Abstract
Typically, cyclooxygenases (COXs) and 5-lipoxygenase (5-LOX), enzymes that generate biologically active lipid molecules termed eicosanoids, are considered inflammatory. Hence, their putative role in Alzheimer’s disease (AD) has been explored in the framework of possible inflammatory mechanisms of AD pathobiology. More recent data indicate that these enzymes and the biologically active lipid molecules they generate could influence the functioning of the central nervous system and the pathobiology of neurodegenerative disorders such as AD via mechanisms different from classical inflammation. These mechanisms include the cell-specific localization of COXs and 5-LOX in the brain, the type of lipid molecules generated by the activity of these enzymes, the type and the localization of receptors selective for a type of lipid molecule, and the putative interactions of the COXs and 5-LOX pathways with intracellular components relevant for AD such as the gamma-secretase complex. Considering the importance of these multiple and not necessarily inflammatory mechanisms may help us delineate the exact nature of the involvement of the brain COXs and 5-LOX in AD and would reinvigorate the search for novel targets for AD therapy.
Recent positron emission tomography (PET) data indicate that the normal human brain consumes 17.8 mg/day of arachidonic acid, and that this consumption increases in the brains of Alzheimer’s disease (AD) patients (for review, see Rapoport, 2008). A portion of intracellular free arachidonic acid is metabolized by cyclooxygenases (e.g., cyclooxygenase-1, COX-1; cyclooxygenase-2, COX-2) and lipoxygenases (e.g., 5-lipoxygenase; 5-LOX) to generate biologically active prostaglandins and leukotrienes, respectively. In the central nervous system (CNS), both COXs and 5-LOX have been associated with pathobiological mechanisms accompanying aging and neurodegeneration (Choi et al., 2009; Chu and Praticò, 2009; Phillis et al., 2006). A recent study of COX-2 and 5-LOX single nucleotide polymorphisms (SNPs) in AD performed on 341 AD patients and 190 controls from Northern Italy found a significant difference in the distribution of the −765G COX-2 and −1708A 5-LOX alleles between AD cases and controls--both alleles were overrepresented in AD patients and underrepresented in controls (Listì et al., 2010). These authors suggested that the identified alleles of COX-2 and 5-LOX could be risk factors for AD.
Since in the periphery, the most prominent function of COXs and 5-LOX is in inflammation, i.e., they are considered “inflammatory” enzymes, findings of the presence and overactivation and overexpression of these enzymes in AD are typically referred to as evidence for an inflammatory basis for AD pathobiology. Furthermore, both COXs and 5-LOX contribute to atherogenesis and it has been proposed that these pathways may be at the core of the co-morbidity of cardiovascular and neurological disorders such as AD (Chu and Praticò, 2009; Praticò and Dogné, 2009). However, both COX and 5-LOX may influence CNS functioning via mechanisms unrelated to the role these proteins play in inflammation. For example, COX-2, which is predominantly expressed in pyramidal neurons in contrast to COX-1, which is mostly present in microglia, regulates neuroplasticity via its conversion of arachidonic acid to classic prostaglandins but also by favoring oxidative metabolism of endocannabinoids to novel prostaglandins (Yang and Chen, 2008). On the other hand, 5-LOX appears to be capable of regulating the brain’s amyloid-beta levels by influencing gamma-secretase (Firuzi et al., 2008).
Additional evidence for a putative role of COXs and 5-LOX in AD derives from pharmacological studies using inhibitors of these enzymes (for review, see Firuzi and Praticò, 2006). In addition to helping delineate the pathobiological mechanisms of AD, these results raise hope for discovering novel therapeutic targets and modalities.
2. Neurotoxic/neuroprotective action of prostaglandins, leukotrienes, and their receptors in the CNS
Initial research into the role of eicosanoids, i.e., prostaglandins and leukotrienes, in brain pathologies had often focused on the so-called “inflammatory” activity of these lipid mediators and had provided evidence that eicosanoids may promote neurodegeneration and neurotoxicity. Recent data suggest a more complex role for these molecules and stress the importance of the type of the lipid molecule and the type of the receptor on which it acts.
The two COX enzymes, COX-1 and COX-2, that are capable of converting arachidonic acid to prostaglandin H2 (PGH2), show a differential cell type-specific expression in adult mammalian brain. COX-1 is constitutively expressed in most tissues and in the brain predominantly in microglia (for review, see Choi et al., 2009). On the other hand, in the brain, COX-2 is constitutively expressed in hippocampal neurons and their dendritic spines. Neuronal COX-2 expression is modified by synaptic activity (Kaufmann et al., 1996) as well as by pathological conditions including the beta-amyloid peptide-triggered neurotoxicity (Ryu et al., 2004). Depending upon the differential cellular localization of COX-1 and COX-2, the subsequent conversion of PGH2 into other active molecules may lead to dissimilar ultimate products of COX-1 vs. COX-2 activity.
