Abstract
Introduction
Little is known about the relationship between gamma-band oscillations prior to the arrival of a target stimulus and subsequent sensory processing and response execution. Although schizophrenia has been associated with abnormalities in gamma-band oscillations, P300, and reaction time (RT), few studies have examined the possible correspondence between these three neurobiological and behavioral markers in schizophrenia. To characterize the relationship between preparatory processes, information processing, and subsequent behavioral performance in schizophrenia, the present study investigated the relationships between pre-stimulus gamma-band power, RT and P300 amplitude.
Methods
EEG and behavioral data were collected from 18 schizophrenia patients and 21 healthy controls during a conventional auditory oddball task.
Results
In controls, single-trial pre-stimulus gamma power was positively correlated with RT, and average P300 amplitude was positively correlated with average pre-stimulus gamma power.
Discussion
We interpret these findings as evidence that gamma power enhancement reflects a state of greater pre-stimulus preparation resulting in fuller evaluation of the target stimulus and therefore slower RT, as proposed by Jokeit and Makieg (1994). Consistent with previous research, schizophrenia patients exhibited RT slowing and P300 amplitude reductions relative to controls. Importantly, neither RT nor P300 amplitude were related to pre-stimulus gamma power in schizophrenia, suggesting a breakdown in the preparatory brain state critical for stimulus processing and later motor execution. The present findings underscore the behavioral significance of gamma-band responses, and provide an additional link between gamma-band oscillations and information processing abnormalities in schizophrenia.
Keywords: ERP, Schizophrenia, P300, gamma power, reaction time
INTRODUCTION
Neural oscillations are a prominent property of neuronal activity that convey information between populations of cells. Neural oscillations arising from synchronous firing of large populations of neurons are seen at the scalp as electroencephalogram (EEG). The EEG signal can be quantified in a number of ways, but it is often decomposed into frequencies in overlapping time windows, using time-frequency analyses. Of particular interest to neuroscientists is the power of the signal, calculated from ensemble averages (evoked power) or from single trials (total power), which is phase-asynchronous with respect to the onset of an event (see (Roach and Mathalon, 2008) for a fuller description of the methods for calculating each). In addition to measures of EEG power, phase-locking factor (Tallon-Baudry et al., 1997) or inter-trial phase coherence (Makeig et al., 2004) describes the consistency of the phase angles (independent of magnitude information) across trials for each time point and frequency bin with respect to the onset of an event. Overall, these EEG time-frequency measures describe different qualities of network oscillations and their synchronization, and often relate to different aspects of neural information processing.
One neuro-oscillatory rhythm that shows particularly strong perceptual and cognitive correlates is the 30–100Hz gamma rhythm (Herrmann et al., 2010) for review). Gamma activity has been most widely associated with top-down attentional processing and object perception (Debener et al., 2003; Gruber and Müller, 2005; Keil et al., 1999; Rodriguez et al., 1999; Singer, 1993; Singer and Gray, 1995; Tallon-Baudry et al., 1997; Tiitinen et al., 1993; von der Malsburg, 1986), but it has also been implicated in a variety of other tasks. For example, mechanisms of bottom-up attention modulate gamma oscillations (Engel et al., 2001), such as spatial (Fries et al., 2001), features-based (Vidal et al., 2006), and object-based (Tallon-Baudry et al., 2005) attention. Gamma activity has also been demonstrated to increase during a delayed visual matching-to-sample task (Tallon-Baudry and Bertrand, 1999; Tallon-Baudry et al., 2005; Tallon-Baudry et al., 1998) and with memory load in working memory tasks (Howard et al., 2003; Jokisch and Jensen, 2007; Medendorp et al., 2007).
A recent focus in the rapidly advancing literature on human gamma-band activity has been on the behavioral relevance of gamma oscillations. These studies have been primarily concerned with the stages of information processing following the onset of a stimulus, highlighting the role of greater gamma power for faster responses as measured by reaction time (RT) (Frund et al., 2007; Jokeit and Makeig, 1994; Schadow et al., 2009) and enhanced performance efficiency as measured by response accuracy (Kaiser et al., 2008a; Kaiser et al., 2008b). Robust correlations have also been shown between shorter post-stimulus gamma-band peak-latency and faster RT in auditory oddball (Haig et al., 1999; Jokeit and Makeig, 1994), and early and late visual Gestalt pattern processing (Schadow et al., 2009), as well as predicting recognition delays in object identification tasks (Martinovic et al., 2008; Martinovic et al., 2007). Evidence from the non-human primate literature further supports the view that greater post-stimulus gamma oscillations and shortened neuronal response latencies may be advantageous for faster information processing and classification processes (e.g. (Womelsdorf et al., 2006). Together these findings suggest that the magnitude, phase, and latency of post-stimulus gamma oscillations reveal important information about the temporal relations in the activities of neural assemblies during the integration of sensory data to ultimately trigger adaptive motor behavior (Herrmann et al., 2010).
An extensive database is now emerging on gamma oscillatory activity during the stages of information processing immediately preceding onset of a stimulus, which may be important for controlling the flow of sensorimotor information by various top-down factors, such as selective attention, anticipation and event prediction prior to behaviorally relevant stimuli (Engel et al., 2001; Liang et al., 2002). Enhanced pre-stimulus gamma activity has been shown to be predictive of response speed (Gonzalez Andino et al., 2005; Schoffelen et al., 2005; Womelsdorf et al., 2006), and modulated by the measure of stimulus predictability (Gross et al., 2006; Kilner et al., 2005; Schoffelen et al., 2005; Summerfield and Mangels, 2006) or the type of warning cue information (Fan et al., 2007; Landau et al., 2007). More recently, greater pre-stimulus gamma activity has been shown to support task performance efficiency (Kaiser et al., 2009), and perceptual performance in both auditory (Sadaghiani et al., 2009) and visual modalities (Hanslmayr et al., 2007). Pre-stimulus gamma-band oscillations therefore appear to represent an efficient mechanism establishing a brain state through which the processing of an approaching stimulus is facilitated.
