Abstract
Plasmodium falciparum, the most important etiological agent of human malaria, is endowed with a highly complex cell cycle that is essential for its successful replication within the host. A number of evidence suggest that changes in parasite Ca2+ levels occur during the intracellular cycle of the parasites and play a role in modulating its functions within the RBC. However, the molecular identification of Plasmodium receptors linked with calcium signalling and the causal relationship between Ca2+ increases and parasite functions are still largely mysterious. We here describe that increases in P. falciparum Ca2+ levels, induced by extracellular ATP, modulate parasite invasion. In particular, we show that addition of ATP leads to an increase of cytosolic Ca2+ in trophozoites and segmented schizonts. Addition of the compounds KN62 and Ip5I on parasites blocked the ATP-induced rise in [Ca2+]c. Besides, the compounds or hydrolysis of ATP with apyrase added in culture drastically reduce RBC infection by parasites, suggesting strongly a role of extracellular ATP during RBC invasion. The use of purinoceptor antagonists Ip5I and KN62 in this study suggests the presence of putative purinoceptor in P. falciparum. In conclusion, we have demonstrated that increases in [Ca2+]c in the malarial parasite P. falciparum by ATP leads to the modulation of its invasion of red blood cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-010-9202-y) contains supplementary material, which is available to authorized users.
Keywords: Purinergic receptor, Malaria, Plasmodium falciparum, Calcium signalling, Red blood cells
Introduction
Malaria is one of the most important infectious diseases in the world, and increased resistance to the classic antimalarial drugs is becoming a major health challenge in the Third World [1, 2]. Decoding Plasmodium signalling pathways is thought to represent a fundamental step in developing new strategies to fight the disease [3, 4]. Malaria parasites are able to sense signalling molecules released by the host: for example, it has been reported that Plasmodium falciparum at the trophozoite stage are sensitive to indol-related molecules such as melatonin or N-acetyl-serotonin, serotonin and tryptamine, and these hormones are capable of modulating the circadian rhythm of both murine and human Plasmodia [5–7]. It has also been shown that infected RBC can release other potentially important signalling molecules, in particular ATP; indeed, P. falciparum-infected RBC release much more ATP when compared to non-infected RBC [8].
Nucleosides and nucleotides (adenosine, ADP, ATP, UDP and UTP) bind to the so-called purinergic receptors and mediate several biological processes in most eukaryotic cells [9]. The purinergic receptors can be divided into major classes: P1 receptors activated by adenosine and P2 receptors activated by ATP and other nucleotides. P2 receptors comprised two receptor families: P2X receptors are ATP-gated ion channels, whereas P2Y receptors belong to the super family of G protein-coupled receptors [10]. In either case, however, the activation of purinergic receptors results in increases of cytosolic Ca2+ concentration [9], and some P2 receptors couple to cAMP accumulation (P2Y11) and others to cAMP inhibition (P2Y12-14) [11–15].
In this paper, we report that ATP elicits cytosolic [Ca2+] increases when added to isolated P. falciparum parasites both at the trophozoite and segmented schizont stages. Most importantly, blocking the ATP receptors (or causing its hydrolysis by adding an exogenous ATPase, apyrase) before invasion strongly inhibits the invasion of RBC by P. falciparum in vitro assays.
Experimental procedures
Parasites
P. falciparum, 3D7 strain, was maintained in continuous in vitro culture in adult human red blood cells [16], and the synchronization was achieved by sorbitol treatment [17]. Parasitemia were determined from Giemsa-stained thin films, as previously described [12].
