Abstract
Drawbacks to current therapies for rheumatoid arthritis and the high cost of many of these drugs have lead to the investigation of novel approaches for treatment of this disease. One such tactic is the targeting of proteins involved in intracellular signal transduction. Inhibitors of p38 kinase have largely failed in clinical trials, due to both lack of efficacy and adverse events. The degree of adverse events may reflect off-target effects or, conversely, may be a mechanism-related event subsequent to successful inhibition of p38. Drugs targeting Janus kinases or spleen tyrosine kinase have shown greater success in clinical trials. A thorough analysis of specificity, as well as publication of both positive and negative results, must be the goal of continuing trials of these and other inhibitors of signal transduction molecules. The success of many clinical trials in this novel class of drugs provides optimism that more cost-effective and improved therapies will soon be available.
Introduction
Rheumatoid arthritis (RA) is a destructive autoimmune disease with an etiology that remains to be fully elucidated, characterized by infiltration of immune cells into the affected joints, release of inflammatory and degradative mediators, and subsequent joint damage and remodeling [1]. Current therapy relies on global suppression of the immune response or specific blockade of inflammatory cytokines. Although effective in many patients, these treatments can lose efficacy over time, cause minor to significant adverse events, and are extremely costly [2•]. Furthermore, some patients find little to no benefit from these therapies. Therefore, in addition to improving currently used drugs, the development of novel therapeutics continues to be the focus of much research. Among these new approaches are drugs targeting components of intracellular signal transduction pathways.
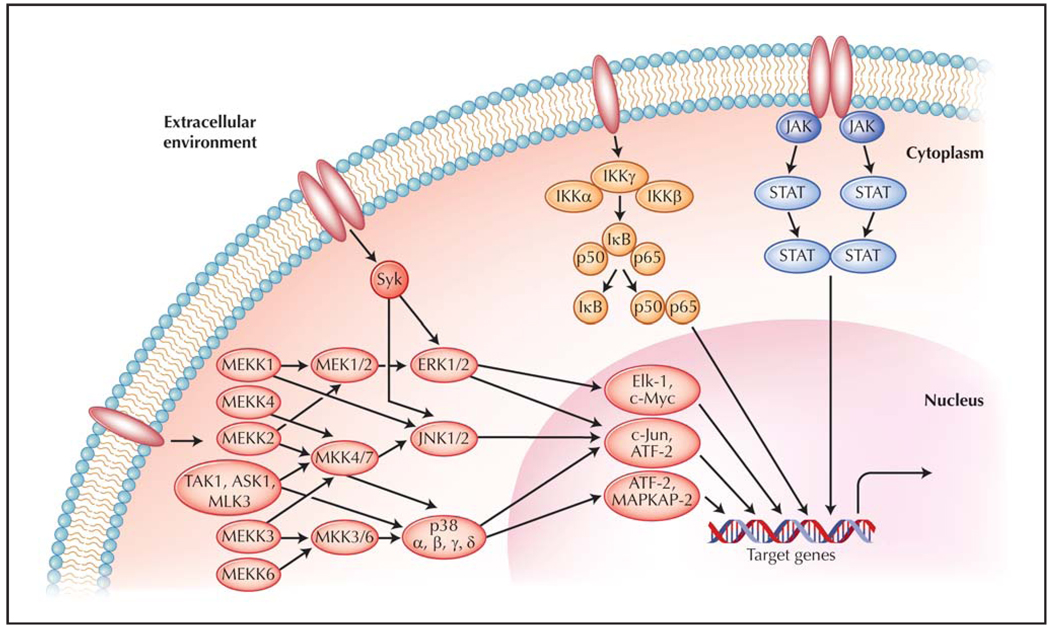
Pathogen-associated molecular patterns, as well as endogenous danger signals, cytokines, chemokines, antibodies, and antigens, bind to receptors on the surface (or in endocytic vesicles) of a variety of cell types [3,4]. This binding typically leads to polymerization of receptors or other structural changes that enable autoactivation or recruitment of binding partners [5]. A cascade of signaling events is then initiated that ultimately converges upon alteration of gene expression or stabilization of mRNA and permits the cell to change its activation status, migrate, or secrete further mediators for a particular response. This subsequent secretion of inflammatory mediators leads to an extracellular milieu abundant in cytokines, chemokines, and other response molecules, resulting in an amplification of the response. Examples of these pathways include the mitogen-activated protein kinase (MAPK) pathway, the Janus kinases (JAK)/signal transducers and activators of transcription (STAT) pathway, spleen tyrosine kinase (Syk) signaling, and the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) pathway (Fig. 1). Cross-signaling frequently exists within and between the pathways, as well as initiation of one pathway by the end-products of another pathway. These signaling cascades are vital for protection of the host from pathogens, but may result in autoimmune disease when aberrantly activated.
Figure 1.