Although some relationships have been demonstrated between pre-stimulus gamma activity and performance speed in healthy humans (Gonzalez Andino et al., 2005; Jokeit and Makeig, 1994; Yordanova et al., 2001) and non-human primates (Womelsdorf et al., 2006), no such studies have addressed these relationships in patients with schizophrenia, despite the contribution such analyses could make to our understanding of the illness. For example, because neural information processing is an interaction between incoming data and the transitory brain state (which might include dimensions such as arousal, emotion, and attention), study of brain states preceding stimulus onset might target the seed point of abnormal processing in schizophrenia and account for a range of experiments where behavioral and ERP abnormalities have been observed in schizophrenia patients. Moreover, schizophrenia symptoms are often characterized as intermittent and spontaneously occurring. Therefore, examination of gamma fluctuations in ongoing neural activity and their relationship to behavior may provide insight into the psychopathology of schizophrenia (Lawrie et al., 2004).
Gamma oscillations and synchrony have been leading candidate mechanisms proposed to accomplish successful integration across functional brain networks in healthy subjects, and are notably impaired in schizophrenia. Abnormal post-stimulus gamma-band responses in patients are evident in tasks engaging different cognitive functions, such as feature binding (Spencer et al., 2003), working memory (Basar-Eroglu C et al., 2007), and attention (Gallinat et al., 2004), as well as motor execution (Ford et al., 2008a), and early visual (Spencer et al., 2008; Uhlhaas et al., 2006) and auditory (Roach and Mathalon, 2008) processing. For this reason, it is plausible to assume that dysfunctional gamma oscillations in schizophrenia will influence the relationship between the pre-stimulus gamma activity underlying the momentary brain state and subsequent behavior.
The aim of the present study was to characterize the relationships between the preparatory brain state, subsequent sensory processing, and ultimately, response execution in schizophrenia. The association between the preparatory brain state and motor behavior was assessed by examining pre-stimulus gamma power and RT on a trial-by-trial basis, which allowed us to directly assess the relationship between moment-to-moment fluctuations in gamma power and RT, within individual subjects. In the growing literature on the behavioral correlates of EEG oscillations, gamma activity has been quantified with different measures, such as inter-trial phase coherence (e.g. (Frund et al., 2007)), evoked power (e.g. (Debener et al., 2003; Frund et al., 2007)), or phase-asynchronous increases or decreases in EEG total power (e.g. (Cho et al., 2006; Jokeit and Makeig, 1994)). However, given our aim to study state-related activity preceding the stimulus, it was important for us to focus on the changes in asynchronous power rather than stimulus-evoked activity as done by others (e.g. (Mazaheri et al., 2009)). RT, a crucial behavioral assessment of the overall efficacy of sensorimotor processing, was also well suited for the present study given its prior link to gamma-range oscillations in healthy participants. Furthermore, RT slowing is a hallmark of schizophrenia and has been called the “closest thing schizophrenia research has to a North Star” (Nuechterlein, 1977).
Analysis of the correspondence between mean pre-stimulus gamma power and the P300 component amplitude of the ERP was also conducted to assess whether gamma power was linked to downstream stimulus processing. P300, a positive voltage deflection in the ERP occurring approximately 300ms after stimulus onset, reflects neurophysiological activity associated with processing infrequent target, novel, or otherwise salient stimuli. P300 amplitude is thought to reflect attentional resource allocation (Isreal et al., 1980; Kramer and Strayer, 1988; Polich, 1989), phasic attentional shifts (Soltani and Knight, 2000), working memory updating of stimulus context (Donchin and Coles, 1988; Johnson, 1986), or stimulus salience (Sutton and Braren, 1965; Sutton and Tueting, 1967). Its latency is thought to reflect processing speed or efficiency during stimulus evaluation (Duncan-Johnson and Donchin, 1977). Furthermore, like RT prolongation, P300 amplitude reduction is one of the most replicated neurobiological abnormalities observed in schizophrenia (Jeon and Polich, 2003). Although no studies have examined pre-stimulus gamma activity associated with P300 amplitude, post-stimulus gamma power increases (Lee et al., 2007) and decreases (Fell et al., 1997; Marshall et al., 1996) have been observed following a target stimulus in auditory oddball tasks. Despite a strong positive correlation between P300 amplitude and gamma activity following target tone onset in controls (Ford et al., 2008b; Gallinat et al., 2004; Haig et al., 2000) no such relationship has been found in patients with schizophrenia.
Accordingly, we tested the following primary hypotheses: patients will exhibit smaller P300 amplitude and slower RT relative to healthy subjects, consistent with findings from the schizophrenia literature (Bramon et al., 2004; Ford, 1999; Pritchard, 1986; Steffy and Waldman, 1993). Healthy subjects will show a positive relationship between pre-stimulus gamma power and RT, also consistent with prior reports utilizing auditory target detection paradigms (Jokeit and Makeig, 1994; Yordanova et al., 2001). Additionally, healthy subjects will exhibit a positive correlation between pre-stimulus gamma power and P300 amplitude, reflecting the role of gamma oscillations during states of attention and expectancy (Engel et al., 2001; Tallon-Baudry et al., 2005). In contrast, both of these correlations were predicted to be absent in patients with schizophrenia based on prior reports of abnormal gamma neural activity in schizophrenia (Herrmann and Demiralp, 2005; Lee et al., 2003; Uhlhaas et al., 2009; Uhlhaas and Singer, 2006). It is likely that inter-relationships among these variables (pre-stimulus gamma power, RT, and P300) will reveal important aspects about the regulation of information processing in the brain and its dysregulation in schizophrenia.
