Abstract
Parent-of-origin specific expression of imprinted genes relies on the differential DNA methylation of specific genomic regions. Differentially methylated regions (DMRs) acquire DNA methylation either during gametogenesis (primary DMR) or after fertilization when allele-specific expression is established (secondary DMR). Little is known about the function of these secondary DMRs. We investigated the DMR spanning Cdkn1c in mouse embryonic stem cells, androgenetic stem cells and embryonic germ stem cells. In all cases, expression of Cdkn1c was appropriately repressed in in vitro differentiated cells. However, stem cells failed to de novo methylate the silenced gene even after sustained differentiation. In the absence of maintained DNA methylation (Dnmt1−/−), Cdkn1c escapes silencing demonstrating the requirement for DNA methylation in long term silencing in vivo. We propose that post-fertilization differential methylation reflects the importance of retaining single gene dosage of a subset of imprinted loci in the adult.
Keywords: DNA methylation, imprinted, secondary DMR, stem cells
Introduction
Studies on the DNA methyl-transferases (Dnmts) Dnmt3a and Dnmt3b and the accessory protein Dnmt3L demonstrate the necessity of de novo DNA methylation for the establishment of allele-specific gene expression.1–5 Maintenance of imprinting by Dnmt1 is only essential for a subset of imprinted genes.6–8 DNA methylation is established in one parental germline at specific cis-acting regulatory elements termed “imprinting centres” (IC).9 Both differentially methylated regions (DMRs) and imprinted regions lacking differential DNA methylation show allele-specific histone modifications suggesting a key role for these modifications in both the establishment and the maintenance of allele-specific gene expression.8,10–17
Mouse chromosome 7 (human chromosome 11p15) contains two IC controlling mechanistically distinct sub domains.7,18,19 One of these domains has a genetically defined IC, known as KvDMR1, which regulates several maternally expressed genes including Cdkn1c.19 The absence of DNA methylation at KvDMR1/IC2 is linked to repression of the normally maternally expressed genes including Cdkn1c.2–4,20–24 Conversely, a targeted deletion of this locus or premature termination of the non-coding, paternally expressed RNA Kcnq1ot1 results in expression of the normally paternally silenced genes.19,25,26
Some DMRs are methylated only after fertilization and are known as secondary, somatic or post-fertilization DMRs.27–32 The maternally expressed Cdkn1c gene is spanned by one such DMR that is DNA methylated on the paternal allele. DNA methylation is present at the Cdkn1c gene only after monoallelic expression of Cdkn1c has been established.28 While DNA methylation catalysed by Dnmt3a and 3L is required to activate Cdkn1c expression,2–4 DNA methylation catalysed by the maintenance Dnmt1 is required for Cdkn1c repression.7,28 The histone methyltransferase, Eed, is required for complete repression of Cdkn1c but not the adjacent imprinted gene, Slc22a18.27 Silencing and DNA methylation of Cdkn1c also requires the SNF2-like protein, lymphoid-specific helicase-1 (Lsh/Hells) that binds to the 5' promoter DMR.33 These data indicate that permanent, heritable silencing of Cdkn1c occurs as a temporal sequence of events involving both histone modification and DNA methylation and requires several trans-acting factors.
Embryonic stem (ES) cell lines are established from blastocyst stage embryos and represent an excellent system for studying epigenetic events in vitro. In biparental ES cell lines, DNA methylation is present at primary DMRs, including KvDMR1, and only expression from the maternal Cdkn1c allele can be detected in in vitro differentiated cells.14 Pluripotent stem cells in the mouse can also be derived from primordial germ cells and these show many similar properties to ES cells.21,34–42 As these cells are epigenetically modified during development, removing DNA methylation and erasing parental imprints, EG cell lines can be derived that lack DNA methylation at all DMRs. In these “imprint-erased” cells, some imprinted genes are released from silencing while others are biallelically repressed. Cdkn1c falls into the latter category. After in vivo differentiation of imprint-erased EG cells in chimeras and derivation as primary embryonic fibroblasts (PEFs), expression of Cdkn1c is biallelically repressed and the gene acquires de novo DNA methylation on both alleles.21 Here, we sought to establish whether these cells would provide an opportunity to study the sequence of events that lead to silencing the Cdkn1c gene and to further understand the processes that result in de novo methylation at a secondary DMR. Contrary to our expectations, the silent Cdkn1c gene did not attract direct DNA methylation even after prolonged differentiation but, nonetheless, silencing of Cdkn1c was maintained.
Results
Silencing of paternal Cdkn1c allele during in vitro differentiation
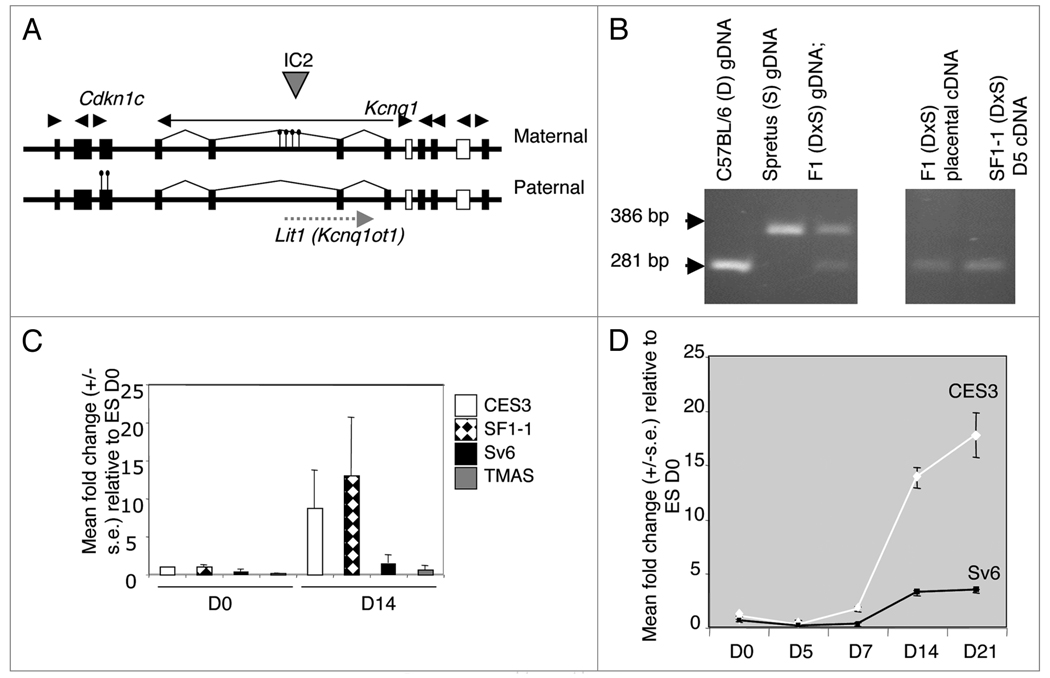
The maternally expressed Cdkn1c gene is located within the IC2 domain on mouse chromosome 7 and is regulated by an imprinting control region, KvDMR1, located more than 200 kb away (Fig. 1A). In somatic cells, Cdkn1c is expressed from the maternal allele and silenced and DNA methylated on the paternal allele. Previous studies demonstrated a similar parental-specific expression pattern in ES cells differentiated in vitro for seven days.14 We used the same hybrid ES cell line, SF1-1 (M. domesticus × M. spretus), to demonstrate that parental-specific expression was apparent as early as day five of in vitro differentiation (Fig. 1B).
