Abstract
Objective
To assess stability of cardiac output, mean arterial pressure, and systemic vascular resistance during biventricular pacing optimization.
Design
Substudy analysis of data collected as part of a randomized controlled study examining the effects of optimized temporary biventricular pacing after cardiopulmonary bypass.
Setting
Single center study at a university affiliated tertiary care hospital.
Participants
Cardiac surgery patients at risk of left ventricular failure following cardiopulmonary bypass (CPB).
Interventions
Biventricular pacing was optimized immediately after CPB. Atrioventricular delay (7 unique settings) was optimized first, followed by left ventricular pacing site (3 unique settings), followed by interventricular delay (9 unique settings) Each setting was tested twice for 10 seconds each time. Vasoactive medication and fluid infusion rates were held constant.
Measurements and Main Results
Aortic flow velocity and radial artery pressure were digitized, recorded, and averaged over single respiratory cycles. Least squares and linear regression/Wilcoxon analysis were applied to the first seven patients studied. Subsequently, curvilinear analysis was applied to 15 patients. Changes in MAP and SVR were statistically insignificant or too small to be meaningful by least squares analysis. During interventricular synchrony optimization, cardiac output and mean arterial pressure decreased (mean changes −5.7% and −2.5%, respectively; with standard errors 2.3% and 1.5%, respectively), whereas SVR increased (mean change 3.1% with standard error 3.4%). Only the change in cardiac output was statistically significant (p =0.043). Curvilinear fits to data for 15 patients demonstrate progressive hemodynamic stability over the total testing period.
Conclusion
BiVP optimization may be safely applied in patients following CPB. With continuous monitoring of MAP and CO the procedure results in no harmful hemodynamic perturbation.
Keywords: Cardiac Function, Cardiopulmonary Bypass, Physiology, Pathophysiology, Circulatory Hemodynamics, Electrophysiology, Pacing, Cardiac Resynchronization Therapy, Biventricular Pacing
Introduction
Congestive heart failure is a major health problem in the United States, with an incidence of over 500,000 patients per year [1]. Biventricular pacing (BiVP) improves objective and subjective measures of heart failure in 70% of patients [4,5]. Benefits of permanently implanted biventricular pacemakers are increased by optimization of atrioventricular delay (AVD), ventricular pacing site (VPS), interventricular pacing delay (VVD), and heart rate (HR).
The effects of temporary BiVP after cardiac surgery and benefits in low output states are not well defined [6-13]. Benefits of BiVP optimization in this setting are unknown [14-23]. Given that 700,000 open-heart procedures are performed in the US alone, the potential impact of temporary BiVP is substantial. Accordingly, the Biventricular Pacing after Cardiac Surgery (BiPACS) trial examines the effects of temporary optimized perioperative BiVP in cardiac surgery patients with risk factors for developing acute postoperative left ventricular (LV) dysfunction. Patients in the trial undergo BiVP optimization at three time points. Phase 1 occurs shortly after separation from cardiopulmonary bypass (CPB), once vasocative medication and fluid/blood product infusion rates are stabilized. Phase 2 occurs after sternal closure (usually 30-60 minutes after conclusion of Phase 1), and Phase 3 occurs on the first post-operative day (12-24 hours after conclusion of Phase 2). During BiVP optimization, in all three phases, changes in the rates of administration of intravascular volume, anesthetics, and vasoactive medications are restricted. This, in concert with BiVP, may in theory lead to extreme hemodynamic lability, especially in the immediate post-CPB period (Phase 1). While studies of ventricular function have been conducted during this time [24-34], little information is available about intrinsic hemodynamic stability in the absence of external interventions. In this sub-study of the BiPACS trial, to asses safety and reliability of BiVP optimization during Phase 1, we evaluated the stability of cardiac output (CO), mean arterial pressure (MAP) and systemic vascular resistance (SVR) during this period.