METHODS
Subjects
All study procedures were approved by the Human Subjects Subcommittee of the Veterans Affairs Healthcare System (VAHS) in West Haven, CT, and the Human Investigations Committee of Yale University School of Medicine in New Haven, CT. 23 healthy controls (HC) (5 female) and 19 schizophrenia patients (SZ) (3 female) matched for age and parental socioeconomic status participated in the study. All subjects were right-handed. HC were recruited from the community and did not meet criteria for a DSM-IV psychiatric or substance abuse diagnosis (First, 1995). SZ were on stable, therapeutic doses of antipsychotic medications, met DSM-IV criteria for schizophrenia or schizoaffective disorder, but did not meet for alcohol or drug abuse within 30 days before the study. Benzodiazepine treatment was also an exclusion criterion based on its potential impact on another measure of interest in this study, to be reported elsewhere. All subjects with a hearing deficit greater than 30dB in both ears, significant head injury (loss of consciousness of >15min), or other medical problems endangering the CNS were excluded.
One patient was unable to perform the task and dropped from further analysis. One non-native English-speaking control who did not fully comprehend the task instructions had RT outlier values (>2 SD’s), and another control was an outlier in the time-frequency domain (>3 SDs). Both subjects were also removed from the sample. Demographic and clinical data from the remaining 21 HC and 18 SZ are summarized in Table 1. Symptom ratings were averaged across two independent raters attending the same rating session, using the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983), and the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984).
Table 1.
Demographic Information
| Healthy Controls (N=21) | Schizophrenia Patients (N=18) | |
|---|---|---|
| Age (years ± SD, min, max) | 36.5 ± 13.9, 21, 64 | 39.9 ± 13.8, 21, 62 |
| Gender | 16M, 5F | 15M, 3F |
| Education (years ± SD) | 16.1 ± 3.1 | 13.2 ± 1.3 |
| Parental SES (score ± SD) | 34.1 ± 14.5 | 37.0 ± 16.9 |
| Illness duration (years ± SD, min, max) | 15.8 ± 11.7, 1, 39 | |
| PANSS total | 56.9 ± 10.2 | |
| PANSS negative | 14.7 ± 8.5 | |
| PANSS positive | 13.7 ± 8.3 | |
| PANSS general psychopathology | 28.6 ± 15.2 | |
| Diagnosis | 9 Paranoid | |
| 3 Undifferentiated | ||
| 3 Schizoaffective | ||
| 2 Residual | ||
| 1 Psychosis not otherwise | ||
| specified |
Stimuli and Experimental Procedures
Each block of trials consisted of 90 standard (1000Hz sinusoidal tone) and 10 target (500Hz sinusoidal tone) stimuli, which were generated using STIM2 digital sound editing software v4.0 (Compumedics Neuroscan, El Paso, TX.) and housed in a Pentium® 4 CPU 3.00GHz PC. Subjects were instructed to fixate on a central cross-hair, and respond with a button-press with their right index finger to the target stimulus only, giving equal importance to speed and accuracy. The same 5 pseudo-random sequences were presented to all subjects with the constraint that standards appear 90% and targets appear 10% per testing block. All stimuli (50ms duration) were randomly jittered between 1067 and 1333ms (average SOA of 1200ms). Auditory stimuli were digitally created using a sine wave function with a 44.1KHz digitization rate. Each tone was passed through a Hanning window, which shaped a 0.5ms rise and fall time to the waveform. Additionally, each tone was set to a 70dB sound pressure level, and routed to subjects through STIM 10 Ohm insert earphones with foam ear tips (ER-3A: Compumedics NeuroMedical Supplies, Charlotte, NC). All stimuli were delivered and experimentally controlled using Presentation software v10.3 (Neurobehavioral Systems, Inc. Albany, CA).
EEG Data Acquisition
EEG data were acquired continuously (0.05–200Hz band-pass filter, 1000Hz digitization rate) from 29 Ag/AgCl ring electrodes, including 7 midline (Fz, FCz, Cz, CPz, Pz, POz, Oz), and 11 lateral pairs (F3/4, C3/4, C7/8, CP3/4, CP5/6, P3/4, P5/6, P7/8, PO3/4, PO7/8, PO9/10), arrayed according to an extended 10–20 system with a linked mastoid reference and a fronto-central (FPz) ground. Bipolar vertical and horizontal electro-oculogram was recorded from electrodes placed above and below the right orbit, and at the outer canthi of the eyes, respectively. Electrode impedances were <10kΩ. Off-line, concatenation of all 5 oddball testing blocks was performed for each group.
ERP Data Analysis
EEG data were digitally low-pass filtered at 30Hz (48dB/octave), and epochs of 900ms (including 100ms pre-stimulus baseline) were extracted. All electrode sites underwent ocular correction based on the widely adopted and improved simple regression method developed by (Gratton et al., 1983), which subtracts any event-related activity from both EEG and EOG recordings and uses only event-unrelated activity to estimate the regression coefficients. EEG trials containing artifact activity exceeding ±100μV were removed. EEG data were baseline-corrected using activity in the 100ms preceding stimulus onset. Target trials with no response were excluded from further analysis. An independent two sample t-test revealed no significant difference across groups in the total number of rejected trials due to artifacts or missed responses (p=0.972). Averages were calculated, time-locked to the onset of the target stimulus. The next step involved automatically identifying the P300 peak amplitude at Fz, Cz, and Pz sites. A search window between 235 and 400ms captured the most positive peak at Pz for each subject. P300 peaks at Fz and Cz were identified within a 50ms search window centered on the peak latency at Pz.