Spectrofluorimetric determinations
Infected red blood cells 108 ml−1 were lysed with 10 mg ml−1 saponin (in PBS); RBC membrane was removed by centrifugation (8,700×g for 10 min at 4°C). The parasites were washed twice in buffer A (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgS04, 5.5 mM d-glucose, 50 mM MOPS and 2 mM CaCl2, pH 7.2) and resuspended in the same buffer containing 40 μM probenecid, an inhibitor of organic anion transport, to prevent Fluo-4 release and sequestration [18]. Fluo-4/AM in DMSO (1 mg ml−1) was added to reach a final concentration of 5 μM, and the suspension was incubated for 1 h at 37°C. The cells were washed three times with buffer A to remove extracellular dye, but in some experiments, calcium was omitted and 3 mM EGTA was added instead. In each experiment, an aliquot of 100 μl (105 cells) was placed in a termostatted cuvette equipped with magnetic stirring. Spectrofluorimetric measurements with Fluo-4/AM were performed using the F-4500 Hitachi spectrofluorimeter (Tokyo, Japan) with excitation at 505 nm and emission at 530 nm (for details, see [5]).
The calibration curves, relating the fluorescence of intact cells to free Ca2+ concentration, were calculated using the Ca2+ software F-4500 Intracellular Cation Measurement System, version 1.02 (Copyright© Hitachi, Ltd., 1994–1995), which takes into account that [Ca2+]c = kd (F − Fmin)/Fmax − F)], where the kd utilized for Fluo-4 was 345 nM, F is the measured fluorescence intensity in the conditions of the experiment, Fmax the fluorescence in the presence of digitonin and Fmin the fluorescence in the presence of digitonin and 8 mM EGTA. All the experiments were performed at 37°C. In experiments with antagonists, Fluo-4 cells were dispensed in a 96-well black clear bottom plates previously treated with polilysine at a density of 5 × 104 cells/well. The cells were incubated with the antagonists for 15 min before the addition of the agonist. The responses were measured immediately after the addition of the ATP (50 μM) as agonist. Fluorescence was measured using a FlexStation® (Molecular Devices, Sunnyvale, CA, USA), a 96-well fluorescence spectrometer, at an excitation wavelength of 485 and emission at 525 nm. All experiments were performed at 37°C. Compounds were added from a 96-well reservoir plate, with the pipette heights, volumes of additions (usually 10–20 μl), rate of addition and mixing protocols optimized to minimize disturbance of the cells whilst ensuring rapid mixing. The data were stored for later analysis by using SoftmaxPro (Molecular Devices). As all other dyes of the tetracarboxylate family, the fluorescence of Fluo-4 and its affinity for Ca2+ are insensitive to pH down to a value of 6.5 or below.
Stock solutions of nucleotides ATP, CTP, GTP, UTP, α,β-methyleneATP and purinoceptor antagonists Ip5I, TNP-ATP, PPADS and suramin (Sigma) were made in buffer A, whilst KN-62 and thapsigargin were dissolved in DMSO. When necessary, stock solutions of ATP were made in buffer A, but without Ca2+.
Assay of merozoite invasion inhibition
Cultures of synchronized parasites at schizont stage were set up in 24-well, flat-bottomed, microculture plates (500 μl/well) and incubated with purinoceptor inhibitors KN-62 (1 μM) and Ip5I (1 μM) or recombinant apyrase (10 nM) from Schistosoma mansoni (SmATPDase). The compounds and enzyme were added separately on microcultures during early segmented-schizont stage and incubated at 37°C for 17 h after the addition of the compounds, and then parasitemia of re-invaded erythrocytes was determined as described [6]. Each experiment was carried out in triplicate. Parasite cultures were also incubated in the presence or absence of ATP or ATP analogues (α,β-methylene ATP) or PPADS for 20 h at 37°C. Solvent blanks in the absence of any drug were performed as controls.
Data analyses
Calcium measurement data were analysed using Prism 4.03 (GraphPad, San Diego, CA, USA). The change in fluorescence was calculated as F (peak fluorescence) minus F0 (baseline fluorescence) after normalization of fluorescence to the baseline. Statistical analyses were carried out using the unpaired Student’s t test. Values of p ≤0.05 were considered to indicate a statistically significant difference.