Figure 1.
Cdkn1c expression in in vitro differentiated stem cells. (A) 800-kb IC2 domain on mouse distal chromosome 7 showing regions of differential DNA methylation in somatic cells as indicated by lollypops. (B) Imprinting of Cdkn1c in stem cells as assessed by the presence or absence of an AvaI restriction enzyme site in Cdkn1c PCR products amplified from cDNA samples as indicated. (C) Relative expression level of Cdkn1c before (D0) and after (D14) differentiation in biparental ES cell lines CES3 and SF1-1 and the EG cell lines Sv6.1 and TMAS21G. Combined results from three independent differentiation using the embryoid bodies protocol (2) or two experiments using the monolayer protocol (1). (D) Expression profile of Cdkn1c over 21 days of in vitro differentiation by the embryoid bodies protocol.
Silencing of non-imprinted Cdkn1c gene during in vitro differentiation
Imprint-erased EG stem cell lines silence Cdkn1c expression when they are differentiated in vivo in chimaeras and selected as PEFs.21 We sought to determine whether this silencing also occurred in vitro in EG stem cells by comparing the relative level of expression of Cdkn1c between two biparental embryonic stem cell lines, SF1-1 and CES3, and two imprint-erased EG stem cell lines, TMAS21G21 and Sv6.1,41 before and after differentiation. To study the initial stages of Cdkn1c regulation, two biparental ES cell lines, CES3 (129/Sv) and SF1-1 (Mus domesticus (BL6/CBA) × Mus spretus), were differentiated in vitro and quantitative real time PCR (QPCR) was used to compare the expression level of Cdkn1c in the undifferentiated cells with expression level in cells differentiated for 14 days. The level of expression of Cdkn1c in the biparental differentiated cells was, respectively, 8.7-fold and 13-fold higher than the level in these cells when they were undifferentiated (Fig. 1C). In a similar experiment using the EG cell lines Sv6.1 and TMAS21G, the level of expression of Cdkn1c was 3-fold higher and 0.3-fold higher, respectively, in the differentiated cells in comparison to undifferentiated cells suggesting relative repression of Cdkn1c (Fig. 1C). A comparison of Cdkn1c expression over 21 days of differentiation between CES3 (129/Sv) and Sv6.1 (129/Sv) was also performed. Very low expression was detected at day five of differentiation, a time point when expression of Cdkn1c from the paternal allele was already known to be restricted (Fig. 1B). In the biparental cells, Cdkn1c expression rose steadily over time to reach a maximum of 18-fold higher than in undifferentiated level, after 21-days of differentiation. Although changes in expression of Cdkn1c in the imprint-erased EG stem cell line followed the same profile, the maximum differentiated level reached was 3.3-fold higher than in the undifferentiated cells (Fig. 1D). Similarly low levels were detected for Phlda2, an adjacent imprinted gene that shares the same imprint control region as Cdkn1c, in differentiated EG cells (data not shown). These data demonstrate that, as in vivo, expression of the maternally expressed genes in the IC2 domain was relatively repressed in the absence of germline DNA methylation.
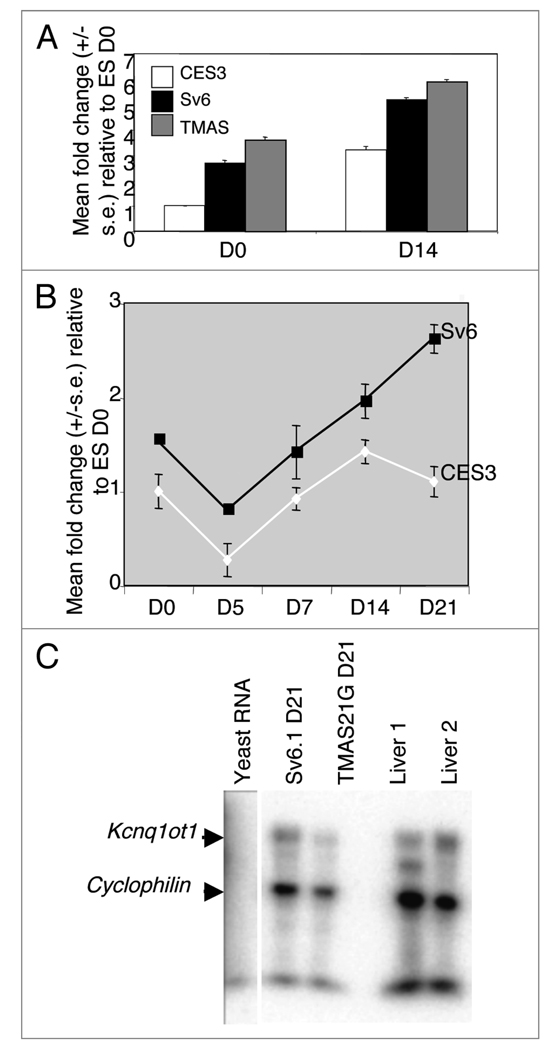
The Kcnq1ot1 transcript was readily detectable in both undifferentiated and differentiated ES and EG cells (Fig. 2A and B). Expression in both EG cell lines was consistently higher than in the biparental ES cell line. The Kcnq1ot1 transcript was also detectable in differentiated EG cells using an RNase protection assay, analogous to a somatic tissue, suggesting that the gene was actively expressed (Fig. 2C).