Schematic of intracellular signaling cascades. Cellular exposure to cytokines, chemokines, growth factors, pathogen-associated molecular patterns or antigens, endogenous danger signals, or stressors such as ultraviolet rays or absence of growth factors results in receptor ligation. Subsequent initiation of signaling cascades leads to altered expression patterns of genes involved in inflammation, degradation of extracellular matrix, apoptosis, and other cellular processes important in mounting an appropriate response to the stimuli. ASK—apoptosis signal-regulating kinase; ATF—activating transcription factor; ERK—extracellular signal-regulated kinase; IκB—inhibitor of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB); IKK—IκB kinase; JAK—Janus tyrosine kinase; JNK—c-Jun N-terminal kinase; MAP-KAP— mitogen-activated protein kinase (MAPK) activated protein; MEK—MAPK/ERK kinase; MEKK—MAPK kinase kinase/MEK kinase; MLK—mixed lineage kinase; MKK—MAPK kinase; STAT—signal transducer and activator of transcription; Syk—spleen tyrosine kinase; TAK—transforming growth factor-β–associated kinase.
Many drugs are in development to target these pathways and eliminate their overactivation that may lead to symptoms and damage associated with RA (Table 1). Because of the cross-signaling and amplification loops involved, targeting a component of one pathway may also lead to inhibition of other pathways. Furthermore, these small molecule inhibitors of signaling pathway proteins can be produced via low-cost methods, compared with complex proteins that require tissue culture or other biological processes for production. This could lead to a dramatic reduction in cost compared with the currently used tumor necrosis factor (TNF)–α blockers and other biologics.
Table 1.
Drugs targeting intracellular signal transduction proteins for the treatment of rheumatoid arthritis and their current status in clinical trials
| Study | Drug | Manufacturer (location) | Target | Phase of clinical trial in RA and status |
|---|---|---|---|---|
| Damjanov et al. [8] | VX-702 | Vertex Pharmaceuticals (Cambridge, MA) |
p38α | 2 (C) |
| Genovese et al. [9] | SCIO-469 | Scios (Mountain View, CA) |
p38α | 2 (A, NR) |
| Hill et al. [10], Cohen et al. [11] |
Pamapimod | Hoffmann-La Roche (Basel, Switzerland) |
p38α | 2 (C) |
| Carter et al. [12], Lee et al. [13] |
ARRY-371797 | Array Biopharma (Boulder, CO) |
p38α | 1 (C) |
| Kaul et al. [15], Wang et al. [16] |
BMS-582949 | Bristol-Myers Squibb (New York, NY) |
p38 | 2 (C) |
| Monahan et al. [17] | PH-797804 | Pfizer (New York, NY) |
p38α | 2 (C) |
| Carter et al. [18,19], Wright et al. [20] |
ARRY-438162 | Array Biopharma (Boulder, CO) |
MEK | 2 (A, NR) |
| Williams et al. [25] | INCB018424 | Incyte Corporation (Wilmington, DE) |
JAK1/2 (TYK2) |
2 (C) |
| Jiang et al. [26], West [27••], Kremer et al. [28••], Silverfield et al. [29], Lawendy et al. [30] |
CP-690,550 | Pfizer (New York, NY) |
JAK3 | 3 (R) |
| Weinblatt et al. [33••] | Fostamatinib | Rigel Pharmaceuticals (South San Francisco, CA) |
Syk | 2 (A, NR) |
| D’Aura Swanson et al. [37•], Paniagua et al. [38], Rosengren and Boyle [39], Eklund and Joensuu [40] |
Imatinib | Novartis (Basel, Switzerland) |
abl, c-kit, PDGF-R |
2 (C) |
| Moss et al. [48] | Apratastat | Wyeth Pharmaceuticals (Madison, NJ) |
MMP/TACE | 2 (A, NR) |
| Kurose et al. [53], Liggins et al. [54] |
Paxceed (micellar paclitaxel) |
Angiotech Pharmaceuticals (Vancouver, Canada) |
Induction of apoptosis |
2 (C) |
A, NR—active, not recruiting; C—completed; JAK—Janus kinases; MEK—mitogen-activated protein kinase extracellular signal-regulated kinase kinase; MMP—matrix metalloproteinase; PDGF-R—platelet-derived growth factor receptor; R—recruiting; RA—rheumatoid arthritis; Syk—spleen tyrosine kinase; TACE—TNF-α–converting enzyme.
Mitogen-Activated Protein Kinases
One signaling pathway that is activated in the context of an immune or autoimmune response is that of the serine/threonine MAPK. Initiated by cytokine receptors, Toll-like receptors, and other danger signals, the pathway begins with the MAPK kinase kinases (MAP3K), which phosphorylate and activate the MAPK kinases (MKK), which then phosphorylate MAPK, ultimately leading to the activation of various transcription factors (Fig. 1) [6]. The MAPK include extracellular signal-regulated kinases (ERK), c-Jun amino-terminal kinases (JNK), and p38 kinase (p38). ERK1 and 2, activated by signaling from growth factor receptors and certain cytokine receptors, activate the transcription factors Elk-1 and c-Myc. JNK1 and JNK2, primarily activated by signaling from cytokine receptors and following other stressors, such as absence of growth factors or ultraviolet ray exposure, phosphorylate activator protein 1 (AP-1), although ERK1/2 and p38 can also activate this transcription factor (contributing to the considerable overlap in the system). p38α and β, typically activated by signaling from Toll-like receptors, as well as in response to oxidative stress, inflammatory cytokines, and osmotic shock, activate activating transcription factor (ATF)-2 and MAPK-activated protein-2. The role of MAPK in transmitting signals from inflammatory cytokines such as TNF-α, which have proven to be successful targets in the treatment of RA, have made the MAPK themselves attractive targets for the development of new therapies.
