Abstract
The major classes of chemicals and brain pathways involved in sexual arousal in mammals are well studied and are thought to be of an ancient, evolutionarily conserved origin. Here we discuss what is known of these neurochemicals and brain circuits in fishes, the oldest and most species-rich group of vertebrates from which tetrapods arose over 200 million years ago. Highlighted are case studies in vocal species where well-delineated sensory and motor pathways underlying reproductive-related behaviors illustrate the diversity and evolution of brain mechanisms driving sexual motivation between (and within) sexes. Also discussed are evolutionary insights from the neurobiology and reproductive behavior of elasmobranch fishes, the most ancient lineage of jawed vertebrates, which are remarkably similar in their reproductive biology to terrestrial mammals.
Keywords: teleost, elasmobranch, POA, AVT, GnRH, monoamines, steroid receptors, vocal-acoustic, neuroethology
Introduction: Evolutionarily conserved neurochemicals and pathways of sexual arousal
Mong et al. (2003) comment that “high arousal is reflected by high sensory responsiveness, high motor activity, and high emotional reactivity”. Ultimately, changes in levels of arousal of any one sensory or motor system depend on some form of modulatory input originating from what Nieuwenhuys et al. (1988) define as the neurochemically rich “core” and “paracore” that together form a neuroendocrine “axis” in the brain. Core regions, like the preoptic area (POA), lie adjacent to the brain's ventricular spaces and contain neuronal populations that synthesize a wide range of neuropeptides, concentrate androgens and estrogens, and are broadly implicated in the control of homeostatic and social behaviors. A laterally positioned “paracore” region at brainstem levels is especially rich in monoamines (serotonin and catecholamines) and interconnected with the core region. These peptidergic-containing nuclei and their fiber tracts can be designated as a caudally extended limbic system that includes the midbrain periaqueductal gray, the nucleus of the solitary tract and area postrema in the caudal medulla, and the central gray of the spinal cord (Nieuwenhuys et al. 1988). The core-paracore regions also include the essential nodes of a proposed “social behavior network” for vertebrates (Goodson, 2005; Newman, 1999). In sum, this highly conserved area of the vertebrate brain suggests its key role in survival by modulating brain arousal to affect behavior in an adaptive fashion, including daily and seasonal extremes in activity.
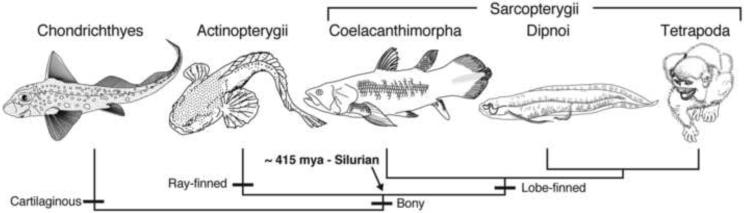
The motivation to reproduce, i.e., sexual arousal, activates physiological processes that can eventually lead to sexual behavior, inclusive of hormonal, genetic and neural-dependent mechanisms (Schober and Pfaff, 2007). The major premise of this article is not to re-review activational effects of steroid hormones or peptides on reproductive behavior in fishes (e.g., Bass and Forlano, 2008; Godwin, 2010; Oliveira and Gonçalves, 2008), but rather to highlight examples where the neurochemically-rich core of the central nervous system interacts with specific sensory and motor systems underlying reproductively motivated behaviors. We focus our discussion on two groups of fishes, teleosts and elasmobranchs. Jawed vertebrates include bony and cartilaginous fishes (Fig. 1) (Nelson, 2006). Bony fishes are divided into two major clades, Actinopterygii and Sarcopterygii. The majority of actinopterygians are teleosts, the largest group of living vertebrates with at least 30,000 species, and the principal focus of this review. Sarcopterygians include lungfish (Dipnoi), coelacanths (Coelacanthimorpha, Latimeria), and tetrapods (we are all fishes!) (Fig 1). The more basal cartilaginous fishes (Chondrichthyes) include holocephalans (chimaeras/ ratfish) and elasmobranchs (sharks, skates and rays).
Fig. 1.
Cladogram of living jawed vertebrates. Adapted from Ma et al. (2010).
What is known of the neural circuitry of “sexual arousal” in fishes?
Connectivity, neurochemistry and function of the POA/anterior hypothalamus
The POA and anterior hypothalamus, neurochemical “core” areas essential for the control of reproductive physiology and hence behavior in all vertebrates, show a highly conserved pattern of anatomical organization (Butler and Hodos, 1996; Meek and Nieuwenhuys, 1998). The POA and anterior hypothalamus can be viewed as one functional unit, the POA, because of their shared developmental origin (Puelles, 2001) and obvious homologies between neuropeptide-containing cell groups in the POA of teleosts (e.g., see Goodson et al., 2003) and the anterior hypothalamus of tetrapods (e.g., the paraventricular and supraoptic nuclei) (see Bass and Forlano, 2008 for further discussion).
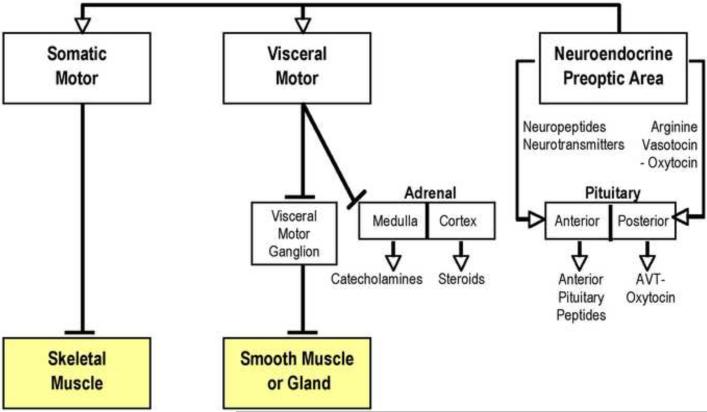
The role of the POA in brain and behavioral arousal is exemplified by its role as a sensorimotor integration center. Its motor outputs include connections with both the somatic and visceral motor systems (Fig. 2). The POA's effect on homeostatic function is enhanced by way of central visceromotor innervation of autonomic ganglia that project to either glands (including the adrenal medulla, a modified autonomic ganglion) or the visceral smooth muscle of multiple organ systems. The POA's pervasive influence on neuroendocrine function is achieved, in part, via its control over the release of several peptides from the anterior pituitary and of arginine vasotocin and isotocin (AVT/IT; arginine vasopression/oxytocin in mammals) from the posterior pituitary. Unlike the majority of vertebrates that have a blood portal system linking POA gonadotropin-releasing hormone (GnRH) neurons and the anterior pituitary, the GnRH POA neurons in teleosts directly innervate the pituitary (Peter and Fryer, 1983). This direct pathway may be a neuro-hormonal adaptation among teleosts allowing for rapid shifts in reproductive function (see Bass and Forlano, 2008 for more discussion). For instance, in the cichlid fish Astatotilapia burtoni, socially dominant territorial males are the only ones that reproduce. When given the opportunity to become dominant, a subordinate male will rapidly (within minutes) change its behavior. Within 30 minutes, neurons show an upregulation of the immediate early gene egr-1 in POA areas of high GnRH-1 expression (Burmeister et al., 2005). The physiological properties of GnRH neurons are also significantly different between territorial males and non-territorial males, which likely translates into less gonadotropin release in non-territorial males (Greenwood and Fernald, 2004). The reader is referred to Bass and Grober (2009) for a recent discussion of the diversity in organization of the POA in relation to the remarkable range of reproductive plasticity exhibited by teleost fishes.
Fig. 2.
Organizational summary of vertebrate somatic motor, visceral motor and neuroendocrine systems (from Bass and Forlano, 2008). The adrenal cortex releases glucocorticoids and mineralcorticoids, while the adrenal medulla releases catecholamines, including epinephrine and norepinephrine. See Bentley (1998) for neurochemical details. Reprinted with permission, Cambridge University press
The presence of the nonapeptide AVT/AVP in magnocellular and parvocellular neurons of the POA in all vertebrates exemplifies the conserved evolution of the neurochemical core of the brain over the last 500 million years or more. Furthermore, in all jawed vertebrates, neuropeptide-containing neurons in the POA project to similar extrahypothalamic brain regions including the ventral telencephalon, midbrain tegmentum, periaqueductal gray, and viscerosensory areas of the medulla (Goodson and Bass, 2001; Saito et al., 2004). AVT/AVP is well documented in its role in mediating vertebrate social behavior (Goodson and Bass, 2001), including AVT manipulations affecting male reproductive displays in teleosts (Carneiro et al., 2003; Salek et al., 2002; Santangelo and Bass, 2010; Semsar and Godwin, 2003; Semsar et al., 2001). In mammals, male penile erection is regulated by androgenic stimulation of oxytocin neurons in the paraventricular hypothalamus (PVN) (Argiolas and Melis, 2005). The magnocellular nucleus of the POA in teleosts is thought to be the PVN homologue, in part due to its abundance of AVT/IT neurons (Gilchriest et al., 2000; Kapsimali et al., 2001; see Forlano and Cone, 2007 for further discussion). Like mammals, these large neuroendocrine neurons also project to the spinal cord in teleosts (Demski and Sloan, 1985; Goodson and Bass, 2000b; Gregory and Tweedle, 1985; see Meek and Nieuwenhuys, 1998; Prasada Rao et al., 1987) and therefore may serve to modulate motor behaviors associated with reproduction (e.g., sperm release; Demski and Knigge, 1971).
There is a large literature on the great diversity and plasticity of hormonal control of reproductive behavior in teleost fishes (see Bass and Grober, 2009; Godwin, 2010; Munakata and Kobayashi, 2010; Oliveira and Gonçalves, 2008). However, with the exception of vocal fish (see later section), little is currently known about the anatomy and physiology of neural circuitry underlying those behaviors. Many studies highlight the diversity of neuropeptide phenotypes in the POA in relation to male social status and reproductive displays. Generally, territorial, displaying males have more or larger GnRH-immunoreactive(ir) cells, while AVT-ir/mRNA relationships are species-specific (for reviews see Bass and Forlano, 2008; Bass and Grober, 2001; Foran and Bass, 1999; Godwin, 2010; Hofmann, 2006; Oliveira and Gonçalves, 2008; Oliveira et al., 2005). There are even fewer functional studies of the teleost POA (or other brain areas) in regards to sexually motivated behavior (but see below and discussion on vocal fishes). AVT-ir and GnRH-ir and/or their receptors are also found in peripheral sensory organs and central sensory processing areas (Dewan et al., 2008; Foran et al., 1997; Goodson and Bass, 2000b; Grens et al., 2005; Maruska, 2009; Maruska and Fernald, 2010; Maruska and Tricas, 2007; Stell et al., 1987), implicating the role of these neuropeptides in modulating levels of “sensory responsiveness”.
Sexual behavior in fishes, like other vertebrates, is often stimulated by olfactory/pheromonal, visual and auditory sensory stimuli or a combination of these cues (for review, see Oliveira and Gonçalves, 2008; also see Satou et al. (1994) for visual and vibrational cues in salmon). All of these sensory systems have inputs to the POA in fishes (e.g., Bass et al., 2000; Becerra et al., 1994; Meek and Nieuwenhuys, 1998), again pointing to the POA as a major central integration site for sensory cues and reproductive behavioral output. Early studies by Demski and Knigge (1971) demonstrated that multiple reproductive behaviors including nest building (sweeping) and courtship circling, and an inhibition of aggression, as well as sperm release could be induced in freely swimming bluegill sunfish by electrical stimulation of the POA. Similar experiments in hime salmon, Onchorhyncus nerka, confirmed the POA as a control center for sexual behavior, both appetitive and spawning (Satou et al., 1984). Interestingly, sweeping behavior in bluegill sunfish (Lepomis macrochirus) could be induced in both males and females by stimulation of the central nucleus of the dorsal division of the telencephalon (Dc), even though females never show this behavior in nature. Thus, sex differences in circulating androgens and/or androgen receptor expression in Dc or POA may account for this sexually dimorphic behavior. Autoradiographic studies of bluegill show testosterone- concentrating cells in the POA, but not in Dc (Demski, 1978). However, more sensitive measures of androgen receptor (AR) binding sites, such as in situ hybridization, might reveal AR mRNA expression in Dc, as has been shown in two other teleost species (Table 1; Forlano et al., 2010; Harbott et al., 2007; Munchrath and Hofmann, 2010). Interestingly, testosterone-stimulation of the POA in male rodents also evokes nest-building behavior (Fisher, 1956).
Table 1.