Figure 2.
Kcnq1ot1 expression. (A) Relative expression level of Kcnq1ot1 before (D0) and after (D14) differentiation in biparental ES cell line CES3 and the EG cell lines Sv6.1 and TMAS21G. (B) Expression profile of Kcnq1ot1 over 21 days of differentiation demonstrating consistently higher Kcnq1ot1 expression in the EG cell line. (C) RNase protection assay against the Kcnq1ot1 transcript. Yeast RNA included as a negative control and riboprobe to cyclophilin included to control for RNA integrity and loading. Differentiation by the embryoid bodies protocol.
Hypomethylation of Cdkn1c after silencing
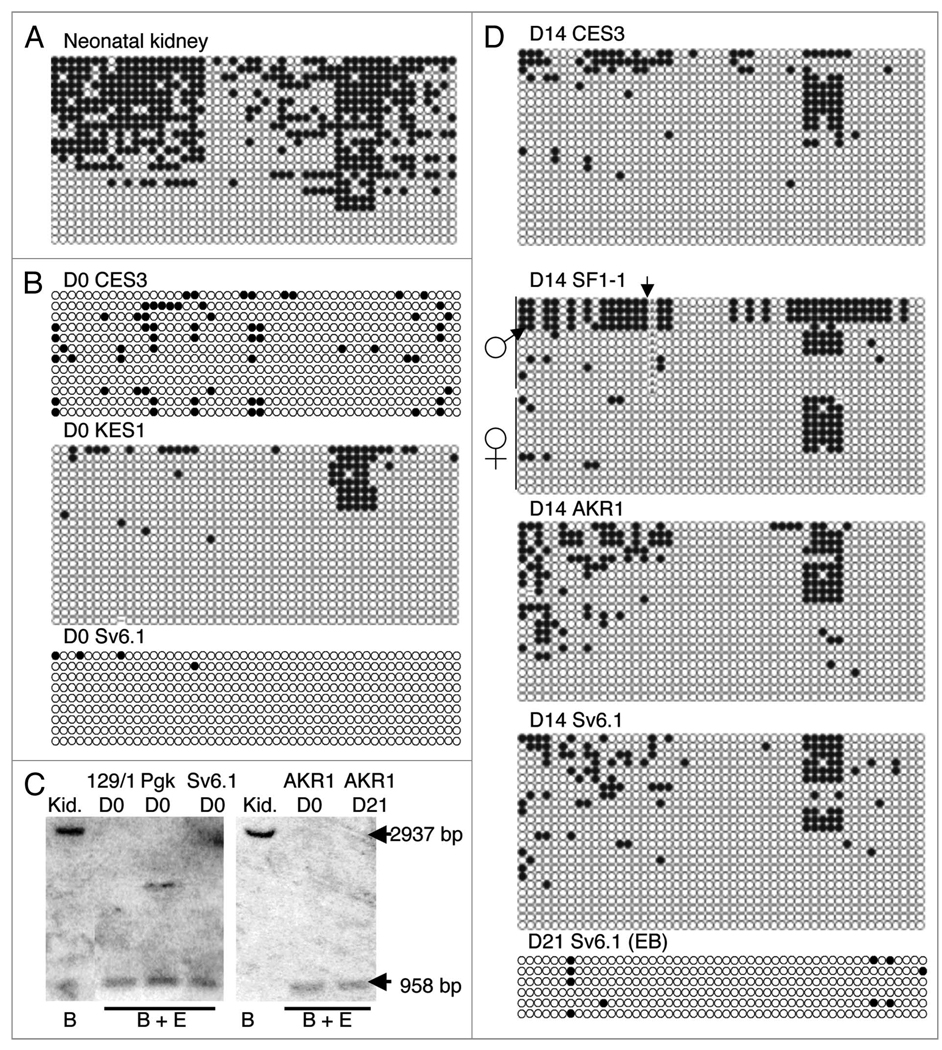
In somatic cells, DNA methylation of the paternal Cdkn1c allele extends from the promoter into the body of the gene as far as intron II.28,43 ES cells are generally derived from E3.5 blastocysts but DNA methylation is not detected at the Cdkn1c gene in vivo until E7.5 suggesting that undifferentiated ES cells should lack DNA methylation at Cdkn1c. We examined the predicted Cdkn1c promoter region in somatic cells by bisulphite sequencing 50 CpG sites just upstream of the predicted transcriptional start site, a region which spans the EagI/Not1 restriction enzyme site described in our previous study.44 In neonatal brain and kidney, Cdkn1c was partially methylated, a pattern consistent with allele-specific DNA methylation (Fig. 3A and data not shown). An unmethylated pattern was present in two independent, undifferentiated (D0) biparental ES cell lines, CES3 and KES1, consistent with timing of derivation of ES cells. The Cdkn1c gene was also unmethylated in the EG stem cell line, Sv6.1 (Fig. 3B) and, as previously reported,21 in TMAS21G cells (data not shown). Southern blotting was used to demonstrate that two additional biparental ES cell lines, 129/1 and Pgk, also lacked detectable DNA methylation at Cdkn1c when undifferentiated (Fig. 3C). We also examined androgenetic stem cells. This type of stem cell is derived from blastocysts that have been engineered to carry two paternal genomes (monoparental).44 We found that the androgenetic AKR1 cell line also contained a hypomethylated Cdkn1c gene when undifferentiated (Fig. 3C).
Figure 3.