Methods
We analyzed data for seven patients (7 male, mean age = 68 ± 11 years) studied as part of the ongoing NIH-funded BiPACS trial. Patients were included in the BiPACS trial if they underwent cardiac surgery on CPB and had a pre-operative LV ejection fraction ≤ 40% and QRS duration ≥100 msec. Patients who underwent concomitant mitral and aortic valve repair, or replacement, were included irrespective of their ejection fraction and QRS duration. Exclusion criteria were intracardiac shunts, re-operation, congenital heart disease, 2° or 3° heart block, post-CPB heart rate > 120 bpm, or atrial fibrillation. Patients were also removed from the BiPACS trial if the attending surgeon and/or anesthesiologist deemed that pausing the surgery to facilitate BiVP optimization would be unsafe due to severe hemodynamic instability, severe bleeding, or other causes. None of the patients analyzed as part of this sub-study were removed from the BiPACS prial at the conclusion of Phase 1. Permission to study each patient was obtained from their attending surgeon prior to enrollment. All patients gave informed consent to participate in the trial, which is approved by our Institutional Review Board and is conducted under an Investigational Device Exemption from the Food and Drug Administration.
Instrumentation
During CPB, before the aortic cross-clamp was removed, paired temporary unipolar epicardial pacing leads (Medtronic, Houston, TX) were sewn to two locations on the LV surface, LV1 and LV2. LV1 was randomly assigned to a posterior-basal or lateral-basal site. LV2 was randomly assigned to a medial-basal, mid-lateral, mid-medial, or apical site. After cross clamp removal, pacing leads were also sewn to the right atrial appendage and the anterior right ventricle (RV). A scissor-type electromagnetic flow probe (Carolina Medical Electronics, King, NC) was applied circumferentially around the ascending aorta to measure instantaneous volume flow. Lead II of the surface electrocardiogram, radial artery pressure, and aortic flow velocity were sampled by an analog-to-digital converter (ADInstruments, Milford, MA) and recorded on a personal computer (IMac, Apple Computer, Cupertino, CA) during Phase 1 optimization.
BiVP Optimization
Temporary BiVP was initiated with a custom temporary, external BiVP unit containing a shock-mounted permanent BiVP generator (InSync III 8042, Medtronic). Pacing parameters were adjusted with a commercial programmer (Model 2090, Medtronic, Inc, Minneapolis, MN). After testing to confirm lead function, patients were weaned from CPB during BiVP, using standard clinical protocols. Default values for initial pacing were atrio-biventricular pacing (DDD mode) with HR = 90 bpm, or 10 bpm above intrinsic heart rate but not exceeding 130 bpm, AVD = 150 msec, VVD = 0 msec, VPS = LV1. All patients were weaned easily from CPB, generally with the assistance of inotropes and vasoactive pharmacologic agents.
After final adjustment of anesthesia, volume status and inotropes, with the patients still cannulated and before administration of Protamine Sulfate, Phase 1 optimization was conducted in three segments. With HR, LV pacing site, and VVD held constant at default values, AVD was randomly varied across seven settings (90 - 270 msec in 30-msec increments). The peak CO for each setting was estimated from the digital readout of the aortic flowmeter. The same seven settings were then repeated, again in random order, resulting in 2 peak CO values for each AVD which were then averaged together to produce one value for each AVD. The AVD resulting in the highest average CO was chosen as the optimal AVD. The optimum LV pacing site was similarly chosen from among 6 settings (3 unique: RV only, RV + LV1, and RV + LV2) with the HR, VVD held constant at default values and AVD held constant at the optimum AVD. Finally, with the AVD and LV pacing site held constant at their optimum values and the HR held constant at the default value, the optimum VVD was chosen based on 18 settings (9 unique: +80 [RV-first] to −80 [LV-first] in 20-msec increments) using the methods described for AVD optimization. A total of 38 settings (14 AVD + 6 LV pacing site + 18 VVD) were tested for 10 seconds each. The total time to complete optimization was approximately eight minutes. No pacing (ODO mode), AAI pacing (AAI mode at the default HR), and optimized BiVP (determined by optimal AVD, LV pacing site and VVD) were then compared in three 30-second intervals. Default values were used when effects on CO were equivocal. Changes in vasoactive drug infusion rates and incremental volume infusion from the heart-lung machine and other sources were avoided during testing but were allowed in intervals between optimization segments.