Results
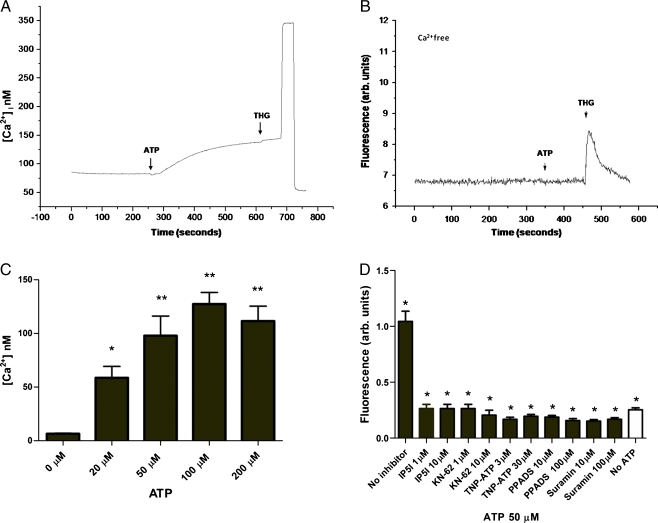
It has been recently demonstrated that Plasmodia-infected erythrocytes can release large amounts of ATP in the medium [8]. ATP and other purines are known to be coupled to numerous signalling pathways (cAMP and Ca2+ in particular) in many eukaryotic cell types. Thus, ATP can potentially modulate the function of both isolated parasites during invasion of RBC and/or parasites within erythrocytes. We thus investigated if extracellular purines (ADP, ATP, CTP, GTP and UTP) could modulate [Ca2+]c in isolated parasites at the trophozoite stage. Isolated P. falciparum trophozoites or schizonts were loaded with Fluo-4 in a Ca2+-containing medium. The addition of CTP, GTP and ADP (from 10 to 100 μM) did not result in appreciable [Ca2+]c rises in the parasites (Electronic supplementary material (ESM) Fig. S1). On the contrary, Fig. 1a shows that addition of ATP induces a clear [Ca2+]c response in trophozoites. This response was maximal at 100 μM ATP, and half-maximal response was obtained at about 20 μM ATP (Fig. 1c). The addition of UTP, a selective agonist for P2Y2 and P2Y4 (10 μM), also led to a [Ca2+]c rise in the parasites (ESM Fig. S1).
Fig. 1.
Effects of ATP on Fluo-4-labelled trophozoites from P. falciparum. a Kinetics of the [Ca2+]c response to ATP added to isolated trophozoites in the presence of 2 mM Ca2+, and thapsigargin (THG) was added after some time. b As in a, but ATP was added in the absence of Ca2+ (and the medium was also supplemented with 3 mM EGTA). Where indicated, ATP (100 μM, arrow) was added and no [Ca2+]c rise was observed. The large rise and drop of fluorescence at the end of the experiment of a represents the addition of digitonin (100 μM) and EGTA (8 mM), respectively, needed for calibrating the signal in terms of [Ca2+]. c Dose-dependent increase of the ATP-induced [Ca2+]c rises, means ± SEM (n ≥ 4). d Isolated trophozoites were treated with ATP (50 μM) upon pretreatment (15 min) with the following purinoceptor inhibitors: Ip5I, KN-62, TNP-ATP, PPADS and suramin at the indicated concentrations. Inhibitors drastically blocked [Ca2+]c responses on parasites. *Data of [Ca2+]c responses from parasites pretreated with inhibitors were significantly different from that only ATP-treated (p < 0.01). The data are presented as means ± SEM (n ≥ 4)
Malaria parasites display calcium pools [19–22], and to investigate whether the [Ca2+]c rise elicited by ATP originates from internal Ca2+ pools or whether it depends on the influx of Ca2+ from the medium, ATP was added in the absence of extracellular Ca2+ (and in the presence of 3 mM EGTA). Figure 1b shows that the removal of external Ca2+ completely prevented the ATP-induced [Ca2+]c rise in trophozoites. However, no significant difference was observed between trophozoites and schizonts (ESM Fig. S2a). Interestingly, addition of UTP (10 μM) in the absence of extracellular calcium did not induce an [Ca2+]c rise in trophozoites (ESM Fig S2b).