Comparative distribution of androgen receptor (AR), aromatase (estrogen synthase) and estrogen receptor (ERα, ERβ1, ERβ2) transcripts and estrogen and androgen concentrating cells in the forebrain of teleosts with putative mammalian homologies and major nodes of the vertebrate social behavior network (*).
| Brain area | AR | ([3H] T/DHT) | Aromatase | ERα | ERβ(1/2) | ([3H] E2) | Putative mammalian homologue |
|---|---|---|---|---|---|---|---|
| Ventral telencephalon | |||||||
| Olfactory bulb (OB) | a | - | m,t,z,p | m,a | a | NR | |
| Ventral nucleus (Vv)*Aud | a | P | m,t,z,p | m,a,s | a, s | t,p,x | Septum2,11,16 |
| Supracommissural nucleus (Vs)* Aud, Voc | m,a | t | m,t,z,p | m,a | a | t,g,x | Basal amygdala2,11 |
| Postcommissural nucleus (Vp) Aud | m,a | - | m,t,z,p | m,a,s | a | t | Basal amygdala2,11 |
| Dorsal nucleus (Vd) | m,a | NR | m,t,z,p | a,s | a, s | NR | Striatum/basal ganglia10,15,16 |
| Intermediate nucleus (Vi) | m | (t) | m,NR | - | - | NR | Basal amygdala13 |
| Dorsal telencephalon | |||||||
| Central zone (Dc) | m,a | NR | m,NR | m,a | a | NR | Pallial amygdala or dorsal pallium1,12,18 |
| Central medial division of medial zone (Dm-cm) | m,a | NR | m,t,z,p | m,a | a | NR | Pallial amygdala or dorsal pallium1,2,12,14,16,18 |
| Posterior division of medial zone (Dm-p) Aud, voc | m | NR | m,NR | m | NR | “ | |
| Dorsal posterior zone (Dp) | a | t | m,z | m,a | a | - | Piriform cortex10,11,12 |
| Preoptic area* | |||||||
| Anterior parvocellular (PPa) And, Voc | m,a,z | t,s,p,g,x | m,t,z,p | m,a,z,t,c,e,s,p | a,c,z,p,s | g,t,p,x | POA7,9 |
| Posterior parvocellular (PPp) Aud, Voc | m,a,z | - | m,t,z,p | a,s,t,c,p | a,s | t | “ |
| Magnocellular (PM/PMg) | m,a | t,p,g,x | m,t,z,p | m,a,t,s,c,p | a,c,s | g,t,p.x | Paraventricular nucleus5,6,8 |
| Ventral hypothalamus | |||||||
| Anterior tuberal (AT)* Aud, Voc | m,a | NR | m,t,z, | m,a,s,t,c | a | t | Ventromedial hypothalamus4,7 |
| Ventral tuberal (vT)* Aud, Voc | m,a | NR | m,z | a,NR | a,NR | NR | Anterior hypothalamus7 |
| Periventricular (Hv/Hd) | m,a,z | t,m,s,p,g,x | m,t,z,p | m,a,s,c,e,z | a,c,z,p,s | g,t,p,x | Arcuate3,5 |
| Thalamus | |||||||
| Central posterior nucleus (CP) Aud | m,a | NR | m,z | m,a,s | a | NR | |
| Dorsal posterior (DPo) | m,a | NR | m,t,z,p | m,a,s | a | NR | |
| Nucleus preglomerulosus (PGl/m) Aud | m,a | t | m,z | a,s | a | t | |
| Periventricular nucleus of posterior tuberal (TPp) | m,a,z | - | m,t,z | m,a | a,s | t | |
| Posterior tuberal nucleus (TP) | m,a,z | m | m,t,z | a,c,z,t | a,c,z,s | - | Ventral tegmental area/substantia nigra15,16 |
| Pineal | - | NR | m,NR | m,NR | NR | NR |
m = midshipman, Porichthys notatus (Forlano et al., 2001; 2005; 2010)
z = zebrafish Danio rerio (Gorelick et al., 2008; Menuet et al., 2002; 2005)
t = trout, Oncorhyncus mykiss (Menuet et al., 2001; 2003)
a = cichlid, Astatotilapia burtoni (Harbott et al., 2007; Munchrath and Hofmann, 2010)
c = croaker, Micropogonias undulatus (Hawkins et al., 2005)
p = pejerrey, Odontesthes bonariensis (Strobl-Mazzulla et al., 2008)
s = seabass, Dicentrarchus labrax (Muriach et al., 2008a; 2008b)
e = eelpout, Zoarces viviparous (Andreassen et al., 2003)
t = oyster toadfish, Opsanus tau (Fine et al., 1996)
m = mormyrid, Brienomyrus brachistius (Bass et al., 1986)
s = sunfish, lepomis cyanellus (Morrell et al., 1975)
p = paradise fish, Macropodus opercularis (Davis et al., 1977)
g = goldfish, Carassius auratus (Kim et al., 1978)
x = platyfish, Xiphophorus maculatus (Kim et al., 1979)
NR = not recorded
Aud, Voc = defined auditory and vocal nuclei in midshipman
GnRH in the terminal nerve ganglion
Teleosts have two or three forms of GnRH in three distinct brain areas: the POA which directly controls gonadotropin release from the pituitary, as well as more poorly understood populations in the midbrain tegmentum and ganglion of the terminal nerve (TN), both thought to serve a neuromodulatory role in the brain (review: Kah et al., 2007). TN neurons are typically clustered near the olfactory bulb-telencephalon junction and have direct projections to both the retina and olfactory epithelium (Demski and Northcutt, 1983; Kah et al., 2007; Schreibman and Margolis-Nunno, 1987). Oka and Matsushima (1993) characterized the oscillatory-like properties of TN neurons in the dwarf gourami, Colisa lalia, and hypothesized a modulatory role based on the TN's physiological properties and widespread projections throughout the brain, including the olfactory bulbs, optic nerves, hypothalamus, optic tectum, and spinal cord. Lesion of the TN depletes GnRH in the brain and negatively affects the frequency of nest building, a sexually motivated behavior stimulated by the presence of a receptive female (Yamamoto et al., 1995). In Nile tilapia, Oreochromis niloticus, removal of the olfactory epithelium preferentially decreased nest building behavior as well as the transcripts encoding GnRH-1 (TN) and GnRH-2 (midbrain), probably by interactions with second order olfactory projections (Uchida et al., 2005). In Japanese medaka, Oryzias latipes exposure to male visual cues suppressed the electrical activity of female TN GnRH neurons. Interestingly, same sex and olfactory cues in general had no effect on modulating TN GnRH activity in this species (Ramakrishnan and Wayne, 2009).
Olfactory pathways and sexual behavior
A role for pheromones in male and female reproductive behavior has been especially well documented by Stacey, Sorensen and colleagues in goldfish (Carassius auratus) (Kobayashi et al., 2002; Munakata and Kobayashi, 2010; Stacey and Liley, 1974; Stacey and Sorensen, 2002). Prior to ovulation, females produce a progestin 17α,20β-dihydroxy-4-pregnen-3-one (17,20-P) in the ovarian follicles that induces oocyte maturation. This progestin functions as a “pre-ovulatory pheromone” that induces an LH surge in the male and subsequent chasing behavior (Dulka et al., 1987). Prostaglandins are produced in the ovary after ovulation and trigger female sexual behavior (e.g., oviposition) that is thought to occur via central mechanisms (likely involving the POA) as injections of the prostaglandin PGF2α in the preoptic recess of the third ventricle of female goldfish effectively trigger this behavior, although the exact mechanism and site of action is unknown (Stacey and Peter, 1979). While PGF2α is essential for female sexual behavior, estrogens are not. Pharmacological increases or decreases in brain GnRH concentration will enhance and inhibit female spawning behavior, respectively (Volkoff and Peter, 1999). However, GnRH within the terminal nerve ganglion appears to function as a “potentiator” to facilitate behavior, but is not essential as transection of the terminal nerve within the olfactory tract only increases the behavioral latency (see Munakata and Kobayashi, 2010). While gonadal steroids do not seem to be necessary for female reproductive behavior, males require androgens (the teleost-specific androgen 11-ketotestosterone/11kT; Bentley, 1998; Borg, 1994) to prime circuitry needed to respond to the female pheromone stimulus. In fact, androgens increase olfactory response in males to female pheromone (15K PGF2α) measured by electro-olfactogram (Cardwell et al., 1995). Interestingly, goldfish adults can be behaviorally sex-changed by prostaglandin injection in a male or 11kT implant in a female followed by 15K PGF2α stimulation (Kobayashi et al., 2002). This example in goldfish, along with nest building in bluegill sunfish (see above), and male-female reversals in sex-changing fishes (see Bass and Grober, 2009) implies bisexual circuitry underlying sexual behavior across diverse species that can be simply activated under a sexually dimorphic hormonal or chemical signal (e.g., androgens, prostaglandins).
Teleosts have a direct connection from the olfactory bulb to the POA (anterior parvocellular) and to the catecholamine-rich posterior tuberal nucleus in the caudal diencephalon. Other secondary olfactory terminal fields that are consistent across teleost species include putative homologues of the lateral septum (Vv, ventral nucleus of the ventral division (V) of the telencephalon), amygdala (Vs, supracommisural nucleus of V), and striatum (Vd, dorsal nucleus of V), as well as nucleus taeniae and the piriform cortex homolog (Dp, posterior nucleus of the dorsal division of the telencephalon) (see Meek and Niewenhuys, 1998; homologies: Mueller and Wullimann, 2009; Northcutt, 1995, 2006; Wullimann and Mueller, 2004). Electrophysiological evidence for the POA as an integration site for olfactory processing in teleosts was demonstrated by the activation of parvocellular POA neurons by electrical stimulation of the olfactory tract in sunfish (Hallowitz et al., 1971). Studies from a diverse range of species (e.g., catfish, goldfish, cod, weakly electric fish, winter flounder) have demonstrated that olfactory bulbs also receive reciprocal input from the ventral and dorsal posterior telencephalon and from a subset of Dc neurons (Bass, 1981; Levine and Dethier, 1985; Prasada Rao and Finger, 1984; Sas et al., 1993; see Meek and Nieuwenhuys, 1998). In some species, the POA and monoamine-containing areas such as the posterior tuberal, locus coeruleus and raphe nuclei were also found to project to the olfactory bulb (Levine and Dethier, 1985; Prasada Rao and Finger, 1984; Sas et al., 1993). The medial olfactory tract, which projects largely to the ventral telencephalon, includes the projections of the GnRH neurons in the terminal nerve ganglion that are thought to preferentially encode sex pheromones (Demski and Northcutt, 1983; Dulka, 1993; Sorensen et al., 1991).
Several lesion studies of Vs and/or posterior Vv have substantiated their importance in male courtship and spawning behavior in goldfish and other species (Koyama et al., 1984; Kyle and Peter, 1982; Kyle et al., 1982). Kyle and Peter (1982), hypothesize that the lesions interfered with critical olfactory (pheromonal) processing necessary for sexual motivation. Anatomical studies have demonstrated reciprocal connections of Vs and the olfactory bulbs, and input to Vs from other olfactory bulb targets (Vv, Vd, POA, posterior tubercle in the diencephalon; Demski and Northcutt, 1983; Meek and Nieuwenhuys, 1998; Shiga et al., 1985a). It is noteworthy that all of the above mentioned areas are targets of steroid hormone receptor expression and dopaminergic input (see Table 1, Fig. 3 and below). In addition, lesions to the anterior POA, and the lateral or medial forebrain bundle greatly impair male sexual behaviors (Koyama et al., 1984). The POA also receives input from Vv, Vd and diencephalic nuclei including the ventral periventricular hypothalamus (Hv/NLT) and anterior tuberal nucleus (Meek and Nieuwenhuys, 1998; Shiga et al., 1985b), proposed homologues of the mammalian arcuate and ventromedial hypothalamic nucleus, respectively (Cerda-Reverter et al., 2003; Forlano and Cone, 2007; Forlano et al., 2005; Goodson, 2005).
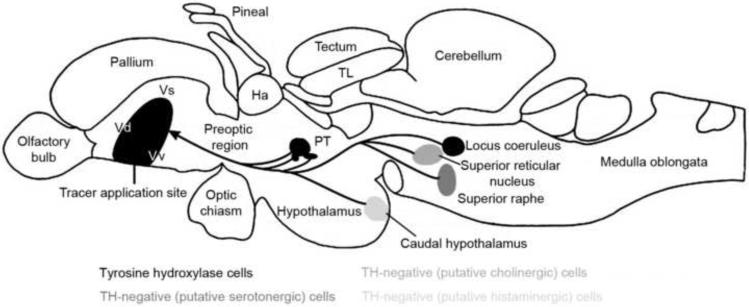
Fig. 3.
Ascending activating systems to the ventral telencephalon in the adult zebrafish brain (modified from Wullimann and Mueller, 2004; permission pending from Wiley-Liss, Inc.). Double-labeling studies identified the posterior tuberculum (PT) to dorsal nucleus of the ventral telencephalon (Vd) projection, proposed as the homologous meso-striatal pathway in mammals. Cholinergic (superior reticular), serotonergic (superior raphe), and histaminergic (caudal hypothalamic) nuclei require neurochemical confirmation. Ha, habenula; TL, torus longitudinalis; Vs, supracommisural nucleus; Vv ventral nucleus of the ventral telencephalon.
Neurochemical arousal pathways in the “paracore”
The median and laterally positioned “paracore” regions include conserved dopamine, norepinephrine, and serotonin-containing neurons (Nieuwenhuys et al., 1988), neuromodulators that together with histamine and acetylcholine ascending pathways are known to increase arousal in mammals (Kapp and Cain, 2001). The neuroanatomical distribution of these pathways in fishes are well documented across several taxa and in many cases show a conserved pattern with tetrapods (Fig. 3; Kaslin and Panula, 2001; Rodriguez-Moldes et al., 2002a; Smeets and Gonzalez, 2000; Wullimann and Mueller, 2004). Tract tracing studies in combination with tyrosine hydroxylase immunocytochemistry revealed an ascending activating system projecting from diencephalic posterior tuber neurons to septal and striatal homolgues in the ventral telencephalon (Vv/Vd) in zebrafish (Danio rerio) (Rink and Wullimann, 2001). Other afferents to this area of the ventral telencephalon arise from cell groups known to express serotonin, acetylcholine and histamine (Bellipanni et al., 2002; Clemente et al., 2004; Kah and Chambolle, 1983; Kaslin and Panula, 2001; Margolis-Kazan et al., 1985; Perez et al., 2000).