All above described procedures and interventions were undertaken as part of the BiPACS Trial. This paper focuses on data analysis for a subset of patients studied as part of that trial.
Data analysis
Aortic flow and arterial pressure data were imported into Matlab (The MathWorks Inc, Natick, MA) and processed with custom routines. CO and MAP for each testing segment were averaged over one respiratory cycle. The beginning and end of a respiratory cycle were defined from minima in MAP. Data near the end of each segment were used allowing time for stabilization of pacing induced hemodynamic changes. SVR was calculated as SVR=MAP/CO.
The slopes of CO, MAP, and SVR vs. time and the validity of the regression were determined for each patient by least square mean regression analysis. Results indicated a need for additional focus on VVD testing (described below).
The slopes were used to calculate absolute changes over the VVD testing period. The absolute changes were compared to the random variability expected between patients, expressed by the standard deviation of variance. Wilcoxon analysis tested for parameter changes more than 5% vs. the mean during VVD optimization.
Subsequent to the initial data analysis, data were reviewed for the first 15 consecutive studies. CO and MAP were averaged for all 38 settings during the three optimizing segments. Data were analyzed graphically with linear or curvilinear fits as indicated by additive mixed effects models.
Results
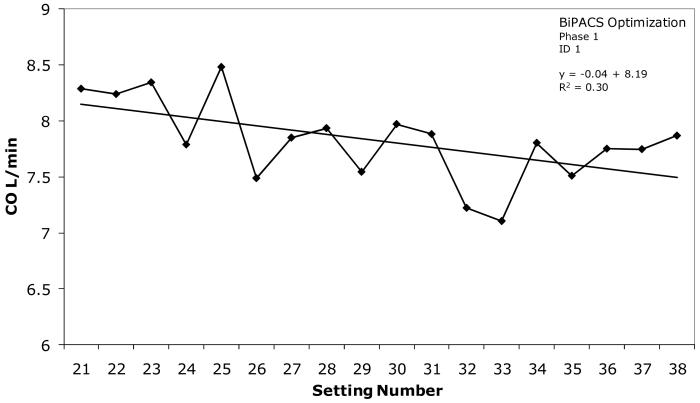
Representative variation of cardiac output during VVD testing in Patient 1 is illustrated in figure 1. At +60 msec (setting numbers 25 and 30) CO was 5.0% higher than the average for the interval and 4.3% higher than the average CO at VVD = 0 msec, the default setting. Phasic variation is apparent. A mean regression line superimposed on the data indicates that CO decreased 8.3% overall during VVD testing in this patient.
Figure 1.
Representative example of phasic variation of cardiac output during testing of 18 VVD settings in Phase 1 of the BiPACS trial. A linear regression line fitting the data is superimposed. The decline in cardiac output indicated by the regression line from 8.1 to 7.5 is 8.3% of the mean value for the interval.
Least squares analyses of CO, MAP, and SVR over the AVD, VVD, and VPS segments of Phase 1 are presented in Tables 1-3. Changes in CO in the AVD and VPS segments are not statistically significant. The decrease during the VVD segment is statistically significant, but the absolute change is less than the standard deviation of the inter-patient variability. MAP declines significantly during the AVD segment, but the absolute change is less than the standard deviation of interpatient variability. MAP changes during the VPS and VVD segments are not statistically significant. SVR changes during all three segments of Phase 1 are not statistically significant.
Table 1.