Due to in vitro and in vivo evidence that this pathway is significantly involved in the pathogenesis of arthritis, it has been the focus of much attention in drug development in recent years [7•]. However, when the highly anticipated results from two 12-week studies on a p38α inhibitor, VX-702 (Vertex Pharmaceuticals; Cambridge, MA), were published, the outcome was disappointing. Despite a trend toward an increased percentage of patients meeting the American College of Rheumatology (ACR) 20% improvement criteria (ACR20) in the treatment groups receiving the drug compared with placebo, the data were not statistically significant [8]. Furthermore, there was no discernable dose-dependent effect of the drug when patients were also treated with methotrexate. Although a transient decrease in the inflammatory markers C-reactive protein (CRP), soluble TNF receptor 1 (sTNFR1), and serum amyloid A (SAA) was observed, levels returned to baseline by week 4. Adverse events included serious infections in 2.4% of patients receiving the drug compared with none in the placebo group in one of the studies, whereas both groups displayed a low rate of adverse events in the other study (2.6% in the VX-702–treated group versus 4.9% in the placebo group). There was also a small dose-dependent QT prolongation in patients receiving the drug. No consistent laboratory abnormalities were seen, including elevations in liver enzymes.
The results of a trial with another p38α inhibitor, SCIO-469 (Scios; Mountain View, CA), were similarly disappointing. A 12-week study, with a 12-week extension phase, was conducted with doses of 100 mg extended release (ER) once a day, 30 mg immediate release (IR) three times a day, or 60 mg IR three times a day. Despite early signs of efficacy, ACR20 results were not statistically significant at week 12 (placebo, 24%; 100 mg ER, 23%; 30 mg IR, 26%; and 60 mg IR, 33%) nor were the differences from placebo in swollen joint count (SJC), tender joint count (TJC), or CRP levels [9]. Furthermore, an increased incidence of adverse events was observed in the treatment groups compared with placebo, particularly skin rash; a slight increase in the percentage of patients with alanine transaminase (ALT) elevation was also noted.
Pamapimod (RO4402257; Hoffmann-La Roche, Basel, Switzerland), a p38α inhibitor with very low p38β activity, was another drug that, despite promising preclinical data, yielded disappointing results in clinical studies [10,11]. Following 12 weeks of treatment, the percentage of patients treated with 50, 150, or 300 mg reaching ACR20 was 23%, 18%, and 31%, respectively, compared with 45% of patients on methotrexate. A higher percentage of patients on pamapimod experienced at least one adverse event compared with methotrexate, typically including dizziness, skin disorders, infections, and gastrointestinal problems.
Despite this rather disappointing track record, ongoing interest in other p38 kinase inhibitors has led to continuing clinical research in this area. The p38α inhibitor ARRY-371797 (ARRY-797; Array BioPharma; Boulder, CO) was shown to be well tolerated for 14 days in phase 1 studies [12]. In laboratory studies, lipopolysaccharide (LPS) stimulation of peripheral blood from treated subjects showed inhibition up to 100% of prostaglandin E2, IL-1β, and TNF compared with matched blood pretreatment [13]. This study, along with in vivo studies using the collagen-induced arthritis (CIA) and adjuvant-induced arthritis rodent models, suggests that the drug may be effective in treating inflammatory diseases such as RA [13]. At this time, however, no phase 2 trials in RA are under way [14].
Another p38 inhibitor, BMS-582949 (Bristol-Myers Squibb; New York, NY), showed promise in its ability to significantly reduce plasma concentrations of TNF-α and IL-1β following LPS treatment of healthy subjects [15]. In another small study, the drug was generally well-tolerated with mild adverse events including rash, dizziness, and headache in RA patients receiving methotrexate [16]. This study was not powered to enable statistical evaluation of efficacy, but a phase 2 study to evaluate efficacy in RA patients is currently recruiting patients [14]. PH-797804, a p38α inhibitor in development by Pfizer (New York, NY), led to reduced TNF-α and IL-6 levels following endotoxin administration [17]. A phase 2 study investigating safety and pharmacokinetics in RA patients on background methotrexate has concluded, but safety and efficacy data have not been published [14].
Other targets in the MAPK pathway are also under investigation. ARRY-438162 (ARRY-162; Array BioPharma; Boulder, CO) is an inhibitor of the MAPK extracellular signal-regulated kinase (MEK). Phase 1 studies demonstrated good tolerability and the drug was able to inhibit 12-O-tetradecanoylphorbol-13-acetate–induced IL-1β, TNF, and IL-6 production ex vivo [18,19]. Phase 2 studies are under way in patients with RA. Some evidence suggests that in addition to inhibition of cytokine production, the drug blocks osteoclast differentiation and reduces bone resorption [20]. Other MEK inhibitors in preclinical studies include RDEA119 (Ardea Biosciences; San Diego, CA) [21] and PD184352 (Pfizer; New York, NY) [22]. A drug targeting both JNK and p38, semapimod (CNI-1493; Cytokine PharmaSciences; King of Prussia, PA), was recently investigated in phase 2 and 3 studies in Crohn’s disease, although it has not yet been studied in RA [14].