In teleosts, a midbrain tegmental population of dopaminergic neurons is missing, but a posterior tuber-Vd pathway is hypothesized to be homologous to the nigro-striatal pathway in mammals since some mammalian dopaminergic (substantia nigra) neurons are found in the embryonic basal diencephalon and, like zebrafish, express the Nurr1 gene and are developmentally regulated by the nr4A2 gene (Kapsimali et al., 2001; Luo et al., 2008; Rink and Wullimann, 2001, 2002). Interestingly, cartilaginous fishes (elasmobranchs, e.g., sharks and rays) have both midbrain and posterior tuber dopaminergic groups (Meredith and Smeets, 1987; Stuesse et al., 1990), suggesting teleosts are exhibiting a derived condition (Wullimann and Mueller, 2004). In general, the widespread neuroanatomical distribution of tyrosine hydroxylase-ir neuronal groups and fiber projections are well documented in diverse taxa of fishes (for review, see Smeets and Gonzalez, 2000), and are in large part found in predicted regions that correspond to putative mammalian homologues.
Teleosts contain adrenergic neurons in the locus coeruleus and caudal medulla, and dopaminergic diencephalic populations in the hypothalamus (including POA), ventral medial thalamus and ventral telencephalon (e.g., Filippi et al., 2010; Ma, 2003; McLean and Fetcho, 2004; Rink and Wullimann, 2001; Smeets and Gonzalez, 2000). Like tetrapods, there is a dense tyrosine hydroxylase-ir somata population in the olfactory bulbs and retina as well as robust fibers within sensory integration centers such as the optic tectum and the olfactory- recipient posterior dorsal telencephalon (Dp) (Meek and Nieuwenhuys, 1998). While dopamine is well documented as a regulator of reproduction in teleosts by inhibiting gonadotropin release in certain species (Dufour et al., 2005; Zohar et al., 2010), little is known regarding its function in sexually motivated behaviors as is well defined in rodents (Hull et al., 2004). However, based on its conserved neuroanatomical distribution and connectivity with brain areas involved in sexual behavior, as well as evidence that pharmacological manipulations of dopamine can affect reward circuitry and motivation (Darland and Dowling, 2001; Lett and Grant, 1989), it is likely dopamine plays a similarly important role in sexual motivation in fishes.
Monoamines are also documented to regulate neuropeptides in the core of the reproductive axis of the teleost brain. Chronic treatment by fluoxetine, a serotonin reuptake inhibitor, decreases AVT mRNA expression in the POA, as well as aggressive behavior in dominant, courting bluehead wrasse males (Thalassoma bifasciatum) (Semsar and Godwin, 2004). Fluoxetine treatment of female goldfish dramatically downregulated isotocin (oxytocin homologue) and ERβ1 mRNA in both the hypothalamus and telencephalon and ERα in the telencephalon, and also decreased plasma estradiol levels (Mennigen et al., 2008). Within the POA, noradrenergic activity increases and serotonergic activity decreases during female to male sex change in the saddleback wrasse, Thalassoma duperrey, changes paralleled within the locus coeruleus and raphe nucleus, respectively (Larson et al., 2003b). Experimentally increasing norepinephrine levels or decreasing serotonin levels can facilitate sex change, while the inverse treatments inhibit sex change in this species (Larson et al., 2003a). Investigating dopaminergic and serotonergic systems in fishes in the context of sexual arousal and their interactions with other neuropeptides remains an interesting comparative topic for further study.
The histaminergic system regulates general brain arousal in daily behavior and physiology, including circadian rhythms of locomotor activity, waking, and feeding (Wada et al., 1991). Since histamines have stimulatory and inhibitory effects on serotonin, noradrenaline, dopamine and acetylcholine in mammals (Schlicker et al., 1994) and have widespread reciprocal connections in teleosts (Kaslin and Panula, 2001), these complex interactions provide a rich potential for uncovering brain and sexual arousal in fish and other vertebrates. In addition, orexin (hypocretin) neurons, another hypothalamic peptidergic system involved in arousal states (such as wakefulness), were found in zebrafish to have largely similar projection patterns as mammals, including innervation of striatal (Vd) and septal (Vv) homologues and the periventricular hypothalamus. Additionally, unlike mammals, an orexin-like cell population was found in the magno- and parvocellular POA. Interestingly, orexin-ir fibers also densely innervated diencephalic dopaminergic neurons (thought homologous to the mammalian ascending system) and the serotonin-containing raphe nucleus, and less so the noradrenergic locus coeruleus and histaminergic neurons in the ventrocaudal hypothalamus. Only moderate contacts were made to mesopontine cholinergic neurons, but all the above systems showed evidence of reciprocal connections with orexin-containing neurons in the POA (Kaslin et al., 2004). These neuroanatomical studies suggest that daily rhythms in general arousal provided by orexin may, in turn, directly activate circuits involved in sexual behavior (e.g., POA; striatal and septal homologues) or indirectly by modulating ascending and descending aminergic systems (Kaslin and Panula, 2001, 2004). A study in goldfish found fewer orexin-ir contacts with aminergic-containing neurons in the tegmentum compared to that reported in zebrafish (Huesa et al., 2005); thus, further studies with other species and localization of orexin on aminergic neurons at the subcellular level may clarify the functional significance of potential interspecific variation. In rodents, there is evidence that orexins may integrate motor activity associated with motivated (reward) behaviors by modulating the ascending dopaminergic system (see Scammell and Saper, 2005); future explorations of these interactions in fishes are also potentially fruitful areas for investigation of neuromodulatory mechanisms of sexual motivation.
Steroid hormone receptors in circuits involved in reproductive behavior
It is beyond the scope of the present essay to review the vast literature of activational effects of steroid hormones on reproductive behavior in fishes (for comprehensive reviews see Godwin, 2010; Oliveira and Gonçalves, 2008; Oliveira et al., 2005). Rather, we provide what is known regarding the distribution of (sex) steroid receptors in the central and peripheral nervous system of teleosts, highlighting areas discussed in the text that are directly linked to reproductive behaviors and/or nuclei that contain ascending arousal pathways. Table 1 shows a comparison of androgen and estrogen receptor expression, steroid-concentrating cells, and the estrogen-synthesizing enzyme aromatase in teleost forebrain nuclei and proposed mammalian homologues if known. Also indicated in the table is expression of steroid receptors in major nodes of the “vertebrate social behavior network” in fishes (i.e., med amygdala/ BnST (Vs); lateral septum (Vv); ventromedial hypothalamus (AT); POA; PAG not listed in Table 1 but see Fig. 4) (Goodson, 2005). Not surprisingly, like all other vertebrates, receptors for androgens and estrogens are robustly expressed in the POA and hypothalamus in teleosts. Thus, steroid receptors are localized within conserved brain areas that control reproductive behavior and/or contain various modulatory peptides that regulate arousal. Unlike mammals, little is known about progesterone receptor (PR) in brain, or role of progesterone in sexual behavior in fishes, however, as expected, it is robustly expressed in the POA (Hanna et al., 2010; Munchrath and Hofmann, 2010).
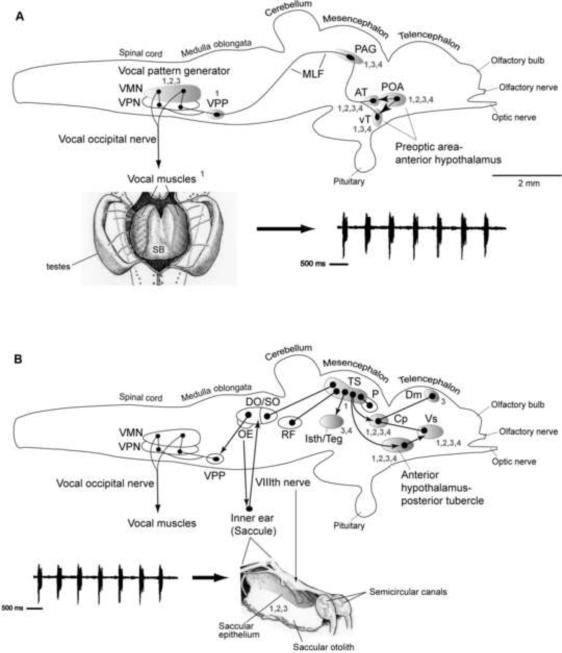
Fig. 4.
Side views of the brain showing vocal motor (A) and auditory (B) systems in batrachoidid fish (midshipman and toadfish) (adapted from Forlano et al., 2010). Solid dots represent somata, and lines represent axonal projection pathways. Two connected dots indicate reciprocal connections. Shaded areas indicate localization of steroid hormone receptor mRNA (1, androgen; 2, estrogen alpha), aromatase (3), and neuropeptide innervation (4, arginine vasotocin/isotocin) (see Forlano et al., 2005; Forlano et al., 2001; Forlano et al., 2010; Goodson and Bass, 2000b; Goodson et al., 2003). A; Descending vocal pathways. Preoptic (POA) and hypothalamic ventral (vT) and anterior (AT) tuberal nuclei project to the midbrain periaqueductal gray (PAG) which innervates, in turn, the vocal pattern generator circuit (VPG) in the hindbrain-spinal cord. The VPG consists of vocal prepacemaker (VPP), pacemaker (VPN) and motor (VMN) nuclei. VMN axons exit the brain via occipital nerve roots (likely homologues of hypoglossal) to sound-producing vocal muscle attached to the swim bladder (ventral view). Also shown is a representative “grunt train” vocalization produced by nest guarding male. B: Central auditory pathways. Vocalizations (e.g., grunt train) are detected by auditory saccule within the inner ear which projects via the VIIIth nerve to auditory nuclei in the hindbrain that innervate the auditory midbrain torus semicircularis (TS). Shown are nuclei interconnected with TS. A dorsal thalamic nucleus (central posterior nucleus, CP) contains reciprocal connections to the telencepahlon (dorsomedial/Dm) and ventral supracommissural/Vs) that receives input from anterior hypothalamus-posterior tubercle (for nomenclature see Braford and Northcutt, 1983). TS and CP also connect to forebrain (anterior hypothalamus, POA) and midbrain (PAG, isthmal/tegmentum) vocal sites, while auditory-recipient hindbrain nuclei connect to the pattern generating VPG circuit (also see Bass et al., 1994; Goodson and Bass, 2002). See Forlano et al. (2010) for additional background references.
While the importance of gonadal steroids on male and female reproductive behavior is quite variable across teleosts (Borg, 1994; Munakata and Kobayashi, 2010; Oliveira and Gonçalves, 2008) and sexual behavior in some fishes has been demonstrated to be independent of gonadal steroids (Godwin et al., 1996; Kobayashi and Stacey, 1993; Semsar and Godwin, 2003), the brain as well as the gonads are both likely sources of steroid hormones which modulate the neural circuits controlling reproductive behavior; gonadal steroids also may function, in part, to upregulate neurosteroid production in the brain (e.g., Forlano and Bass, 2005; see reviews in Diotel et al., 2010; Forlano et al., 2006). High levels of brain aromatase in teleosts (the highest among all vertebrates) may regulate the ratio of androgens to estrogens that reach behavioral circuits on a rapid or long-term timescale (Forlano et al., 2006; Bass and Forlano, 2008; also, see discussion below for vocal fishes). For instance, within minutes to hours after female to male sex change in the bluebanded goby, Lythrypnus dalli, an increase in aggressive behavior and decrease in brain aromatase activity occurs (Black et al., 2005). In male goldfish, aromatase inhibition blocks the rapid effects of testosterone on visually mediated approach behavior toward females (Lord et al., 2009). In guppies, Poecilia reticulata, two of three male sexual displays were reduced by aromatase inhibition (Hallgren et al., 2006), suggesting testosterone aromatization into estradiol facilitates these behaviors. Nesting males of the peacock blenny, Salaria pavo, have higher brain aromatase activity than males that adopt a sneaking reproductive tactic (Goncalves et al., 2008). Sex differences in aromatase activity or expression in various brain regions including the POA of teleosts is found in several species (reviewed in Forlano et al., 2006; Goncalves et al., 2010; Goncalves et al., 2008), and thus similar levels of circulating testosterone between sexes could be seen very differently in brain areas where androgen metabolism is sexually dimorphic. It is interesting to note that while the vast majority of teleost fishes reproduce by external fertilization and estrogen does not appear necessary for female sexual behavior (Munakata and Kobayashi, 2010), guppies are internal fertilizers, and like mammals, female receptivity is regulated by estradiol (Liley, 1972). Although most female teleosts have low circulating estradiol levels during the spawning period (see Sisneros et al., 2004b and review within), the production of estrogen within the brain itself may still be high and important in regulating female sexual behavior in some species.
Brain arousal and reproductive behavior in vocal fishes
Vocal teleost fish species have a long history as models for studying the neural basis of behavior due to their simple, stereotyped, and easily quantified behaviors that are controlled by a discrete population of neurons whose anatomical and physiological properties are well defined (Bass and Zakon, 2005). These fish have also provided excellent models to study how steroids and neuropeptides rapidly modulate plasticity in neural circuits controlling behavior (Bass and Remage-Healey, 2008; Forlano et al., 2007; Goodson and Bass, 2000a). In species such as the midshipman fish (Porichthys notatus), where the production and perception of sound is essential for reproduction (Bass and McKibben, 2003), we can ask questions regarding the arousal of sexual motivation at the level of both vocal and auditory circuits, in the male and female, respectively. Although many teleosts use sound communication for courtship and aggressive interactions (e.g., Ladich et al., 2006; Maruska et al., 2007; Santangelo and Bass, 2006; Simoes et al., 2008), little is known regarding the neural pathways and mechanisms of sound production (and reception) outside of the toadfish family (Batrachoididae) that includes midshipman.