Least Squares Analysis of Cardiac Output in Phase I BiVP Testing
| Slope | P value | S.D. | AbsΔ | |
|---|---|---|---|---|
| AVD | −0.02 | 0.07 | 2.29 | −0.41 |
| VPS | −0.02 | 0.40 | 2.29 | −0.093 |
| VVD | −0.01 | 0.01 | 2.29 | −0.15 |
S.D. = Standard Deviation over all segments of Phase 1
AbsΔ = Absolute Change
AVD = Atrioventricular Delay
VPS = Ventricular Pacing Site
VVD = Interventricular Delay
Table 3.
Least Squares Analysis of Systemic Vascular Resistance in Phase I BiVP Testing
| Slope | P value | Std. Dev. | AbsΔ | |
|---|---|---|---|---|
| AVD | −0.12 | 0.19 | 11.90 | −2.61 |
| VPS | 0.14 | 0.22 | 11.90 | 0.83 |
| VVD | 0.08 | 0.06 | 11.90 | 1.42 |
S.D. = Standard Deviation over all segments of Phase 1
AbsΔ = Absolute Change
AVD = Atrioventricular Delay
VPS = Ventricular Pacing Site
VVD = Interventricular Delay
Because CO, the ultimate end point of the BiPACS trial, changed significantly during VVD testing, stability during this segment was analyzed further. Table 4 presents linear regression analysis of changes in CO, MAP, and SVR. MAP changed by less than 1% in 4 of 7 patients, and less than 10% in all patients. CO changed less than 10% in 6 of 7 patients, but more than 15% in one patient. SVR changed less than 10% in 5 of 7 patients and less than 15% in 6 of 7 patients. For patient #2, SVR and CO changed more than 15%. On average, CO decreased 5.7±2.3%, MAP decreased 2.5±1.5%, and SVR increased 3.1±3.4%. Only the change in CO was statistically significant (p = 0.043).
Table 4.
Percent Change vs. Mean of Hemodynamic Variables During Phase 1 BiVP Testing
| Patient ID | CO | MAP | SVR |
|---|---|---|---|
| 1 | −8.3 | −3.0 | 5.2 |
| 2 | −15.1 | 0.2 | 15.1 |
| 3 | −4.9 | −8.0 | −2.9 |
| 4 | −0.8 | 0.6 | 1.6 |
| 5 | −5.8 | 0.6 | 6.2 |
| 6 | 4.2 | −8.1 | −12.4 |
| 7 | −9.0 | 0.3 | 9.3 |
| Mean Change* | −5.7 ± 2.3 | −2.5 ± 1.5 | 3.1 ± 3.4 |
Mean ± Standard Error of Mean
ID = Identification Number
CO = Cardiac Output
MAP = Mean Arterial Pressure
SVR = Systemic Vascular Resistance
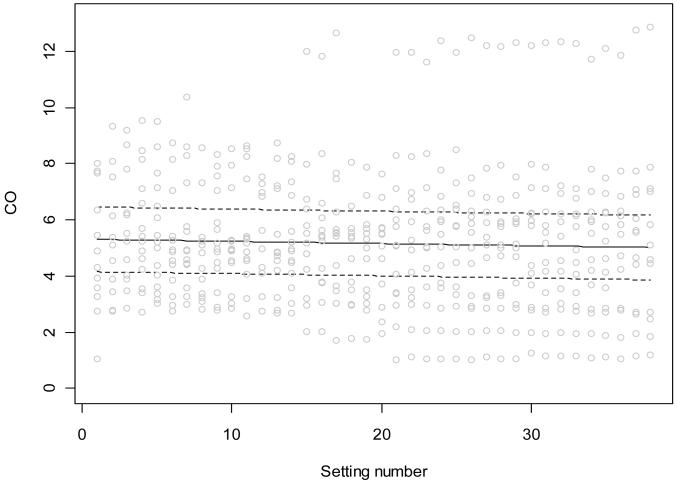
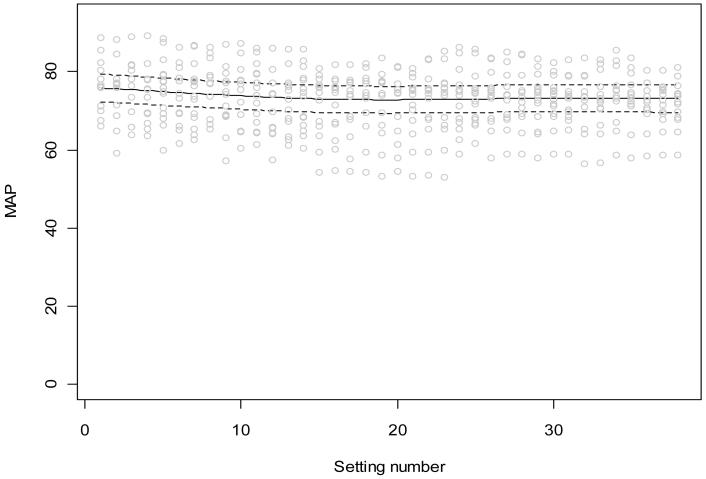
Figures 2 and 3 show CO and MAP data in 15 patients, respectively, including all 38 10-second data segments. Figure 2 superimposes a linear regression and error limits, as defined by a linear mixed effects model. An overall downward trend in CO is demonstrated, but the change is too small to be clinically important. Figure 3 includes a curvilinear fit required by the additive mixed effects model. Again, there is a statistically significant downward trend that is too small to be considered clinically important.
Figure 2.
