Abstract
VACTERL association involves the presence of specific congenital, multi-organ malformations that tend to co-occur. Clinical and research efforts typically center on pediatric patients, and there is a scarcity of information in the literature regarding VACTERL-related issues and outcomes in adulthood. We describe here 11 adults with features of VACTERL association ascertained through our research study on the condition. In our cohort of adult patients, approximately 25% of medically significant malformations that are component features of VACTERL association, including 40% of vertebral, 50% of cardiac, and 50% of renal anomalies, were not identified during childhood. Additionally, medical sequelae of many of the primary malformations identified in infancy or early childhood persist or are first reported in adulthood. These sequelae can involve challenging medical and surgical management in adulthood. As most adults with VACTERL association are not specifically followed for VACTERL-related issues, a more uniform diagnostic work-up and a low threshold for investigation of medical sequelae of the primary disorder may enhance the quality of clinical management in these patients.
Keywords: VACTERL, VACTERL association, VATER, VATER association
1. Introduction
VACTERL association, estimated to occur in approximately 1 in 10,000 live births [7], is a recognizable group of congenital malformations that tend to coexist in a single patient. In 1973, Quan and Smith initially named the condition VATER association, which included vertebral defects (V), anal atresia (A), tracheoesophageal fistula (TE) with esophageal atresia, renal defects (R), and radial dysplasia (R) [26]. The condition was soon redefined as VACTERL association, with the inclusion of cardiac defects (C) and additional limb anomalies (L) [23, 26, 35]. Uncertainty persists regarding exact diagnostic criteria. For example, some studies, such as the relatively large clinical series described by Weaver et al. in 1986 [39], required the presence of two component features for diagnosis, while others, including a follow-up study on that series [40], require at least three component features [3, 4, 14, 29]. While several genetic causes have been implicated in a small number of human patients or in animal models [6, 11, 16, 27, 30, 34, 36], evidence of causality has not been uniform, and no consistent etiology has been identified.
Because VACTERL association is defined by congenital anomalies, clinical and research efforts tend to center around pediatric patients. Several large studies have obtained patient information from databases on congenital malformations in infants [3, 4, 14, 15, 29]; other sizeable cohorts have been described by pediatric geneticists or by pediatric surgical specialists, and focus mainly on infants and children [7, 32, 39]. While the outcomes of each of the component features have been studied separately, we located only one study examining long-term prognosis of adults with VACTERL association [40]; this study included many patients previously described as children, some of whom were ultimately found to have alternate diagnoses. The results of this study highlighted the fact that while patients with “classical” VACTERL association may have medical challenges, long-term outcomes generally tend to be positive [40].
Despite this, in our experience, many children diagnosed with VACTERL association are seen by pediatric geneticists and other pediatric specialists, but are not followed for issues related to VACTERL association in adulthood. The paucity of information regarding VACTERL-related issues that persist or begin in adulthood is becoming increasingly problematic: children with VACTERL association now survive to adulthood more frequently than do children diagnosed decades ago, due to increased availability and quality of surgical treatments for TE fistula [9, 18, 25], imperforate anus [24, 28], and congenital heart defects [8], as well as enhanced ventilatory support, neonatal anesthesia, and intensive care management [9, 18]. Treatment of adults with VACTERL association is also complicated by the fact that although recurrence has been demonstrated in some families [2, 22, 32], the majority of patients do not have an affected relative whose medical care can highlight pertinent clinical issues [16].
Our research shows that many medically significant malformations may not be ascertained until after a patient’s care has moved beyond the pediatric realm. Additionally, sequelae of malformations identified in infancy may not appear until later in life.
To address the need for information regarding clinical issues in adults with features of VACTERL association, we describe 11 adult patients diagnosed with VACTERL association.
2. Materials and Methods
Over the course of approximately two years, our IRB-approved research protocol at the National Human Genome Research Institute (National Institutes of Health, Bethesda, MD, United States) has accumulated a cohort of 107 patients, consisting of fetuses, infants, children, and adults previously diagnosed with multiple features of VACTERL association. Inclusion criteria for the overall research study includes at least one of the following: at least three core component features of VACTERL association, at least two core component features and another congenital anomaly, or at least two core component features and a first-degree relative with at least one component feature. Patients were excluded if, either at first screening, or on later analysis, they were felt to likely meet criteria for an overlapping condition, either because of clinical features or results of genetic testing.
From this cohort, 11 adult patients were identified, with appropriate consent obtained from participants. All patients described here had been previously diagnosed or suspected to have VACTERL association (in Patients 1 and 10, who both had affected relatives, formal diagnosis was made upon participation in the study along with their affected family members). Participants were referred by clinicians or self-referred. Eight of the 11 patients were available to be contacted via telephone and/or e-mail for an extensive medical history. Six of the 11 patients, indicated by an asterisk (*) in section 3.2 of the Results, were additionally seen in person at the National Institutes of Health. For patients not seen in person, the telephone interview was supplemented by review of all available medical records provided by patients as well as those provided by referring clinicians in order to validate patient-reported information.