Methylation analysis of the secondary Cdkn1c-DMR in undifferentiated and differentiated stem cells. (A) Bisulphite sequence data for neonatal kidney. Each row corresponds to an individual sequenced DNA clone. Each circle represents a CpG on the strand, filled circles and open circles indicate methylated and unmethylated sites, respectively. (B) Bisulphite sequence data for the undifferentiated biparental ES stem cell lines, CES3 and KES1 and the undifferentiated, imprint-erased EG stem cell line, Sv6.1. (C) Southern blot data for undifferentiated biparental stem cell lines 129/1 and Pgk, undifferentiated EG stem cell line Sv6.1 and undifferentiated and D21 differentiated androgenetic stem cell line AKR1. DNAs were digested with BamHI and EagI. (D) Bisulphite sequence data for stem cell lines CES3, SF1-1, AKR1 and Sv6.1 differentiated for 14 days by the monolayer protocol and data for Sv6.1 differentiated for 21 days by the embryoid bodies protocol. Arrow marks the position of a polymorphism between M. domesticus (C57BL/6) and M. spretus.
In vivo, the Cdkn1c gene acquires paternal allele-specific DNA methylation within two days of establishing of allele-specific expression at E7.5.28 ES cells carry both parental alleles and show allele-specific expression of Cdkn1c after five days of differentiation in vitro (Fig. 1B). However, when we examined DNA methylation at the Cdkn1c gene after 14 days of in vitro differentiation as a monolayer, we found no evidence for differential de novo DNA methylation (Fig. 3D). EG stem cells, which are imprint-erased and lack DNA methylation at ICs, acquire de novo methylation at the Cdkn1c gene when these cells are differentiated in chimaeras and then isolated as PEFs.21 When we differentiated the imprint-erased EG cell line, Sv6.1, the Cdkn1c gene remained predominantly hypomethylated (Fig. 3D). In AKR1 cells, both Cdkn1c alleles are paternal in origin (predicted biallelic DNA methylation) but these cells showed a hypomethylated pattern (Fig. 3D). A region within the second intron of the gene was examined and was also found to be hypomethylated after differentiation (data not shown). There was small region of DNA methylation within the 50 CpG scanned that showed a degree of DNA methylation in some samples. However, in the SF1-1 cells, this methylation was present on both parental alleles indicating that it was not an allele-specific modification. To explore the possibility that the absence of methylation at the gene was due to the method of differentiation, the EG cell line Sv6.1 and the androgenetic cell line AKR1 were in vitro differentiated for 21 days exclusively by the embryoid body method with similar results (Fig. 3C and D).
H3K27 trimethylation at Cdkn1c during in vitro differentiation of EG stem cells
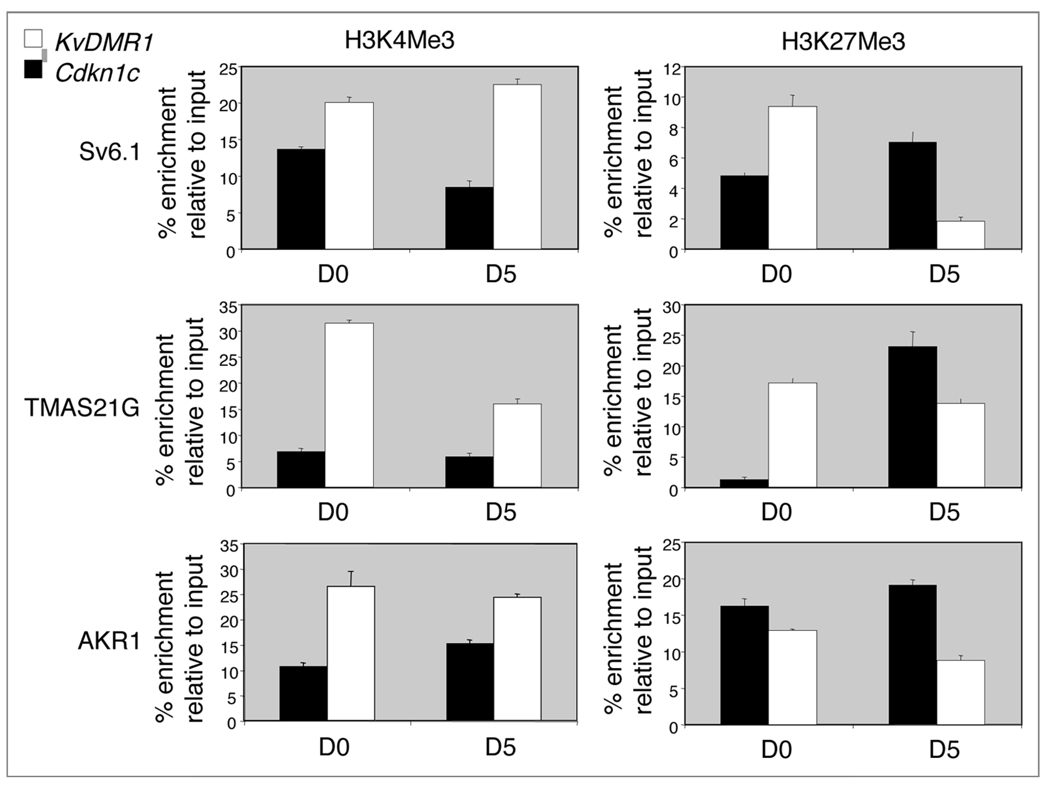
Differential histone modifications have been reported in somatic cells and in SF1-1 ES cells at the Cdkn1c gene. In particular, the silent paternal Cdkn1c allele is enriched for histone H3 trimethylation at lysine 27 (H3K27me3) while the active allele is enriched for histone H3 lysine 4 (H3K4Me3).14 We quantified the relative level of these marks between the Cdkn1c promoter region and the Kcnq1ot1 promoter region in AKR1 cells, where both alleles carry the paternal imprint, and in the two EG cells lines, Sv6.1 and TMAS21G, where both alleles are imprint-erased. The Kcnq1ot1 promoter region was relatively enriched for the active mark, H3K4Me3, in both undifferentiated and differentiated cells (Fig. 4) consistent with expression of Kcnqtot1 (Fig. 2). Conversely, the Cdkn1c promoter region was relatively enriched for H3K27me3 in differentiated AKR1 and EG cells (Fig. 4). However, only undifferentiated AKR1 cells were enriched for this repressive mark at Cdkn1c. This suggested that H3K27me3 was acquired as the EG cells differentiated and that imprint-erased EG cells might represent a more rudimentary silent state for Cdkn1c than AKR1 cells.