We further investigated the Ca2+ response by performing similar experiments in the presence of the following purinoceptor antagonists: Ip5I (a selective antagonist for P2X1 and P2X3); KN-62 (a selective antagonist of P2X7 receptors in humans but not rat); TNP-ATP (a highly effective antagonist at P2X1, P2X2/3 and P2X3 receptors with very weak actions at P2X4 receptors); and PPADS and suramin (non-selective P2X and P2Y receptor antagonists) [23–25]. Figure 1d shows that the addition of ATP (50 μM) to trophozoites that have been previously incubated with the purinoceptor antagonists Ip5I (1 or 10 μM), KN-62 (1 or 10 μM), TNP-ATP (3 or 30 μM) and PPADS (10 or 100 μM) failed to elicit any [Ca2+]c rise in Plasmodium parasites. Collectively, the data are consistent with a primary role of purinoceptor in ATP-elicited [Ca2+]c rise in P. falciparum.
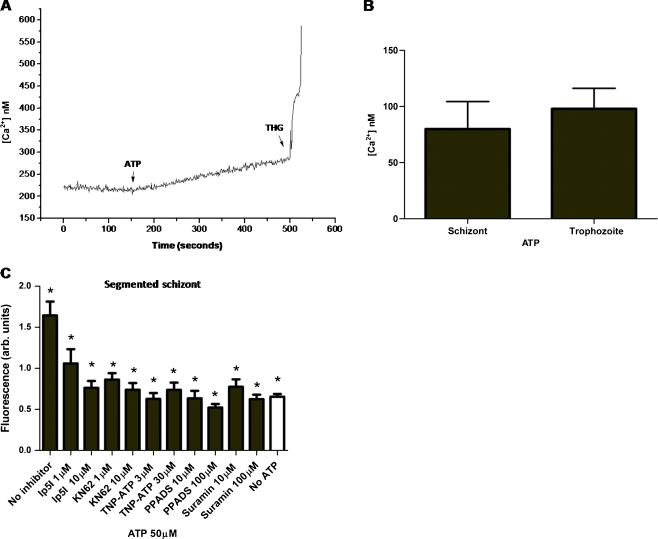
We next investigated whether the ATP-dependent [Ca2+]c increases were a unique feature of trophozoites or whether they could be observed also at other parasite stages. Figure 2a shows an experiment in segmented schizonts loaded with Fluo-4 (in Ca2+ medium). Addition of ATP (50 μM) leads to a [Ca2+]c rise also in parasites at this developmental stage (Fig. 2b). The increase in [Ca2+]c caused by 50 μM ATP in schizonts (79.9 ± 24.4 nM) is similar to that elicited in parasites at the trophozoite stage (97.88 ± 18.24 nM, Fig. 2b).
Fig. 2.
Effects of ATP on [Ca2+]c in P. falciparum segmented schizonts. Stimulation of Fluo-4-labelled isolated segmented schizont with the addition of ATP (50 μM) in the presence of Ca2+ (2 mM) induced fluorescence increases. a Kinetics of the [Ca2+]c response to ATP. b Histogram showing [Ca2+]c response obtained with ATP (50 μM) on the schizont and trophozoite stages. The data are presented as mean ± SEM (n ≥ 4). c Isolated shizonts were treated with ATP (50 μM) upon pretreatment (15 min) with the following purinoceptor inhibitors: Ip5I, KN-62, TNP-ATP, PPADS and suramin at the indicated concentrations. Inhibitors drastically blocked [Ca2+]c responses on parasites. *Data of [Ca2+]c responses from parasites pretreated with inhibitors were significantly different from that only ATP-treated (p < 0.01). The data are presented as means ± SEM (n ≥ 4)
Similarly to trophozoite stage parasites, calcium experiments were performed in the presence of the compounds Ip5I, KN-62, TNP-ATP, PPADS and suramin at segmented schizonts. Figure 2c shows that addition of ATP (50 μM) to schizonts that have been previously incubated with the purinoceptor antagonists Ip5I (1 or 10 μM), KN-62 (1 or 10 μM), TNP-ATP (3 or 30 μM) and PPADS (10 or 100 μM) failed to elicit any [Ca2+]c rise in Plasmodium parasites.