3. Results
3.1 Aggregate Results
Eleven patients (10%) out of our total cohort of 107 patients with features of VACTERL association were adults (see Table I for a summary of adult patients and Figure 1 for illustrations of selected findings). The adult group included five males and six females, with ages ranging from 28 to 64 years (mean 40 years). The average height in males was 170.2 centimeters; the average height in females was 159 cm. Eight patients (73%) demonstrated three or more component features of VACTERL association. Two patients (18%) had two component features of VACTERL association as well as clinical evidence of likely renal anomalies, but no further proof of that type of malformation. One patient (9%) had only two component features. In terms of specific component features, 10 patients (91%) had vertebral malformations, 5 (45%) had imperforate anus/anal atresia, 4 (36%) had cardiac malformations, 6 (55%) had a tracheoesophageal fistula (TEF), 4 (36%) had clear evidence of renal anomalies, and 4 (36%) had limb malformations. Three patients (27%) had genital malformations.
Table I. Summary of the 11 reported adults.
More detailed descriptions are contained in Section 3.2 of the text. Adult sequelae suspected to be linked to the VACTERL anomalies are also listed, and the age of diagnosis of each anomaly is listed under the specific anomaly.
| # | Age at study ascertainment (approximate age at VACTERL diagnosis) (y) | Sex | Height (cm) Weight (kg) BMI (kg/m2) |
V | A | C | TE | R | L | Adult sequelae of anomalies | Family history | Genetic testing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 64 (64) | M | 166 66.4 24.1 |
S1 SBO, mild mid-lumbar dextrotatory scoliosis (20 y) | – | VSD (infancy) | – | Ureteral stricture, numerous episodes of nephrolithiasis (20s) | – | Severe back pain, nephrolithiasis | VACTERL (daughter; Patient 2) | Normal microarray |
| 2* | 35 (34) | F | 156 47.4 19.5 |
Cervico-thoracic scoliosis, dysplastic C7-T3 vertebrae (26 y) | – | – | TEF (infancy) | U: numerous UTIs, though unclear if there is an underlying structural defect (throughout life) | – | Back, shoulder, and neck pain; dysphagia; asthma; recurrent UTIs | VACTERL (father; Patient 1) | Normal microarray |
| 3 | 29 (17) | F | 160 64.4 25.2 |
Mild scoliosis, supernumerary rib; other details unknown (early childhood) | Imperforate anus (infancy) | – | – | U: multiple hospitalizations for UTIs, pyelonephritis; though unclear if there is an underlying structural defect (throughout life) | – | Back pain, constipation, regurgitation, choking, recurrent UTIs | Negative | Not performed |
| 4 | 28 (early childhood) | F | 158 47.6 19.1 |
Multiple thoracic hemivertebrae, vertebral fusions and dysplastic vertebrae, scoliosis (childhood) | Anal atresia (infancy) | Ostium secundum ASD (infancy) | Type B TEF (infancy) | Unilateral renal agenesis (infancy) | Right thumb hypoplasia, bilateral thenar hypoplasia, bony wrist anomalies (infancy) | Constipation, exercise intolerance, hand/wrist pain | Negative | Normal karyotype |
| 5* | 28 (infancy) | M | 178 100.0 31.6 |
Multiple cervical, thoracic, and lumbar vertebral anomalies, including hemivertebrae, block vertebrae, and dysplastic vertebrae; mild kyphosis (5 y) | – | – | Type III TEF (Vogt class- ification) (infancy) | – | – | Severe back pain, tracheomalacia, reactive airway disease | “Collapsing trachea” (mat. uncle, pat. grand-mother); vertebral anomalies (mat. uncle, mat. grandmother, father) | Normal microarray |
| 6* | 28 (18) | F | 155 54.4 22.6 |
Cervical and thoracic block vertebrae, lumbar pedicle dysplasia, scoliosis (childhood) | – | Persistent left superior vena cava (28 y) | Type C TEF (infancy) | – | – | Hip dysplasia, dysphagia, GER, poor esophageal motility, nephrolithiasis | Negative | Not performed |
| 7* | 52 (late 30s) | M | 165 79.4 29.2 |
Cervical and lumbar block vertebrae, cervical and lumbar hemi- vertebrae, lumbar scoliosis (infancy) | Imperforate anus (infancy) | – | – | Horsehoe kidney (infancy) | – | GI obstructions and adhesions, GI bleeding, fecal incontinence, nephrolithiasis | Negative | Normal microarray |
| 8 | 40 (infancy) | F | 166 68.0 24.7 |
Scoliosis; other details unknown (early childhood) | – | – | TEF (infancy) | – | Bilateral radial aplasia (infancy) | Dysphagia, GER, asthma, poor esophageal motility | Unknown congenital malformation (infant born to pat. great- uncle) | U |
| 9 | 42 (32) | M | 176.3 102.3 31.8 |
– | Imperforate anus (infancy) | LV dilatation; aortic enlargement (32 y) | – | Unilateral renal atresia, dysplastic kidney (30 y) | – | Pyelonephritis, GI obstructions | Negative | Not performed |
| 10* | 53 (53) | M | 165 75 26.6 |
C3-C4 block vertebrae, T1-T3 hemi-vertebrae (adolescence) | Imperforate anus/anal atresia (infancy) | – | – | – | Left thenar hypoplasia (infancy) | Neck/back stiffness, constipation | VACTERL (grandson) | Not performed |
| 11 | 39 (22) | F | U | Cervical vertebral fusions (adolescence) | – | – | TEF (infancy) | – | Left thumb agenesis, right postaxial polydactyly (infancy) | Choking, poor esophageal motility | U | U |
A: anal malformations; BMI: body mass index, C: cardiac malformations, F: female; GER: gastroesophageal reflux; GI: gastrointestinal; L: limb malformations; M: male; mat.: maternal; pat.: paternal; R: renal anomalies; SBO: spina bifida occulta; TE: tracheoesophageal fistula; U: unknown; UTI: urinary tract infection; V: vertebral malformations; y: years; +: present; –: absent;
: seen in person at the NIH.