Figure 4.
ChIP analysis of the Kcnq1ot1 and Cdkn1c promoter regions in undifferentiated and differentiated stem cells. Chromatin immunoprecipitation (ChIP) was performed using antibodies to detect trimethylated H3K4 (H3K4me3), a mark associated with active chromatin and trimethylated H3K27 (H3K27me3), a mark associated with silent chromatin, in undifferentiated and differentiated AKR1 and EG stem cells. Results are expressed as the % enrichment relative to input chromatin. Quantitative PCR was performed for regions within 600 bp of the transcription start sites of Cdkn1c and Kcnq1ot1. Embryoid bodies protocol.
In summary, we have shown that stem cells cultured in vitro are able to repress Cdkn1c expression until at least 21 days of differentiation but do not acquire DNA methylation at the Cdkn1c secondary DMR.
Discussion
In this study we demonstrate that expression of Cdkn1c is suppressed in in vitro differentiated stem cells in the absence of DNA methylation. The absence of direct DNA methylation at the Cdkn1c gene in ES cells is a novel finding but consistent with the time point at which ES cells are derived. The Cdkn1c genes acquires differential DNA methylation from E7.5, four days after ES cells are normally derived.28 The absence of significant DNA methylation at the Cdkn1c gene after differentiation in vitro of biparental ES cells was unexpected.21 EG cells (two imprint-erased genomes, unmethylated KvDMR1) and androgenetic stem cells (two paternal genomes, unmethylated KvDMR1) also silenced Cdkn1c expression but failed to directly de novo methylate the Cdkn1c gene.
Role of direct DNA methylation in regulating Cdkn1c expression
We have shown that silencing of Cdkn1c can be maintained in vitro without DNA methylation for at least 21 days. In vivo, the maintenance DNA methylase, Dnmt1, is required to keep the paternal Cdkn1c allele repressed in E9.5 embryos and the ectoplacental cone.7,28 This loss of imprinted expression is apparent three days after imprinted expression is detectable in wild type embryos and two days after the silent allele normally acquires DNA methylation.28 This demonstrates that DNA methylation is required to keep the paternal Cdkn1c allele silent in vivo.
Role of histone modification in regulating Cdkn1c expression
Cdkn1c is located more than 200 kb from the imprinting centre that regulates its imprinted expression. Termination of Kcnq1ot1 leads to inappropriate activation of the paternal Cdkn1c allele suggesting that the Kcnq1ot1 transcript participates in the long range silencing of Cdkn1c. Bhogal et al.28 demonstrated that silencing of Cdkn1c takes place prior to direct DNA methylation at the Cdkn1c gene in vivo. Our finding that there is a relative enrichment of the H3K27me3 mark in stem cells after five days of differentiation demonstrates that this modification also precedes de novo methylation at Cdkn1c. The low abundance of H3K27me3 at Cdkn1c in undifferentiated EG cells suggests that this mark is recruited to Cdkn1c at the very earliest stages of silencing.
Other genes
Cdkn1c is not the only imprinted gene spanned by a secondary DMR. Similar DMRs are located over the transcriptional start sites for Igf2r, Nesp55 and Gtl2.29,30,45 These all acquire methylation on the paternal allele after fertilization and, at least in the case of Gtl2, within a similar time frame to Cdkn1c. Paternal repression of Gtl2 and Cdkn1c is lost in Eed-deficient embryos whereas Igf2r expression appears to be unaffected.27 Another scenario in which there is altered imprinted expression of the Cdkn1c gene is in lymphoid specific helicase (Lsh)-deficient embryos.33 Lsh (official symbol Hells) is involved in reinforcing DNA methylation and silencing of polycomb repressive complex targets and Lsh-deficiency leads to loss of repression of Cdkn1c but not H19, Igf2, Igf2r, Zac1 or Meg9/Mirg.33 The fact that Cdkn1c and Igf2r do not respond in a similar way in either of these models argues against a common mechanism involving either Lsh (Hells) or Eed. However, it will be important to determine the expression status of Nesp55 in Eed-deficient embryos and both Nesp55 and Gtl2 in Hells-deficient embryos and also to identify any commonalties with other genes encompassed by secondary DMRs as this could provide support for a common mechanism for their establishment and maintenance.
Long term silencing
Once differential methylation is established at the IC, histone modifications appear to be sufficient to transmit the imprint signal to adjacent genes within an imprinted domain and to maintain this imprint, at least within the placenta.8 Lewis and colleagues suggested that the differences in imprinted gene expression between the placenta and the embryo might reflect the existence of an evolutionarily older imprinting mechanism based on histone modifications with DNA methylation recruited in the embryo as a more stable epigenetic mark for use in specifically in embryonic lineages. Our data suggest that Cdkn1c can also be effectively silenced in the short term through just the action of histone modifications in stem cells. One possibility is that signalling from the IC and the recruitment of histone modifications is a continual process in placental lineages and in stem cells and that direct DNA methylation is only required for the long term silencing of genes that lie at a distance from their IC. This in turn suggests that the dosage of genes spanned by post fertilization DMRs, which include Cdkn1c, Igf2r, Gtl2 and Nesp55, is critical not just during embryonic development but also during the life span of the organism. Identifying any commonalities in the postnatal function of the imprinted genes in this category may provide insight into the rational for regulating gene dosage in the adult mammal.
CDKN1C in humans
The human CDKN1C gene also exhibits imprinted expression but, in contrast to the mouse gene, there is no evidence for direct DNA methylation of the paternal allele and this allele is not fully silenced.24,43,46–48 In some respects, this scenario is similar to our in vitro differentiated stem cells. Either humans have lost the ability to directly methylate the CDKN1C locus or mice have specifically acquired a secondary DMR. Either way, this could suggest that the evolutionary necessity to fully repress paternal Cdkn1c expression differs between the two species.