Niemoller and Bazan (2010) stressed the complex nature of the downstream cyclooxygenase signaling pathways that may be responsible for both neurodegeneration and neuroprotection. These include PGE2, a product of COX-2 that activates the G protein-coupled receptors (GPCRs) EP1, EP2, EP3 and EP4. It appears that activation of the G-alpha-coupled EP2 receptor by PGE2 is neuroprotective and drug discovery efforts have been directed toward finding compounds that could potentiate this action of PGE2, i.e., the positive allosteric modulators (potentiators) of the EP2 receptors (Jiang et al., 2010). On the other hand, PGE2 is capable of stimulating the production of amyloid-beta by a coordinated action on both EP2 and EP4 receptors (Hoshino et al., 2009). In addition, it has been suggested that cell type-specific targeting of EP2 receptors may be needed to reduce the amyloid-beta-induced neurodegeneration (Shie et al., 2005). Furthermore, activation of EP1 receptors leads to neurodegeneration that can be reduced by selective EP1 antagonists (Abe et al., 2009).
In vitro, the synthesis of 5-LOX metabolites of arachidonic acid has been demonstrated in microglia (Matsuo et al., 1995) and neuronal precursors (Wada et al., 2006). The CNS is capable of producing 5-LOX metabolites (Chinnici et al., 2007; Hynes et al., 1991), possibly via a transcellular synthesis of cysteinyl leukotrienes in neurons and glia (Farias et al., 2007). 5-LOX products play a critical role in neuroplasticity such as the hedgehog-dependent neurite projection (Bijlsma et al., 2008). Generally, the conversion of arachidonic acid by 5-LOX leads to production of leukotrienes and, under certain conditions, lipoxins. These lipid mediators affect cell functioning via corresponding GPCRs (Wada et al., 2006). Various leukotriene receptors are expressed by both neurons and microglia (Okubo et al., 2010). It was shown that 5-LOX pathway plays a significant role in neuronal precursors (e.g., the immature cerebellar granule cells) (Uz et al., 2001) and neural stem cell (NSC) (Wada et al., 2006). Thus, proliferation of NSCs was stimulated by LTB4 and blocked by a LTB4 receptor antagonist, which also caused apoptosis and cell death. In contrast, LXA4 attenuated growth of NSCs (Wada et al., 2006). In addition to proliferation, LTB4 induced differentiation of NSCs into neurons as monitored by neurite outgrowth. These authors suggested that LTB4 and LXA4 directly regulate proliferation and differentiation of NSCs and demonstrated the opposing actions of two different 5-LOX metabolites on NSCs. Recent data indicate that leukotrienes could influence neuronal survival and differentiation via novel types of receptors, e.g., the P2Y-like receptor GPR17 (Daniele et al., 2010).
It has been reported that leukotrienes and their receptors, e.g. the cysteinyl leukotriene receptor 1 (CysLT1) may promote brain injury (Ding et al., 2007) and that increased 5-LOX expression and activity lead to production of brain-toxic molecules (Khan et al., 2010). However, differential effects of leukotrienes (e.g., LTB4) and lipoxins (e.g., LXA4) were observed with respect to neuroprotection. Sobrado et al. (2009) found that rosiglitazone, an anti-diabetic drug, induced 5-LOX expression in ischemic rat brain concomitant with neuroprotection. This type of drug-induced 5-LOX upregulation was accompanied by increased cerebral levels of LXA4 and by inhibited ischemia-induced production of LTB4. Although 5-LOX inhibitors are neuroprotective in models of brain ischemia (Tu et al., 2010), pharmacological inhibition and/or genetic deletion of 5-LOX inhibited the observed rosiglitazone-induced LXA4-mediated neuroprotection. The neuroprotective effect of LXA4 appears to be mediated by the agonistic activity of this molecule on the peroxisome proliferator-activated receptor gamma (PPARgamma) (Sobrado et al., 2009). Hence, an increase of 5-LOX expression/activity may lead to production of both, putatively neurotoxic mediators such as leukotrienes or neuroprotective mediators such as LXA4.