Intracellular Tyrosine Kinases
Janus kinases
Ligation of interferon (IFN) and IL-6 receptors, as well as those of many other cytokines and growth factors, results in receptor crosslinking and phosphorylation of JAK bound to the receptor (Fig. 1) [23•]. These kinases then phosphorylate the receptors to which they are bound, enabling the subsequent binding of signal transducers and activators of transcription (STATs). The STATs are phosphorylated by JAK, dissociate, dimerize via their Src homology 2 (SH2) domains, and translocate to the nucleus, where they initiate transcription of target genes. Whereas common γ-chain receptors use a combination of JAK1 and 3, the IFN-γ receptor uses JAK1/2, and receptors involved in hematopoietic cell development and proliferation employ JAK2.
Because of the significant role IL-6 plays in RA pathogenesis, and other evidence suggesting the JAK/STAT pathway contributes to the disease, several JAK inhibitors have been developed and clinical trials are under way [24]. Small early studies of INCB018424 (Incyte Corporation; Wilmington, DE), an inhibitor of JAK1/2 with some activity against TYK2 and less against JAK3, showed clinical improvement in multiple parameters including ACR20, ACR 50% improvement criteria (ACR50), ACR 70% improvement criteria (ACR70), and ACR 90% improvement criteria, as well as 28 joint Disease Activity Score (DAS28) with mild adverse events [25].
The most studied candidate in this pathway is CP-690,550 (Pfizer; New York, NY), a small molecule that predominantly blocks JAK3 [26,27••]. Results from phase 2 clinical trials demonstrated an ACR20 response in up to 60.6% of CP-690,550–treated subjects compared with 37.7% in subjects receiving placebo following 12 weeks of treatment, with an ACR50 of up to 46.7% compared with 17.4% in placebo and an ACR70 of up to 25.3% compared with 5.8% in placebo [28••]. DAS28 remission rates of up to 37.7% compared with 8.8% in placebo were also observed. Mild side effects occurred in a dose-dependent manner, and a small number of patients had reversible ALT increases of greater than three times the upper limit of normal. Currently, the long-term drug safety in combination with methotrexate is under investigation, with interim data demonstrating that the drug is generally well-tolerated (the most common adverse events were urinary tract infections and diarrhea) [29]. Laboratory parameters, including serum creatinine levels, absolute neutrophil counts, and hemoglobin levels, all remained within normal limits. CP-690,550 has now entered phase 3 trials with at least six currently active trials [14]. Importantly, in a study of healthy volunteers, this drug was shown to have no effect on creatinine clearance, effective renal plasma flow, or glomerular filtration rate [30].
Spleen tyrosine kinase
The ligation of fragment crystallizable–γ (Fc-γ) receptors and B-cell receptors results in crosslinking and activation of the Src family kinases, which phosphorylate the intracellular domain of the receptors leading to the recruitment of Syk (Fig. 1) [31]. The subsequent cascade of phosphorylation, in part involving phosphoinositide 3-kinase (PI3K) activation, leads to calcium mobilization and MAPK activation. This pathway has also been of interest in developing drugs for the treatment of RA [32••]. Phase 2 trials of R788 (fostamatinib) from Rigel Pharmaceuticals (South San Francisco, CA) in patients with active RA despite methotrexate treatment demonstrated promising results [33••]. The percentage of patients treated with 100 mg or 150 mg twice a day achieving an ACR20, ACR50, or ACR70 was 65% and 72%, 49% and 57%, and 33% and 40%, respectively, compared with 38%, 19%, and 4% in the placebo group. A similar percentage of patients reported at least one adverse event in the placebo and treatment groups; however, the percentage of patients developing the most common adverse events, diarrhea and neutropenia, were elevated in the patients receiving the study drug compared with placebo, in a dose-dependent manner. Multiple phase 2 studies are ongoing. PI3K inhibitors are also under investigation in preclinical studies [34–36].
Other tyrosine kinases
Imatinib mesylate (Gleevec; Novartis, Basel, Switzerland) is a tyrosine kinase inhibitor specific for Abelson murine leukemia viral oncogene homolog 1 (abl), c-kit (also known as CD117), colony-stimulating factor 1 receptor, leukocyte-specific protein tyrosine kinase, and platelet-derived growth factor receptor currently used to treat various cancers [37•]. This drug reduced inflammatory mediator production and arthritis severity in preclinical in vitro and in vivo studies [38,39]. A report on three patients with refractory RA treated with imatinib suggested that the drug resulted in symptomatic improvement, despite withdrawal of one patient due to rash [40]. The drug was also investigated in a phase 2 trial in combination with methotrexate, but no results were published following the conclusion of the study [14].