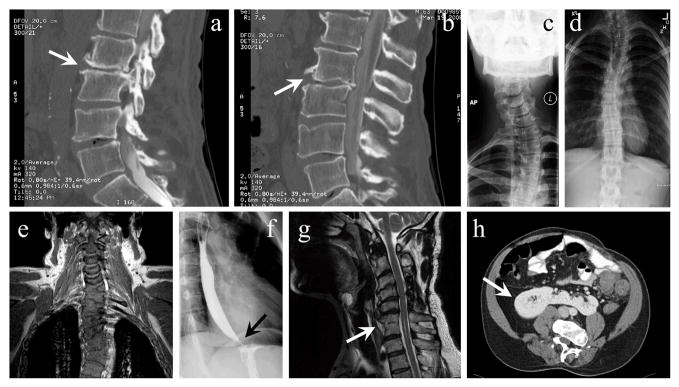
Figure 1. Radiographic images of selected patients.
These images, of various patients described in section 3.2 of the text, show their malformations attributed to VACTERL association. (a–b) Sagittal CT sections of Patient 1, a 64-year-old male with severe back pain, showing degenerative disc changes related to relatively mild congenital vertebral malformations. The most severely affected areas are indicated with arrows. (c) X-ray (anterior-posterior view) of Patient 2, a 35-year-old female with neck, shoulder, and back pain, demonstrating cervical scoliosis and moderate degenerative arthritis at the C6-C7 level with degenerative disc narrowing. Costovertebral anomalies in Patient 2 were unknown until X-rays were performed in adulthood after an unrelated accident. (d) X-ray (anterior-posterior view) of Patient 2, demonstrating thoracolumbar scoliosis. (e) Coronal MR section of Patient 5, a 28-year-old male with progressive back pain, showing complex vertebral malformations, including scoliosis, multilevel ankylosis, block vertebrae, and hemivertebrae. (f) X-ray image (lateral view) of barium swallow in Patient 6, a 28-year-old female with reduced esophageal motility and gastroesophageal reflux, demonstrates terminal esophageal narrowing (arrow) consistent with mild early achalasia as well as minimal deformity due to prior TEF repair procedure. (g) Sagittal MR section of Patient 7, a 52-year-old male, displaying congenital cervical spine anomalies accompanied by degenerative changes, including posterior fusion of C3-C4 and C5 and congenital block vertebrae at C6-C7 (indicated by the arrow). (h) Axial CT section of Patient 7 demonstrating the presence of a horseshoe kidney (indicated by the arrow); this patient had normal renal function but a history of nephrolithiasis and urinary tract infections possibly related to congenital rectal anomalies.
Five of the 11 patients (45%) were diagnosed with VACTERL association in childhood, and 8/11 (73%) were diagnosed at least 10 years before participation in our study. While the presence of imperforate anus/anal atresia, TEF, and limb anomalies were all diagnosed in infancy, vertebral anomalies were not diagnosed until adolescence/adulthood in 40% (4/10) of the patients with vertebral anomalies, cardiac anomalies were not diagnosed until adulthood in 50% (2/4) of patients with cardiac malformations, and renal anomalies were not diagnosed until adulthood in 50% (2/4) of patients with renal anomalies. Overall, 24% (8/33) of the core component features of VACTERL association in this cohort were not diagnosed until after childhood.
Karyotype testing was performed, with a normal result, in one patient (9%); microarray analysis (Illumina Omni1-Quad BeadChip) was performed, with a normal result, in 4 patients (36%); and no genetic testing had been reportedly performed (or known) for 6 patients (55%). No patients had evidence of developmental delay or neurocognitive impairment, or specific evidence for hydrocephalus. No patients described here had features highly suggestive of an alternate diagnosis.
Apart from Patients 1 and 2, who were father and daughter, patients were unrelated to each other. Patient 10 was the only other patient with a relative who also had VACTERL association. In Patients 1 and 2, inheritance appeared to be autosomal dominant, while X-linked inheritance was most likely in Patient 10’s family. Patient 5 has multiple relatives with features of VACTERL association, though complete details are not available as the patient was adopted.
In terms of adult sequelae, 6 patients (55% of the total patient cohort, and 60% of the patients with vertebral anomalies) reported back pain associated with vertebral malformations. Five patients (45% of the total cohort and 100% of patients with imperforate anus/anal atresia) had adult sequelae of imperforate anus/anal atresia. Five patients (45% of the total cohort and 4/6, or 67% of those with TEF) reported dysphagia and/or reflux associated with TEF, as one patient who reported this issue did not have a TEF. Three patients (27% of the total cohort and 50% of patients with TEF) had pulmonary symptoms consistent with reactive airway disease, and 5 (45% of the total cohort, with 75% of patients who had a clear renal anomaly) had a history of UTIs and/or nephrolithiasis likely related to subtle renal anomalies, though only 1 (9% of the total cohort and 25% of patients with renal anomalies) had evidence of impaired renal function.