The type of the 5-LOX metabolite produced may be influenced by factors such as 5-LOX phosphorylation. For example, 5-LOX phosphorylation at Ser523 determines whether certain drugs (e.g., pioglitazone and atorvastatin) induce production of LTB4 or LXA4 (Ye et al., 2008). Interestingly, Ser523-phosphorylated 5-LOX content is increased in post-mortem brain samples of suicide victims compared to controls (Uz et al., 2008). Regarding AD, the available data suggest that amyloid-beta is capable of increasing the release of microglia-derived leukotrienes (Paris et al., 1999).
3. Aging and brain expression of COX and 5-LOX
AD is a prototype of aging-associated neurodegenerative disorders. Although certain COX-2 and 5-LOX SNPs have been associated with AD (Listì et al., 2010), other studies point to a lack of such associations (Alvarez et al., 2008). Factors other than DNA sequence variations could influence COX and 5-LOX expression in the brain and could play a role in determining the pathobiological involvement of the COX and 5-LOX pathways in AD. These include aging and possibly epigenetic modifications.
Aid and Bosetti (2007) characterized the effects of aging on COX-1 and COX-2 expression in the hippocampus and cerebral cortex of 4-, 12-, 24- and 30-month-old rats. They found increased hippocampal COX-1 mRNA levels at 12, 24, and 30 months, whereas COX-2 mRNA expression was significantly decreased only at 30 months. In the cerebral cortex, mRNA levels of both COX-1 and COX-2 were not significantly changed. On the other hand, in humans, the neuronal expression (i.e., immunoreactivity) of COX-2, which was observed in the CA3 subdivision of the hippocampus, subiculum, entorhinal cortex and transentorhinal cortex correlated (i.e., increased) with age in the post-mortem brains of nondemented subjects (Fujimi et al., 2007).
In the rat (Qu et al., 2000; Uz et al., 1998) and mouse (Chinnici et al., 2007; Dzitoyeva et al., 2009) brain, the expression of 5-LOX increases during aging. This effect was confirmed using both mRNA and protein measurements. Nevertheless, some differences were observed regarding the brain regions in which aging-associated increased 5-LOX expression was significant.
Epigenetic mechanisms, which encompass the modification of chromatin structure that leads to regulation of the gene expression, are influenced by environmental factors including aging. For example, Siegmund et al. (2007) studied DNA methylation at the sites of CpG dinucleotides in human cerebral cortex samples in aging subjects and found a progressive aging-associated rise in the DNA methylation of CpG islands of certain CNS genes. In a study using methylation-sensitive restriction endonucleases (AciI, BstUI, HpaII, and HinP1I) to assess 5-LOX DNA methylation in brain and heart tissue samples from young (2 months) and old (22 months) mice, it was found that in young mice, the 5-LOX mRNA content was significantly greater in the heart compared to the brain; 5-LOX DNA methylation was lower, except in the AciI assay in which it was higher in the heart (Dzitoyeva et al., 2009). Furthermore, in mice, aging decreased 5-LOX mRNA content in the heart and increased it in the brain while increasing 5-LOX DNA methylation and this effect was site- (i.e., enzyme) and tissue-specific. Currently, there is more information available on the epigenetic mechanisms involved in the regulation of human 5-LOX (ALOX5) gene (Katryniok et al., 2010) than on the regulation of the expression of COXs. Studies in the field of oncology have established that COX-2 gene expression is regulated by epigenetic mechanisms such as DNA methylation (de Maat et al., 2007). Further research is needed to determine whether epigenetic regulation plays a role in neuronal COX-2 expression.
4. COX and 5-LOX in postmortem AD brain
Minghetti (2004) reviewed the findings on COX-2 mRNA levels in AD brains, pointing out that the available evidence demonstrated either decreased or increased levels, possibly because of the short half-life of COX-2 transcripts or individual variability. Histological analyses of AD brains have also produced apparently conflicting results. For example, Kitamura et al. (1999) found that in AD brains, protein levels of COX-1 were increased in both cytosolic and particulate fractions and that COX-2 protein was also increased in the particulate fraction. In a study analyzing the postmortem brains of 45 autopsy subjects without dementia and 25 AD patients from the town of Hisayama, Japan, it was found that in AD patients, neurons of CA1 exhibited increased COX-2 immunoreactivity that correlated with the severity of AD pathology (Fujimi et al., 2007). Furthermore, it appears that in AD, changes in COX-1 and COX-2 expression depend on the stage of the disease and the type of cells expressing these enzymes (for review, see Choi et al., 2009; Hoozemans et al., 2008; Minghetti et al., 2004).