Other Targets
Transcription factors
The direct targeting of transcription factors is an intriguing approach that has not yet moved as far into the clinical realm as targeting signal transduction pathway proteins. However, a number of transcription factor inhibitors have shown promise in preclinical studies. The Toyama Chemical drug T-5224 (Tokyo, Japan), an inhibitor of c-Fos/AP-1, reduced CIA through reduction of inflammatory cytokine and matrix metalloproteinase (MMP)-1 levels [41]. Another logical transcription factor target would be NF-κB, given its role in the transcription of cytokines and adhesion molecules important in arthritis and in protection against TNF-α–mediated cytotoxicity (Fig. 1) [42]. Indeed, evidence suggests that overactivation of NF-κB, via overexpression of inhibitor of NF-κB kinase β (IKKβ), results in synovial inflammation [43]. Supporting this, IKKβ blockade with several different inhibitors has been shown to reduce joint inflammation and destruction in animal models of arthritis [44–46]. Despite this preclinical data, no regulators of the NF-κB signal transduction pathway are currently being investigated in human trials [14].
Other proteins regulating cytokine production
Following the success of extracellular TNF-α inhibitors in the treatment of RA, many drugs inhibiting TNF-α converting enzyme (TACE) were examined in preclinical or phase 1 studies; most were not pursued due to apparent hepatotoxicity [47]. A combined MMP-TACE inhibitor, apratastat (TMI-005; Wyeth Pharmaceuticals, Madison, NJ), displayed no hepatotoxicity, but was also relatively ineffective in phase 2 trials [48]. Our laboratory recently demonstrated that the cyclin-dependent kinase (CDK) inhibitor p21(WAF1/CIP1) (p21) plays a role in the suppression of inflammatory cytokine production and development of arthritis in vivo and that peptidomimetics to this protein reduce the production of inflammatory cytokines in vitro [49,50]. Further investigation of the inhibitory properties of these p21 peptidomimetics may yield beneficial therapeutics in the treatment of inflammatory diseases, such as RA. In addition, because the mechanism by which p21 regulates cell cycle progression is via inhibition of the function of CDKs, the possibility exists that p21 also acts through CDKs in the inhibition of inflammatory cytokine production. As small molecular inhibitors of CDKs are already in use clinically in the treatment of cancer, these drugs may also prove useful in the treatment of RA.
Inducers of apoptosis
Programmed cell death is executed through highly regulated signaling pathways initiated by ligation of death receptors on the cell surface (extrinsic pathway) or an imbalance in the cytoplasmic levels of proapoptotic and antiapoptotic proteins (intrinsic pathway) [51]. Synoviocytes and immune cells present in the synovial lining of RA-affected joints display a resistance to apoptosis, suggesting that drugs which induce apoptosis may have a role in the treatment of this disease [51,52]. Paclitaxel, an antimitotic that stabilizes microtubules and induces apoptosis, is in use clinically for cancer chemotherapy. Preclinical data showed efficacy in reducing the severity of antigen- and carrageenan-induced rabbit models of arthritis and in inducing apoptosis in human synoviocytes [53,54]. Phase 2 clinical trials investigating the use of paxceed (Angiotech Pharmaceuticals, Vancouver, BC, Canada), a micellar form of paclitaxel, in RA have recently concluded, although the results have not yet been published [14]. Recently, we have investigated the efficacy of a peptidomimetic to the proapoptotic BH3-only Bim protein in an animal model of arthritis (unpublished data and [55]). Similar to ABT-737 (Abbott Laboratories; Abbott Park, IL), a small molecule inhibitor of the antiapoptotic proteins Bcl-2, Bcl-xL, and Bcl-w, we demonstrated that treatment with the Bim peptidomimetic ameliorated arthritis development, reduced the number of myeloid cells in the joint, and enhanced apoptosis [56]. Furthermore, treatment with the Bim peptidomimetic did not result in the significant lymphopenia and thrombocytopenia observed with ABT-737 treatment, suggesting that treatment with the Bim peptidomimetic may result in fewer adverse events.
Conclusions
The lack of clinical efficacy and the high rate of adverse events seen in the p38 MAPK inhibitor trials highlight several problems in designing drugs that target these critical intracellular signaling pathways. First, the structural similarity among many kinases calls into question the true specificity of the drugs that target them. Off-target effects may account for many of the side effects observed. Second, the importance of these pathways in host defense against disease has naturally resulted in redundancy in signaling. Thus, inhibition of one signaling component may be compensated for by increased signaling through a complementary pathway. This leaves investigators with a conundrum—lack of specificity may result in off-target effects causing increased side effects, but too much specificity may result in lack of efficacy due to redundancy in signaling. Conversely, adverse events may also be the result of successful inhibition of the target. The similar adverse event profile of the p38 MAPK inhibitors suggests that p38 may play a significant role in homeostasis, as well as in disease states, and its inhibition therefore results in unacceptable side effects. Despite these limitations, p38 inhibitors are still being actively investigated in clinical trials, though some believe their outlook is not promising.
Another problem in designing drugs to treat RA is the lack of an animal model that truly recapitulates human disease. Although many models share some features with human RA, no model encompasses all of the characteristics of human disease. Furthermore, drugs that show great efficacy in animal models often fail in clinical trials. Therefore, an important focus of research remains the search for an improved disease model. One novel approach with great potential is the development of a “humanized mouse” in which hematopoietic stem cells from RA patients are used to repopulate a lethally irradiated mouse. The strengths of this model are the ability to monitor changes of the human immune system before and during disease progression, as well as to determine the relative contributions of genetics and environmental factors in the development of RA. Additionally, this model will provide a novel approach to assessing potential new therapies that can be employed with mice possessing competent human immune systems from patients.