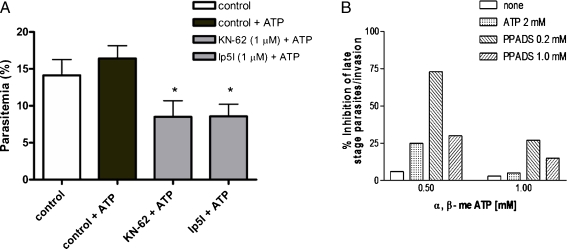
The question then arises as to the functional role of these [Ca2+]c increases elicited by ATP and UTP. In order to address this, we performed invasion assays with parasites in in vitro cultures in the presence of exogenous ATP or upon addition of the compounds KN-62 (1 μM) or Ip5I (1 μM). Figure 3 shows that ATP added to the medium during infection resulted in a small, non-significant increase of RBC invasion, whilst the purinergic antagonists strongly reduced the invasion process: the number of newly invaded cells was 16.41 ± 1.73% in controls and 8.5 ± 2.18% and 8.57 ± 1.65% upon KN-62 and Ip5I treatments, respectively. We have also investigated the effect of a selective agonist of P2X1, P2X3 and P2X2/3 receptors, α,β-methylene ATP, on P. falciparum cultures. Figure 3b shows the inhibition of parasite invasion in the presence of different concentrations of the antagonist PPADS (0.2 and 1 mM) and α,β-methylene ATP (0.5 and 1 mM). Therefore, it is clear that ATP had effects in malaria parasites, but the receptor subtype or subtypes involved in the response need to be investigated.
Fig. 3.
Effects of the purinergic receptor inhibitors KN-62 and Ip5I on P. falciparum merozoite invasion of human erythrocytes. a Cultures of Plasmodia during segment schizont stage were incubated in the presence of ATP (50 μM) with 1 μM KN-62 or 1 μM Ip5I for 17 h at 37°C. Percentage of parasitemia presented as mean ± SEM are based on three independent experiments in triplicate. *Data from cultures treated with compounds were significantly different from cultures ATP-treated (p < 0.05). b Effect of ATP and PPADS in reversing, α,β-methylene ATP effect on P. falciparum invasion of RBCs. The figure shows the inhibition of parasite invasion in the presence of different concentrations of the antagonist PPADS (0.2 and 1 mM) and agonists α,β-methylene ATP (0.5 and 1 mM) or ATP (2 mM). Bars represent the numbers of rings (average), expressed as a percentage of control
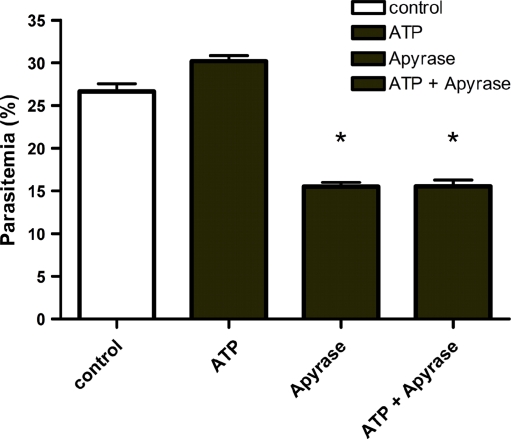
To further support the conclusion that extracellular ATP plays an important role in the invasion process (and that inhibition by purinoceptor blockers is not a side effect of the drugs), the invasion assay was carried out in the presence of recombinant apyrase from S. mansoni. Apyrase hydrolyzes extracellular ATP and thus prevents purinoceptor activation by locally released ATP. When the invasion assay was carried out in the presence of apyrase, the number of newly invaded cells was reduced from 26.0 ± 0.9% to 15.8 ± 0.5%, whereas parasitemia in the presence of ATP was reduced from 30.2 ± 0.6% to 15.5 ± 0.7% (p < 0.05, Fig. 4). Clearly, the target of the purinoceptor inhibitors, or of apyrase, are the parasites and not the RBC: the drugs, in fact, had no effect on the percentage of invaded cells (measured 17 h later) when they were added 1 h after the initiation of invasion, i.e. when the parasites are inside the RBC (ESM Fig. S3). Similarly, the addition of extracellular ATP after invasion was without effects (ESM Fig. S3).