Behavioral and neurophysiological studies demonstrate the extreme seasonal and daily rhythms of brain arousal related to social vocal communication in midshipman fish, mechanisms that are potently influenced by both peptidergic and steroid hormones. Although not as well studied compared to hormone-driven male courtship behaviors in fishes, examples where females actively seek out and choose a male mate are good models for identifying brain-behavioral neuroendocrinological mechanisms underlying brain arousal and the motivation to reproduce. Midshipman females use the advertisement calls of males to find their nest sites during the late spring-summer breeding season (Brantley and Bass, 1994; McKibben and Bass, 1998). Underwater playbacks show that females distinguish between calls based on their frequency, duration, amplitude and patterns of amplitude modulation (McKibben and Bass, 1998, 2001), traits encoded by central auditory pathways (see Bass and McKibben, 2003). Only females filled with mature eggs (i.e., gravid) show a positive phonotactic response to advertisement calls. Once females release all of their eggs, they leave the nest and males remain to guard developing embryos/ larvae (Brantley and Bass, 1994); they also no longer respond to playbacks of advertisement calls (McKibben and Bass, 1998). Female phonotaxis behavior, a directed motor response stimulated by a single sensory modality, is an unambiguous proxy for high sexual arousal in this species. The apparent loss of any motivation by previously gravid females to attend to advertisement calls led to studies of shifts in auditory sensitivity that might be linked to this dramatic behavioral change. Subsequent investigations showed steroid-dependent enhancement in the ability of the peripheral auditory system to encode the frequency content of the advertisement call (Sisneros, 2009; Sisneros and Bass, 2003; Sisneros et al., 2004a). While this mechanism can account for seasonal changes in auditory encoding ability that parallel changes in circulating steroid levels (i.e., steroids “prime” the auditory system to encode advertisement calls), the neural mechanisms leading to the sudden change in female phonotaxis remain to be shown. The steroid dependent plasticity of auditory encoding is a specific example of estrogen-induced peripheral nervous system arousal (Lee and Pfaff, 2008) that might be analogous to estrogen-dependent enlarging of the genitosensory field of tactile receptors in rodents (Bereiter and Barker, 1980).
Midshipman fish have two male reproductive morphs; type I, territorial/nesting males acoustically court females while type II, sneak/satellite spawning males steal fertilizations from the type I males during spawning behaviors (Brantley and Bass, 1994). Type I and II males diverge in a large suite of somatic, neural and neuroendocrine traits (for recent reviews see Bass and Forlano, 2008; Bass and Grober, 2009; Bass and Remage-Healey, 2008). The sensitivity of the central vocal circuit to the nonapeptides AVT/IT and steroids (androgens and glucocorticoids) shows both sex and male morph-specific variation (Goodson and Bass, 2000a; Remage-Healey and Bass, 2004, 2007). The steroid phenotype parallels morph-specific patterns in circulating levels of androgens and estrogen (Remage-Healey and Bass, 2007). A series of field studies of the closely related toadfish (Opsanus beta) show how rapid shifts in circulating androgen levels can account for similarly rapid changes in nesting male calling behavior in response to behavioral playbacks of conspecific calls (Remage-Healey and Bass, 2005, 2006). The most recent neurophysiological studies show a nocturnal, reproductive dependence in the ability of the central vocal network to generate long duration advertisement and aggressive calls that only type I males generate during the breeding season (Rubow and Bass, 2009). A potential modulatory role of peptidergic and steroid hormones on these events, in this case on a daily basis, seems likely given the above studies.
Together, the above studies show how both peptides and steroids have powerful modulatory influence over both vocal and auditory mechanisms in toadfishes. Goodson and Bass (2002) outlined major nodes of interface between vocal-auditory network and the neuroendocrine axis, largely focusing on the projections of POA- nonapeptide containing neurons (also see Goodson et al., 2003). The integration of more recent studies showing the central expression pattern of aromatase as well as estrogen and androgen receptors (Fig. 4; Table 1), now reveals patterns of both peptidergic and steroid receptor containing systems that are conserved across the major vertebrate lineages and, more specifically, have co-evolved with vocal-acoustic networks. Figure 4 shows steroid receptors, aromatase and AVT/IT in vocal and auditory circuits in the midshipman brain. With these maps in place, neurophysiological studies can now more fully explore how neuroendocrine signaling pathways modulate vocal-auditory neuronal integration that leads to changes in brain arousal levels related specifically to vocal-acoustic behaviors and more generally to seasonal patterns of reproduction.
Neural substrates for sexual arousal and reproductive behavior in elasmobranch fishes
Elasmobranch fishes are an important group for comparative and evolutionary studies, as they belong to the oldest lineage of extant jawed vertebrates (Chondrichthyes, see Fig. 1), and serve as an outgroup to bony vertebrates (actinopterygians and tetrapods) and may therefore provide further insights into the ancestral vertebrate condition in brain and behavior. In many respects, their reproductive physiology (Maruska and Gelsleichter, 2010) and, in some cases, chemical neuroanatomy (below) is more similar to mammals than to the more recently derived teleosts. Out of the almost 30,000 species of teleosts, only a very small percentage exhibit internal fertilization (i.e., the Poecilid family: guppies, swordtails and platyfish) (Nelson, 2006). In contrast, all chondrichthyans have intromittent copulatory organs (claspers) derived from the medial section of each pelvic fin used for internal fertilization (Pratt and Carrier, 2001). Out of the approximate 940 species of elasmobranchs (Nelson 2006), the modes of reproduction are quite diverse: the majority of taxa (70% of sharks and all rays) are viviparous (livebearers) often with long gestation periods where embryos receive placental or aplacental (e.g. yolk) nourishment, to oviparous (egg laying) skates (Rajiformes), and some small bodied sharks (Wourms and Demski, 1993). Documented reproductive behaviors in this group include complex interactions such as the establishment of dominance hierarchies, group mating, and even cooperative breeding (Pratt and Carrier, 2001, 2005).
Interesting comparative questions arise when one asks how might the mode of the act of reproduction (internal vs. external fertilization) and its evolutionary adaptations shape the neuroendocrine control of reproductive behavior across vertebrates. For instance, are mechanisms underlying sexual physiology and arousal in sharks and guppies more similar to each other and rodents than either are to other teleosts? While some studies in elasmobranchs correlate circulating steroid hormones with reproductive state, few have investigated hormonal and neural mechanisms that directly control reproductive behavior. We summarize below some research on stingrays that approaches this subject of sexual arousal from a behavioral, neuroecological perspective. For a recent comprehensive review on chondrichthyan hormones and reproduction, see Maruska and Gelsleichter (2010).
Unlike some male teleosts which employ dramatic color change, intricate swimming displays and acoustic signals to court females, the precopulatory behaviors of elasmobranch males include following, nosing, and aggressive tactile assaults on the female, in the form of biting, and oral grasping (reviewed in Pratt and Carrier, 2001), which was proposed as a releasing mechanism to facilitate female cooperation in mating (Springer, 1960). Elevated circulating androgens (namely testosterone and dihydrotestosterone) are thought to drive aggressive courtship behavior in some species (Rasmussen et al., 1999; Tricas et al., 2000). The resulting wounds that appear on the female skin are a reliable indicator of reproductive activity (Kajiura et al., 2000). Thus, for successful copulation, males must be highly motivated to seek out females (populations are often sexually segregated) and physically stimulate her to receptivity, generally by gripping the female pectoral fin in its jaws; receptive females may arch their back, flare or cup their pelvic fins to expose their cloaca to allow insertion of the clasper for fertilization (Pratt and Carrier, 2001, 2005). However, females are often not receptive to the male's advances and are instead highly motivated to avoid copulation by struggling free, shielding their cloaca against the substrate, or fleeing to areas not conducive to mating (shallow water) (Pratt and Carrier, 2001, 2005). This type of behavior suggests female mate choice and assessment via strong somatosensory stimuli (see below).
Some female elasmobranchs may have a more active role in precopulatory behavior, as documented in skates, where females display back arching and pectoral fin undulations to attract males (Luer and Gilbert, 1985). Based on the observation that males of some shark species follow females closely prior to copulation, pheromones are hypothesized to play a role in stimulating mating behavior (Demski, 1990; Heuter et al., 2004; Johnson and Nelson, 1978); however, at this time, there are no experimental data to support this. An elegant set of field experiments demonstrated that male round stingrays, Urolophus halleri, utilize electroreception to precisely orient and pinpoint buried females for mating during the reproductive season (Tricas et al., 1995). In an analogous example to the seasonal plasticity and hormone regulation of the female midshipman peripheral auditory system, the frequency tuning of electroreceptors in male Atlantic stingrays, Dasyatis sabina, changes with the natural cycling of circulating androgens (Tricas et al., 2000) and can be induced by experimentally increasing levels of serum dihydrotestosterone (Sisneros and Tricas, 2000). The significance of this change in frequency tuning is that this sensory modality becomes optimized to detect the natural bioelectric field of the female (Sisneros and Tricas, 2000; see Bass and Zakon, 2005 for recent review of comparable steroid-dependent, electrosensory-motor coupling mechanisms among teleosts). Unreceptive female round stingrays also use electroreception to find each other, as they are found buried in groups for refuge late in the mating season (Sisneros and Tricas, 2002; Tricas et al., 1995). These studies and those in midshipman exemplify striking examples of adaptive coordination of natural seasonal changes in reproductive steroid hormones that optimize sensory responsiveness to locate mates.
Atlantic stingrays are documented to have the longest continuous mating season (8–9 months) of any elasmobranch (Kajiura et al., 2000; Maruska et al., 1996; Snelson et al., 1988); ovulation and fertilization occur at least 7 months after mating begins. Mating for long periods without fertilization may function to stimulate ova development in this species (Maruska et al., 1996). The results parallel studies in mammals that indicate copulatory activity is necessary in some species for triggering ovulation or stimulating neuroendocrine regulation of reproductive behavior such as estrogen-dependent release of oxytocin in spinal cord (Komisaruk and Steinman, 1986; Sansone et al., 2002). Kajiura et al. (2000) proposed that stimulation of cutaneous receptors by male biting and grasping may trigger reproductive induction in females. Dermal thickness, which does not vary seasonally, is 50% greater in females, and may protect them from serious injury during the reproductive season (Kajiura et al., 2000). In addition, female stingrays have a lower density of free nerve endings within the dermis on the disc margin where the greatest proportion of bite wounds is located. These receptors, which may encode mechanical stimuli are well positioned to transmit information to assess mate quality (e.g., size and strength of bite) (Callahan et al., 1999). Whether this sexual dimorphism is dependent on sex differences in steroid hormone levels, or the sensitivity of these receptors peak with serum estrogen levels around the time of ovulation (Tricas et al., 2000), are interesting questions for future investigations that may reveal parallels with estrogen modulation of the somatosensory-induced lordosis behavior (Kow et al., 1979; Pfaff et al., 1977) or enlargement of mechanoreceptive sensory fields in female rodents (Bereiter and Barker, 1980).
Ascending projection systems are remarkably conserved between elasmobranchs and other vertebrates (Cruce et al., 1999; Ebbesson and Hodde, 1981; Sneddon, 2004), including abundance of the natural opioid, enkephalin (Snow et al., 1996; Stuesse et al., 1991a, b), and serotonergic (Carrera et al., 2008; Ritchie et al., 1983; Stuesse and Cruce, 1992; Stuesse et al., 1995), catecholaminergic (Meredith and Smeets, 1987; Stuesse and Cruce, 1992; Stuesse et al., 1990) and cholinergic neuronal groups (Anadon et al., 2000). As mentioned previously, elasmobranchs exhibit an ascending dopaminergic system consistent with tetrapods that includes a ventral tegmental (A9–A10) dopaminergic cell population (unlike teleosts) (Meredith and Smeets, 1987; reviewed in Smeets and Gonzalez, 2000). While elasmobranchs, teleosts and tetrapods contain catacholaminergic cell groups in the POA and anterior hypothalamus, elasmobranchs are again similar to mammals (and different from teleosts) with the absence of catacholaminergic neurons in the pretectum and presence in the habenula and cortical equivalent groups (reviewed in Smeets and Gonzalez, 2000). Furthermore, a comparison of cholinergic cell groups across vertebrates revealed patterns of highly conserved cell groups in the habenulointerpeduncular system, cranial and spinal motor nuclei, magnocellular preoptic area or supraoptic nucleus in all vertebrates, but distribution in the olfactory bulb/tubercle pallium/cortex and octaval nuclei are all found in an elasmobranch and mammals but not teleosts (reviewed in Rodriguez-Moldes et al., 2002b). Unfortunately, these comparisons are based on a single species of elasmobranch, Scyliorhynus canicula, so further comparisons with other species would be necessary to support this pattern. In addition, histamines are present in the brain of all vertebrate groups, and the spiny dogfish, Squalus acanthias, was found to have highest levels of histamines in the midbrain, similar to mammals (Almeida and Beaven, 1981).