3.2 Individual Patient Descriptions
Patient 1*
Patient 1 is a 64-year-old male with VACTERL association with vertebral, cardiac, and possible renal anomalies. Adult sequelae include a ureteral stricture requiring surgical correction and numerous episodes of nephrolithiasis (>3,000 stones) secondary to idiopathic hypercalciuria and resulting in renal papillary dilation, calyceal damage, and impaired renal function. At age 26, he began experiencing severe lower back pain, which was refractory to laminectomy, medication, and implantation of a spinal cord stimulator, and which greatly limits his activities. He also has a history of a resting tremor, multiple hernias, chronic obstructive pulmonary disease (COPD) ascribed to tobacco use, and pulmonary hypertension. Family history is notable for VACTERL association in his daughter (Patient 2), but no similar findings in other relatives. Microarray (Illumina Omni1-Quad) was normal.
Patient 2*
Patient 2, the 35-year-old daughter of Patient 1, had vertebral anomalies and a TEF, as well as multiple (>30) UTIs but no obvious renal anomalies on imaging. Her TEF was repaired at birth, but required a repeat operation at age 4 years due to recurrence; she has had progressive difficulty swallowing both liquids and solids in her thirties, for which antireflux medication and balloon dilatation were ineffective. She has a history of asthma and a tremor similar to that of her father (and other paternal relatives). In adulthood, she was noted to have associated degenerative joint disease of the cervical spine, and reports back, shoulder, and neck pain, but it is unclear whether this is secondary to the vertebral anomalies, the tremor, or both. Microarray (Illumina Omni1-Quad) was normal.
Patient 3
Patient 3 is a 29-year-old female with vertebral anomalies, imperforate anus, recurrent UTIs with multiple hospitalizations for pyelonephritis, urinary incontinence, and the presence of uterus didelphys, for which she underwent reconstructive surgery. She does not have a true TEF, but a mild tracheoesophageal anomaly is suggested by numerous episodes of “spitting up” and “choking” as a child, which occasionally still occur. The scoliosis did not require bracing or surgery, but is associated with lower back pain exacerbated by lengthy standing. Sequelae of rectourinary anomalies are her main adult issue: she had a colostomy and pull-through procedure at age 2 years, one episode of intestinal blockage requiring hospitalization in her mid-twenties, and requires twice daily enemas. She had never seen a geneticist, has not had any genetic testing, and has no contributory family history.
Patient 4
Patient 4 is a 28-year-old female with vertebral anomalies, anal atresia with rectovaginal fistula, a cardiac defect, TEF, right renal agenesis, and limb anomalies. The scoliosis does not interfere with daily activities, but has resulted in asymmetry, making weight bearing difficult. The anal atresia was repaired during infancy without a colostomy, and she currently reports no issues with incontinence, though she has had to treat constipation medically. The ASD was repaired at age 4 years; she reports that despite copious physical activity during childhood, she is now relatively easily fatigued. She also has a history of bilateral inguinal hernias repaired at 3 months of age. She currently has pain in her wrists/hands, and has difficulty writing. She has no family history of similar features, and she had not seen a geneticist or obtained genetic testing other than a normal karyotype in early childhood.
Patient 5*
Patient 5 is a 28-year-old male with vertebral anomalies and a TEF. He has severe recurrent lower back pain lasting 1–3 days per month, limiting both work and recreational activities, and which is refractory to medical management. The TEF was repaired at two days of age; subsequent gastroesophageal reflux was managed with a Nissen fundoplication. He was diagnosed with tracheomalacia and reactive airway disease, both of which were severe during childhood; he currently reports occasional wheezing and inability to keep up with others during physical activity. Family history is notable for vertebral anomalies in his father, maternal uncle, and maternal grandmother and “collapsing trachea” in another maternal uncle and paternal grandmother (further specifics unavailable); and consideration of a mild, subtle variant of TEF in his 1-year-old son. Microarray (Illumina Omni1-Quad) was normal.
Patient 6*
Patient 6 is a 28-year-old female with vertebral anomalies, a cardiac malformation, and a TEF. Bilateral trochanteric surgery, required for hip dysplasia possibly related to scoliosis and characterized by short femoral necks and trochanteric overgrowth, was performed to improve motion. She reports back pain with exertion. An echocardiogram, performed as part of her research participation, revealed a persistent left superior vena cava. TEF sequelae include poor esophageal motility, difficulty swallowing, and occasional symptomatic gastroesophageal reflux. A barium enema demonstrated mild achalasia. She has had one episode of nephrolithiasis, but no known renal anomalies. Additional issues include hearing loss, thought to be related to childhood medication toxicity, and recurrent pneumonia in childhood. She had never previously seen a geneticist or had genetic testing, and family history is noncontributory.
Patient 7*
Patient 7 is a 52-year-old male with vertebral anomalies, imperforate anus, renal anomalies, and mild hypospadias. His imperforate anus was corrected immediately at birth without a colostomy. He is unable to work due to gastrointestinal issues, which have included obstructions, pseudo-obstructions, adhesions, and inadequate bowel control. Since adolescence, he reports episodes lasting several hours during which the frequency and volume of stools increase progressively; approximately twice annually, these episodes are accompanied by emesis, painful cramping, symptomatic anemia, light-headedness, and hematochezia of unknown etiology, requiring hospitalization. A defecogram revealed a small-caliber rectum with no muscular contractility, and pelvic MRI demonstrated absent muscle around the surgically created pseudo-rectum. Other medical issues include otosclerosis, for which he received bilateral stapedectomies; COPD ascribed to tobacco use; a history of an inguinal hernia; and infertility of unknown etiology. His family history is noncontributory. Microarray (Illumina Omni1-Quad) was normal.