Variation of cardiac output during BiVP optimization using 38 settings in Phase 1 of the BiPACS trial. Data for 15 patients; we first used an additive mixed effects model allowing a flexible trend. When this model suggested a linear trend, we used a linear mixed effects model. Random subject effects were included in both models to account for the within-subject correlation due to repeated measures from the same subject. The trend suggests an overall gradual decline in cardiac output (p=0.014).
Figure 3.
Phasic variation of the mean arterial pressure during BiVP optimization using 38 settings in Phase 1 of the BiPACS trial. Data for 15 patients; we first used an additive mixed effects model allowing a flexible trend. If this model suggested a linear trend, we used a linear mixed effects model. Random subject effects were included in both models to account for the within-subject correlation due to repeated measures from the same subject. There is a significant nonlinear trend (p<0.001).
Discussion
Potential lability of hemodynamics immediately after weaning from CPB is affected by the interplay of inotropes, anesthetics, vasoconstrictors and vasodilators. Changes in contractility are critical. For some patients, contractility returns to normal after CPB, whereas others require a combination of pharmacological support, electrical pacing, mechanical support and surgical intervention to maintain contractility [35]. Changes in core and blood temperature, delayed onset of action of vasoactive drugs, and the depth of anesthesia are also potentially confounding variables.
Hemodynamic instability after CPB might interfere with BiVP optimization and could make it difficult to assess independent effects of BiVP. However, the linear regression in Figure 1 and the regressions in Figures 2 and 3 are consistent with the clinical impression that changes in cardiac output and mean arterial pressure occur relatively slowly in the absence of specific interventions. Thus, we attribute rapid phasic changes in the figures to the effect of randomized changes in pacemaker settings. Indeed, when such data are rearranged in a linear sequence of the independent variable, changes in CO and MAP are also found to occur gradually, in recognizable patterns [14].
Potential benefits of temporary BiVP are likely to be most important in Phase 1, emphasizing the potential importance of optimization at this time. Furthermore, since 30% of patients do not respond to permanent biventricular pacing, it is imperative to understand the physiology and details of optimization.
It is unlikely that complex protocols will be required for general application of temporary postoperative BiVP, but the present study is designed to define the value and risks from optimization. This interim sub-study is intended to objectively assess variation in hemodynamics during Phase 1, in order to confirm that continued Phase 1 testing is justified.
