Abstract
Hepatitis C virus genome replication occurs in endoplasmic reticulum-derived membrane compartments, but it is unknown how these structures arise. In this issue, Reiss and colleagues show that the virus recruits a specific lipid kinase to replication sites, stimulates its kinase activity, and alters the phospholipid profile of replication compartments.
All positive-strand RNA viruses replicate their genomes in the cytoplasm of infected cells with virally-encoded enzymes and host factors that associate with cellular membranes. For hepatitis C virus (HCV), a major cause of acute and chronic hepatitis, RNA replication takes place in an endoplasmic reticulum (ER)-derived structure called the “membranous web” (Moradpour et al., 2007). Based on the resistance of isolated HCV replication complexes to protease and nuclease treatment (Quinkert et al., 2005), and their partial sensitivity to detergents, replication is thought to take place within invaginations of a cholesterol- and sphingomyelin-rich membrane (Fig. 1A).
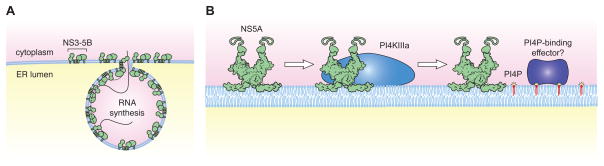
Figure 1. PI4KIIIα Is Needed for HCV Replication Complex Assembly.
(A and B) HCV RNA replication is thought to take place within invaginations of an ER-derived membrane. The viral NS3-4A, NS4B, NS5A, and NS5B proteins are all essential for this process (A). NS5A binds and activates PI4KIII, leading to the local production of PI4P (B). One possibility is that PI4P then recruits viral or cellular factors that are essential for forming the replication compartment.
While it is not yet known how the membranous web forms, expression of the viral nonstructural (NS) protein NS4B can induce similar structures in uninfected cells. Once a functional replication complex forms, the viral genome is copied by the NS5B RNA-dependent RNA polymerase (RdRP), which likely functions in concert with the NS3-4A RNA helicase and NS5A RNA-binding phosphoprotein.
In addition to viral NS proteins, host factors that participate in HCV replication have been identified through several genome-wide and targeted siRNA screens (for instance, see (Berger et al., 2009; Borawski et al., 2009; Li et al., 2009; Tai et al., 2009; Vaillancourt et al., 2009)). Due to differences in experimental design and analysis, few studies identified host factors in common, and specific roles in viral replication have not been determined for most of these host factors. Nevertheless, one essential HCV host factor that was reported across several studies is phosphatidylinositol 4-kinase, subtype III (PI4KIII). This lipid kinase localizes to the ER, Golgi complex, and plasma membrane, and is responsible for phosphorylating the 4 position of the inositol ring in phosphatidylinositol (PI), to generate PI4P. Other PI kinases phosphorylate PI at the 2, 3, 4, and 5 positions, and the action of multiple PI kinases can give rise to mono-, bi- and triphosphorylated species. Thus, the subcellular localization of PI kinases and regulation of their activity determine the profile of phosphatidylinositol phosphates (PIPs) found in different cellular membranes. Since many membrane trafficking proteins are PIP-binding proteins that can discriminate between different PIP species, PI kinases (and PI phosphatases) play important roles in determining the dynamic phospholipid and protein composition of cellular membranes.
In the present study, Reiss and colleagues performed a genome-wide siRNA screen to identify cellular kinases that enhance or inhibit HCV replication. The notable features of this screen were that: i) it was comprehensive for all annotated kinases within the human genome; ii) it was performed with infectious virus; and iii) extensive validation experiments were performed. A total of 13 kinases were identified that, when knocked down, decreased HCV RNA replication. Of these, PI4KIII had the strongest validation scores and was therefore pursued further. The authors showed that PI4KIII colocalized to sites of HCV RNA replication and was required in other cell culture models of HCV RNA replication. In contrast, knockdown of PI4KIIIβ had only minor inhibitory effects on a subset of HCV isolates tested. Importantly, off-target effects of the siRNA knockdown experiments were excluded by showing that HCV replication could be complemented by expression of an siRNA-resistant form of PI4KIII, and that complementation depended on expression of a catalytically active kinase.