EEG Time-Frequency Analysis
EEG raw data were digitally high-pass filtered at 1Hz (48dB/octave). Single-trials were segmented into 2000ms epochs (1000ms pre-stimulus and 1000ms post-stimulus) and corrected for eye movements and blinks (Gratton et al., 1983). EEG epochs containing artifacts were removed on the basis of a ±100μV rejection criterion. Target trials with no response were excluded, and there was no significant difference across groups in the total number of rejected trials due to artifacts or missed responses (p=0.943). Time-frequency analysis of these data was done with a Morlet wavelet decomposition using FieldTrip software in Matlab. The Morlet Wavelet has a Gaussian shape that is defined by a ratio (σf = f/C) and a wavelet duration (6σt), where f is the center frequency and σt = 1/(2πσf). In this analysis, C was set to 7, which produced wavelets over 6 cycles long. After obtaining complex time-frequency data points for every individual trial, these data were transformed into a total power measure using previously described calculations (Ford et al., 2008a). An average of total power single-trial values without baseline correction was calculated to capture “state” aspects of ongoing EEG oscillations. For each subject, Pearson’s correlations were calculated across trials (~45 trials) between single-trial target reaction time (RT) and single-trial power on that trial, at each frequency between 10 and 60Hz for every time point between 200ms pre-stimulus onset to 200ms post-stimulus onset. Separate two-dimensional correlation coefficient matrices were obtained for midline electrodes (Fz, Cz, Pz) for each subject. Pearson’s correlation coefficients (r) between RT and power were extracted for the gamma-band by averaging r-values across 40 to 55Hz and −200 to −100ms for a pre-stimulus bin and 0 to 100ms for a post-stimulus bin for each subject. To stabilize the variance, these mean correlation coefficients were Fisher r-to-z transformed using the following formula and entered into statistical analyses:
Statistical Analysis
Behavioral performance
Median reaction times were analyzed in a 1-way analysis of variance (ANOVA) for Group (SZ, HC).
Between-subjects P300 ERP analysis
P300 amplitudes were analyzed in a 2-way repeated measures ANOVA for Group (SZ, HC) and Site (Fz, Cz, Pz).
Between-subjects gamma power - RT analyses
Gamma – RT correlation coefficients were assessed in a 3-way repeated measures ANOVA for Group (SZ, HC), Site (Fz, Cz, Pz), and Time (pre-stimulus, post-stimulus). Significant interactions were parsed with follow-up tests.
Within-subjects gamma power - RT analyses
In addition to analyses of Group or Time effects, z-transformed correlation coefficients were tested against the null hypothesis to determine if extracted values were significantly different from zero. The pre- and post-stimulus periods for SZ and HC were tested, using four individual t-tests.
Between-subjects relationship of P300 and gamma power
The relationship between P300 peak amplitude and gamma power was tested in a regression analysis, which included a Group * Gamma Power interaction term to test for a Group difference in the slope of the regression line modeling the relationship between P300 peak amplitude and pre-stimulus gamma power. If the interaction term were significant, suggesting that the relationship between P300 amplitude and gamma was different in the two groups, the relationship would be tested separately in each group.
RESULTS
Behavioral performance
There was a significant effect of Group (F(1,37)=10.95, p=0.002) with controls responding faster to targets than patients (327ms vs. 384ms). Analysis of the percentage of correct responses yielded no significant Group effect (p>0.05) as subjects made few errors (HC: 99.8% correct, SZ: 99.5% correct) due to the simplicity of the task.
Between-subjects P300 ERP analysis
As expected, P300 was smaller in patients than controls (F(1,37)=11.58, p=0.002). There was an effect of Site (F(1,37)=18.47, p<0.001, Greenhouse-Geisser adjusted) driven by a significant increase in P300 from frontal to parietal electrodes tested by within subjects contrasts (Fz vs. Cz: p=0.001, Cz vs. Pz: p=0.001).
Between-subjects gamma power - RT analyses
As can be seen in Table 2, there was a Time * Group interaction that was parsed by separately testing for a Group effect in the pre-stimulus (−200 to −100) and post-stimulus (0 to 100) periods. While there was no Group effect in the post-stimulus period, there was a significant Group effect in the pre-stimulus period, with controls showing greater z-transformed correlation coefficients than patients (figure 3). There was not an overall Group difference in the correlation between gamma power and RT. There was a trend towards an effect of Site. This trend appeared to be driven by larger values in posterior than anterior electrode sites (figure 2 top vs. bottom), but single degree of freedom contrasts within subjects failed to reach significance (Fz vs. Cz: p=0.082, Cz vs. Pz: p=0.17).
Table 2.
Tests of Gamma Band-Response Reaction Time Relationships
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Site |
.017 | 1.294 | .013 | 3.315 | .064 |
| Site * Group |
.002 | 1.294 | .001 | .300 | .645 |
| Time |
.004 | 1.000 | .004 | .551 | .462 |
| Time * Group |
.067 | 1.000 | .067 | 8.884 | .005 |
| pre-stimulus: Group | .044 | 1 | .044 | 4.278 | .046 |
| post-stimulus: Group |
.000 | 1 | .000 | .000 | .987 |
| Time * Site |
.003 | 1.607 | .002 | .755 | .448 |
| Time * Site * Group |
.010 | 1.607 | .006 | 2.547 | .098 |
| Group | .011 | 1 | .011 | 1.293 | .263 |
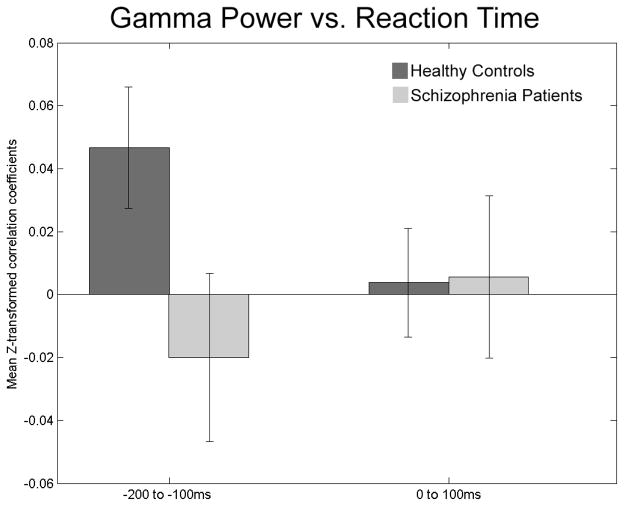
Figure 3.
Means of the z-transformed correlation coefficients (shown in Figure 2) between single-trial gamma power and RT are plotted separately for healthy controls and patients with schizophrenia for gamma power preceding the stimulus onset (−200 to −100 ms) and immediately following it (0 to 100 ms). Healthy control subjects (dark gray) show a significantly greater positive relationship than patients (light gray) before the target but not after it. Standard error bars illustrate that only controls are significantly different from 0 in the pre-stimulus bin.