Patient 8
Patient 8 is a 40-year-old female with possible vertebral anomalies, TEF, radial anomalies, and septated uterus. She required bracing for scoliosis for one year and does not currently report frequent back pain or impairment of daily activities. Her TEF required two repairs in infancy, as the first repair failed and was complicated by scarring and strictures. She currently has poor esophageal motility, problems swallowing, gastroesophageal reflux, and a diagnosis of mild asthma. She has no affected relatives other than one female infant, her first cousin once removed, who had congenital malformations further details of which are unknown. She was originally seen by Quan and Smith as a neonate, but no genetic testing was reportedly done (and it is unclear if she were one of the patients originally described by these clinicians).
Patient 9
Patient 9 is a 52-year-old male with imperforate anus, cardiac anomalies, and renal anomalies. He underwent a colostomy repair in infancy with a subsequent pull-through procedure, and remains affected in adulthood by constipation, with over 10 hospitalizations for obstipation. His renal function has remained normal, as has cardiac function. His history also includes multiple lipomas requiring surgical excision and hearing loss ascribed to working in a noisy environment. His family history is noncontributory, and no genetic testing has been performed.
Patient 10*
Patient 10 is a 53 year-old male with vertebral anomalies, imperforate anus/anal atresia (details unclear), and limb anomalies. Adult sequelae include decreased range of motion in his neck and back, as well as bowel irregularity managed medically. His grandson, through his daughter, has similar vertebral anomalies and imperforate anus, and he has other maternal relatives with similar malformations in an overall pattern consistent with X-linked inheritance. No genetic testing has been performed, though linkage analysis coupled with high-throughput sequencing of minimal critical regions via whole-exome sequencing has been initiated. Though Townes-Brocks is in the differential diagnosis (and SALL1 has not yet been tested) neither he nor his affected relatives have ear anomalies or hearing loss, and his relatives have features that are not typically seen in this condition.
Patient 11
Patient 11 is a 39-year-old female with vertebral anomalies, TEF, limb anomalies, and right postaxial polydactyly (further specific details unknown, though the patient is African-American, and the description appears consistent with the polydactyly variant more commonly observed in individuals of this ethnicity [41]). She reported difficulty swallowing in childhood, with several choking episodes, persisting occasionally through adulthood. Additionally, she limits her choices of foods and notices mucus buildup during cold weather and dysphagia associated with respiratory infections. This patient is lost to follow-up, and further details are unknown.
4. Discussion
The patients we describe shed light on VACTERL association outside of the pediatric period. In our experience, patients and families with features of VACTERL association are told very little about long-term prognoses and outcomes, perhaps due to a dearth of published information. Because of this, we feel that a descriptive case series such as is presented here can be a valuable resource for both medical caregivers and for affected patients and families.
Overall, the patients reported here demonstrate two central points. First, medically significant malformations that are component features of VACTERL association, especially those affecting the vertebrae, heart, and kidneys, may not be ascertained until adulthood. Strikingly, almost one-quarter of malformations that are core component features of VACTERL association were not identified until after childhood, and in over half of patients, the diagnosis of VACTERL association was not made until adulthood, though this may very well be due to the fact that the condition now termed VACTERL association was first described after many patients described here were born. Currently, greater awareness of VACTERL association and similar conditions may result in improved ascertainment of malformations. Some anomalies, such as TEF or imperforate anus, are usually obvious in the neonatal period, but in many of our patients, other malformations were only noted later in life as an incidental finding, as in one case when an X-ray was performed after a coincidental traumatic injury. In our cohort, these late-diagnosed malformations resulted in medically significant issues later in life, such as debilitating back pain related to vertebral anomalies, or unilateral renal agenesis with a dysplastic remaining kidney or the presence of a cardiac malformation necessitating careful follow-up of renal or cardiac function. It is thus important for medical professionals such as practitioners of general/family medicine, internal medicine, and other specialists who regularly see adult patients to be aware of conditions like VACTERL association that have traditionally been the purview of pediatricians and pediatric subspecialists.
Second, the experiences of adult patients with features of VACTERL association indicate that in many patients, medical sequelae of the primary malformations persist or are first reported in adulthood. The majority of patients with vertebral malformations continued to experience back, shoulder, and/or neck pain, which was particularly severe in two patients. The typical age of onset of back pain in our cohort was lower than that in the general population [10], and though our cohort is small, the severity of the initial vertebral malformations did not appear qualitatively greater in patients with severe back pain. In view of these observations, we recommend that all patients with vertebral anomalies continue to have this issue carefully followed in adulthood, with a low threshold for referral for further management.
In terms of anal anomalies, it is well established that sequelae of imperforate anus/anal atresia can be debilitating [20]. Several patients report complications such as severe constipation and intestinal blockage requiring hospitalizations. Functional stooling problems and poor quality of life persisted in many children treated for anorectal malformations, and quality of life may worsen with age [13]. Attention to these issues in adults with VACTERL association, as well as up-to-date knowledge of the continuously emerging surgical options for incontinence [5] and non-surgical options such as biofeedback therapy [42], may be helpful.
While a spectrum of renal issues exists in many patients – and admittedly, some patients have renal issues but no identified structural renal anomaly – the most commonly observed medical problems are nephrolithiasis and UTIs (in both genders). This may be explained by findings in a small previous study, in which 43% of pediatric patients with genitourinary involvement had severe urinary tract reflux, which can cause kidneys stones and UTIs [37]. Although our group only included one patient with impaired renal function, many patients with VACTERL association survive into adulthood with renal anomalies that can cause chronic kidney disease and associated morbidities [1].
