellsig.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Lancaster B, O'Connor V, Wheal HV. Protein kinase signalling requirements for metabotropic action of kainate receptors in rat CA1 pyramidal neurones. J. Physiol. 2007;579:363–373. doi: 10.1113/jphysiol.2006.122051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr. Opin. Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res. Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch. Ital. Biol. 2004;142:379–396. [PubMed] [Google Scholar]
- Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Kim SA, Hudmon A, Volmer A, Waxham MN. CaM-kinase II dephosphorylates Thr(286) by a reversal of the autophosphorylation reaction. Biochem. Biophys. Res. Commun. 2001;282:773–780. doi: 10.1006/bbrc.2001.4651. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Greenberg ME. A kinase to remember: dual roles for MAP kinase in long-term memory. Neuron. 1997;18:839–842. doi: 10.1016/s0896-6273(00)80322-0. [DOI] [PubMed] [Google Scholar]
- Lim YG, Kim KK, Park KS. ECG recording on a bed during sleep without direct skin-contact. IEEE Trans. Biomed. Eng. 2007;54:718–725. doi: 10.1109/TBME.2006.889194. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Acetylcholine modulates sleep and wakefulness: a synaptic perspective. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. Neurochemistry of sleep and wakefulness. Cambridge: Cambridge University Press; 2008. pp. 109–143. [Google Scholar]
- Mao L, Tang Q, Samdani S, Liu Z, Wang JQ. Regulation of MAPK/ERK phosphorylation via ionotropic glutamate receptors in cultured rat striatal neurons. Eur. J. Neurosci. 2004;19:1207–1216. doi: 10.1111/j.1460-9568.2004.03223.x. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat. Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Connor JA. NR2B-NMDA receptor-mediated increases in intracellular Ca2+ concentration regulate the tyrosine phosphatase, STEP, and ERK MAP kinase signaling. J. Neurochem. 2010;114:1107–1118. doi: 10.1111/j.1471-4159.2010.06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M, Kalenderova S, Miteva L, Poumay Y, Mitev V. Ca(2+)/calmodulin-dependent protein kinase (CaM-kinase) inhibitor KN-62 suppresses the activity of mitogen-activated protein kinase (MAPK), c-myc activation and human keratinocyte proliferation. Arch. Dermatol. Res. 2002;294:198–202. doi: 10.1007/s00403-002-0312-4. [DOI] [PubMed] [Google Scholar]
- Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. Embo. J. 1998;17:7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Richer M, David M, Villeneuve LR, Trieu P, Ethier N, Petrin D, Mamarbachi AM, Hebert TE. GABA-B(1) receptors are coupled to the ERK1/2 MAP kinase pathway in the absence of GABA-B(2) subunits. J. Mol. Neurosci. 2009;38:67–79. doi: 10.1007/s12031-008-9163-6. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Saxena M, Mustelin T. Extracellular signals and scores of phosphatases: all roads lead to MAP kinase. Semin. Immunol. 2000;12:387–396. doi: 10.1006/smim.2000.0219. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Tasken K, Mustelin T. Crosstalk between cAMP-dependent kinase and MAP kinase through a protein tyrosine phosphatase. Nat. Cell Biol. 1999;1:305–311. doi: 10.1038/13024. [DOI] [PubMed] [Google Scholar]
- Socodato RE, Magalhaes CR, Paes-de-Carvalho R. Glutamate and nitric oxide modulate ERK and CREB phosphorylation in the avian retina: evidence for direct signaling from neurons to Muller glial cells. J. Neurochem. 2009;108:417–429. doi: 10.1111/j.1471-4159.2008.05778.x. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Stack EC, Desarnaud F, Siwek DF, Datta S. A novel role for calcium/calmodulin kinase II within the brainstem pedunculopontine tegmentum for the regulation of wakefulness and rapid eye movement sleep. J. Neurochem. 2010;112:271–281. doi: 10.1111/j.1471-4159.2009.06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J. Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J. Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tu H, Rondard P, Xu C, Bertaso F, Cao F, Zhang X, Pin JP, Liu J. Dominant role of GABAB2 and Gbetagamma for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cell Signal. 2007;19:1996–2002. doi: 10.1016/j.cellsig.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J. Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- Vanhoose AM, Emery M, Jimenez L, Winder DG. ERK activation by G-protein-coupled receptors in mouse brain is receptor identity-specific. J. Biol. Chem. 2002;277:9049–9053. doi: 10.1074/jbc.M108309200. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol. Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Sebben M, Dumuis A, Bockaert J. Neurotransmitter regulation of MAP kinase signaling in striatal neurons in primary culture. Synapse. 1998;29:29–36. doi: 10.1002/(SICI)1098-2396(199805)29:1<29::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J. Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, Yang L, Mao L. Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol. Neurobiol. 2004;29:1–14. doi: 10.1385/MN:29:1:01. [DOI] [PubMed] [Google Scholar]
- Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat. Rev. Mol. Cell Biol. 2003;4:651–657. doi: 10.1038/nrm1173. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Feng J, Fienberg AA, Greengard P. D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc. Natl. Acad. Sci. USA. 1999;96:11607–11612. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]