Figure 2.
Grand averages of single-trial power-RT correlation matrices for healthy controls (left) and schizophrenia patients (right) are plotted for electrodes Fz (top), Cz (middle), and Pz bottom). Dark red indicates a negative correlation while light yellow indicates a positive correlation. Dashed green boxes outline the gamma-band (40–55Hz) correlations for the pre- and post-stimulus averaging windows, −200 to −100ms and 0 to 100ms, respectively.
Within-subjects gamma power - RT analyses
As can be seen in Table 3, only gamma power-RT correlations in the control group were significantly different from zero. Controls showed a positive correlation between gamma-band total power and RT such that greater gamma-band power preceding stimulus onset was associated with longer RTs.
Table 3.
One-Sample Tests
| group | Test Value = 0 | ||||
|---|---|---|---|---|---|
| t | df | Sig. (2-tailed) | Mean Difference | ||
| HC | pre-stimulus | 2.399 | 20 | .026 | .04670 |
| post-stimulus | .258 | 20 | .799 | .00444 | |
| SZ | pre-stimulus | −.765 | 17 | .455 | −.02046 |
| post-stimulus | .191 | 17 | .851 | .00495 | |
Between-subjects relationship of P300 and pre-stimulus gamma power
The model revealed a significant difference in the relationship between P300 and gamma power in SZ compared to HC (p = .048). Follow up correlations showed a significant positive relationship between P300 peak amplitude and pre-stimulus gamma power in HC (r = .485, p = .026) and no significant relationship in SZ (r = .103, p = .685). As can be seen in figure 4, two outliers drove the SZ relationship and dropping them changed the correlation (r = −.297, p = .264), but the difference in slope of the two measures remained significantly different (p = .013).
Figure 4.
Scatter plots show the relationship between P300 peak amplitude and pre-stimulus gamma total power for controls (top) and patient with schizophrenia (bottom). Regression equations, Pearson correlation coefficients, and two-tailed significance levels are shown. The regression line for the patients was calculated without the outliers (indicated by an asterisk).
DISCUSSION
As anticipated, patients with schizophrenia exhibited prolonged RTs as compared with healthy subjects. This finding confirms what is considered to be one of the most established neurocognitive abnormalities seen in schizophrenia (Steffy and Waldman, 1993), dating back to early research on the slowing of movements in the illness (Bleuler, 1950; Kraepelin, 1971). Also consistent with the schizophrenia literature (Bramon et al., 2004; Ford, 1999; Pritchard, 1986) patients had auditory P300 amplitude reductions relative to controls during a conventional auditory oddball task. Importantly, neither RT prolongation nor P300 amplitude reduction was related to pre-stimulus gamma power in patients. In contrast, healthy controls did show a significant positive correlation between P300 amplitude and mean pre-stimulus gamma power, indicating that the subjects with more gamma power produced larger amplitude P300 peaks. Furthermore, on a single-trial basis, pre-stimulus gamma power was positively correlated with RT, supporting the view that pre-stimulus gamma-band activity has behaviorally meaningful correlates. This effect was primarily a phenomenon of the 40 to 55Hz band, −200 to −100ms prior to stimulus onset, and maximal over the parietal midline region.
Although our single-trial analysis differed from analyses used by other investigators, our data from healthy controls are consistent with their reports showing the behavioral significance of gamma-band responses during the pre-stimulus (Gonzalez Andino et al., 2005; Jokeit and Makeig, 1994; Yordanova et al., 2001) or post-stimulus time period (Frund et al., 2007; Haig et al., 1999; Jokeit and Makeig, 1994; Martinovic et al., 2008; Martinovic et al., 2007; Schadow et al., 2009). Jokeit and Makeig (Jokeit and Makeig, 1994) showed that response preparation and execution are represented in total power gamma-band activity. In particular, they showed that fast responders had less pre-stimulus gamma-band activity than slow responders, and suggested that gamma-band activity reflects the implementation of a stimulus-oriented, or “accuracy” strategy. That is, pre-stimulus gamma power may reflect anticipation of behaviorally relevant stimuli, leading to a fuller evaluation of the target stimulus, and thus slower RT. Earlier investigations in healthy subjects have similarly concluded that greater gamma-range EEG activity is involved in top-down attentional processing (Debener et al., 2003; Tallon-Baudry et al., 2005; Tiitinen et al., 1993), and sensorimotor processing during states of expectancy (Bastiaansen et al., 2001; Bastiaansen and Brunia, 2001; Gonzalez Andino et al., 2005), planning, and execution in humans (Pfurtscheller and Lopes da Silva, 1999) and non-human primates (Bressler et al., 1993; Lee, 2003).
Healthy controls in the present study show a similar relationship between gamma power and RT, but on a single-trial level. Unlike Jokeit and Makeig, who reported the RT and gamma power effects of inter-individual variability, we identified a gamma power/RT relationship within healthy subjects, across trials, suggesting that RT relies on the strength of gamma power in a moment-to-moment, state-dependent manner. That is to say, in the healthy brain the ability to rapidly shift states may be an important feature of cortical brain dynamics, predicting fluctuations in behavioral responses. However, even though gamma power is related to the timing of subsequent motor responses in control participants, we are not able to draw conclusions about causality.
Further support for the view that greater gamma power preceding a stimulus reflects attention to stimulus processing comes from the findings we obtained with P300 amplitude, an indicator of an engaged processing state, and its relation to pre-stimulus gamma power. Perhaps gamma power readies the brain to engage endogenous aspects of stimulus processing. This observation is in accord with previous findings showing that pre-stimulus or ongoing background activity levels in sensory areas impact how forthcoming input will be perceived (Hanslmayr et al., 2007; Sadaghiani et al., 2009). Others have similarly concluded that pre-stimulus or ongoing baseline activity acts as a carrier of dynamic predictions of future events (Fox and Raichle, 2007; Mazaheri et al., 2009).