Despite the disappointment of p38 inhibition to date, the targeting of intracellular signaling proteins may still prove successful in the treatment of RA. Both the JAK3 inhibitor CP-690,550 and the Syk inhibitor R788 (fostamatinib) have displayed encouraging efficacy and acceptable tolerability profiles in clinical studies, and new targets are continually being pursued. Important considerations for these studies must include a thorough analysis of specificity, which can guide interpretation of both positive and negative results as well as the development of adverse events. Without such evaluations (and publication of all positive and negative data), the results from clinical trials may be misinterpreted and the progress of the field may suffer. Genovese [57••] recently published an excellent commentary on the importance of publication of both positive and negative results in clinical trials of intracellular signaling modifiers, as well as the current status of inhibition of p38 in the treatment of arthritis.
As basic science continues to elucidate the molecular mechanisms of various intracellular pathways involved in physiologic and pathologic inflammatory responses, new therapeutic targets are constantly identified. The current success of clinical trials involving inhibitors of many of these targets provides hope that more effective therapeutics for RA available at a lower cost than current treatments are on the horizon.
Acknowledgment
This work was supported by grants to Dr. Harris Perlman from the National Institutes of Health: National Institute of Arthritis and Musculoskeletal and Skin Diseases (5R01AR054796-03) and National Institute of Allergy and Infectious Diseases (7R21AI067590-0308).
Footnotes
Disclosure
Dr. Ruderman has been a consultant for UCB, Abbott, and Genentech, and has received research funding from Abbott, Array Pharma, Targeted Genetics, and Biogen Idec.
No further potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2. Donahue KE, Gartlehner G, Jonas DE, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148:124–134. doi: 10.7326/0003-4819-148-2-200801150-00192.This article reviews studies of head-to-head comparisons of disease-modifying drugs for efficacy or adverse event outcomes. It also highlights the dearth of such studies, which prevents drawing conclusions about the superiority of treatment regimens.
- 3.O’Neill LAJ. Primer: Toll-like receptor signaling pathways–what do rheumatologists need to know? Nat Clin Pract Rheumatol. 2008;4:319–327. doi: 10.1038/ncprheum0802. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rincón M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 6.Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 7. Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–916. doi: 10.1136/ard.2007.074278. This review provides an excellent description of the p38 MAPK pathway and summarizes the evidence that this pathway plays a role in the pathogenesis of RA.
- 8.Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009;60:1232–1241. doi: 10.1002/art.24485. [DOI] [PubMed] [Google Scholar]
- 9.Genovese MC, Cohen SB, Wofsy D, et al. A randomized, double-blind, placebo-controlled phase 2 study of an oral p38α MAPK inhibitor, SCIO-469, in patients with active rheumatoid arthritis [abstract 715]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 10.Hill RJ, Dabbagh K, Phippard D, et al. Pamapimod, a novel p38 mitogen-activated protein kinase inhibitor: preclinical analysis of efficacy and selectivity. J Pharmacol Exp Ther. 2008;327:610–619. doi: 10.1124/jpet.108.139006. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SB, Cheng T-T, Chindalore V, et al. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 2009;60:335–344. doi: 10.1002/art.24266. [DOI] [PubMed] [Google Scholar]
- 12.Carter L, Litwiler K, Neale J, et al. ARRY-797, a novel p38 MAP kinase inhibitor: results of a 14-day phase 1 study [abstract 359]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 13.Lee P, Pheneger J, Brown S, et al. ARRY-797, a selective, potent inhibitor of p38, promotes disease normalization in animal models of rheumatoid arthritis and significantly reduces ex vivo IL-1 beta, PGE2, and TNF-alpha production in normal human subjects [abstract SAT0052] Ann Rheum Dis. 2007;66 Suppl 2:445. [Google Scholar]
- 14.National Institutes of Health: Clinicaltrials.gov registry. [Accessed May 2009]; Available at http://clinicaltrials.gov.
- 15.Kaul S, Hess H, Ji P, et al. Anti-inflammatory effects of BMS-582949, a P38 mitogen activated protein kinase (MAPK) inhibitor, during experimental endotoxemia in healthy male subjects [abstract 354]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 16.Wang J, Kaul S, Campanha H, et al. Multiple ascending dose study of a potent p38 MAPK inhibitor BMS-582949 in subjects with stable RA receiving concomitant methotrexate [abstract 356]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 17.Monahan JB, Hope H, Schindler J, et al. Anti-inflammatory properties of a novel N-phenyl pyridinone inhibitor of p38 MAP kinase: preclinical to clinical translation [abstract FRI0001]. Presented at the European League Against Rheumatism Annual European Congress of Rheumatology; June 10–13; Copenhagen, Denmark. 2009. [Google Scholar]
- 18.Carter L, Brown S, Klopfenstein N, et al. ARRY-162, a novel inhibitor of MEK kinase: phase 1A-1B pharmacokinetic and pharmacodynamic results [abstract OP-0124] Ann Rheum Dis. 2008;67 Suppl 2:87. [Google Scholar]
- 19.Carter L, Brown S, Klopfenstein N, et al. ARRY-162, a novel MEK inhibitor: results of a 14-day phase 1a study in healthy subjects and a 28-day phase 1b study in rheumatoid arthritis patients [abstract 358]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 20.Wright AD, Pheneger J, Gomez A, et al. ARRY-162, a potent and selective inhibitor of the MEK1/2, inhibits osteoclast differentiation and bone resorption in adjuvant-induced arthritis [abstract FRI0063]. Presented at the European League Against Rheumatism Annual European Congress of Rheumatology; June 10–13; Copenhagen, Denmark. 2009. [Google Scholar]
- 21.Miampamba M, Elow D, Eide L, et al. Selective MEK1/2 inhibitors RDEA436 and RDEA119 exhibit anti-inflammatory efficacy in mono and combination therapy with methotrexate in pristane-induced arthritis in rats [abstract SAT0138]. Presented at the European League Against Rheumatism Annual European Congress of Rheumatology; June 10–13; Copenhagen, Denmark. 2009. [Google Scholar]
- 22.Thiel MJ, Schaefer CJ, Lesch ME, et al. Central role of the MEK/ERK MAP kinase pathway in a mouse model of rheumatoid arthritis: potential proinflammatory mechanisms. Arthritis Rheum. 2007 56;:3347–3357. doi: 10.1002/art.22869. [DOI] [PubMed] [Google Scholar]
- 23. Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. This review presents an in-depth description of the JAK-STAT signaling pathway and touches on the future of research related to this pathway.