Fig. 4.
Effects of extracellular apyrase on the invasion of human red blood cells by P. falciparum merozoite. Recombinant apyrase from S. mansoni (SmATPDase2) was added to the culture of plasmodia during segment schizont stage in the presence and absence of ATP (50 μM). The graph shows clearly a reduction of parasitemia using apyrase, suggesting strongly the importance of P. falciparum purinergic receptor actived by ATP during the invasion process of parasites to human RBC. Data are presented as mean ± SEM of three independent experiments in triplicate. Mean percentage of parasitemia of the groups treated with apyrase were significantly different from their control groups (without apyrase, p < 0.05). Control culture media without ATP, ATP culture media with ATP
We then asked whether the early [Ca2+]c increases elicited by ATP only affected the efficiency of RBC invasion or whether it had any late effect during the intraerythrocytic cell cycle. We thus investigated whether the intracellular development of P. falciparum was affected by the pre-incubation with either the purinoceptor inhibitors or with apyrase. Although the percentage of infected cells was reduced by these treatments (see above), the intracellular development of the parasites was normal, as determined by Giemsa staining (ESM Fig S4).
Discussion
Recently, it has been demonstrated that erythrocytes infected by Plasmodia are capable of releasing large amounts of ATP in the medium [8]. Given the potential signalling properties of ATP, we have thus investigated whether it could affect a key second messenger signalling, i.e. [Ca2+]c of P. falciparum. The results in the isolated parasites are straightforward: P. falciparum, both at the schizont and trophozoite stages, express ATP receptors, probably more than one subtype [9], and these receptors involved in the response need to be investigated. We also found that these purinergic receptors play a key role during Plasmodium invasion of RBC as blocking these receptors impairs invasion, probably abolishing the Ca2+ rises induced by ATP. The lack of significant effects of exogenously added ATP on the efficiency of RBC infection suggests that the erythrocytes release enough ATP in the local environment to saturate the parasite ATP receptors.
Indeed, the addition of apyrase, a potent ATP-consuming enzyme, to the medium during infection drastically reduces RBC invasion. The effects of purinoceptor blockers and of apyrase are clearly due to their interfering with ATP modulation of parasite and not to RBC functions, given that the addition of these drugs, after the engulfment of the Plasmodia, is totally ineffective. The molecular target of the early Ca2+ rise caused by ATP in segmented schizonts remains undetermined, and it may be speculated that the Plasmodium cytoskeleton could be involved, given the major importance that cytoskeletal proteins play in the invasion process. On the contrary, the normal development of the parasites, pretreated with purinoceptor inhibitors, within the RBC suggests that ATP receptors have no major role at the later stage of the intraerythrocytic cycle. This conclusion remains, however, to be directly tested, given that it remains to be established whether or not the inhibitors remain at sufficient concentration within the parasitophorous vacuole and whether they can bind to the purinergic receptors in that environment. The impermeability of the RBC membrane to the inhibitors does not allow at present to directly test this possibility.
It is well established that malaria parasites utilize calcium for cell signalling [26–35]. Melatonin [6], its precursor N-acetylserotonin, tryptamine, serotonin and AFMK are able to affect parasite cell cycle [5, 7, 36–38]. These molecules induce calcium release from stores in P. falciparum and in the rodent malaria parasite Plasmodium chabaudi. In P. falciparum, melatonin increases cytosolic cAMP and Ca2+ concentration,[Ca2+]c, with both second messengers interacting with each other in a synergistic manner [29, 39], as in many other eukaryotes [40]. A molecular target of Ca2+ in Plasmodia has been described by us recently [41], i.e. a calcium-dependent protease activated at the throphozoite stage in P. chabaudi and P. falciparum.