To clarify the mechanism by which PI4KIII contributes to viral replication, the authors showed that PI4KIII knockdown inhibited viral NS protein-induced formation of the membranous web. Instead, NS protein expression caused a profusion of double-membrane vesicles, which are often seen adjacent to but distinct from, the membranous web. Thus, PI4KIII was necessary for proper formation of the viral replication compartment. The authors further showed that PI4P accumulates in viral replication compartments within infected cultured cells and in HCV-positive regions of liver biopsies from chronically infected patients. Finally, the most insightful experiment of this paper was the demonstration that the viral NS5A protein binds PI4KIII and stimulates its kinase activity. Taken together, these results show that the interaction between NS5A and PI4KIII plays an essential role in forming the HCV replication complex, most likely by generating PI4P.
A big unanswered question is “how does PI4P contribute to HCV replication?” As stated above, the profile of PIPs within a given membrane helps to regulate the spectrum of cellular proteins that associate with that membrane. Thus, PI4P might be needed to recruit an essential cellular effector protein (Fig. 1B). As noted by Reiss and colleagues in their Discussion, one likely candidate is oxysterol binding protein (OSBP). OSBP binds PI4P though a pleckstrin homology domain, and was previously shown to be essential for HCV replication (Amako et al., 2009). OSBP is itself involved in lipid metabolism, acting as a transporter of cholesterol and oxidized cholesterol, and could be directly involved in producing the sterol-rich membranous web. Thus, PI4KIII -generated PI4P could recruit OSBP to form sites of RNA replication. Clearly more experiments are needed to test this hypothesis and to determine whether additional PI4P-binding proteins are necessary for HCV replication.
In addition to cellular factors, PI4KIII-generated PI4P could serve to mediate the membrane association of viral proteins. For instance, Hsu and colleagues recently showed that enteroviruses, a very distantly related group of positive-strand RNA viruses that includes poliovirus and coxsackievirus, recruit and activate PI4KIIIβ to sites of RNA replication (Hsu et al., 2010). Intriguingly, these authors showed that the poliovirus RdRP preferentially binds PI4P, suggesting that PI4P is need for replication complex assembly. While the HCV RdRP, NS5B, associates with membranes via a C-terminal tail-anchor (Moradpour et al., 2007), it is possible that NS5B has an uncharacterized affinity for PI4P that could reflect a deep evolutionary relationship among positive-strand RNA viruses.
Finally, it is interesting that NS5A is the HCV factor that mediates interaction with PI4KIII. Since NS5A can be phosphorylated on multiple serine residues, it would be interesting to know whether NS5A phosphorylation affects its ability to recruit and activate PI4KIII. If so, this could reflect a dynamic role for NS5A in integrating and coordinating multiple replication processes, including replication site assembly. Furthermore, a new class of NS5A-binding compounds have recently proven to be extremely potent at inhibiting HCV replication, with a half-maximal effectiveness in the picomolar range (Gao et al., 2010). While the molecular basis of their inhibition remains unclear, it is tempting to speculate that they could inhibit NS5A’s ability to recruit PI4KIII and initiate replication complex assembly.
Despite years of study, we have only just begun to scratch the surface in understanding how positive-stand RNA viruses manipulate membrane biology. With this publication, Reiss and colleagues have made a major stride forward, and the field welcomes these insights.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Amako Y, Sarkeshik A, Hotta H, Yates J, 3rd, Siddiqui A. Role of oxysterol binding protein in hepatitis C virus infection. Journal of virology. 2009;83:9237–9246. doi: 10.1128/JVI.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KL, Cooper JD, Heaton NS, Yoon R, Oakland TE, Jordan TX, Mateu G, Grakoui A, Randall G. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borawski J, Troke P, Puyang X, Gibaja V, Zhao S, Mickanin C, Leighton-Davies J, Wilson CJ, Myer V, Cornellataracido I, et al. Class III phosphatidylinositol 4-kinase alpha and beta are novel host factor regulators of hepatitis C virus replication. Journal of virology. 2009;83:10058–10074. doi: 10.1128/JVI.02418-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O’Boyle DR, 2nd, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brass AL, Ng A, Hu Z, Xavier RJ, Liang TJ, Elledge SJ. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci U S A. 2009;106:16410–16415. doi: 10.1073/pnas.0907439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nature reviews. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Quinkert D, Bartenschlager R, Lohmann V. Quantitative analysis of the hepatitis C virus replication complex. Journal of virology. 2005;79:13594–13605. doi: 10.1128/JVI.79.21.13594-13605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ, Chung RT. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell host & microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt FH, Pilote L, Cartier M, Lippens J, Liuzzi M, Bethell RC, Cordingley MG, Kukolj G. Identification of a lipid kinase as a host factor involved in hepatitis C virus RNA replication. Virology. 2009;387:5–10. doi: 10.1016/j.virol.2009.02.039. [DOI] [PubMed] [Google Scholar]