It is important to note that enhanced gamma oscillations in the period between a warning cue and visual stimulus are correlated with shorter reaction times to the stimulus on a trial-by-trial basis (e.g. (Gonzalez Andino et al., 2005)), suggesting that the greater gamma power, the faster the response, which is the opposite of our pattern. In addition, Cho et al. (Cho et al., 2006) showed that post-cue, pre-target gamma power increases were associated with greater task accuracy in healthy controls, but not patients with schizophrenia. These studies and others (e.g. (Fan et al., 2007; Womelsdorf et al., 2006)) that have reported a correlation between behavioral performance and enhanced gamma oscillations during the period between a warning cue and an imperative stimulus are consistent with the hypothesized role of gamma in top-down attentional processing (e.g. (Debener et al., 2003; Fell et al., 2003). However, research employing a warning cue is difficult to fit into the context of the present study due to differences in task parameters. Brain activity sustained over a cue-target interval as compared with brain activity in response to target events in a simple oddball paradigm represents different aspects of dynamic brain function (Fernandez-Duque and Posner, 1997; Grice, 1968; Hackley and Valle-Inclán, 2003; Reddi, 2001; Reddi et al., 2003). For example, with regard to sensory processing, the warning cue is known to reduce RT (Jahanshahi and Brown, 1992) and may speed up the rate at which the target is registered by the brain or increase the magnitude of its signal. In terms of motor function, the warning cue may decrease the threshold required to initiate a response or speed up the rate at which activity accumulates to reach threshold. Although RT is fastest with an intermediate level of arousal (e.g. during a cued stimulus trial), nonetheless the increase in pre-stimulus gamma power observed in both cued and uncued experimental settings reveals the attentiveness and anticipation induced by proximity of the imperative stimulus.
For the patient group, the absence of a pre-stimulus gamma/RT correlation suggests a breakdown in the relationship between behavioral performance and a gamma oscillatory brain state, which is consistent with comparisons of average total gamma power and task performance (Cho et al., 2006). Since groups did not differ in uncorrected pre- or post-stimulus gamma power, lack of gamma power could not be responsible for the absence of a gamma/RT link in patients. Rather the observed absence of a behaviorally-relevant brain state in patients may represent a response preparation abnormality in the fashion described by Jokeit and Makeig. More support for this view comes from the finding that mean pre-stimulus gamma power did not correspond to P300 amplitude in schizophrenia, perhaps due to aberrant attentional processing during response preparation. This interpretation fits with a range of findings on default-mode network (DMN) brain dysfunction in schizophrenia (see (Broyd et al., 2009) for review), specifically in which temporal connectivity of the DMN is altered during auditory oddball tasks (Calhoun et al., 2008). In these studies, increased anti-correlations between the DMN and task-positive network in schizophrenia suggest extreme competition between the psychological processes and underlying neural circuits of these networks. The antagonistic relationship between pre- and post-stimulus brain networks might account for the observed disconnect between pre-stimulus gamma power and post-stimulus P300 and RT. However, it is also possible that this relationship is actually intact in patients, but the correlation is obscured by other neural activity, which is unrelated to gamma power but does predict slower responses in schizophrenia.
It is important to note that other breakdowns between gamma activity and behavior have been noted in pathological groups. For example, Lenz et al. (2008) (Lenz et al., 2008) reported that evoked gamma-band responses during stimulus encoding were enhanced in ADHD patients during a task, but this enhancement was not associated with subsequent recognition performance on a cognitive task; healthy subjects did exhibit a strong positive correlation between evoked gamma activity during stimulus encoding and recognition performance. They interpreted this finding as evidence of enhanced excitation levels and non-specific activation of processing resources in ADHD patients.
Future studies are needed to elucidate a variety of matters in connection with gamma/RT linked abnormalities in schizophrenia. For example, different patterns of abnormal neural oscillatory activity may map onto symptomatic profiles within schizophrenia (Peled, 1999; Spencer and Niznikiewicz, 2009). However, we were unable to find significant relationships between clinical symptoms and the abnormal gamma/RT link, perhaps due to the composition of our patient sample which included chronic patients and patients early in the illness. Second, using paradigms with more trials would allow for an evoked time-frequency analysis of gamma/RT relationships as well as a more direct comparison between accuracy and pre-stimulus gamma power, which may help to further clarify their association. Third, the relationship between disturbed gamma/RT correlations in schizophrenia and demographic characteristics, such as gender, requires attention given that although the prevalence of schizophrenia is approximately equal in men and women (see (Saha et al., 2005) for review) gamma-band activity has been shown to vary with gender (Slewa-Younan et al., 2004). The present report suggests that pre-stimulus gamma power and reaction time correlations may turn out to be an important key for understanding both the behavioral significance of gamma-band responses as well as preparatory processing failures in neuropsychiatric disorders, such as schizophrenia.
Figure 1.