Our finding that purinergic receptors are expressed in P. falciparum and that they can trigger Ca2+ rise essential for the invasion process [42, 43] opens the possibility of screening for molecules that would selectively inhibit these purinoceptors. Additionally, the role of ATP in P. falciparum and adenosine in P. yoelii have been previously reported [44, 45, 46]. The phylogenetic distance between humans and P. falciparum (and the lack of sequences homologous to those of human purinoceptors in the P. falciparum genome) [9, 47] may allow the development of inhibitors specific to the form expressed in the parasites, leaving unaffected the human receptors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Addition of nucleosides CTP, GTP and ADP has no effect on calcium rise in trophozoites of P. falciparum. However, UTP (10 μM) is able to increase [Ca2+]c. Each experiment was carried out in triplicates at least three times. Student’s t test was applied, *p = 0.0322 (DOC 231 kb)
a ATP does not lead to a Ca2+ rise in absence of external calcium (adding 3 mM EGTA) in schizont parasites. Where indicated, ATP (100 μM) was added. b Addition of UTP (10 μM) does not induce an increase [Ca2+]c in trophozoite parasites in the absence extracellular of calcium. Thapsigargin addition as control is able to raise [Ca2+]c (DOC 95 kb)
Cultures of Plasmodium 1 h post-invasion were incubated in the presence of ATP (50 μM), KN-62 (1 μM) and apyrase (10 nM) for 17 h at 37°C. Percentage of parasitemia presented as mean ± SEM are based on three independent experiments in triplicate (DOC 262 kb)
Development of parasites after in vitro treatment with KN62, Ip5I or apyrase during 24 or 48 h. P. falciparum-infected RBCs do not change morphology after treatment for 24 or 48 h, showing that these inhibitors act on parasite invasion but do not have any action on parasite development inside the RBC. Slides were fixed with methanol and stained with Giemsa (DOC 1833 kb)
Acknowledgements
We are grateful to Dr. Sergio Verjovski de Almeida (Chemistry Institute, São Paulo University) for kindly providing the recombinant apyrase from S. mansoni. This work was supported by grants from Brazilian Agencies FAPESP, CNPq (to C.R.S.G)-INCT-InBqmed, and Pronex Malaria-MS-DECIT.
Abbreviations
- THG
Thapsigargin
- [Ca2+]c
Calcium cytosolic concentration
- RBC
Red blood cell
- SmATPDase
S. mansoni ATP-diphosphohydrolase
References
- 1.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7:341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 2.Haldar K, Hiller NL, Ooij C, Bhattacharjee S. Plasmodium parasite proteins and the infected erythrocyte. Trends Parasitol. 2005;21:402–403. doi: 10.1016/j.pt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doerig C. Protein kinases as targets for anti-parasitic chemotherapy. Biochim Biophys Acta. 2004;1697:155–168. doi: 10.1016/j.bbapap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Beraldo FH, Garcia CRS. Products of tryptophan catabolism induce Ca2+ release and modulate the cell cycle of Plasmodium falciparum malaria parasites. J Pineal Res. 2005;39:224–230. doi: 10.1111/j.1600-079X.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 6.Hotta CT, Gazarini ML, Beraldo FH, Varotti FP, Lopes C, Markus RP, Pozzan T, Garcia CRS. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat Cell Biol. 2000;2:466–468. doi: 10.1038/35017112. [DOI] [PubMed] [Google Scholar]
- 7.Hotta CT, Markus RP, Garcia CRS. Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites. Braz J Med Biol Res. 2003;36:1583–1587. doi: 10.1590/S0100-879X2003001100016. [DOI] [PubMed] [Google Scholar]
- 8.Akkaya C, Shumilina E, Bobballa D, Brand VB, Mahmud H, Lang F, Huber SM. The Plasmodium falciparum-induced anion channel of human erythrocytes is an ATP-release pathway. Pflügers Arch Eur J Physiol. 2009;457:1035–1047. doi: 10.1007/s00424-008-0572-8. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol. 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyden L, Adams DJ, Luttrell BM, Conigrave AD, Morris MB. Pharmacological characterisation of the P2Y11 receptor in stably transfected haematological cell lines. Mol Cell Biochem. 2000;213:75–81. doi: 10.1023/A:1007168215748. [DOI] [PubMed] [Google Scholar]