Quantitative Western blot assays were used to investigate 5-LOX protein content in the hippocampus and frontal cortex of humans with AD and in matching controls. 5-LOX levels were significantly increased in AD brain samples (Firuzi et al., 2008). Another study investigated the distribution and cellular localization of 5-LOX in the medial temporal lobe from AD and control subjects (Ikonomovic et al., 2008) and found that in AD subjects, 5-LOX immunoreactivity is elevated relative to controls and that its localization is dependent on the antibody-targeted portion of the 5-LOX amino acid sequence. Thus, carboxy terminus-directed antibodies detected 5-LOX in glial cells and neurons but less frequently in neurons with dystrophic morphology, whereas immunoreactivity observed using 5-LOX amino terminus-directed antibodies was absent in neurons and abundant in neurofibtillary tangles, neuritic plaques, and glia. Furthermore, double-labeling studies showed a close association of 5-LOX-immunoreactive processes and glial cells with amyloid beta immunoreactive plaques and vasculature.
5. AD and transgenic COX and 5-LOX mouse models
Various models of transgenic mice have been used to investigate the contribution of COX and 5-LOX to AD. Typically, the intent of the models has been to demonstrate that the absence or the overexpression of COX/5-LOX influences AD-like phenotypes. A microarray analysis of gene expression in the cerebral cortex and hippocampus of mice deficient in either COX-1 or COX-2 revealed that the majority (>93%) of the differentially expressed genes in both the cortex and hippocampus were altered in one COX isoform knockout mouse but not the other (Toscano et al., 2007), suggesting that COX-1 and COX-2 differentially modulate brain gene expression and that this type of modulation could impact the development/progression of AD.
For example, Choi and Bosetti (2009) investigated the effect of COX-1 genetic deletion on neurodegeneration induced by beta-amyloid peptide 1–42, which was centrally injected in the lateral ventricle of COX-1-deficient mice and their respective wild-type controls. These authors found that in the absence of COX-1 the beta-amyloid-induced damage was attenuated. On the other hand, Melinkova et al. (2006) showed that overexpression of COX-2 in APPswe-PS1dE9 mice (a model of AD pathology) leads to deficits in spatial working memory in female but not male mice. This COX-2-dependent sex-specific deficit was abolished by pharmacological inhibition of COX-2. Furthermore, these authors reported that the effects of COX-2 and amyloid-beta peptides on cognition occurred in a sex-specific manner in the absence of significant changes in amyloid burden.
Firuzi et al. (2008) investigated the effect of 5-LOX deficiency on the amyloid-beta pathology of a transgenic mouse model of AD-like amyloidosis, the Tg2576 mice. These authors generated a double transgenic model crossing 5-LOX-deficient with Tg2576 mice and found that the genetic disruption of 5-LOX reduced amyloid-beta deposits and amyloid-beta 42 levels. Furthermore, these changes occurred in the absence of alteration in amyloid-beta precursor protein processing or amyloid-beta catabolism. Additional in-vitro studies showed that 5-LOX activation and its metabolites increased whereas 5-LOX inhibition decreased amyloid-beta formation by modulating the gamma-secretase complex activity (Firuzi et al., 2008). These authors suggested that 5-LOX could participate in AD pathobiology through a mechanism that involves the modulation of the gamma-secretase activity.
6. AD and COX/5-LOX pharmacology
Early studies on the role of cyclooxygenases in AD were inspired by epidemiological data suggesting that COX inhibitors such as nonsteroidal anti-inflammatory drugs (NSAIDs) could be beneficial in AD patients (Lucca et al., 1994; McGeer et al., 1990). This line of research led to the development and trials of selective COX-2 inhibitors as a putative therapy for AD. As summarized in several recent reviews (Choi et al., 2009; Imbimbo, 2009), it appears that selective COX-2 inhibitors may not be an effective therapy in AD patients with mild to severe cognitive impairment. It has been proposed that these drugs and possibly selective COX-1 inhibitors might be beneficial if administered before the onset of AD symptoms (Choi et al., 2009).
Some NSAIDs, e.g., aspirin, trigger a peculiar interplay between the COXs and 5-LOX pathways that ultimately shifts the 5-LOX end-products from leukotrienes to lipoxins. Thus, by causing the acetylation of COX-2, aspirin triggers the production of (15R)-15-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid (15R-HETE) that is converted by 5-LOX to 15-epi-LXA4 (Claria and Serhan, 1955). Recently, similar pathway to the production of 15-epi-LXA4 has been described for some non-NSAIDs (Ye et al., 2008). Furthermore, it appears that certain NSAIDs could influence AD pathobiology independent of their COX-inhibitory activity, i.e. by interacting with the gamma-secretase complex (Kukar and Golde, 2008).