Like other vertebrate groups, elasmobranchs contain GnRH neural populations in the POA and basal forebrain, the terminal nerve ganglia, and the midbrain tegmentum (Demski et al., 1997; Sherwood and Lovejoy, 1993). GnRH neurons in the POA of elasmobranchs have neither a direct neural connection, nor a blood portal system to reach the region of the pituitary where gonadotropins are released to induce steroidogenesis in the gonad (Dodd et al., 1983). Instead, there is anatomical and physiological evidence for GnRH release from the TN into the general circulation or the cerebrospinal fluid (Demski et al., 1997; Demski et al., 1987; Moeller and Meredith, 1998). The terminal nerve in elasmobranchs consists of several ganglia that are distinct from but proximal to the olfactory tract and bulb, have direct projections to the retina, and are a major source of GnRH throughout the forebrain. Thus, TN GnRH is anatomically positioned to modulate and/or integrate sensory cues with reproductive centers of the brain such as the POA and basal forebrain (Demski et al., 1997; Forlano et al., 2000). The most ancestral and evolutionarily conserved GnRH2 form found in the midbrain tegmentum in all jawed vertebrate groups comprises a much greater population of neurons in elasmobranchs compared to teleosts, although its function remains speculative (Forlano et al., 2000; Maruska and Gelsleichter, 2010). In the Atlantic stingray, projections from this large cell population innervate the hindbrain electrosensory nucleus and therefore may serve as a central modulator of sensory systems used for mate localization described above (Forlano et al., 2000). Descending projections to motor centers in the spinal cord may also function to control locomotor activity and/or clasper movements (Forlano et al., 2000; Wright and Demski, 1991). Mandado et al. (2001) demonstrated a pineal connection to the midbrain GnRH population which could synchronize GnRH activity with photoperiod and thus seasonal reproductive behaviors.
Neuroanatomical steroid receptor expression is limited to one of estrogen concentrating neurons in POA-hypothalamic regions (Jenkins et al., 1980). Like mammals, they express a single aromatase gene (teleosts have two) and adults have low relative brain aromatase activity compared to teleosts (Callard, 1984; Ijiri et al., 2000). Teleosts express brain aromatase mRNA and protein in glial cells, whereas mammalian brain aromatase is predominantly neuronal (reviewed in Diotel et al., 2010; Forlano et al., 2006), and therefore one might predict largely neuronal expression of aromatase in elasmobranchs. Elevated levels of brain aromatase in teleosts may be a derived character related to the unusually high plasticity and diversity of sexual behaviors and phenotypes in teleosts (see Forlano et al., 2006; Godwin, 2010) not evident in elasmobranchs, which appear more similar to mammals in mechanisms of sexual differentiation (Maruska and Gelsleichter, 2010).
Concluding comments
Interdisciplinary approaches combining behavioral endocrinology with molecular neuroanatomy (location and abundance of steroid synthesizing enzymes and receptors, peptidergic pathways) and neurophysiology have established both teleosts and elasmobranch fishes as powerful model systems to investigate cellular mechanisms of brain arousal, especially in the context of reproduction. The output of a highly conserved core-paracore region, inclusive of the POA-anterior hypothalamus and brainstem cell groups giving rise to ascending arousal pathways, is integrated with sensory (e.g., olfactory, auditory, electrogenic) and motor systems that directly mediate the effects of biotic (conspecific) and non-biotic (the physical environment) stimuli on ongoing behaviors. To date, these mechanisms have been most widely studied in teleost fishes, the largest group of living vertebrates that display a remarkable range of reproductive tactics and plasticity of sexual phenotypes (e.g., environmental sex determination, adult sex change, alternative male reproductive morphs). Elasmobranch fishes, by virtue of their remarkable similarities to mammals and differences to teleosts in reproductive physiology and behavior (e.g., somatosensory stimulation, internal fertilization), circulating steroids (e.g., dihydrotestosterone) and brain neurochemistry (e.g., low brain aromatase), represent a promising new model to further investigate the evolution of hormonal and neural control of sexual arousal in vertebrates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida AP, Beaven MA. Phylogeny of histamine in vertebrate brain. Brain Res. 1981;208:244–250. doi: 10.1016/0006-8993(81)90642-9. [DOI] [PubMed] [Google Scholar]
- Anadon R, Molist P, Rodriguez-Moldes I, Lopez JM, Quintela I, Cervino MC, Barja P, Gonzalez A. Distribution of choline acetyltransferase immunoreactivity in the brain of an elasmobranch, the lesser spotted dogfish (Scyliorhinus canicula) J. Comp. Neurol. 2000;420:139–170. doi: 10.1002/(sici)1096-9861(20000501)420:2<139::aid-cne1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Andreassen TK, Skjoedt K, Anglade I, Kah O, Korsgaard B. Molecular cloning, characterisation, and tissue distribution of oestrogen receptor alpha in eelpout (Zoarces viviparus) Gen. Comp. Endocrinol. 2003;132:356–368. doi: 10.1016/s0016-6480(03)00101-1. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog. Neurobiol. 2005;76:1–21. doi: 10.1016/j.pneurobio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Bass AH. Telencephalic efferents in channel catfish, Ictalurus punctatus: projections to the olfactory bulb and optic tectum. Brain Behav. Evol. 1981;19:1–16. doi: 10.1159/000121631. [DOI] [PubMed] [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J. Comp. Neurol. 2000;419:505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bass AH, Forlano PM. Neuroendocrine mechanisms of alternative reproductive tactics: the chemical language of social plasticity. In: Oliveira R, Taborsky M, Brockmann J, editors. Alternative Reproductive Tactics: An Integrative Approach. Cambridge University Press; Cambridge, UK: 2008. [Google Scholar]
- Bass AH, Grober MS. Social and neural modulation of sexual plasticity in teleost fish. Brain Behav. Evol. 2001;57:293–300. doi: 10.1159/000047247. [DOI] [PubMed] [Google Scholar]
- Bass AH, Grober MS. Reproductive Plasticity in Fish: Evolutionary Lability in the Patterning of Neuroendocrine and Behavioral Traits Underlying Divergent Sexual Phenotypes. In: Pfaff D, Arnold A, Etgen A, Rubin R, Fahrbach S, editors. Hormones, Brain, and Behavior. Elsevier; 2009. [Google Scholar]
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog. Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm. Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Segil N, Kelley DB. Androgen binding in the brain and electric organ of a mormyrid fish. J.Comp. Phys. A- Sen. Neural Behav. Phys. 1986;159:535–544. doi: 10.1007/BF00604173. [DOI] [PubMed] [Google Scholar]
- Bass AH, Zakon HH. Sonic and electric fish: At the crossroads of neuroethology and behavioral neuroendocrinology. Horm. Behav. 2005;48:360–372. doi: 10.1016/j.yhbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Becerra M, Manso MJ, Rodriguez-Moldes I, Anadon R. Primary olfactory fibres project to the ventral telencephalon and preoptic region in trout (Salmo trutta): a developmental immunocytochemical study. J. Comp. Neurol. 1994;342:131–143. doi: 10.1002/cne.903420112. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Rink E, Bally-Cuif L. Cloning of two tryptophan hydroxylase genes expressed in the diencephalon of the developing zebrafish brain. Gene Expr. Pattern. 2002;2:251–256. doi: 10.1016/s1567-133x(02)00056-x. [DOI] [PubMed] [Google Scholar]
- Bentley PJ. Comparative Vertebrate Endocrinology. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Bereiter DA, Barker DJ. Hormone-induced enlargement of receptive fields in trigeminal mechanoreceptive neurons. I. Time course, hormone, sex and modality specificity. Brain Res. 1980;184:395–410. doi: 10.1016/0006-8993(80)90808-2. [DOI] [PubMed] [Google Scholar]
- Black MP, Balthazart J, Baillien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, Lythrypnus dalli. Proc. Royal Soc. Lond. B-Biol. Sci. 2005;272:2435–2440. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg B. Androgens in teleost fishes. Comp. Biochem. Physiol. A. 1994;109C:219–245. [Google Scholar]
- Braford MR., Jr. Comparative aspects of forebrain organization in the ray-finned fishes: touchstones or not? Brain Behav. Evol. 1995;46:259–274. doi: 10.1159/000113278. [DOI] [PubMed] [Google Scholar]
- Braford MR, Jr., Northcutt RG. Organization of the diencephalon and pretectum of the ray-finned fishes. In: Davis RE, Northcutt RG, editors. Fish neurobiology. University of Michigan Press; Ann Arbor: 1983. pp. 117–164. [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic-signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Bruce LL, Braford MR. Evolution of the limbic system. In: Squire LR, editor. Encyclopedia of neuroscience. Academic Press; Oxford: 2009. pp. 43–55. [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3:e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Hodos W. Comparative vertebrate neuroanatomy. John Wiley & Sons, Inc.; New York: 1996. [Google Scholar]
- Callahan M, Forlano PM, Tricas TC. Cutaneous receptor density in the Atlantic stingray: sexual dimorphisms and functional significance during courtship and mating. Soc. Neurosci. Abstr. 1999;25:1363. [Google Scholar]
- Callard GV. Aromatization in brain and pituitary: an evolutionary prespective. In: Celotti F NF, Martini L, editors. Metabolism of Hormonal Steroids in the Neuroendocrine Structures. Raven Press; New York: 1984. pp. 79–102. [Google Scholar]
- Cardwell JR, Stacey NE, Tan ESP, McAdam DSO, Lang SC. Androgen increases olfactory receptor response to a vertebrate sex pheromone. J. Comp. Physiol. A. 1995;176:55–61. [Google Scholar]
- Carneiro LA, Oliveira RF, Canario AVM, Grober MS. The effect of arginine vasotocin on courtship behavior in a bleniid fish with alternative reproductive tactics. Fish physiol. biochem. 2003;28:241–243. [Google Scholar]
- Carrera I, Molist P, Anadon R, Rodriguez-Moldes I. Development of the serotoninergic system in the central nervous system of a shark, the lesser spotted dogfish Scyliorhinus canicula. J. Comp. Neurol. 2008;511:804–831. doi: 10.1002/cne.21857. [DOI] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Ringholm A, Schioth HB, Peter RE. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology. 2003;144:2336–2349. doi: 10.1210/en.2002-0213. [DOI] [PubMed] [Google Scholar]
- Clemente D, Porteros A, Weruaga E, Alonso JR, Arenzana FJ, Aijon J, Arevalo R. Cholinergic elements in the zebrafish central nervous system: Histochemical and immunohistochemical analysis. J. Comp. Neurol. 2004;474:75–107. doi: 10.1002/cne.20111. [DOI] [PubMed] [Google Scholar]
- Cruce WL, Stuesse SL, Northcutt RG. Brainstem neurons with descending projections to the spinal cord of two elasmobranch fishes: thornback guitarfish, Platyrhinoidis triseriata, and horn shark, Heterodontus francisci. J. Comp. Neurol. 1999;403:534–560. doi: 10.1002/(sici)1096-9861(19990125)403:4<534::aid-cne8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc. Natl. Acad. Sci. U S A. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Morrell JI, Pfaff DW. Autoradiographic localization of sex steroid-concentrating cells in the brain of the teleost Macropodus opercularis (Osteichthyes: Belontiidae) Gen. Comp. Endocrinol. 1977;33:496–505. doi: 10.1016/0016-6480(77)90108-3. [DOI] [PubMed] [Google Scholar]
- Demski LS. Neuroanatomical substrates of reproductive behavior in male sunfish (Genus Lepomis) Ann. Biol. Anim. Bioch. Biophys. 1978;18:831–836. [Google Scholar]
- Demski LS. Neuroendocrine mechanism controlling the sexual development and behavior of sharks and rays. J. Aquacult. Aqua. Sci. 1990;5:53–67. [Google Scholar]
- Demski LS, Beaver JA, Sudberry JJ, Custis JR. GnRH systems in cartilaginous fishes. In: Parhar IS, Sakuma Y, editors. GnRH neurons: gene to behavior. Brain Shuppan Publishing Co.; Tokyo: 1997. pp. 123–143. [Google Scholar]
- Demski LS, Fields RD, Bullock TH, Schriebman MP, Margolis-Nunno H. The terminal nerve of sharks and rays: EM, immunocytochemical and electrophysiolgical studies. Ann. NY. Acad. Sci. 1987;519:15–32. [Google Scholar]
- Demski LS, Knigge KM. The telencephalon and hypothalamus of the bluegill (Lepomis macrochirus): evoked feeding, aggressive and reproductive behavior with representative frontal sections. J. Comp. Neurol. 1971;143:1–16. doi: 10.1002/cne.901430102. [DOI] [PubMed] [Google Scholar]
- Demski LS, Northcutt RG. The terminal nerve: a new chemosensory system in vertebrates? Science. 1983;220:435–437. doi: 10.1126/science.6836287. [DOI] [PubMed] [Google Scholar]
- Demski LS, Sloan HE. A direct magnocellular-preopticospinal pathway in goldfish: implications for control of sex behavior. Neurosci. Lett. 1985;55:283–288. doi: 10.1016/0304-3940(85)90449-5. [DOI] [PubMed] [Google Scholar]