Summary
We have shown here that the silencing of Cdkn1c can be established and maintained in differentiated stem cells without direct DNA methylation. However, the requirement for Dnmt1 in vivo demonstrates that DNA methylation is critical for the long term silencing of Cdkn1c. We suggest that this reflects the importance of the controlled dosage of this gene in the adult animal as well as during embryogenesis. In addition, the failure of stem cells to complete their full silencing program could have bearing on their usefulness in in vitro differentiation studies and stem cell-based therapies.
Materials and Methods
Stem cell lines
Biparental ES cell stem lines KES1 and CES3 were derived from 129/Sv embryos and were a kind gift from M. A. Surani. SF1-1, AKR1, TMAS21G and Sv6.1 were described previously.21,41,44 Cells were maintained in the undifferentiated state on a SNL (mouse fibroblast STO cell line transformed with neomycin resistance and murine LIF genes) feeder layer as described previously.43 Stem cell lines were all XY and were reconfirmed to be Oct4 positive with a full chromosomal compliment when undifferentiated at the end of the study. Feeders were removed from the undifferentiated cells prior to RNA, DNA or chromatin preparation by panning for 20 minutes. Cells were differentiated by plating at low density in the absence of LIF and feeders on non adherent (embryoid bodies) or adherent (monolayer) plates as indicated. Medium was changed every 1–2 days.
RNA analysis
Expression levels were determined using real-time quantitative RT-PCR as described previously49 on cDNA from three independent differentiations for CES3, Sv6.1 and TMAS21G and a single differentiation for SF1-1. Primers Cdkn1c 5'-AGA GAA CTG CGC AGG AGA AC-3' and 5'-TCT GGC CGT TAG CCT CTA AA-3' and Kcnq1ot1 5'-TCC AAT CGG GTA GAG ATT CG-3' and 5'-AGA CCA TCG GAA AAC ACA GG-3'. For the ribonuclease protection assay, the Kcnq1ot1 RPA probe was located approximately 1,000 bp downstream of the Kcnq1ot1 promoter (nucleotides 144,718 to 145,568 from sequence AJ271885). RNase protection was performed as previously described.25 Essentially, radioactive RNA probes were synthesized using the MaxiScript T7/T6 kit (Ambion) and 32PUTP (800 Ci/mmol). RPA was performed using the RPAIII kit (Ambion), with hybridization at 45°C (Kcnq1ot1 plus Cyclophillin). For restriction fragment length polymorphism analysis, 35 cycles of PCR was performed using the RED Genomic Template PCR system (Sigma) at an annealing temperature of 64.5°C using the published primers that span a polymorphic AvaI restriction site within exon 3 of the Cdkn1c gene.50
DNA analysis
Genomic DNA was prepared as described previously.43 Bisulphite sequencing was performed as described51 using primers 5'-TGG GTG TAG AGG GTG GAT TTA GTT A-3's and 5'-CCC ACA AAA ACC CTA CCC CC-3' and hemi-nested primer 5'-GTA TTG TTA GGA TTA GGA TTT AGT TGG TAG TAG TAG. Southern blotting and hybridisation was performed with a 0.53 kb XhoI-EagI probe fragment from the Cdkn1c cDNA as described.43
Chromatin preparation and analysis
Chromatin immunoprecipitation (ChIP) assays were carried out using the Orange ChIP assay kit (Diagenode) according to the manufacturer’s instructions, with a few modifications. For undifferentiated stem cells, the cells were separated from the feeder layer and then resuspended in cold phosphate-buffered saline supplemented with protease inhibitors (Sigma). Cross-linking for all cells was performed with 0.8% formaldehyde (Sigma) for 10 minutes at room temperature and the reactions were quenched with 0.125 M glycine for 5 minutes at room temperature. Cells were resuspended in lysis buffer at 10,000 cells/µl and incubated on ice for 5 minutes. Following lysis, sonication was carried out with a Diagenode Bioruptor. For each ChIP, 10 µl of the sonicated cell supernatant (equivalent to 100,000 cells) was diluted 10-fold in ChIP dilution buffer (Diagenode). 100 µl of the diluted material was removed prior to the addition of antibodies (input). 100 µl of diluted chromatin was used for each IP reaction with antibodies against H3K4me3 (Kch-403-020, Diagenode) and H3K27me3 (Ab6002, Abcam). IgG (Ab6697, Abcam) was used as the negative control. For the final step, samples and input chromatin were heat treated to reverse the crosslinks and the DNA was precipitated and resuspended in 50 µl of Tris-EDTA. 5 µl was used for each qPCR reaction. Percentage enrichment was calculated using the formula A^(Input CT − IP CT) × 100 where A is the amplification efficiency of the qPCR. Two independent differentiations were performed. Cdkn1c promoter region primers 5'-GCG GTG TTG TTG AAA CTG AA-3' and 5'-GTC TGG ATC GCT TGT CCT GT and Kcnq1 promoter region primers 5'-AAG CTC ACC CAA TCC AAA TG and 5'-CTC CTA GCG ACA ACG GGT AG.
Acknowledgements
M. W. was supported by a BBSRC Epigenetic Initiative studentship. We would like to thank Dr. Nick Allen, Professor Azim Surani and Dr. Gabriella Durcova-Hills for providing the stem cell lines and Dr. Tatyana Nesterova for help with Southern blotting.