Least squares analysis of individual testing segments in Phase 1 compared standard deviation of hemodynamic parameters to variance between patients. While some of hemodynamic changes were statistically significant, the absolute values of the observed variations were too small to jeopardize patient welfare.
Small but statistically significant changes in CO were revealed by least squares analysis during VVD optimization and led to more detailed analysis. Wilcoxon analysis revealed a 2.5% decrease in MAP, a 5.7% decrease in CO, and a 3.1% increase in SVR. While these small changes seem acceptable overall, the change in CO exceeds the 5% change we arbitrarily defined as an upper limit. Furthermore, patient 2, for whom MAP did not change, exhibited a 15% decrease in cardiac output during VVD testing. No clinical deterioration was observed in this patient, and the possibility of artifacts in the data cannot be completely excluded. Nevertheless, we have developed and deployed new technology to accurately assess changes in cardiac output in real time [36].
Figure 2 demonstrates a diminishing decrease in cardiac output totaling less than 5% during the entire testing period. Figure 3 demonstrates an initial decrease in mean arterial pressure that reverses with as systemic vascular resistance increases.
Based on these data, we have established decreases in MAP of 10% and in CO of 15% as the safe upper limit in our studies of patients after cardiopulmonary bypass.
In summary, the present results support the view that hemodynamic stability over ten minutes following conclusion of cardiopulmonary bypass is sufficient for studies of cardiac function in most patients. Such studies should be conducted with careful hemodynamic monitoring, however, with established limits to protect patient welfare.
Table 2.
Least Squares Analysis of Mean Arterial Pressure in Phase 1 BiVP Testing
| Slope | p value | S.D. | AbsΔ | |
|---|---|---|---|---|
| AVD | −0.23 | <0.0001 | 6.53 | −4.82 |
| VPS | −0.10 | 0.23 | 6.53 | −0.58 |
| VVD | 0.23 | 0.093 | 6.53 | 4.16 |
S.D. = Standard Deviation over all segments of Phase 1
AbsΔ = Absolute Change
AVD = Atrioventricular Delay
VPS = Ventricular Pacing Site
VVD = Interventricular Delay
Aknowledgments
Dr. Henry Spotnitz is the George H. Humphreys, II, Professor of Surgery.
Research Support: Supported in part by USHPHS grant # NHLBI Research Grant HL-080152, “Biventricular Pacing after Cardiopulmonary Bypass”. Dr. Daniel Y. Wang is supported by an NIH Training Grant, 5 T32 HL007854-13. Matthew Spotnitz was supported by a Dean’s Summer Research Fellowship from the Columbia University College of Physicians and Surgeons. Dr. Alexander Rusanov is supported by an NIH Training Grant, 5 T32 GM008464-17.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46:2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 2--issues during and after device implantation and unresolved questions. J Am Coll Cardiol. 2005;46:2168–2182. doi: 10.1016/j.jacc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Auricchio A, Stellbrink C, Sack S. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026–2033. doi: 10.1016/s0735-1097(02)01895-8. et a. [DOI] [PubMed] [Google Scholar]
- 6.Foster AH, Gold MR, McLaughlin JS. Acute hemodynamic effects of atrio-biventricular pacing in humans. Ann Thorac Surg. 1995;59:294–300. doi: 10.1016/0003-4975(94)00878-b. [DOI] [PubMed] [Google Scholar]
- 7.Pham PP, Balaji S, Shen I, et al. Impact of conventional versus biventricular pacing on hemodynamics and tissue Doppler imaging indexes of resynchronization postoperatively in children with congenital heart disease. J Am Coll Cardiol. 2005;46:2284–2289. doi: 10.1016/j.jacc.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Pichlmaier M, Bagaev E, Lichtenberg A, et al. Four-chamber pacing in patients with poor ejection fraction but normal QRS durations undergoing open heart surgery. Pacing Clin Electrophysiol. 2008;31:184–191. doi: 10.1111/j.1540-8159.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Okishige K, Mizuno T, et al. Temporary and permanent biventricular pacing via left ventricular epicardial leads implanted during primary cardiac surgery. Jpn J Thorac Cardiovasc Surg. 2002;50:284–289. doi: 10.1007/BF03032296. [DOI] [PubMed] [Google Scholar]