- Dewan AK, Maruska KP, Tricas TC. Arginine vasotocin neuronal phenotypes among congeneric territorial and shoaling reef butterflyfishes: species, sex and reproductive season comparisons. J. Neuroendocrinol. 2008;20:1382–1394. doi: 10.1111/j.1365-2826.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- Diotel N, Le Page Y, Mouriec K, Tong SK, Pellegrini E, Vaillant C, Anglade I, Brion F, Pakdel F, Chung BC, Kah O. Aromatase in the brain of teleost fish: expression, regulation and putative functions. Front. Neuroendocrinol. 2010;31:172–192. doi: 10.1016/j.yfrne.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Dodd JM, Dodd MHI, Duggan RT. Control of reproduction in elasmobranch fishes. In: Rankin JC, Pitcher TJ, Duggan RT, editors. Control processes in fish physiology. Croon Helm; London: 1983. pp. 221–287. [Google Scholar]
- Dufour S, Weltzien FA, Sebert ME, Le Belle N, Vidal B, Vernier P, Pasqualini C. Dopaminergic inhibition of reproduction in teleost fishes: ecophysiological and evolutionary implications. Ann. N Y Acad. Sci. 2005;1040:9–21. doi: 10.1196/annals.1327.002. [DOI] [PubMed] [Google Scholar]
- Dulka JG. Sex pheromone systems in goldfish: comparisons to vomeronasal systems in tetrapods. Brain Behav. Evol. 1993;42:265–280. doi: 10.1159/000114166. [DOI] [PubMed] [Google Scholar]
- Dulka JG, Stacey NE, Sorensen PW, Van Der Kraak GJ. A steroid sex pheromone synchronizes male–female spawning readiness in goldfish. Nature. 1987;325:251–253. [Google Scholar]
- Ebbesson SO, Hodde KC. Ascending spinal systems in the nurse shark, Ginglymostoma cirratum. Cell Tiss. Res. 1981;216:313–331. doi: 10.1007/BF00233622. [DOI] [PubMed] [Google Scholar]
- Filippi A, Mahler J, Schweitzer J, Driever W. Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J. Comp. Neurol. 2010;518:423–438. doi: 10.1002/cne.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine ML, Chen FA, Keefer DA. Autoradiographic localization of dihydrotestosterone and testosterone concentrating neurons in the brain of the oyster toadfish. Brain Res. 1996;709:65–80. doi: 10.1016/0006-8993(95)01275-3. [DOI] [PubMed] [Google Scholar]
- Fisher AE. Maternal and sexual behavior induced by intracranial chemical stimulation. Science. 1956;124:228–229. doi: 10.1126/science.124.3214.228-a. [DOI] [PubMed] [Google Scholar]
- Foran CM, Bass AH. Preoptic GnRH and AVT: axes for sexual plasticity in teleost fish. Gen. Comp. Endocrinol. 1999;116:141–152. doi: 10.1006/gcen.1999.7357. [DOI] [PubMed] [Google Scholar]
- Foran CM, Myers DA, Bass AH. Modification of gonadotropin releasing hormone (GnRH) mRNA expression in the retinal-recipient thalamus. Gen. Comp. Endocrinol. 1997;106:251–264. doi: 10.1006/gcen.1997.6875. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Steroid regulation of brain aromatase expression in glia: Female preoptic and vocal motor nuclei. J. Neurobiol. 2005;65:50–58. doi: 10.1002/neu.20178. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Cone RD. Conserved neurochemical pathways involved in hypothalamic control of energy homeostasis. J. Comp. Neurol. 2007;505:235–248. doi: 10.1002/cne.21447. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neurol. 2005;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J. Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Marchaterre M, Deitcher DL, Bass AH. Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish. J. Comp. Neurol. 2010;518:493–512. doi: 10.1002/cne.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Maruska KP, Sower SA, King JA, Tricas TC. Differential distribution of gonadotropin-releasing hormone-immunoreactive neurons in the stingray brain: functional and evolutionary considerations. Gen. Comp. Endocrinol. 2000;118:226–248. doi: 10.1006/gcen.2000.7467. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Remage-Healey L, Sisneros JA, Bass AH. Steroid-induced plasticity in the auditory and vocal motor system: Recent studies in a teleost fish. In: Canonaco M, Facciolo RM, editors. Evolutionary Molecular Strategies and Plasticity. Research Signpost; Kerala, India: 2007. [Google Scholar]
- Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front. Neuroendocrinol. 2006;27:247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Gilchriest BJ, Tipping DR, Hake L, Levy A, Baker BI. The effects of acute and chronic stresses on vasotocin gene transcripts in the brain of the rainbow trout (Oncorhynchus mykiss) J. Neuroendocrinol. 2000;12:795–801. doi: 10.1046/j.1365-2826.2000.00522.x. [DOI] [PubMed] [Google Scholar]
- Godwin J. Neuroendocrinology of sexual plasticity in teleost fishes. Front. Neuroendocrinol. 2010;31:203–216. doi: 10.1016/j.yfrne.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J, Crews D, Warner RR. Behavioural sex change in the absence of gonads in a coral reef fish. Proc. Royal Soc. Lond. B-Biol. Sci. 1996;263:1683–1688. doi: 10.1098/rspb.1996.0246. [DOI] [PubMed] [Google Scholar]
- Goncalves D, Saraiva J, Teles M, Teodosio R, Canario AV, Oliveira RF. Brain aromatase mRNA expression in two populations of the peacock blenny Salaria pavo with divergent mating systems. Horm. Behav. 2010;57:155–161. doi: 10.1016/j.yhbeh.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Goncalves D, Teles M, Alpedrinha J, Oliveira RF. Brain and gonadal aromatase activity and steroid hormone levels in female and polymorphic males of the peacock blenny Salaria pavo. Horm. Behav. 2008;54:717–725. doi: 10.1016/j.yhbeh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000a;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J.Comp. Neurol. 2000b;422:363–379. doi: 10.1002/1096-9861(20000703)422:3<363::aid-cne4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res. Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. plus 236:291–294. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J. Comp. Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: Relation to vasotocin and vocal-acoustic circuitry. J. Comp. Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Watson W, Halpern ME. Androgen receptor gene expression in the developing and adult zebrafish brain. Dev. Dyn. 2008;237:2987–2995. doi: 10.1002/dvdy.21700. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Fernald RD. Social regulation of the electrical properties of gonadotropin-releasing hormone neurons in a cichlid fish (Astatotilapia burtoni) Biol. Reprod. 2004;71:909–918. doi: 10.1095/biolreprod.104.030072. [DOI] [PubMed] [Google Scholar]
- Gregory WA, Tweedle CD. Horseradish peroxidase evidence for a spinal projection from the preoptic area of the goldfish, a light and electron microscopic study. Brain Res. 1985;341:82–91. doi: 10.1016/0006-8993(85)91475-1. [DOI] [PubMed] [Google Scholar]
- Grens KE, Greenwood AK, Fernald RD. Two visual processing pathways are targeted by gonadotropin-releasing hormone in the retina. Brain Behav. Evol. 2005;66:1–9. doi: 10.1159/000085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren SL, Linderoth M, Olsen KH. Inhibition of cytochrome p450 brain aromatase reduces two male specific sexual behaviours in the male Endler guppy (Poecilia reticulata) Gen. Comp. Endocrinol. 2006;147:323–328. doi: 10.1016/j.ygcen.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hallowitz RA, Woodward DJ, Demski LS. Forebrain activation of single units in preoptic area of sunfish. Comp. Biochem. Physiol. A Comp. Physiol. 1971;40:733–741. doi: 10.1016/0300-9629(71)90258-1. [DOI] [PubMed] [Google Scholar]
- Hanna RN, Daly SC, Pang Y, Anglade I, Kah O, Thomas P, Zhu Y. Characterization and expression of the nuclear progestin receptor in zebrafish gonads and brain. Biol. Reprod. 2010;82:112–122. doi: 10.1095/biolreprod.109.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbott LK, Burmeister SS, White RB, Vagell M, Fernald RD. Androgen receptors in a cichlid fish, Astatotilapia burtoni: structure, localization, and expression levels. J. Comp. Neurol. 2007;504:57–73. doi: 10.1002/cne.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins MB, Godwin J, Crews D, Thomas P. The distributions of the duplicate oestrogen receptors ER-beta a and ER-beta b in the forebrain of the Atlantic croaker (Micropogonias undulatus): evidence for subfunctionalization after gene duplication. Proc. Biol. Sci. 2005;272:633–641. doi: 10.1098/rspb.2004.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuter RE, Mann DA, Maruska KP, Sisneros JA, Demski LS. Sensory biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. CRC Press; Boca Raton: 2004. pp. 325–368. [Google Scholar]
- Hofmann HA. Gonadotropin-releasing hormone signaling in behavioral plasticity. Curr. Opin. Neurobiol. 2006;16:343–350. doi: 10.1016/j.conb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Huesa G, van den Pol AN, Finger TE. Differential distribution of hypocretin (orexin) and melanin-concentrating hormone in the goldfish brain. J. Comp. Neurol. 2005;488:476–491. doi: 10.1002/cne.20610. [DOI] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol. Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Ijiri S, Berard C, Trant JM. Characterization of gonadal and extra-gonadal forms of the cDNA encoding the Atlantic stingray (Dasyatis sabina) cytochrome P450 aromatase (CYP19) Mol. Cell. Endocrinol. 2000;164:169–181. doi: 10.1016/s0303-7207(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Jenkins N, Joss JP, Dodd JM. Biochemical and autoradiographic studies on the oestradiol-concentrating cells in the diencephalon and pituitary gland of the female dogfish (Scyliorhinus canicula L.) Gen. Comp. Endocrinol. 1980;40:211–219. doi: 10.1016/0016-6480(80)90124-0. [DOI] [PubMed] [Google Scholar]
- Johnson RH, Nelson DR. Copulation and possible olfaction-mediated pair formation in two species of carcharhinid sharks. Copeia. 1978;3:539–542. [Google Scholar]
- Kah O, Chambolle P. Serotonin in the brain of the goldfish, Carassius auratus - an immunocytochemical study. Cell Tiss. Res. 1983;234:319–333. doi: 10.1007/BF00213771. [DOI] [PubMed] [Google Scholar]
- Kah O, Lethimonier C, Somoza G, Guilgur LG, Vaillant C, Lareyre JJ. GnRH and GnRH receptors in metazoa: a historical, comparative, and evolutive perspective. Gen. Comp. Endocrinol. 2007;153:346–364. doi: 10.1016/j.ygcen.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Kajiura SM, Sebastian AP, Tricas TC. Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina. Environ. Biol. Fish. 2000;58:23–31. [Google Scholar]
- Kapp B, Cain M. The neural basis of arousal. In: Smelser N, Baltes P, editors. The international encylopedia of social and behavioral sciences. Elsevier Science Ltd; Oxford: 2001. pp. 1463–1466. [Google Scholar]
- Kapsimali M, Bourrat F, Vernier P. Distribution of the orphan nuclear receptor Nurr1 in medaka (Oryzias latipes): cues to the definition of homologous cell groups in the vertebrate brain. J. Comp. Neurol. 2001;431:276–292. doi: 10.1002/1096-9861(20010312)431:3<276::aid-cne1070>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Nystedt JM, Ostergard M, Peitsaro N, Panula P. The orexin/hypocretin system in zebrafish is connected to the aminergic and cholinergic systems. J. Neurosci. 2004;24:2678–2689. doi: 10.1523/JNEUROSCI.4908-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslin J, Panula P. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio) J. Comp. Neurol. 2001;440:342–377. doi: 10.1002/cne.1390. [DOI] [PubMed] [Google Scholar]
- Kim YS, Stumpf WE, Sar M. Topography of estrogen target cells in forebrain of goldfish, Carassius auratus. J.Comp. Neurol. 1978;182:611–620. doi: 10.1002/cne.901820404. [DOI] [PubMed] [Google Scholar]
- Kim YS, Stumpf WE, Sar M. Topographical distribution of estrogen target cells in the forebrain of platyfish, Xiphophorus maculatus, studied by autoradiography. Brain Res. 1979;170:43–59. doi: 10.1016/0006-8993(79)90939-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Sorensen PW, Stacey NE. Hormonal and pheromonal control of spawning behavior in goldfish. Fish Physiol. Biochem. 2002;26:71–84. [Google Scholar]
- Kobayashi M, Stacey N. Prostaglandin-induced female spawning behavior in goldfish (Carassius auratus) appears independent of ovarian influence. Horm. Behav. 1993;27:38–55. doi: 10.1006/hbeh.1993.1004. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Steinman JL. Genital stimulation as a trigger for neuroendocrine and behavioral control of reproduction. Ann. N Y Acad. Sci. 1986;474:64–75. doi: 10.1111/j.1749-6632.1986.tb27999.x. [DOI] [PubMed] [Google Scholar]
- Kow LM, Montgomery MO, Pfaff DW. Triggering of lordosis reflex in female rats with somatosensory stimulation: quantitative determination of stimulus parameters. J. Neurophysiol. 1979;42:195–202. doi: 10.1152/jn.1979.42.1.195. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Satou M, Oka Y, Ueda K. Involvement of the telencephalic hemispheres and the preoptic area in sexual behavior of the male goldfish, Carassius auratus: a brain-lesion study. Behav. Neural Biol. 1984;40:70–86. doi: 10.1016/s0163-1047(84)90182-1. [DOI] [PubMed] [Google Scholar]