Healthy control (dark) and schizophrenia patient (light) grand average auditory ERPs time-locked to target stimulus onset. Time is shown on the x-axis and voltage on the y-axis. P300 is reduced in patients relative to controls at all three midline electrode sites in the standard Fz<Cz<Pz distribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen N. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1983. [Google Scholar]
- Andreasen N. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City: 1984. [Google Scholar]
- Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes BCS. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MCM, Böcker KBE, Brunia CHM, de Munck JC, Spekreijse H. Event-related desynchronization during anticipatory attention for an upcoming stimulus: a comparative EEG/MEG study. Clinical Neurophysiology. 2001;112:393–403. doi: 10.1016/s1388-2457(00)00537-x. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MCM, Brunia CHM. Anticipatory attention: an event-related desynchronization approach. International Journal of Psychophysiology. 2001;43:91–107. doi: 10.1016/s0167-8760(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or Group of Schizophrenia [1911] International Universities Press; New York, NY: 1950. [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Coppola R, Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature. 1993;366:153–156. doi: 10.1038/366153a0. [DOI] [PubMed] [Google Scholar]
- Broyd S, Demanuele C, Debener S, Helps S, James C, Sonuga-Barke E. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport. 2003;14:683–686. doi: 10.1097/00001756-200304150-00005. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles M. Is the P300 component a manifestation of context updating? (Commentary on Verleger’s critique of the context updating model) Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: The variation in event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI. The relation of brain oscillations to attentional networks. J Neurosci. 2007;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Fernandez G, PK, Elger CE, Fries P. Is synchronized neuronal gamma activity relevant for selective attention? Brain Research Reviews. 2003;42:265–272. doi: 10.1016/s0165-0173(03)00178-4. [DOI] [PubMed] [Google Scholar]
- Fell J, Hinrichs H, Roschke J. Time course of human 40 Hz EEG activity accompanying P3 responses in an auditory oddball paradigm. Neuroscience Letters. 1997:121–124. doi: 10.1016/s0304-3940(97)00730-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Posner MI. Relating the mechanisms of orienting and alerting. Neuropsychologia. 1997;35:477–486. doi: 10.1016/s0028-3932(96)00103-0. [DOI] [PubMed] [Google Scholar]
- Ford J. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Ford J, Roach B, Faustman W, Mathalon D. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol Psychiatry. 2008a;63:736–743. doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Hoffman RS, Mathalon DH. The dependence of P300 amplitude on gamma synchrony breaks down in schizophrenia. Brain Res. 2008b;1235:133–142. doi: 10.1016/j.brainres.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds J, Rorie ARD. Modulation of oscillatory neuronal synchronization by selective visual attention. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Frund I, Busch NA, Schadow J, Korner U, Herrmann CS. From perception to action: phase-locked gamma oscillations correlate with reaction times in a speeded response task. BMC Neurosci. 2007;8:27. doi: 10.1186/1471-2202-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez Andino SL, Michel CM, Thut G, Landis T, Grave de Peralta R. Prediction of response speed by anticipatory high-frequency (gamma band) oscillations in the human brain. Human Brain Mapping. 2005;24:50–58. doi: 10.1002/hbm.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grice GR. Stimulus intensity and response evocation. Psychol Rev. 1968;75:359–373. doi: 10.1037/h0026287. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Anticipatory control of long-range phase synchronization. Eur J Neurosci. 2006;24:2057–2060. doi: 10.1111/j.1460-9568.2006.05082.x. [DOI] [PubMed] [Google Scholar]
- Gruber T, Müller M. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb Cortex. 2005;15:109–116. doi: 10.1093/cercor/bhh113. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Valle-Inclán F. Which stages of processing are speeded by a warning signal? Biological Psychology. 2003;64:27–45. doi: 10.1016/s0301-0511(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Haig AR, Gordon E, De Pascalis V, Meares RA, Bahramali H, Harris A. Gamma activity in schizophrenia: evidence of impaired network binding? Clin Neurophysiol. 2000;111:1461–1468. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- Haig AR, Pascalis VD, Gordon E. Peak gamma latency correlated with reaction time in a conventional oddball paradigm. Clin Neurophysiol. 1999;110:158–165. doi: 10.1016/s0013-4694(98)00112-6. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev. 2010;34:981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Isreal JB, Chesney GL, Wickens CD, Donchin E. P300 and tracking difficulty: Evidence for multiple resources in dual-task performance. Psychophysiology. 1980;17:259–273. doi: 10.1111/j.1469-8986.1980.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Brown RG, et al. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson’s disease. Brain. 1992;115:539–564. doi: 10.1093/brain/115.2.539. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Jokeit H, Makeig S. Differing event-related patterns of gamma band power in brain waves of fast and slow reacting subjects. Proceedings of the National Academy of Sciences. 1994:6339–6343. doi: 10.1073/pnas.91.14.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Heidegger T, Lutzenberger W. Behavioral relevance of gammaband activity for short-term memory-based auditory decision-making Eur. J Neurosci. 2008a;27:3322–3328. doi: 10.1111/j.1460-9568.2008.06290.x. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Heidegger T, Wibral M, Altmann CF, Lutzenberger W. Distinct gamma-band components reflect the short-term memory maintenance of different sound lateralization angles. Cereb Cortex. 2008b;18:2286–2295. doi: 10.1093/cercor/bhm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Rahm B, Lutzenberger W. Temporal dynamics of stimulus-specific gamma-band activity components during auditory short-term memory. NeuroImage. 2009;44:257–264. doi: 10.1016/j.neuroimage.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Keil A, Müller M, Ray W, Gruber T, Elbert T. Human Gamma Band Activity and Perception of a Gestalt. The Journal of Neuroscience. 1999;19:7152–7161. doi: 10.1523/JNEUROSCI.19-16-07152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J, Bott L, Posada A. Modulations in the degree of synchronization during ongoing oscillatory activity in the human brain. Eur J Neurosci. 2005;21:2547–2554. doi: 10.1111/j.1460-9568.2005.04069.x. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and paraphrenia [1919] Krieger, Robert E; New York, NY: 1971. [Google Scholar]
- Kramer AF, Strayer DL. Assessing the development of automatic processing: An application of dual-task and event-related brain potential methodologies. Biological Psychology. 1988;26:231–267. doi: 10.1016/0301-0511(88)90022-1. [DOI] [PubMed] [Google Scholar]