In patients with repaired TEF, problems such as dysphagia, choking, and gastroesophageal reflux can be attributed to poor esophageal motility, while respiratory issues may include reactive airway disease and tracheomalacia. These issues may relate to sequelae of the TEF itself, to surgical repair of the TEF, or to underlying cellular differences. The presence of these issues is unsurprising: in several analyses of morbidity and mortality associated with TEF/EA, many patients have been documented with recurrent respiratory infections, dyspnea, dysphagia, gastroesophageal reflux, tracheomalacia, and an increased risk of esophageal malignancy [9, 12, 21, 31, 33]. The presence of formally diagnosed asthma in patients with repaired TEF is less well established: while wheezing and other respiratory symptoms are often documented, it is not always clear whether patients meet criteria for the diagnosis of asthma or if the symptoms are attributable to effects of the TEF [19]. Several quality of life studies show that most survivors of TEF/EA repair enjoy a normal quality of life [12, 17, 38].
Despite these conclusions, major limiting factors of this study include its retrospective nature, which may create an undue emphasis on more severe cases, and lack of uniform examination, both in contrast to the cohort followed by Wheeler and Weaver approximately 20 years after initial ascertainment [40]. In addition, in the absence of uniformly applied diagnostic criteria, it is difficult to ascertain how accurately the patients in our cohort and those in Wheeler and Weaver’s cohort represent the entire population of affected patients. In agreement with the conclusions of Wheeler and Weaver, our patients have normal intelligence and can live independently. Our findings echo common themes regarding complications of anal and genital anomalies that could lead to infertility. Unlike Wheeler and Weaver’s study, our analysis highlights other sequelae, such as those relating to vertebral malformations (e.g., back pain), TEF (e.g. gastroesopheal reflux and reactive airway disease), and renal anomalies (e.g. nephrolithiasis and UTIs). The patients in our cohort were not smaller in size than average. Interestingly, as in the previous study, hearing loss was reported in several patients in our cohort, though explanations (e.g., neonatal medication toxicity, working in environments with loud noises) did not necessarily suggest a pathogenic process related to VACTERL association or overlapping disorders among our patients.
We hope that improved care will result from anticipation of the issues described here. Because VACTERL association may be less familiar to adult practitioners, it is important for older patients to continue seeing caregivers who are knowledgeable about the condition. Additionally, as medically significant findings in patients with VACTERL association may be missed unless a comprehensive clinical work-up is performed, we suggest that patients in whom a diagnosis of VACTERL association is considered undergo testing and/or examination for the presence of each of the core component features, with the following initial testing at a minimum: a thorough history and physical examination by a clinician familiar with the condition, X-rays of the entire spine with consideration of spinal MRI and/or ultrasound, echocardiogram, and renal ultrasound with blood and urine testing for renal function. As previous studies have suggested, and which is echoed in the patients described here, clinicians should also be aware that component features of VACTERL association are more common in relatives of patients than in the general population; thus, a careful family history with further work-up as medically indicated is advised in family members of affected patients [32]. Finally, as patients age, clinicians should continue to maintain a low threshold for considering and working up the common medical issues described in our cohort.
5. Conclusions
Medically significant malformations in patients with VACTERL association may not be ascertained until adulthood. Patients may experience long-term sequelae that may not be evident until adulthood or which are different in nature from the issues they experienced in childhood. These outcomes are not completely predictable by the type and initial severity of component features of VACTERL association in each patient. A more uniform diagnostic work-up is advised in patients in whom a diagnosis of VACTERL association is considered.
Acknowledgments
We would like to express our gratitude to the patients described in this article for their willingness to take part in this study. This research was supported in part by the Howard Hughes Medical Institute and by the Division of Intramural Research at the National Human Genome Research Institute (National Institutes of Health, Department of Health and Human Services, United States of America).
Abbreviations
- ASD
atrial septal defect
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- EA
esophageal atresia
- GER
gastroesophageal reflux
- TEF
tracheoesophageal fistula
- UTI
urinary tract infection
- VACTERL
vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula with esophageal atresia, renal defects, limb anomalies
- VATER
vertebral defects, anal atresia, tracheoesophageal fistula with esophageal atresia, renal defects, radial dysplasia
- VSD
ventricular septal defect
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest or disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Manu S. Raam, Email: raamm@ccf.org.
Daniel E. Pineda-Alvarez, Email: pinedaad@mail.nih.gov.
Donald W. Hadley, Email: dhadley@nhgri.nih.gov.
Benjamin D. Solomon, Email: solomonb@mail.nih.gov.