- Kyle AL, Peter RE. Effects of forebrain lesions on spawning behaviour in the male goldfish. Physiol. Behav. 1982;28:1103–1109. doi: 10.1016/0031-9384(82)90183-4. [DOI] [PubMed] [Google Scholar]
- Kyle AL, Stacey NE, Peter RE. Ventral telencephalic lesions: effects on bisexual behavior, activity, and olfaction in the male goldfish. Behav. Neural Biol. 1982;36:229–241. doi: 10.1016/s0163-1047(82)90855-x. [DOI] [PubMed] [Google Scholar]
- Ladich F, Collin S, Moller P, Kapoor BG. Communication in Fishes. Science Publishers; Enfield: 2006. [Google Scholar]
- Larson ET, Norris DO, Gordon Grau E, Summers CH. Monoamines stimulate sex reversal in the saddleback wrasse. Gen. Comp. Endocrinol. 2003a;130:289–298. doi: 10.1016/s0016-6480(02)00622-6. [DOI] [PubMed] [Google Scholar]
- Larson ET, Norris DO, Summers CH. Monoaminergic changes associated with socially induced sex reversal in the saddleback wrasse. Neurosci. 2003b;119:251–263. doi: 10.1016/s0306-4522(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Lee AW, Pfaff DW. Hormone effects on specific and global brain functions. J. Physiol. Sci. 2008;58:213–220. doi: 10.2170/physiolsci.RV007008. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL. The hedonic effects of amphetamine and pentobarbital in goldfish. Pharmacol. Biochem. Behav. 1989;32:355–356. doi: 10.1016/0091-3057(89)90254-2. [DOI] [PubMed] [Google Scholar]
- Levine RL, Dethier S. The connections between the olfactory bulb and the brain in the goldfish. J. Comp. Neurol. 1985;237:427–444. doi: 10.1002/cne.902370402. [DOI] [PubMed] [Google Scholar]
- Liley NR. The effects of estrogens and other steroids on the sexual behavior of the female guppy Poecilia reticulata. Gen. Comp. Endocrinol. 1972;(Suppl 3):542–552. [Google Scholar]
- Lord LD, Bond J, Thompson RR. Rapid steroid influences on visually guided sexual behavior in male goldfish. Horm. Behav. 2009;56:519–526. doi: 10.1016/j.yhbeh.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luer CA, Gilbert PW. mating behavior, egg deposition, incubation period, and hatching in the clearnose skate, Raja eglanteria. Environ. Biol. Fish. 1985;13:161–171. [Google Scholar]
- Luo GR, Chen Y, Li XP, Liu TX, Le WD. Nr4a2 is essential for the differentiation of dopaminergic neurons during zebrafish embryogenesis. Mol. Cell. Neurosci. 2008;39:202–210. doi: 10.1016/j.mcn.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Ma L-H, Gilland E, Bass AH, Baker R. Ancestry of innervation to pectoral fin and forelimb. Nat. Comm. 2010;1:1–8. doi: 10.1038/ncomms1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PM. Catecholaminergic systems in the zebrafish. IV. Organization and projection pattern of dopaminergic neurons in the diencephalon. J. Comp. Neurol. 2003;460:13–37. doi: 10.1002/cne.10544. [DOI] [PubMed] [Google Scholar]
- Mandado M, Molist P, Anadon R, Yanez J. A DiI-tracing study of the neural connections of the pineal organ in two elasmobranchs (Scyliorhinus canicula and Raja montagui) suggests a pineal projection to the midbrain GnRH-immunoreactive nucleus. Cell Tiss. Res. 2001;303:391–401. doi: 10.1007/s004410000328. [DOI] [PubMed] [Google Scholar]
- Margolis-Kazan H, Halpern-Sebold LR, Schreibman MP. Immunocytochemical localization of serotonin in the brain and pituitary gland of the platyfish, Xiphophorus maculatus. Cell Tiss. Res. 1985;240:311–314. doi: 10.1007/BF00222340. [DOI] [PubMed] [Google Scholar]
- Maruska KP. Sex and temporal variations of the vasotocin neuronal system in the damselfish brain. Gen. Comp. Endocrinol. 2009;160:194–204. doi: 10.1016/j.ygcen.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Boyle KS, Dewan LR, Tricas TC. Sound production and spectral hearing sensitivity in the Hawaiian sergeant damselfish, Abudefduf abdominalis. J. Exp. Biol. 2007;210:3990–4004. doi: 10.1242/jeb.004390. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Cowie EG, Tricas TC. Periodic gonadal activity and protracted mating in elasmobranch fishes. J. Exp. Zool. 1996;276:219–232. [Google Scholar]
- Maruska KP, Fernald RD. Reproductive status regulates expression of sex steroid and GnRH receptors in the olfactory bulb. Behav. Brain Res. 2010;213:208–217. doi: 10.1016/j.bbr.2010.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Gelsleichter J. Hormones and Reproduction in Chondrichthyan Fishes. In: Norris DO, Lopez K, editors. Hormones and Reproduction in Vertebrates. Elsevier; 2010. [Google Scholar]
- Maruska KP, Tricas TC. Gonadotropin-releasing hormone and receptor distributions in the visual processing regions of four coral reef fishes. Brain Behav. Evol. 2007;70:40–56. doi: 10.1159/000101068. [DOI] [PubMed] [Google Scholar]
- McKibben JR, Bass AH. Behavioral assessment of acoustic parameters relevant to signal recognition and preference in a vocal fish. J. Acoustic Soc. Am. 1998;104:3520–3533. doi: 10.1121/1.423938. [DOI] [PubMed] [Google Scholar]
- McKibben JR, Bass AH. Effects of temporal envelope modulation on acoustic signal recognition in a vocal fish, the plainfin midshipman. J. Acoustic Soc. Am. 2001;109:2934–2943. doi: 10.1121/1.1373441. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J. Comp. Neurol. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- Meek J, Nieuwenhuys R. Holosteans and teleosts. In: Nieuwenhuys R, Donkelaar HJ, Hans J, Nicholson C, editors. The central nervous system of vertebrates. Springer-Verlag; Berlin: 1998. [Google Scholar]
- Mennigen JA, Martyniuk CJ, Crump K, Xiong H, Zhao E, Popesku J, Anisman H, Cossins AR, Xia X, Trudeau VL. Effects of fluoxetine on the reproductive axis of female goldfish (Carassius auratus) Physiol. Genom. 2008;35:273–282. doi: 10.1152/physiolgenomics.90263.2008. [DOI] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Flouriot G, Pakdel F, Kah O. Tissue-specific expression of two structurally different estrogen receptor alpha isoforms along the female reproductive axis of an oviparous species, the rainbow trout. Biol. Reprod. 2001;65:1548–1557. doi: 10.1095/biolreprod65.5.1548. [DOI] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: Comparison with estrogen receptor alpha. J. Comp. Neurol. 2003;462:180–193. doi: 10.1002/cne.10726. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Anglade I, Blaise O, Laudet V, Kah O, Pakdel F. Molecular characterization of three estrogen receptor forms in zebrafish: Binding characteristics, transactivation properties, and tissue distributions. Biol. Reprod. 2002;66:1881–1892. doi: 10.1095/biolreprod66.6.1881. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, Kah O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol. 2005;485:304–320. doi: 10.1002/cne.20497. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Smeets WJ. Immunocytochemical analysis of the dopamine system in the forebrain and midbrain of Raja radiata: evidence for a substantia nigra and ventral tegmental area in cartilaginous fish. J. Comp. Neurol. 1987;265:530–548. doi: 10.1002/cne.902650407. [DOI] [PubMed] [Google Scholar]
- Moeller JF, Meredith M. Increase in gonadotropin-releasing hormone (GnRH) levels in CSF after stimulation of the nervus terminalis in Atlantic stingray, Dasyatis sabina. Brain Res. 1998;806:104–107. doi: 10.1016/s0006-8993(98)00683-0. [DOI] [PubMed] [Google Scholar]
- Mong J, Easton A, Kow LM, Pfaff D. Neural, hormonal and genetic mechanisms for the activation of brain and behavior. Eur. J. Pharmacol. 2003;480:229–231. doi: 10.1016/j.ejphar.2003.08.109. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Kelley DB, Pfaff DW. Sex steroid binding in the brains of vertebrates. In: Knigge KM, Scott DE, Kobayashi H, Miura-shi S, editors. Brain-Endocrine Interaction II. The Ventricular System. S. Karger; Basel: 1975. pp. 230–256. [Google Scholar]
- Mueller T, Wullimann MF. An evolutionary interpretation of teleostean forebrain anatomy. Brain Behav. Evol. 2009;74:30–42. doi: 10.1159/000229011. [DOI] [PubMed] [Google Scholar]
- Munakata A, Kobayashi M. Endocrine control of sexual behavior in teleost fish. Gen. Comp. Endocrinol. 2010;165:456–468. doi: 10.1016/j.ygcen.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Munchrath LA, Hofmann HA. Distribution of sex steroid hormone receptors in the brain of an African cichlid fish, Astatotilapia burtoni. J. Comp. Neurol. 2010;518:3302–3326. doi: 10.1002/cne.22401. [DOI] [PubMed] [Google Scholar]
- Muriach B, Carrillo M, Zanuy S, Cerda-Reverter JM. Distribution of estrogen receptor 2 mRNAs (Esr2a and Esr2b) in the brain and pituitary of the sea bass (Dicentrarchus labrax) Brain Res. 2008a;1210:126–141. doi: 10.1016/j.brainres.2008.02.053. [DOI] [PubMed] [Google Scholar]
- Muriach B, Cerda-Reverter JM, Gomez A, Zanuy S, Carrillo M. Molecular characterization and central distribution of the estradiol receptor alpha (ERalpha) in the sea bass (Dicentrarchus labrax) J. Chem. Neuroanat. 2008b;35:33–48. doi: 10.1016/j.jchemneu.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Nelson JS. Fishes of the World. Wiley; New York: 2006. [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N Y Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Veening JG, van Domburg P. Core and paracores; some new chemoarchitectural entities in the mammalian neuraxis. Acta. Morphol. Neerl. Scand. 1988;26:131–163. [PubMed] [Google Scholar]
- Northcutt RG. The forebrain of gnathostomes: in search of a morphotype. Brain Behav. Evol. 1995;46:275–318. doi: 10.1159/000113279. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J. Comp. Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Braford MR., Jr. New observations on the organization and evolution of the telencephalon of of actinopterygian fishes. In: Ebbesson SOE, editor. Comparative Neurology of the Telencephalon. Plenum; New York: 1980. pp. 41–98. [Google Scholar]
- Oka Y, Matsushima T. Gonadotropin-releasing hormone (GnRH)-immunoreactive terminal nerve cells have intrinsic rhythmicity and project widely in the brain. J. Neurosci. 1993;13:2161–2176. doi: 10.1523/JNEUROSCI.13-05-02161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, Gonçalves DM. Hormones and social behaviour of teleost fish. In: Magnhagen C, Braithwaite VA, Forsgren E, Kapoor BG, editors. Fish Behaviour. Science Publishers Inc.; Enfield: 2008. pp. 61–150. [Google Scholar]
- Oliveira RF, Ros AF, Goncalves DM. Intra-sexual variation in male reproduction in teleost fish: a comparative approach. Horm. Behav. 2005;48:430–439. doi: 10.1016/j.yhbeh.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Perez SE, Yanez J, Marin O, Anadon R, Gonzalez A, Rodriguez-Moldes I. Distribution of choline acetyltransferase (ChAT) immunoreactivity in the brain of the adult trout and tract-tracing observations on the connections of the nuclei of the isthmus. J. Comp. Neurol. 2000;428:450–474. doi: 10.1002/1096-9861(20001218)428:3<450::aid-cne5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Peter RE, Fryer JN. Endocrine functions of the hypothalamus of actinopterygians. In: Davis RE, Northcutt RG, editors. Fish Neurobiology. University of Michigan Press; Ann Arbor: 1983. pp. 165–201. [Google Scholar]
- Pfaff D, Montgomery M, Lewis C. Somatosensory determinants of lordosis in female rats: behavioral definition of the estrogen effect. J. Comp. Physiol. Psychol. 1977;91:134–145. doi: 10.1037/h0077305. [DOI] [PubMed] [Google Scholar]
- Portavella M, Torres B, Salas C. Avoidance response in goldfish: emotional and temporal involvement of medial and lateral telencephalic pallium. J. Neurosci. 2004;24:2335–2342. doi: 10.1523/JNEUROSCI.4930-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasada Rao PD, Finger TE. Asymmetry of the olfactory system in the brain of the winter flounder, Pseudopleuronectes americanus. J. Comp. Neurol. 1984;225:492–510. doi: 10.1002/cne.902250403. [DOI] [PubMed] [Google Scholar]
- Prasada Rao PD, Jadhao AG, Sharma SC. Descending projection neurons to the spinal cord of the goldfish, Carassius auratus. J. Comp. Neurol. 1987;265:96–108. doi: 10.1002/cne.902650107. [DOI] [PubMed] [Google Scholar]
- Pratt HL, Carrier JC. A review of elasmobranch reproductive behavior with a case study on the nurse shark, Ginglymostoma cirratum. Environ. Biol. Fish. 2001;60:157–188. [Google Scholar]
- Pratt HL, Carrier JC. Elasmobranch courtship and mating behavior. In: Hamlett WC, editor. Reproductive biology and phylogeny of chondrichthyes. Sharks, batoids and chimaeras. Science Publishers, Inc.; Enfield: 2005. pp. 129–169. [Google Scholar]
- Puelles L. Brain segmentation and forebrain development in amniotes. Brain Res. Bull. 2001;55:695–710. doi: 10.1016/s0361-9230(01)00588-3. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Wayne NL. Social cues from conspecifics alter electrical activity of gonadotropin-releasing hormone neurons in the terminal nerve via visual signals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R135–141. doi: 10.1152/ajpregu.00143.2009. [DOI] [PubMed] [Google Scholar]