- 10.Weisse U, Isgro F, Werling C, et al. Impact of atrio-biventricular pacing to poor left-ventricular function after CABG. J Thorac Cardiovasc Surg. 2002;50:131–135. doi: 10.1055/s-2002-32403. [DOI] [PubMed] [Google Scholar]
- 11.Dzemali O, Bakhtiary F, Dogan S, et al. Perioperative biventricular pacing leads to improvement of hemodynamics in patients with reduced left-ventricular function-interim results. Pacing Clin Electrophysiol. 2006;29:1341–1345. doi: 10.1111/j.1540-8159.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman FJ, Starr JP, Koenig PR, et al. Acute hemodynamic benefit of multisite ventricular pacing after congenital heart surgery. Ann Thorac Surg. 2003;75:1775–1780. doi: 10.1016/s0003-4975(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 13.Dzemali O, Bakhtiary F, Israel CW, et al. Impact of different pacing modes on left ventricular function following cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2008;56:87–92. doi: 10.1055/s-2007-989395. [DOI] [PubMed] [Google Scholar]
- 14.Berberian G, Cabreriza SE, Quinn TA, et al. Left ventricular pacing site-timing optimization during biventricular pacing using a multi-electrode patch. Ann Thorac Surg. 2006;82:2292–2294. doi: 10.1016/j.athoracsur.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 15.Berberian G, Kanter JP, Quinn TA, et al. Optimized perioperative biventricular pacing in setting of right heart failure. Europace. 2005;7:385–387. doi: 10.1016/j.eupc.2005.02.119. [DOI] [PubMed] [Google Scholar]
- 16.Berberian G, Quinn TA, Kanter JP, et al. Optimized biventricular pacing in atrioventricular block after cardiac surgery. Ann Thorac Surg. 2005;80:870–875. doi: 10.1016/j.athoracsur.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 17.Berberian G, Quinn TA, Cabreriza SE, et al. Load dependence of cardiac output in biventricular pacing: left ventricular volume overload in pigs. J Thorac Cardiovasc Surg. 2006;131:666–670. doi: 10.1016/j.jtcvs.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Berberian G, Quinn TA, Cabreriza SE, et al. Left ventricular pacing site and timing optimization during biventricular pacing using a multielectrode patch in pigs. J Thorac Cardiovasc Surg. 2007;134:574–578. doi: 10.1016/j.jtcvs.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Quinn TA, Berberian G, Cabreriza SE, et al. Effects of sequential biventricular pacing during acute right ventricular pressure overload. Am J Physiol Heart Circ Physiol. 2006;291:H2380–2387. doi: 10.1152/ajpheart.00446.2006. [DOI] [PubMed] [Google Scholar]
- 20.Rabkin DG, Cabreriza SE, Curtis LJ, et al. Load dependence of cardiac output in biventricular pacing: right ventricular pressure overload in pigs. J Thorac Cardiovasc Surg. 2004;127:1713–1722. doi: 10.1016/s0022-5223(03)01319-9. [DOI] [PubMed] [Google Scholar]
- 21.Rabkin DG, Cabreriza SE, Curtis LJ, et al. Mechanisms of optimized biventricular pacing in pulmonary stenosis: effects on left ventricular geometry in swine. Pacing Clin Electrophysiol. 2004;27:1060–1071. doi: 10.1111/j.1540-8159.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- 22.Rabkin DG, Curtis LJ, Cabreriza SE, et al. Load dependence of cardiac output in biventricular pacing: right ventricular volume overload in pigs. J Thorac Cardiovasc Surg. 2004;128:98–102. doi: 10.1016/j.jtcvs.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Spotnitz HM. Optimizing temporary perioperative cardiac pacing. J Thorac Cardiovasc Surg. 2005;129:5–8. doi: 10.1016/j.jtcvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Wong CYH, Spotnitz HM. Systolic and diastolic properties of the human left ventricle during valve replacement for chronic mitral regurgitation. Am J Cardiol. 1981;47:40–50. doi: 10.1016/0002-9149(81)90287-3. [DOI] [PubMed] [Google Scholar]
- 25.Dubroff JM, Clark M, Wong CYH, et al. Left ventricular ejection fraction during cardiac surgery: a two-dimensional echocardiographic study. Circulation. 1983;68:95–103. doi: 10.1161/01.cir.68.1.95. [DOI] [PubMed] [Google Scholar]
- 26.Nicolosi AC, Spotnitz HM. Quantitative analysis of regional systolic function with left ventricular aneurysm. Circulation. 1988;78:856–862. doi: 10.1161/01.cir.78.4.856. also Surg Forum. 39:254–256. 188. extended abstract.