References
- 1.Ahn SY, Mendoza S, Kaplan G, Reznik V. Chronic kidney disease in the VACTERL association: clinical course and outcome. Pediatr Nephrol. 2009;24:1047–1053. doi: 10.1007/s00467-008-1101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auchterlonie IA, White MP. Recurrence of the VATER association within a sibship. Clin Genet. 1982;21:122–124. doi: 10.1111/j.1399-0004.1982.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 3.Botto LD, Khoury MJ, Mastroiacovo P, Castilla EE, Moore CA, Skjaerven R, Mutchinick OM, Borman B, Cocchi G, Czeizel AE, Goujard J, Irgens LM, Lancaster PAL, Martínez-Frías ML, Merlob P, Ruusinen A, Stoll C, Sumiyoshi Y. The spectrum of congenital anomalies of the VATER association: an international study. Am J Med Genet. 1997;71:8–15. doi: 10.1002/(sici)1096-8628(19970711)71:1<8::aid-ajmg2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Czeizel A, Ludányi I. An aetiological study of the VACTERL-association. Eur J Pediatr. 1985;144:331–337. doi: 10.1007/BF00441773. [DOI] [PubMed] [Google Scholar]
- 5.da Silva GM, Jorge JMN, Belin B, Nogueras JJ, Weiss EG, Vernava AM, III, Habr-Gama A, Wexner SD. New surgical options for fecal incontinence in patients with imperforate anus. Dis Colon Rectum. 2004;47:204–209. doi: 10.1007/s10350-003-0039-0. [DOI] [PubMed] [Google Scholar]
- 6.Damian MS, Seibel P, Schachenmayr W, Reichmann H, Dorndorf W. VACTERL with the mitochondrial NP 3243 point mutation. Am J Med Genet. 1996;62:398–403. doi: 10.1002/(SICI)1096-8628(19960424)62:4<398::AID-AJMG13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.de Jong EM, Felix JF, Deurloo JA, van Dooren MF, Aronson DC, Torfs CP, Heij HA, Tibboel D. Non-VACTERL-type anomalies are frequent in patients with esophageal atresia/tracheo-esophageal fistula with full or partial VACTERL association. Birth Defects Res A. 2008;82:92–97. doi: 10.1002/bdra.20437. [DOI] [PubMed] [Google Scholar]
- 8.Dorfman AT, Marino BS, Wernovsky G, Tabbutt S, Ravishankar C, Godinez RI, Priestley M, Dodds KM, Rychik J, Gruber PJ, Gaynor JW, Levy RJ, Nicolson SC, Montenegro LM, Spray TL, Dominguez TE. Critical heart disease in the neonate: presentation and outcome at a tertiary care center. Pediatr Crit Care Med. 2008;9:193–202. doi: 10.1097/PCC.0b013e318166eda5. [DOI] [PubMed] [Google Scholar]
- 9.Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer RT., III Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Arch Surg. 1995;130:502–508. doi: 10.1001/archsurg.1995.01430050052008. [DOI] [PubMed] [Google Scholar]
- 10.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Barceló M-M, Wong KK-y, Lui VC-h, Yuan Z-w, So M-t, Ngan ES-w, Miao X-p, Chung PH-y, Khong P-l, Tam PK-h. Identification of a HOXD13 mutation in a VACTERL patient. Am J Med Genet A. 2008;146A:3181–3185. doi: 10.1002/ajmg.a.32426. [DOI] [PubMed] [Google Scholar]
- 12.Goyal A, Jones MO, Couriel JM, Losty PD. Oesophageal atresia and tracheo-oesophageal fistula. Arch Dis Child Fetal Neonatal Ed. 2006;91:F381–F384. doi: 10.1136/adc.2005.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashish MS, Dawoud HH, Hirschl RB, Bruch SW, El Batarny AM, Mychaliska GB, Drongowski RA, Ehrlich PF, Hassaballa SZ, El-Dosuky NI, Teitelbaum DH. Long-term functional outcome and quality of life in patients with high imperforate anus. J Pediatr Surg. 2010;45:224–230. doi: 10.1016/j.jpedsurg.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Källén K, Mastroiacovo P, Castilla EE, Robert E, Källén B. VATER non-random association of congenital malformations: study based on data from four malformation registers. Am J Med Genet. 2001;101:26–32. doi: 10.1002/ajmg.1201. [DOI] [PubMed] [Google Scholar]
- 15.Khoury MJ, Cordero JF, Greenberg F, James LM, Erickson JD. A population study of the VACTERL association: evidence for its etiologic heterogeneity. Pediatrics. 1983;71:815–820. [PubMed] [Google Scholar]
- 16.Kim JH, Kim PCW, Hui C-c. The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet. 2001;59:306–315. doi: 10.1034/j.1399-0004.2001.590503.x. [DOI] [PubMed] [Google Scholar]
- 17.Koivusalo A, Pakarinen MP, Turunen P, Saarikoski H, Lindahl H, Rintala RJ. Health-related quality of life in adult patients with esophageal atresia–a questionnaire study. J Pediatr Surg. 2005;40:307–312. doi: 10.1016/j.jpedsurg.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Konkin DE, O’Hali WA, Webber EM, Blair GK. Outcomes in esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2003;38:1726–1729. doi: 10.1016/j.jpedsurg.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 19.Kovesi T, Rubin S. Long-term complications of congenital esophageal atresia and/or tracheoesophageal fistula. Chest. 2004;126:915–925. doi: 10.1378/chest.126.3.915. [DOI] [PubMed] [Google Scholar]
- 20.Levitt MA, Peña A. Outcomes from the correction of anorectal malformations. Curr Opin Pediatr. 2005;17:394–401. doi: 10.1097/01.mop.0000163665.36798.ac. [DOI] [PubMed] [Google Scholar]