- Rasmussen LE, Hess DL, Luer CA. Alterations in serum steroid concentrations in the clearnose skate, Raja eglanteria: correlations with season and reproductive status. J. Exp. Zool. 1999;284:575–585. doi: 10.1002/(sici)1097-010x(19991001)284:5<575::aid-jez13>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Horm. Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. From social behavior to neural circuitry: Steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm. Behav. 2006;50:432–441. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. Connections of the ventral telencephalon and tyrosine hydroxylase distribution in the zebrafish brain (Danio rerio) lead to identification of an ascending dopaminergic system in a teleost. Brain Res. Bull. 2002;57:385–387. doi: 10.1016/s0361-9230(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Ritchie TC, Livingston CA, Hughes MG, McAdoo DJ, Leonard RB. The distribution of serotonin in the CNS of an elasmobranch fish: immunocytochemical and biochemical studies in the Atlantic stingray, Dasyatis sabina. J Comp Neurol. 1983;221:429–443. doi: 10.1002/cne.902210406. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moldes I, Molist P, Adrio F, Pombal MA, Yanez SE, Mandado M, Marin O, Lopez JM, Gonzalez A, Anadon R. Organization of cholinergic systems in the brain of different fish groups: a comparative analysis. Brain Res. Bull. 2002a;57:331–334. doi: 10.1016/s0361-9230(01)00700-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moldes I, Molist P, Adrio F, Pombal MA, Yanez SE, Mandado M, Marin O, Lopez JM, Gonzalez A, Anadon R. Organization of cholinergic systems in the brain of different fish groups: a comparative analysis. Brain Res Bull. 2002b;57:331–334. doi: 10.1016/s0361-9230(01)00700-6. [DOI] [PubMed] [Google Scholar]
- Rubow TK, Bass AH. Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. J. Exp. Biol. 2009;212:3252–3262. doi: 10.1242/jeb.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito D, Komatsuda M, Urano A. Functional organization of preoptic vasotocin and isotocin neurons in the brain of rainbow trout: central and neurohypophysial projections of single neurons. Neurosci. 2004;124:973–984. doi: 10.1016/j.neuroscience.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Salek SJ, Sullivan CV, Godwin J. Arginine vasotocin effects on courtship behavior in male white perch (Morone americana) Behav. Brain Res. 2002;133:177–183. doi: 10.1016/s0166-4328(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Sansone GR, Gerdes CA, Steinman JL, Winslow JT, Ottenweller JE, Komisaruk BR, Insel TR. Vaginocervical stimulation releases oxytocin within the spinal cord in rats. Neuroendocrinology. 2002;75:306–315. doi: 10.1159/000057340. [DOI] [PubMed] [Google Scholar]
- Santangelo N, Bass AH. New insights into neuropeptide modulation of aggression: field studies of arginine vasotocin in a territorial tropical damselfish. Proc. Biol. Sci. 2006;273:3085–3092. doi: 10.1098/rspb.2006.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo N, Bass AH. Individual behavioral and neuronal phenotypes for arginine vasotocin mediated courtship and aggression in a territorial teleost. Brain Behav. Evol. 2010;75:282–291. doi: 10.1159/000316867. [DOI] [PubMed] [Google Scholar]
- Sas E, Maler L, Weld M. Connections of the olfactory bulb in the gymnotiform fish, Apteronotus leptorhynchus. J. Comp. Neurol. 1993;335:486–507. doi: 10.1002/cne.903350403. [DOI] [PubMed] [Google Scholar]
- Satou M, Oka Y, Kusunoki M, Matsushima T, Kato M, Fujita I, Ueda K. Telencephalic and preoptic areas integrate sexual behavior in hime salmon (landlocked red salmon, Oncorhynchus nerka): results of electrical brain stimulation experiments. Physiol. Behav. 1984;33:441–447. doi: 10.1016/0031-9384(84)90167-7. [DOI] [PubMed] [Google Scholar]
- Satou M, Takeuchi HA, Takei K, Hasegawa T, Matsushima T, Okumoto N. Characterization of vibrational and visual signals which elicit spawning behavior in the male hime salmon (landlocked red salmon, Oncorhynchus nerka) J. Comp. Physiol. 1994;174A:527–537. [Google Scholar]
- Scammell TE, Saper CB. Orexin, drugs and motivated behaviors. Nat. Neurosci. 2005;8:1286–1288. doi: 10.1038/nn1005-1286. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Malinowska B, Kathmann M, Gothert M. Modulation of neurotransmitter release via histamine H3 heteroreceptors. Fundam. Clin. Pharmacol. 1994;8:128–137. doi: 10.1111/j.1472-8206.1994.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Schober JM, Pfaff D. The neurophysiology of sexual arousal. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:445–461. doi: 10.1016/j.beem.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Schreibman MP, Margolis-Nunno H. Reproductive biology of the terminal nerve (nucleus olfactoretinalis) and other LHRH pathways in teleost fishes. Ann. N Y Acad. Sci. 1987;519:60–68. doi: 10.1111/j.1749-6632.1987.tb36286.x. [DOI] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. J. Neurosci. 2003;23:4386–4393. doi: 10.1523/JNEUROSCI.23-10-04386.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Horm. Behav. 2004;45:345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Semsar K, Kandel FL, Godwin J. Manipulations of the AVT system shift social status and related courtship and aggressive behavior in the bluehead wrasse. Horm. Behav. 2001;40:21–31. doi: 10.1006/hbeh.2001.1663. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Lovejoy DA. Gonadotropin-releasing hormone in cartilaginous fishes: structure, location, and transport. Environ. Biol. Fish. 1993;38:197–208. [Google Scholar]
- Shiga T, Oka Y, Satou M, Okumoto N, Ueda K. Efferents from the supracommissural ventral telencephalon in the hime salmon (landlocked red salmon, Oncorhynchus nerka): an anterograde degeneration study. Brain Res. Bull. 1985a;14:55–61. doi: 10.1016/0361-9230(85)90177-7. [DOI] [PubMed] [Google Scholar]
- Shiga T, Oka Y, Satou M, Okumoto N, Ueda K. An HRP study of afferent connections of the supracommissural ventral telencephalon and the medial preoptic area in hime salmon (landlocked red salmon, Oncorhynchus nerka) Brain Res. 1985b;361:162–177. doi: 10.1016/0006-8993(85)91286-7. [DOI] [PubMed] [Google Scholar]
- Simoes JM, Fonseca PJ, Turner GF, Amorim MCP. Courtship and agonistic sounds by the cichlid fish Pseudotropheus zebra. J. Acoustic Soc. Am. 2008;124:1332–1339. doi: 10.1121/1.2945712. [DOI] [PubMed] [Google Scholar]
- Sisneros JA. Seasonal plasticity of auditory saccular sensitivity in the vocal plainfin midshipman fish, Porichthys notatus. J. Neurophysiol. 2009;102:1121–1131. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. J. Neurosci. 2003;23:1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004a;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen. Comp. Endocrinol. 2004b;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Tricas TC. Androgen-induced changes in the response dynamics of ampullary electrosensory primary afferent neurons. J. Neurosci. 2000;20:8586–8595. doi: 10.1523/JNEUROSCI.20-22-08586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Tricas TC. Neuroethology and life history adaptations of the elasmobranch electric sense. J. Physiol-Paris. 2002;96:379–389. doi: 10.1016/S0928-4257(03)00016-0. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Gonzalez A. Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res. Brain Res. Rev. 2000;33:308–379. doi: 10.1016/s0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Sneddon LU. Evolution of nociception in vertebrates: comparative analysis of lower vertebrates. Brain Res. Brain Res. Rev. 2004;46:123–130. doi: 10.1016/j.brainresrev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Snelson FF, Williams-Hooper SE, Schmid TH. Reproduction and ecology of the Atlantic stingray, Dasyatis sabina, in Florida coastal lagoons. Copeia. 1988;1988:729–739. [Google Scholar]
- Snow PJ, Renshaw GM, Hamlin KE. Localization of enkephalin immunoreactivity in the spinal cord of the long-tailed ray Himantura fai. J. Comp. Neurol. 1996;367:264–273. doi: 10.1002/(SICI)1096-9861(19960401)367:2<264::AID-CNE8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sorensen PW, Hara TJ, Stacey NE. Sex pheromones selectively stimulate the medial olfactory tracts of male goldfish. Brain Res. 1991;558:343–347. doi: 10.1016/0006-8993(91)90790-3. [DOI] [PubMed] [Google Scholar]
- Springer S. Natural history of the sandbar shark, Eulamia milberti. US Fish. Bull. 1960;61:1–38. [Google Scholar]
- Stacey NE, Liley NR. Regulation of spawning behavior in the female goldfish. Nature. 1974;247:71–72. [Google Scholar]
- Stacey NE, Peter RE. Central action of prostaglandins in spawning behaviour of female goldfish. Physiol. Behav. 1979;22:1191–1196. doi: 10.1016/0031-9384(79)90275-0. [DOI] [PubMed] [Google Scholar]
- Stacey NE, Sorensen PW. Fish hormonal pheromones. In: Pfaff DW, Arnold AP, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain, and Behavior. Academic Press; New York: 2002. pp. 375–435. [Google Scholar]
- Stell WK, Walker SE, Ball AK. Functional-anatomical studies on the terminal nerve projection to the retina of bony fishes. Ann. N Y Acad. Sci. 1987;519:80–96. doi: 10.1111/j.1749-6632.1987.tb36288.x. [DOI] [PubMed] [Google Scholar]
- Strobl-Mazzulla PH, Lethimonier C, Gueguen MM, Karube M, Fernandino JI, Yoshizaki G, Patino R, Strussmann CA, Kah O, Somoza GM. Brain aromatase (Cyp19A2) and estrogen receptors, in larvae and adult pejerrey fish Odontesthes bonariensis: Neuroanatomical and functional relations. Gen. Comp. Endocrinol. 2008;158:191–201. doi: 10.1016/j.ygcen.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Cruce WL. Distribution of tyrosine hydroxylase, serotonin, and leuenkephalin immunoreactive cells in the brainstem of a shark, Squalus acanthias. Brain Behav. Evol. 1992;39:77–92. doi: 10.1159/000114106. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Cruce WL, Northcutt RG. Distribution of tyrosine hydroxylase- and serotonin-immunoreactive cells in the central nervous system of the thornback guitarfish, Platyrhinoidis triseriata. J. Chem. Neuroanat. 1990;3:45–58. [PubMed] [Google Scholar]
- Stuesse SL, Cruce WL, Northcutt RG. Localization of serotonin, tyrosine hydroxylase, and leu-enkephalin immunoreactive cells in the brainstem of the horn shark, Heterodontus francisci. J. Comp. Neurol. 1991a;308:277–292. doi: 10.1002/cne.903080211. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Cruce WL, Northcutt RG. Serotoninergic and enkephalinergic cell groups in the reticular formation of the bat ray and two skates. Brain Behav. Evol. 1991b;38:39–52. doi: 10.1159/000114378. [DOI] [PubMed] [Google Scholar]
- Stuesse SL, Stuesse DC, Cruce WL. Raphe nuclei in three cartilaginous fishes, Hydrolagus colliei, Heterodontus francisci, and Squalus acanthias. J. Comp. Neurol. 1995;358:414–427. doi: 10.1002/cne.903580308. [DOI] [PubMed] [Google Scholar]
- Tricas TC, Maruska KP, Rasmussen LE. Annual cycles of steroid hormone production, gonad development, and reproductive behavior in the Atlantic stingray. Gen. Comp. Endocrinol. 2000;118:209–225. doi: 10.1006/gcen.2000.7466. [DOI] [PubMed] [Google Scholar]
- Tricas TC, Michael SW, Sisneros JA. Electrosensory optimization to conspecific phasic signals for mating. Neurosci. Lett. 1995;202:129–132. doi: 10.1016/0304-3940(95)12230-3. [DOI] [PubMed] [Google Scholar]
- Uchida H, Ogawa S, Harada M, Matushita M, Iwata M, Sakuma Y, Parhar IS. The olfactory organ modulates gonadotropin-releasing hormone types and nest-building behavior in the tilapia Oreochromis niloticus. J. Neurobiol. 2005;65:1–11. doi: 10.1002/neu.20156. [DOI] [PubMed] [Google Scholar]
- Volkoff H, Peter RE. Actions of two forms of gonadotropin releasing hormone and a GnRH antagonist on spawning behavior of the goldfish Carassius auratus. Gen. Comp. Endocrinol. 1999;116:347–355. doi: 10.1006/gcen.1999.7377. [DOI] [PubMed] [Google Scholar]
- Wada H, Inagaki N, Yamatodani A, Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci. 1991;14:415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- Wourms JP, Demski LS. The reproduction and development of sharks, skates, rays, and ratfishes: introduction, history, overview, and future prospects. Environ. Biol. Fish. 1993;38:7–21. [Google Scholar]
- Wright DE, Demski LS. Gonadotropin hormone-releasing hormone (GnRH) immunoreactivity in the mesencephalon of sharks and rays. J. Comp. Neurol. 1991;307:49–56. doi: 10.1002/cne.903070105. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 2004;475:143–162. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ishikawa Y, Yoshimoto M, Xue HG, Bahaxar N, Sawai N, Yang CY, Ozawa H, Ito H. A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav. Evol. 2007;69:96–104. doi: 10.1159/000095198. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Oka Y, Amano M, Aida K, Hasegawa Y, Kawashima S. Multiple gonadotropin-releasing hormone (GnRH)-immunoreactive systems in the brain of the dwarf gourami, Colisa lalia: immunohistochemistry and radioimmunoassay. J. Comp. Neurol. 1995;355:354–368. doi: 10.1002/cne.903550303. [DOI] [PubMed] [Google Scholar]
- Zohar Y, Munoz-Cueto JA, Elizur A, Kah O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010;165:438–455. doi: 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]