Compared to the pharmacology of COX inhibition, the clinical pharmacological tools for 5-LOX inhibition are scarce. Except for drugs in various stages of pre-clinical and early clinical development, zileuton appears to be the only choice. With respect to CNS pathologies, it has been shown in a rat model of stroke that zileuton is capable of reducing the extent of brain damage (Tu et al., 2010). Based on the finding that zileuton acts as an inhibitor of the gamma-secretase activity in-vitro (Firuzi et al., 2008), it is reasonable to expect that this drug would be tested pre-clinically and clinically for its potential to alter the course of AD. Indirect support for possible benefits of 5-LOX inhibition in AD is provided by findings that drugs that act as functional 5-LOX inhibitors, e.g., minocycline (Chu et al., 2010; Song et al., 2006), are beneficial in AD animal models (Cuello et al., 2010). More commonly used drugs that affect the 5-LOX pathway are the leukotriene receptor inhibitors. Recently, it has been noted that that these drugs may affect CNS functioning because their use has been associated with neuropsychiatric side effects. Although leukotriene receptor inhibitors appear to be neuroprotective in animal models of stroke (Yu et al., 2005) no data are available on their possible effects in AD.
7. Conclusion
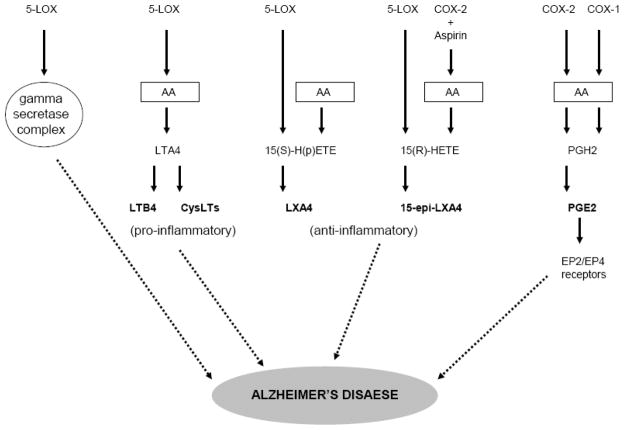
Early work regarding the putative role of COXs and 5-LOX in AD was based on the concept of possible inflammatory mechanisms of AD pathobiology. More recent data indicate that these enzymes and biologically active lipid molecules resulting from their activity could influence CNS and neurodegenerative disorders such as AD via mechanisms unlike classical inflammation (Fig. 1). These mechanisms include the cell-specific localization of CNS COX and 5-LOX, the type of lipid molecules generated by these enzymes, the type and the localization of GPCRs selective for a type of lipid molecule, and the putative interactions of the COX and 5-LOX pathways with intracellular components such as the gamma-secretase complex. Surprisingly, little research has been directed toward understanding the role of eicosanoid receptors in AD. Furthermore, interplay between COXs and 5-LOX pathways, as exemplified by the generation of anti-inflammatory/neuroprotective 15-epi-LXA4, may provide novel insights into the role of these pathways in neurodegenerative disorders. Considering the importance of these multiple mechanism may help us delineate the exact nature of the involvement of COX and 5-LOX in AD and would reinvigorate the search for novel targets for AD therapy.
Figure 1.
Putative mechanisms linking 5-LOX and COX pathways to Alzheimer’s disease (AD). 5-LOX could influence AD by producing pro-inflammatory leukotrienes (LTB4 and CysLTs) and anti-inflammatory lipoxins (naturally occurring LHA4 and aspirin-triggered 15-epi-LXA4) and by an interaction with the gamma-secretase complex. Cyclooxygenases could influence AD by acting on arachidonic acid (AA) and producing PGH2 and PGE2 (see text for details).
Research Highlights.
Typically, cyclooxygenases (COXs) and 5-lipoxygenase (5-LOX), enzymes that generate biologically active lipid molecules termed eicosanoids, are considered inflammatory. Hence, their putative role in Alzheimer’s disease (AD) has been explored in the framework of possible inflammatory mechanisms of AD pathobiology. More recent data indicate that these enzymes and the biologically active lipid molecules they generate could influence the functioning of the central nervous system and the pathobiology of neurodegenerative disorders such as AD via mechanisms different from classical inflammation. These mechanisms include the cell-specific localization of COXs and 5-LOX in the brain, the type of lipid molecules generated by the activity of these enzymes, the type and the localization of receptors selective for a type of lipid molecule, and the putative interactions of the COXs and 5-LOX pathways with intracellular components relevant for AD such as the gamma-secretase complex. Considering the importance of these multiple and not necessarily inflammatory mechanisms may help us delineate the exact nature of the involvement of the brain COXs and 5-LOX in AD and would reinvigorate the search for novel targets for AD therapy.