- 21.Little DC, Rescorla FJ, Grosfeld JL, West KW, Scherer LR, Engum SA. Long-term analysis of children with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2003;38:852–856. doi: 10.1016/s0022-3468(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 22.Nezarati MM, McLeod DR. VACTERL manifestations in two generations of a family. Am J Med Genet. 1999;82:40–42. [PubMed] [Google Scholar]
- 23.Nora AH, Nora JJ. A syndrome of multiple congenital anomalies associated with teratogenic exposure. Arch Environ Health. 1975;30:525–526. doi: 10.1080/00039896.1975.10666626. [DOI] [PubMed] [Google Scholar]
- 24.Peña A, Hong A. Advances in the management of anorectal malformations. Am J Surg. 2000;180:370–376. doi: 10.1016/s0002-9610(00)00491-8. [DOI] [PubMed] [Google Scholar]
- 25.Ponsky TA, Rothenberg SS. Minimally invasive surgery in infants less than 5 kg: experience of 649 cases. Surg Endosc. 2008;22:2214–2219. doi: 10.1007/s00464-008-0025-7. [DOI] [PubMed] [Google Scholar]
- 26.Quan L, Smith DW The VATER association. Vertebral defects, anal atresia, T-E fistula with esophageal atresia, radial and renal dysplasia: a spectrum of associated defects. J Pediatr. 1973;82:104–107. doi: 10.1016/s0022-3476(73)80024-1. [DOI] [PubMed] [Google Scholar]
- 27.Reardon W, Zhou X-P, Eng C. A novel germline mutation of the PTEN gene in a patient with macrocephaly, ventricular dilatation, and features of VATER association. J Med Genet. 2001;38:820–823. doi: 10.1136/jmg.38.12.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rintala RJ, Pakarinen MP. Imperforate anus: long- and short-term outcome. Semin Pediatr Surg. 2008;17:79–89. doi: 10.1053/j.sempedsurg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63:529–536. doi: 10.1002/(SICI)1096-8628(19960628)63:4<529::AID-AJMG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Shaw-Smith C. Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. J Med Genet. 2006;43:545–554. doi: 10.1136/jmg.2005.038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sistonen SJ, Koivusalo A, Nieminen U, Lindahl H, Lohi J, Kero M, Kärkkäinen P, Färkkilä M, Sarna S, Rintala RJ, Pakarinen MP. Esophageal morbidity and function in adults with repaired esophageal atresia with tracheoesophageal fistula: a population-based long-term follow-up. Ann Surg. 2010;251:1167–1173. doi: 10.1097/SLA.0b013e3181c9b613. [DOI] [PubMed] [Google Scholar]
- 32.Solomon BD, Pineda-Alvarez DE, Raam MS, Cummings DAT. Evidence for inheritance in patients with VACTERL association. Hum Genet. 2010;127:731–733. doi: 10.1007/s00439-010-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somppi E, Tammela O, Ruuska T, Rahnasto J, Laitinen J, Turjanmaa V, Järnberg J. Outcome of patients operated on for esophageal atresia: 30 years’ experience. J Pediatr Surg. 1998;33:1341–1346. doi: 10.1016/s0022-3468(98)90003-3. [DOI] [PubMed] [Google Scholar]
- 34.Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, Ou Z, Wiszniewska J, Driscoll DJ, Bolivar J, Bauer M, Zackai EH, McDonald-McGinn D, Nowaczyk M, Murray M, Shaikh TH, Martin V, Tyreman M, Simonic I, Willatt L, Paterson J, Mehta S, Rajan D, Fitzgerald T, Gribble S, Prigmore E, Patel A, Shaffer LG, Carter NP, Cheung SW, Langston C, Shaw-Smith C. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temtamy SA, Miller JD. Extending the scope of the VATER association: definition of the VATER syndrome. J Pediatr. 1974;85:345–349. doi: 10.1016/s0022-3476(74)80113-7. [DOI] [PubMed] [Google Scholar]
- 36.Thauvin-Robinet C, Faivre L, Huet F, Journeau P, Glorion C, Rustin P, Rötig A, Munnich A, Cormier-Daire V. Another observation with VATER association and a complex IV respiratory chain deficiency. Eur J Med Genet. 2006;49:71–77. doi: 10.1016/j.ejmg.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Uehling DT, Gilbert E, Chesney R. Urologic implications of the VATER association. J Urol. 1983;129:352–354. doi: 10.1016/s0022-5347(17)52093-5. [DOI] [PubMed] [Google Scholar]
- 38.Ure BM, Slany E, Eypasch EP, Weiler K, Troidl H, Holschneider AM. Quality of life more than 20 years after repair of esophageal atresia. J Pediatr Surg. 1998;33:511–515. doi: 10.1016/s0022-3468(98)90100-2. [DOI] [PubMed] [Google Scholar]
- 39.Weaver DD, Mapstone CL, Yu P-l. The VATER association: analysis of 46 patients. Am J Dis Child. 1986;140:225–229. doi: 10.1001/archpedi.1986.02140170051027. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler PG, Weaver DD. Adults with VATER association: long-term prognosis. Am J Med Genet A. 2005;138A:212–217. doi: 10.1002/ajmg.a.30938. [DOI] [PubMed] [Google Scholar]
- 41.Woolf CM, Myrianthopoulos NC. Polydactyly in American negroes and whites. Am J Hum Genet. 1973;25:397–404. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhengwei Y, Weilin W, Yuzuo B, Weisong C, Wei W. Long-term outcomes of individualized biofeedback training based on the underlying dysfunction for patients with imperforate anus. J Pediatr Surg. 2005;40:555–561. doi: 10.1016/j.jpedsurg.2004.11.034. [DOI] [PubMed] [Google Scholar]