Abbreviations
- IC
imprinting centre
- DMR
differentially methylated region
- PEFs
primary embryonic fibroblasts
References
- 1.Okano M, Bell DW, Haber DA, Li E. DNA methyl-transferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 2.Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyl-transferases to establish maternal imprints in mice. Development. 2002;129:1983–1993. doi: 10.1242/dev.129.8.1983. [DOI] [PubMed] [Google Scholar]
- 3.Bourc’his D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–2539. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- 4.Kaneda M, Okano M, Hata K, Sado T, Tsujimoto N, Li E, Sasaki H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature. 2004;429:900–902. doi: 10.1038/nature02633. [DOI] [PubMed] [Google Scholar]
- 5.Arima T, Hata K, Tanaka S, Kusumi M, Li E, Kato K, et al. Loss of the maternal imprint in Dnmt3Lmat−/− mice leads to a differentiation defect in the extraembryonic tissue. Dev Biol. 2006;297:361–373. doi: 10.1016/j.ydbio.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Li E, Beard C, Jaenisch R. Role of DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- 7.Caspary T, Cleary MA, Baker CC, Guan XJ, Tilghman SM. Multiple mechanisms regulate imprinting of the mouse distal chromosome 7 gene cluster. Mol Cell Biol. 1998;18:3466–3474. doi: 10.1128/mcb.18.6.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis A, Mitsuya K, Umlauf D, Smith P, Dean W, Walter J, et al. Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat Genet. 2004;36:1291–1295. doi: 10.1038/ng1468. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson-Smith AC, Surani MA. Imprinting and the epigenetic asymmetry between parental genomes. Science. 2001;293:1086–1089. doi: 10.1126/science.1064020. [DOI] [PubMed] [Google Scholar]
- 10.Verona RI, Thorvaldsen JL, Reese KJ, Bartolomei MS. The transcriptional status but not the imprinting control region determines allele-specific histone modifications at the imprinted H19 locus. Mol Cell Biol. 2008;28:71–82. doi: 10.1128/MCB.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regha K, Sloane MA, Huang R, Pauler FM, Warczok KE, Melikant B, et al. Active and repressive chromatin are interspersed without spreading in an imprinted gene cluster in the mammalian genome. Mol Cell. 2007;27:353–366. doi: 10.1016/j.molcel.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaval K, Govin J, Cerqueira F, Rousseaux S, Khochbin S, Feil R. Differential histone modifications mark mouse imprinting control regions during spermatogenesis. EMBO J. 2007;26:720–729. doi: 10.1038/sj.emboj.7601513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fournier C, Goto Y, Ballestar E, Delaval K, Hever AM, Esteller M, Feil R. Allele-specific histone lysine methylation marks regulatory regions at imprinted mouse genes. EMBO J. 2002;21:6560–6570. doi: 10.1093/emboj/cdf655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umlauf D, Goto Y, Cao R, Cerqueira F, Wagschal A, Zhang Y, Feil R. Imprinting along the Kcnq1 domain on mouse chromosome 7 involves repressive histone methylation and recruitment of Polycomb group complexes. Nat Genet. 2004;36:1296–1300. doi: 10.1038/ng1467. [DOI] [PubMed] [Google Scholar]
- 15.Redrup L, Branco MR, Perdeaux ER, Krueger C, Lewis A, Santos F, et al. The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development. 2009;136:525–530. doi: 10.1242/dev.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terranova R, Yokobayashi S, Stadler MB, Otte AP, van Lohuizen M, Orkin SH, Peters AH. Polycomb group proteins Ezh2 and Rnf2 direct genomic contraction and imprinted repression in early mouse embryos. Developmental cell. 2008;15:668–679. doi: 10.1016/j.devcel.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Mohammad F, Pandey RR, Nagano T, Chakalova L, Mondal T, Fraser P, Kanduri C. Kcnq1ot1/Lit1 noncoding RNA mediates transcriptional silencing by targeting to the perinucleolar region. Mol Cell Biol. 2008;28:3713–3728. doi: 10.1128/MCB.02263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinberg AP. The two-domain hypothesis in Beckwith-Wiedemann syndrome. J Clin Invest. 2000;106:739–740. doi: 10.1172/JCI10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzpatrick GV, Soloway PD, Higgins MJ. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat Genet. 2002;32:426–431. doi: 10.1038/ng988. [DOI] [PubMed] [Google Scholar]
- 20.Kato Y, Iii WM, Hilton K, Barton SC, Tsunoda Y, Surani MA. Developmental potential of mouse primordial germ cells. Development. 1999;126:1823–1832. doi: 10.1242/dev.126.9.1823. [In Process Citation] [DOI] [PubMed] [Google Scholar]
- 21.Tada T, Tada M, Hilton K, Barton SC, Sado T, Takagi N, Surani MA. Epigenotype switching of imprintable loci in embryonic germ cells. Dev Genes Evol. 1998;207:551–561. doi: 10.1007/s004270050146. [DOI] [PubMed] [Google Scholar]
- 22.Kono T, Obata Y, Yoshimzu T, Nakahara T, Carroll J. Epigenetic modifications during oocyte growth correlates with extended parthenogenetic development in the mouse. Nature Genetics. 1996;13:91–94. doi: 10.1038/ng0596-91. [DOI] [PubMed] [Google Scholar]
- 23.Obata Y, Kaneko-Ishino T, Koide T, Takai Y, Ueda T, Domeki I, et al. Disruption of primary imprinting during oocyte growth leads to the modified expression of imprinted genes during embryogenesis. Development. 1998;125:1553–1560. doi: 10.1242/dev.125.8.1553. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Meyer N, Day CD, Khatod K, Maher ER, Cooper W, Reik W, et al. Silencing of CDKN1C (p57KIP2) is associated with hypomethylation at KvDMR1 in Beckwith-Wiedemann syndrome. J Med Genet. 2003;40:797–801. doi: 10.1136/jmg.40.11.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin JY, Fitzpatrick GV, Higgins MJ. Two distinct mechanisms of silencing by the KvDMR1 imprinting control region. EMBO J. 2008;27:168–178. doi: 10.1038/sj.emboj.7601960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancini-Dinardo D, Steele SJ, Levorse JM, Ingram RS, Tilghman SM. Elongation of the Kcnq1ot1 transcript is required for genomic imprinting of neighboring genes. Genes Dev. 2006;20:1268–1282. doi: 10.1101/gad.1416906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mager J, Montgomery ND, de Villena FP, Magnuson T. Genome imprinting regulated by the mouse Polycomb group protein Eed. Nat Genet. 2003;33:502–507. doi: 10.1038/ng1125. [DOI] [PubMed] [Google Scholar]