Acknowledgments
This research was supported in part by grant R01 AG015347 from the National Institute on Aging (NIA) and grant 1R21 DA024099-01 from the National Institute on Drug Abuse (NIDA). NIA and NIDA had no role in the preparation, review, or approval of this commentary. The content is solely the responsibility of the authors and does not necessary represent the official views of the NIA and NIDA.
List of abbreviations
- AA
arachidonic acid
- AD
Alzheimer’s disease
- CNS
central nervous system
- COXs
cyclooxygenases
- CysLT1
cysteinyl leukotriene 1
- EP
prostaglandin E receptor
- GPCRs
G protein-coupled receptors
- 5-LOX
5-lipoxygenase
- LTB4
leukotriene B4
- LXA4
lipoxin A4
- NSAIDs
nonsteroidal anti-inflammatory drugs
- NSC
neural stem cell
- PET
recent positron emission tomography
- PGE2
prostaglandin E2
- PGH2
prostaglandin H2
- PPAR
peroxisome proliferator-activated receptor
- 15R-HETE
(15R)-15-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid
- SNPs
single nucleotide polymorphisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kunz A, Shimamura M, Zhou P, Anrather J, Iadecola C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29(1):66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aïd S, Bosetti F. Gene expression of cyclooxygenase-1 and Ca2+-independent phospholipase A(2) is altered in rat hippocampus during normal aging. Brain Res Bull. 2007;73(1–3):108–13. doi: 10.1016/j.brainresbull.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez V, González P, Corao AI, Menéndez M, Lahoz CH, Martínez C, et al. The Sp1/Egr1-tandem repeat polymorphism in the 5-lipoxygenase gene promoter is not associated with late onset Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(2):177–180. doi: 10.1097/WAD.0b013e3181572046. [DOI] [PubMed] [Google Scholar]
- Bijlsma MF, Peppelenbosch MP, Spek CA, Roelink H. Leukotriene synthesis is required for hedgehog-dependent neurite projection in neuralized embryoid bodies but not for motor neuron differentiation. Stem Cells. 2008;26(5):1138–45. doi: 10.1634/stemcells.2007-0841. [DOI] [PubMed] [Google Scholar]
- Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28(9):1457–62. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Choi SH, Bosetti F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging (Albany NY) 2009;1(2):234–44. doi: 10.18632/aging.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30(4):174–81. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Praticò D. The 5-lipoxygenase as a common pathway for pathological brain and vascular aging. Cardiovasc Psychiatry Neurol. 2009;2009:174657. doi: 10.1155/2009/174657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LS, Fang SH, Zhou Y, Yin YJ, Chen WY, Li JH, et al. Minocycline inhibits 5-lipoxygenase expression and accelerates functional recovery in chronic phase of focal cerebral ischemia in rats. Life Sci. 2010;86(5–6):170–7. doi: 10.1016/j.lfs.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92(21):9475–9. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Ferretti MT, Leon WC, Iulita MF, Melis T, Ducatenzeiler A, et al. Early-stage inflammation and experimental therapy in transgenic models of the Alzheimer-like amyloid pathology. Neurodegener Dis. 2010;7(1–3):96–8. doi: 10.1159/000285514. [DOI] [PubMed] [Google Scholar]
- Daniele S, Lecca D, Trincavelli ML, Ciampi O, Abbracchio MP, Martini C. Regulation of PC12 cell survival and differentiation by the new P2Y-like receptor GPR17. Cell Signal. 2010;22(4):697–706. doi: 10.1016/j.cellsig.2009.12.006. [DOI] [PubMed] [Google Scholar]
- de Maat MF, van de Velde CJ, Umetani N, de Heer P, Putter H, van Hoesel AQ, et al. Epigenetic silencing of cyclooxygenase-2 affects clinical outcome in gastric cancer. J Clin Oncol. 2007;25(31):4887–94. doi: 10.1200/JCO.2006.09.8921. [DOI] [PubMed] [Google Scholar]