- 27.Hart JP, Cabreriza SE, Walsh R, et al. Echocardiographic Analysis of Ventricular Geometry and Function During Repair of Congenital Septal Defects. Ann Thorac Surg. 2004;77:53–60. doi: 10.1016/s0003-4975(03)01328-6. [DOI] [PubMed] [Google Scholar]
- 28.Quinn TA, Curtis LJ, Cabreriza SE, et al. Regional functional depression immediately following ventricular septal defect closure. J Am Soc Echocardiogr. 2004;17:1066–72. doi: 10.1016/j.echo.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Berberian G, Quinn TA, Kanter JP, et al. Optimized biventricular pacing in atrioventricular block after cardiac surgery. Ann Thorac Surg. 2005;80:870–5. doi: 10.1016/j.athoracsur.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 30.Garofalo CA, Cabreriza SE, Quinn TA, et al. Diastolic properties predict short-term postoperative risk and duration of pleural effusions after the Fontan operation. Circulation. 2006;114(1 Suppl):I56–61. doi: 10.1161/CIRCULATIONAHA.105.001396. [DOI] [PubMed] [Google Scholar]
- 31.Berberian G, Cabreriza SE, Quinn TA, et al. Left ventricular pacing site-timing optimization during biventricular pacing using a multielectrode patch. Ann Thorac Surg. 2006;82(6):2292–4. doi: 10.1016/j.athoracsur.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 32.Richmond ME, Cabreriza SE, Van Batavia JP, et al. Direction of pre-operative ventricular shunting affects ventricular mechanics after tetralogy of Fallot repair. Circulation. 2008;118(23):2338–44. doi: 10.1161/CIRCULATIONAHA.107.761080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YH, Cowan DB, Moran AM, et al. Myocyte apoptosis occurs early during the development of pressure-overload hypertrophy in infant myocardium. J Thorac Cardiovasc Surg. 2009;137(6):1356–62. e3. doi: 10.1016/j.jtcvs.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaura K, Hoka S, Okamoto H, et al. Noninvasive assessment of left ventricular pressure-area relationship using transesophageal echocardiography and tonometry during cardiac and abdominal aortic surgery. J Anesth. 2005;19(2):106–11. doi: 10.1007/s00540-004-0296-7. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan JA, Reich DL, Lake CL, Konstadt SN, editors. Kaplan’s Cardiac Anesthesia. 4th Edition WB Saunders; Philadelphia, PA: 1999. pp. 1060–1089. [Google Scholar]
- 36.George E, Cabreriza S, Quinn TA, et al. Validation of Automated Monitoring of Biventricular Pacing Optimization. Artif Organs. 2009;33(5):A23. doi: 10.1097/MAT.0b013e3181cf882a. [DOI] [PMC free article] [PubMed] [Google Scholar]