- 28.Bhogal B, Arnaudo A, Dymkowski A, Best A, Davis TL. Methylation at mouse Cdkn1c is acquired during postimplantation development and functions to maintain imprinted expression. Genomics. 2004;84:961–970. doi: 10.1016/j.ygeno.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Stoger R, Kubicka P, Liu CG, Kafri T, Razin A, Cedar H, Barlow DP. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993;73:61–71. doi: 10.1016/0092-8674(93)90160-r. [DOI] [PubMed] [Google Scholar]
- 30.Takada S, Paulsen M, Tevendale M, Tsai CE, Kelsey G, Cattanach BM, Ferguson-Smith AC. Epigenetic analysis of the Dlk1-Gtl2 imprinted domain on mouse chromosome 12: implications for imprinting control from comparison with Igf2-H19. Hum Mol Genet. 2002;11:77–86. doi: 10.1093/hmg/11.1.77. [DOI] [PubMed] [Google Scholar]
- 31.Yatsuki H, Joh K, Higashimoto K, Soejima H, Arai Y, Wang Y, et al. Domain regulation of imprinting cluster in Kip2/Lit1 subdomain on mouse chromosome 7F4/F5: large-scale DNA methylation analysis reveals that DMR-Lit1 is a putative imprinting control region. Genome Res. 2002;12:1860–1870. doi: 10.1101/gr.110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson CM, Turner MD, Ball ST, Nottingham WT, Glenister P, Fray M, et al. Identification of an imprinting control region affecting the expression of all transcripts in the Gnas cluster. Nat Genet. 2006;38:350–355. doi: 10.1038/ng1731. [DOI] [PubMed] [Google Scholar]
- 33.Fan T, Hagan JP, Kozlov SV, Stewart CL, Muegge K. Lsh controls silencing of the imprinted Cdkn1c gene. Development. 2005;132:635–644. doi: 10.1242/dev.01612. [DOI] [PubMed] [Google Scholar]
- 34.Labosky PA, Barlow DP, Hogan BL. Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development. 1994;120:3197–3204. doi: 10.1242/dev.120.11.3197. [DOI] [PubMed] [Google Scholar]
- 35.Matsui Y, Zsebo K, Hogan BLM. Derivation of pluri-potential embryonic stem cells from murinre primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- 36.Resnick JL, Bixler LS, Cheng L, Donovan PJ. Longterm proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- 37.Stewart CL, Gadi I, Bhatt H. Stem cells from primordial germ cells can reenter the germ line. Developmental Biology. 1994;161:626–628. doi: 10.1006/dbio.1994.1058. [DOI] [PubMed] [Google Scholar]
- 38.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Inoue K, Ono R, Ogonuki N, Kohda T, Kaneko-Ishino T, et al. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development. 2002;129:1807–1817. doi: 10.1242/dev.129.8.1807. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki Y, Low EW, Marikawa Y, Iwahashi K, Bartolomei MS, McCarrey JR, Yanagimachi R. Adult mice cloned from migrating primordial germ cells. Proc Natl Acad Sci USA. 2005;102:11361–11366. doi: 10.1073/pnas.0504943102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durcova-Hills G, Ainscough J, McLaren A. Pluripotential stem cells derived from migrating primordial germ cells. Differentiation. 2001;68:220–226. doi: 10.1046/j.1432-0436.2001.680409.x. [DOI] [PubMed] [Google Scholar]
- 42.Durcova-Hills G, Wianny F, Merriman J, Zernicka-Goetz M, McLaren A. Developmental fate of embryonic germ cells (EGCs), in vivo and in vitro. Differentiation. 2003;71:135–141. doi: 10.1046/j.1432-0436.2003.710204.x. [DOI] [PubMed] [Google Scholar]
- 43.John RM, Hodges M, Little P, Barton SC, Surani MA. A human p57(KIP2) transgene is not activated by passage through the maternal mouse germline. Hum Mol Genet. 1999;8:2211–2219. doi: 10.1093/hmg/8.12.2211. [DOI] [PubMed] [Google Scholar]
- 44.Allen ND, Barton SC, Hilton K, Norris ML, Surani MA. A functional analysis of imprinting in mouse parthenogenetic embryonic stem cells. Development. 1994;120:1473–1482. doi: 10.1242/dev.120.6.1473. [DOI] [PubMed] [Google Scholar]
- 45.Coombes C, Arnaud P, Gordon E, Dean W, Coar EA, Williamson CM, et al. Epigenetic properties and identification of an imprint mark in the Nesp-Gnasxl domain of the mouse Gnas imprinted locus. Mol Cell Biol. 2003;23:5475–5488. doi: 10.1128/MCB.23.16.5475-5488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuoka S, Thompson JS, Edwards MC, Bartletta JM, Grundy P, Kalikin LM, et al. Imprinting of the gene encoding a human cyclin-dependent kinase inhibitor, p57KIP2, on chromosome 11p15. Proc Natl Acad Sci USA. 1996;93:3026–3030. doi: 10.1073/pnas.93.7.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatada I, Inazawa J, Abe T, Nakayama M, Kaneko Y, Jinno Y, et al. Genomic imprinting of human p57KIP2 and its reduced expression in Wilms’ tumors. Hum Mol Genet. 1996;5:783–788. doi: 10.1093/hmg/5.6.783. [DOI] [PubMed] [Google Scholar]
- 48.Monk D, Arnaud P, Apostolidou S, Hills FA, Kelsey G, Stanier P, et al. Limited evolutionary conservation of imprinting in the human placenta. Proc Natl Acad Sci USA. 2006;103:6623–6628. doi: 10.1073/pnas.0511031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrews SC, Wood MD, Tunster SJ, Barton SC, Surani MA, John RM. Cdkn1c (p57Kip2) is the major regulator of embryonic growth within its imprinted domain on mouse distal chromosome 7. BMC Dev Biol. 2007;7:53. doi: 10.1186/1471-213X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li T, Vu TH, Ulaner GA, Littman E, Ling JQ, Chen HL, et al. IVF results in de novo DNA methylation and histone methylation at an Igf2-H19 imprinting epigenetic switch. Mol Hum Reprod. 2005;11:631–640. doi: 10.1093/molehr/gah230. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi H, Hiura H, John RM, Sato A, Otsu E, Kobayashi N, et al. DNA methylation errors at imprinted loci after assisted conception originate in the parental sperm. Eur J Hum Genet. 2009;17:1582–1591. doi: 10.1038/ejhg.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]