- Ding Q, Fang SH, Zhou Y, Zhang LH, Zhang WP, Chen Z, et al. Cysteinyl leukotriene receptor 1 partially mediates brain cryoinjury in mice. Acta Pharmacol Sin. 2007;28(7):945–52. doi: 10.1111/j.1745-7254.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Dzitoyeva S, Imbesi M, Ng LW, Manev H. 5-Lipoxygenase DNA methylation and mRNA content in the brain and heart of young and old mice. Neural Plast. 2009;2009:209596. doi: 10.1155/2009/209596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias SE, Zarini S, Precht T, Murphy RC, Heidenreich KA. Transcellular biosynthesis of cysteinyl leukotrienes in rat neuronal and glial cells. J Neurochem. 2007;103(4):1310–8. doi: 10.1111/j.1471-4159.2007.04830.x. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Praticò D. Coxibs and Alzheimer’s disease: should they stay or should they go? Ann Neurol. 2006;59(2):219–28. doi: 10.1002/ana.20774. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22(4):1169–78. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimi K, Noda K, Sasaki K, Wakisaka Y, Tanizaki Y, Iida M, et al. Altered expression of COX-2 in subdivisions of the hippocampus during aging and in Alzheimer’s disease: the Hisayama Study. Dement Geriatr Cogn Disord. 2007;23(6):423–31. doi: 10.1159/000101957. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Rozemuller JM, van Haastert ES, Veerhuis R, Eikelenboom P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr Pharm Des. 2008;14(14):1419–27. doi: 10.2174/138161208784480171. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Namba T, Takehara M, Nakaya T, Sugimoto Y, Araki W, et al. Prostaglandin E2 stimulates the production of amyloid-beta peptides through internalization of the EP4 receptor. J Biol Chem. 2009;284(27):18493–502. doi: 10.1074/jbc.M109.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N, Bishai I, Lees J, Coceani F. Leukotrienes in brain: natural occurrence and induced changes. Brain Res. 1991;553(1):4–13. doi: 10.1016/0006-8993(91)90222-h. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J Histochem Cytochem. 2008;56(12):1065–73. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbimbo BP. An update on the efficacy of non-steroidal anti-inflammatory drugs in Alzheimer’s disease. Expert Opin Investig Drugs. 2009;18(8):1147–68. doi: 10.1517/13543780903066780. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ganesh T, Du Y, Thepchatri P, Rojas A, Lewis I, et al. Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci USA. 2010;107(5):2307–12. doi: 10.1073/pnas.0909310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katryniok C, Schnur N, Gillis A, von Knethen A, Sorg BL, Looijenga L, et al. Role of DNA methylation and methyl-DNA binding proteins in the repression of 5-lipoxygenase promoter activity. Biochim Biophys Acta. 2010;1801(1):49–57. doi: 10.1016/j.bbalip.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93(6):2317–21. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Singh J, Gilg AG, Uto T, Singh I. Very long chain fatty acid accumulation causes lipotoxic response via 5-lipoxygenase in cerebral adrenoleukodystrophy. J Lipid Res. 2010 doi: 10.1194/jlr.M002329. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Koike H, Kakimura J, Matsuoka Y, Nomura Y, et al. Increased expression of cyclooxygenases and peroxisome proliferator-activated receptor-gamma in Alzheimer’s disease brains. Biochem Biophys Res Commun. 1999;254(3):582–6. doi: 10.1006/bbrc.1998.9981. [DOI] [PubMed] [Google Scholar]
- Kukar T, Golde TE. Possible mechanisms of action of NSAIDs and related compounds that modulate gamma-secretase cleavage. Curr Top Med Chem. 2008;8(1):47–53. doi: 10.2174/156802608783334042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listì F, Caruso C, Lio D, Colonna-Romano G, Chiappelli M, Licastro F, et al. Role of cyclooxygenase-2 and 5-lipoxygenase polymorphisms in Alzheimer’s disease in a population from northern Italy: implication for pharmacogenomics. J Alzheimers Dis. 2010;19(2):551–7. doi: 10.3233/JAD-2010-1260. [DOI] [PubMed] [Google Scholar]
- Lucca U, Tettamanti M, Forloni G, Spagnoli A. Nonsteroidal antiinflammatory drug use in Alzheimer’s disease. Biol Psychiatry. 1994;36(12):854–6. doi: 10.1016/0006-3223(94)90598-3. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Hamasaki Y, Fujiyama F, Miyazaki S. Eicosanoids are produced by microglia, not by astrocytes, in rat glial cell cultures. Brain Res. 1995;685(1–2):201–4. doi: 10.1016/0006-8993(95)00490-h. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer E, Rogers J, Sibley J. Anti-inflammatory drugs and Alzheimer disease. Lancet. 1990;335(8696):1037. doi: 10.1016/0140-6736(90)91101-f. [DOI] [PubMed] [Google Scholar]
- Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, et al. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer’s disease in a sex-dimorphic pattern. Neuroscience. 2006;141(3):1149–62. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63(9):901–10. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Niemoller TD, Bazan NG. Docosahexaenoic acid neurolipidomics. Prostaglandins Other Lipid Mediat. 2010;91(3–4):85–9. doi: 10.1016/j.prostaglandins.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo M, Yamanaka H, Kobayashi K, Noguchi K. Leukotriene synthases and the receptors induced by peripheral nerve injury in the spinal cord contribute to the generation of neuropathic pain. Glia. 2010;58(5):599–610. doi: 10.1002/glia.20948. [DOI] [PubMed] [Google Scholar]
- Paris D, Town T, Parker TA, Tan J, Humphrey J, Crawford F, et al. Inhibition of Alzheimer’s beta-amyloid induced vasoactivity and proinflammatory response in microglia by a cGMP-dependent mechanism. Exp Neurol. 1999;157(1):211–21. doi: 10.1006/exnr.1999.7055. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev. 2006;52(2):201–43. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Praticò D, Dogné JM. Vascular biology of eicosanoids and atherogenesis. Expert Rev Cardiovasc Ther. 2009;7(9):1079–89. doi: 10.1586/erc.09.91. [DOI] [PubMed] [Google Scholar]
- Qu T, Uz T, Manev H. Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats. Neurobiol Aging. 2000;21(5):647–52. doi: 10.1016/s0197-4580(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):153–6. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Franciosi S, Sattayaprasert P, Kim SU, McLarnon JG. Minocycline inhibits neuronal death and glial activation induced by beta-amyloid peptide in rat hippocampus. Glia. 2004;48(1):85–90. doi: 10.1002/glia.20051. [DOI] [PubMed] [Google Scholar]
- Schulte EC, Slawik H, Schüle R, Gunther T, Hüll M. Alterations in excitotoxicity and prostaglandin metabolism in a transgenic mouse model of Alzheimer’s disease. Neurochem Int. 2009;55(7):689–96. doi: 10.1016/j.neuint.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 as a new target to increase amyloid beta phagocytosis and decrease amyloid beta-induced damage to neurons. Brain Pathol. 2005;15(2):134–8. doi: 10.1111/j.1750-3639.2005.tb00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, et al. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One. 2007;2(9):e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernández-López D, Pradillo JM, et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29(12):3875–84. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wei EQ, Zhang WP, Ge QF, Liu JR, Wang ML, et al. Minocycline protects PC12 cells against NMDA-induced injury via inhibiting 5-lipoxygenase activation. Brain Res. 2006;1085(1):57–67. doi: 10.1016/j.brainres.2006.02.042. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Prabhu VV, Langenbach R, Becker KG, Bosetti F. Differential gene expression patterns in cyclooxygenase-1 and cyclooxygenase-2 deficient mouse brain. Genome Biol. 2007;8(1):R14. doi: 10.1186/gb-2007-8-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Wang CH, Shi SS, Zhang YL, Chen CM, et al. Zileuton reduces inflammatory reaction and brain damage following permanent cerebral ischemia in rats. Inflammation. 2010 doi: 10.1007/s10753-010-9191-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 1998;12(6):439–49. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- Uz T, Manev R, Manev H. 5-Lipoxygenase is required for proliferation of immature cerebellar granule neurons in vitro. Eur J Pharmacol. 2001;418(1–2):15–22. doi: 10.1016/s0014-2999(01)00924-4. [DOI] [PubMed] [Google Scholar]
- Uz T, Dwivedi Y, Pandey GN, Roberts RC, Conley RR, Manev R, et al. 5-lipoxygenase in the prefrontal cortex of suicide victims. Open Neuropsychopharmacol J. 2008;1:1–5. doi: 10.2174/1876523800801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y, et al. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20(11):1785–92. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des. 2008;14(14):1443–51. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Lin Y, Perez-Polo JR, Uretsky BF, Ye Z, Tieu BC, et al. Phosphorylation of 5-lipoxygenase at ser523 by protein kinase A determines whether pioglitazone and atorvastatin induce proinflammatory leukotriene B4 or anti-inflammatory 15-epi-lipoxin a4 production. J Immunol. 2008;181(5):3515–23. doi: 10.4049/jimmunol.181.5.3515. [DOI] [PubMed] [Google Scholar]
- Yu GL, Wei EQ, Zhang SH, Xu HM, Chu LS, Zhang WP, et al. Montelukast, a cysteinyl leukotriene receptor-1 antagonist, dose- and time-dependently protects against focal cerebral ischemia in mice. Pharmacology. 2005;73(1):31–40. doi: 10.1159/000081072. [DOI] [PubMed] [Google Scholar]