- 24.Walker JG, Smith MD. The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol. 2005;32:1650–1653. [PubMed] [Google Scholar]
- 25.Williams W, Scherle P, Shi J, et al. A randomized placebo-controlled study of INCB018424, a selective Janus kinase1& 2 (JAK1&2) inhibitor in rheumatoid arthritis (RA) [abstract 714]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 26.Jiang J-k, Ghoreschi K, Deflorian F, et al. Examining the chirality, conformation and selective kinase inhibition of 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile (CP-690,550) J Med Chem. 2008;51:8012–8018. doi: 10.1021/jm801142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. West K. CP-690550, a JAK3 inhibitor as an immuno-suppressant for the treatment of rheumatoid arthritis, transplant rejection, psoriasis and other immune-mediated disorders. Curr Opin Investig Drugs. 2009;10:491–504. This review offers an outstanding summary of the preclinical data on CP-690550, as well as the clinical development of the drug for use in RA, transplant rejection, psoriasis, and other diseases.
- 28. Kremer J, Cohen S, Wilkinson B, et al. The Oral Jak inhibitor CP-690,550 (CP) in combination with methotrexate (MTX) is efficacious, safe and well tolerated in patients with active rheumatoid arthritis (RA) with an inadequate response to methotrexate alone [abstract L13]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. This study presents results from a double-blind placebo-controlled phase 2B trial of 509 RA patients receiving a range of CP-690,550 doses for 12 weeks, including data on efficacy (ACR20, 50, and 70; health assessment questionnaire disability index; and DAS28) and adverse events.
- 29.Silverfield J, Connell C, Bloom B, et al. CP-690,550, an oral JAK inhibitor, is a well-tolerated and effective long-term treatment for patients with moderate to severe rheumatoid arthritis [abstract 716]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 30.Lawendy N, Krishnaswami S, Wang R, et al. Effect of CP-690,550, an orally active Janus kinase inhibitor, on renal function in healthy adult volunteers. J Clin Pharmacol. 2009;49:423–429. doi: 10.1177/0091270008330982. [DOI] [PubMed] [Google Scholar]
- 31.Sada K, Takano T, Yanagi S, Yamamura H. Structure and function of Syk protein-tyrosine kinase. J Biochem. 2001;130:177–186. doi: 10.1093/oxfordjournals.jbchem.a002970. [DOI] [PubMed] [Google Scholar]
- 32. Bajpai M, Chopra P, Dastidar SG, Ray A. Spleen tyrosine kinase: a novel target for therapeutic intervention of rheumatoid arthritis. Expert Opin Investig Drugs. 2008;17:641–659. doi: 10.1517/13543784.17.5.641. This description of the role of Syk in RA pathogenesis also highlights the inhibitors of Syk currently under investigation. Importantly, it further addresses adverse effects that have resulted or may occur due to Syk inhibition.
- 33. Weinblatt ME, Kavanaugh A, Burgos-Vargas R, et al. Treatment of rheumatoid arthritis with a syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:3309–3318. doi: 10.1002/art.23992. This article provides data from a phase 2 study of 189 RA patients treated with various doses of the Syk kinase inhibitor R788, including information regarding efficacy (ACR20, 50, and 70; DAS28; and serum IL-6 and MMP-3 levels) and adverse events.
- 34.Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. Eur J Immunol. 2008;38:1215–1224. doi: 10.1002/eji.200838266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camps M, Ruckle T, Ji H, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 36.Tohyama S, Tamura N, Haruta K, et al. Anti-rheumatic effect of ZSTK474, a novel phosphoinositide 3-kinase inhibitor, by inhibiting osteoclast formation [abstract 1788]. Presented at the American College of Rheumatology Annual Scientific Meeting; November 6–11; Boston, MA. 2007. [Google Scholar]
- 37. D’Aura Swanson C, Paniagua RT, Lindstrom TM, Robinson WH. Tyrosine kinases as targets for the treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:317–324. doi: 10.1038/nrrheum.2009.82. This article describes the roles of many tyrosine kinases in the pathogenesis of RA, including JAK, Syk, and platelet-derived growth factor, among others. It also enumerates the inhibitors of these targets currently being tested as treatments for RA.