- Landau AN, Esterman M, Robertson LC, Bentin S, Prinzmetal W. Different effects of voluntary and involuntary attention on EEG activity in the gamma band. J Neurosci. 2007;27:11986–11990. doi: 10.1523/JNEUROSCI.3092-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Weinberger DR, Johnstone EC. Schizophrenia: from neuroimaging to neuroscience. Oxford University Press; New York: 2004. [Google Scholar]
- Lee B, Park KS, Kang DH, Kang KW, Kim YY, Kwon JS. Generators of the gamma-band activities in response to rare and novel stimuli during the auditory oddball paradigm. Neurosci Lett. 2007;413:210–215. doi: 10.1016/j.neulet.2006.11.066. [DOI] [PubMed] [Google Scholar]
- Lee D. Coherent oscillations in neuronal activity of the supplementary motor area during a visuomotor task. Journal of Neuroscience. 2003:6798–6809. doi: 10.1523/JNEUROSCI.23-17-06798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous Gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Research Reviews. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Lenz D, Krauel K, Schadow J, Baving L, Duzel E, Herrmann CS, Res B. Enhanced gamma-band activity in ADHD patients lacks correlation with memory performance found in healthy children. Brain Res. 2008;1235:117–132. doi: 10.1016/j.brainres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Liang H, Bressler SL, Ding M, Truccolo WA, Nakamura R. Synchronized activity in prefrontal cortex during anticipation of visuomotor processing. Neuroreport. 2002;13:2011–2015. doi: 10.1097/00001756-200211150-00004. [DOI] [PubMed] [Google Scholar]
- Makeig S, Delorme A, Westerfield M, Jung TP, Townsend J, Courchesne E, Sejnowski TJ. Electroencephalographic brain dynamics following manually responded visual targets. PLoS Biol. 2004:e176. doi: 10.1371/journal.pbio.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Molle M, Bartsch P. Event-related gamma band activity during passive and active oddball tasks. Neuroreport. 1996:1517–1520. doi: 10.1097/00001756-199606170-00016. [DOI] [PubMed] [Google Scholar]
- Martinovic J, Gruber T, Hantsch A, Muller MM. Induced gamma-band activity is related to the time point of object identification. Brain Research. 2008:93–106. doi: 10.1016/j.brainres.2007.12.050. [DOI] [PubMed] [Google Scholar]
- Martinovic J, Gruber T, Müller MM. Induced gamma-band responses predict recognition delays during object identification. The Journal of Neuroscience. 2007:1–14. doi: 10.1162/jocn.2007.19.6.921. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis I, van Dijk H, Jensen O. A Top-down drive Modulates Oscillatory Brain States Predictive of Motor Performance. NeuroImage. 2009;47:S180–S180. [Google Scholar]
- Medendorp WP, Kramer GF, Jensen O, Oostenveld R, Schoffelen JM, Fries P. Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb Cortex. 2007;17:2364–2374. doi: 10.1093/cercor/bhl145. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K. Reaction time and attention in schizophrenia: A critical evaluation of the data and theories. Schizophr Bull. 1977:373–436. doi: 10.1093/schbul/3.3.373. [DOI] [PubMed] [Google Scholar]
- Peled A. Multiple constraint organization in the brain: A theory for schizophrenia. Brain Research Bulletin. 1999;49:245–250. doi: 10.1016/s0361-9230(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical Neurophysiology. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 from a passive auditory paradigm. Electroencephalography and Clinical Neurophysiology. 1989;74:312–320. doi: 10.1016/0168-5597(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Pritchard W. Cognitive event-related potential correlates of schizophrenia. Psychol Bull. 1986:43–66. [PubMed] [Google Scholar]
- Reddi BA. Decision making: the two stages of neuronal judgement. Curr Biol. 2001;11:R603–606. doi: 10.1016/s0960-9822(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Reddi BA, Asrress KN, Carpenter R. Accuracy, information, and response time in a saccadic decision task. J Neurophysiol. 2003;90:3538–3546. doi: 10.1152/jn.00689.2002. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux J, Martinerie J, Renault B, Varela F. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and Antagonistic Contributions of Ongoing Activity Fluctuations to Auditory Stimulus Detection. The Journal of Neuroscience. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. 2005:2–e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadow J, Dettler N, Paramei GV, Lenz D, Frund I, Sabel BA, Herrmann CS. Impairments of Gestalt perception in the intact hemifield of hemianopic patients are reflected in gamma-band EEG activity. Neuropsychologia. 2009;47:556–568. doi: 10.1016/j.neuropsychologia.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Evian G, Harris AW, Haig AR, Brown KJ, Flor-Henry P, Williams LM. Sex Differences in Functional Connectivity in First-Episode and Chronic Schizophrenia Patients. Am J Psychiatry. 2004:1595–1602. doi: 10.1176/appi.ajp.161.9.1595. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14:199–224. [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, et al. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-Evoked Gamma Oscillations in Chronic Schizophrenia. Biological Psychiatry. 2008;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffy RA, Waldman I. Schizophrenic’s reaction time: north star or shooting star? In: Cromwell RL, Snyder CR, editors. Schizophrenia: Origins, Processes, Treatment, and Outcome. Oxford University Press; 1993. pp. 111–134. [Google Scholar]
- Summerfield C, Mangels JA. Dissociable neural mechanisms for encoding predictable and unpredictable events. J Cogn Neurosci. 2006;18:1120–1132. doi: 10.1162/jocn.2006.18.7.1120. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, et al. Evoked potential correlates of stimulus uncertainty. Science. 1965:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Sutton S, Tueting P, et al. Information delivery and the sensory evoked potential. Science. 1967;155:1436–1439. doi: 10.1126/science.155.3768.1436. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C. Attention Modulates Gamma-band Oscillations Differently in the Human Lateral Occipital Cortex and Fusiform Gyrus. Cerebral Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci. 2009 doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural Synchrony in Brain Disorders: Relevance for Cognitive Dysfunctions and Pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vidal JR, Chaumon M, O’Regan JK, Tallon-Baudry C. Visual grouping and selective attention induce gamma-band oscillations at different frequencies in human MEG signals. J Cogn Neurosci. 2006;18:1850–1862. doi: 10.1162/jocn.2006.18.11.1850. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C. Am I thinking assemblies? In: Palm G, Aertsen A, editors. Brain Theory, ser Proceedings of First Trieste Meeting on Brain Theory. Springer-Verlag; Berlin Heidelberg New York Tokyo: 1986. pp. 161–176. [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Banaschewski T, Kolev V, Woerner W, Rothenberger A. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder - evidence from event-related gamma oscillations. Clinical Neurophysiology. 2001;112:1096–1108. doi: 10.1016/s1388-2457(01)00524-7. [DOI] [PubMed] [Google Scholar]