- 38.Paniagua RT, Sharpe O, Ho PP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116:2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosengren S, Boyle DL. Imatinib inhibits synergistic effects of PDGF and TGFβ on inflammatory mediator synthesis by synoviocytes and limits arthritis [abstract 2026]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 40.Eklund KK, Joensuu H. Treatment of rheumatoid arthritis with imatinib mesylate: clinical improvement in three refractory cases. Ann Med. 2003;35:362–367. doi: 10.1080/07853890310001339. [DOI] [PubMed] [Google Scholar]
- 41.Aikawa Y, Morimoto K, Yamamoto T, et al. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat Biotechnol. 2008;26:817–823. doi: 10.1038/nbt1412. [DOI] [PubMed] [Google Scholar]
- 42.Miagkov AV, Kovalenko DV, Brown CE, et al. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tak PP, Gerlag DM, Aupperle KR, et al. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 2001;44:1897–1907. doi: 10.1002/1529-0131(200108)44:8<1897::AID-ART328>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.McIntyre KW, Shuster DJ, Gillooly KM, et al. A highly selective inhibitor of IkappaB kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 2003;48:2652–2659. doi: 10.1002/art.11131. [DOI] [PubMed] [Google Scholar]
- 45.Tas SW, Vervoordeldonk MJ, Hajji N, et al. Local treatment with the selective IkappaB kinase beta inhibitor NEMO-binding domain peptide ameliorates synovial inflammation. Arthritis Res Ther. 2006;8:R86. doi: 10.1186/ar1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mbalaviele G, Sommers CD, Bonar SL, et al. A novel, highly selective, tight binding I{kappa}B kinase-2 (IKK-2) inhibitor: a tool to correlate IKK-2 activity to the fate and functions of the components of the nuclear factor-{kappa}B pathway in arthritis-relevant cells and animal models. J Pharmacol Exp Ther. 2009;329:14–25. doi: 10.1124/jpet.108.143800. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Hegen M, Xu J, et al. Characterization of (2R, 3S)-2-( [4-(2-butynyloxy)phenyl]sulfonyl amino)-N,3-dihydroxybutanamide, a potent and selective inhibitor of TNF-[alpha] converting enzyme. Int Immunopharmacol. 2004;4:1845–1857. doi: 10.1016/j.intimp.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Moss ML, Sklair-Tavron L, Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008;4:300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 49.Scatizzi JC, Mavers M, Hutcheson J, et al. The CDK domain of p21 is a suppressor of IL-1beta-mediated inflammation in activated macrophages. Eur J Immunol. 2009;39:820–825. doi: 10.1002/eji.200838683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mavers M, Balomenos D, Perlman H. The cyclin dependent kinase inhibitor p21(WAF1/CIP1) is a suppressor of inflammatory cytokine production and inflammatory arthritis [abstract 2083]. Presented at the American College of Rheumatology Annual Scientific Meeting; October 24–29; San Francisco, CA. 2008. [Google Scholar]
- 51.Perlman H, Pagliari LJ, Volin MV. Regulation of apoptosis and cell cycle activity in rheumatoid arthritis. Curr Mol Med. 2001;1:597–608. doi: 10.2174/1566524013363429. [DOI] [PubMed] [Google Scholar]
- 52.Korb A, Pavenstädt H, Pap T. Cell death in rheumatoid arthritis. Apoptosis. 2009;14:447–454. doi: 10.1007/s10495-009-0317-y. [DOI] [PubMed] [Google Scholar]
- 53.Kurose A, Yoshida W, Yoshida M, Sawai T. Effects of paclitaxel on cultured synovial cells from patients with rheumatoid arthritis. Cytometry. 2001;44:349–354. doi: 10.1002/1097-0320(20010801)44:4<349::aid-cyto1126>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 54.Liggins RT, Cruz T, Min W, et al. Intra-articular treatment of arthritis with microsphere formulations of paclitaxel: biocompatibility and efficacy determinations in rabbits. Inflamm Res. 2004;53:363–372. doi: 10.1007/s00011-004-1273-1. [DOI] [PubMed] [Google Scholar]
- 55.Scatizzi JC, Hutcheson J, Perlman H. Bim-BH3 peptide mimetic therapy is effective at preventing development of inflammatory arthritis [abstract 1770]. Presented at the American College of Rheumatology Annual Scientific Meeting; November 6–11; Boston, MA. 2007. [Google Scholar]
- 56.Bardwell PD, Gu J, McCarthy D, et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol. 2009;182:7482–7489. doi: 10.4049/jimmunol.0802813. [DOI] [PubMed] [Google Scholar]
- 57. Genovese MC. Inhibition of p38: has the fat lady sung? Arthritis Rheum. 2009;60:317–320. doi: 10.1002/art.24264. This editorial summarizes the current status of targeting p38 in the treatment of RA. However, the importance of this article also comes from its analysis of the development of this field and emphasis on the importance of publishing both positive and negative results from clinical trials to point researchers and patients toward therapies that truly hold potential.