- 12.Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- 13.Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to G(i) J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 14.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 15.Zhang FL, Luo L, Gustafson E, Palmer K, Qiao X, Fan X, Yang S, Laz TM, Bayne M, Monsma F., Jr P2Y(13): identification and characterization of a novel Galphai-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther. 2002;301:705–713. doi: 10.1124/jpet.301.2.705. [DOI] [PubMed] [Google Scholar]
- 16.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science (New York, N.Y) 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 17.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 18.Virgilio F, Steinberg TH, Silverstein SC. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990;11:57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- 19.Varotti FP, Beraldo FH, Gazarini ML, Garcia CR. Plasmodium falciparum malaria parasites display a THG-sensitive Ca2+ pool. Cell Calcium. 2003;33:137–144. doi: 10.1016/S0143-4160(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 20.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. doi: 10.1016/S0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 21.Biagini GA, Bray PG, Spiller DG, White MR, Ward SA. The digestive food vacuole of the malaria parasite is a dynamic intracellular Ca2+ store. J Biol Chem. 2003;278:27910–27915. doi: 10.1074/jbc.M304193200. [DOI] [PubMed] [Google Scholar]
- 22.Garcia CRS, Dluzewski AR, Catalani LH, Burting R, Hoyland J, Mason WT. Calcium homeostasis in intraerythrocytic malaria parasites. Eur J Cell Biol. 1996;71:409–413. [PubMed] [Google Scholar]
- 23.Wildman SS, King BF. P2X receptors: epithelial ion channels and regulators of salt and water transport. Nephron Physiol. 2008;108:p60–p67. doi: 10.1159/000122028. [DOI] [PubMed] [Google Scholar]
- 24.Bavan S, Straub VA, Blaxter ML, Ennion SJ. A P2X receptor from the tardigrade species Hypsibius dujardini with fast kinetics and sensitivity to zinc and copper. BMC Evol Biol. 2009;9:17. doi: 10.1186/1471-2148-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 26.Moreno SN, Docampo R. Calcium regulation in protozoan parasites. Curr Opin Microbiol. 2003;6:359–364. doi: 10.1016/S1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 27.Vaid A, Sharma P. PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum: II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. J Biol Chem. 2006;281:27126–27133. doi: 10.1074/jbc.M601914200. [DOI] [PubMed] [Google Scholar]
- 28.Gazarini ML, Thomas AP, Pozzan T, Garcia CRS. Calcium signaling in a low calcium environment: how the intracellular malaria parasite solves the problem. J Cell Biol. 2003;161:103–110. doi: 10.1083/jcb.200212130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia CRS, Azevedo MF, Wunderlich G, Budu A, Young JA, Bannister L. Plasmodium in the postgenomic era: new insights into the molecular cell biology of malaria parasites. Int Rev Cell Mol Biol. 2008;266(266):85−+. doi: 10.1016/S1937-6448(07)66003-1. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman M, Chaparro J. Intraerythrocytic calcium chelators inhibit the invasion of Plasmodium falciparum. Parasitol Res. 1996;82:102–107. doi: 10.1007/s004360050078. [DOI] [PubMed] [Google Scholar]
- 31.McCallum-Deighton N, Holder AA. The role of calcium in the invasion of human erythrocytes by Plasmodium falciparum. Mol Biochem Parasitol. 1992;50:317–323. doi: 10.1016/0166-6851(92)90229-D. [DOI] [PubMed] [Google Scholar]
- 32.LaGreca N, Hibbs AR, Riffkin C, Foley M, Tilley L. Identification of an endoplasmic reticulum-resident calcium-binding protein with multiple EF-hand motifs in asexual stages of Plasmodium falciparum. Mol Biochem Parasitol. 1997;89:283–293. doi: 10.1016/S0166-6851(97)00134-5. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto Y, Perry G, Scheibel LW, Aikawa M. Role of calmodulin in Plasmodium falciparum: implications for erythrocyte invasion by the merozoite. Eur J Cell Biol. 1987;45:36–43. [PubMed] [Google Scholar]
- 34.Scheibel LW, Colombani PM, Hess AD, Aikawa M, Atkinson CT, Milhous WK. Calcium and calmodulin antagonists inhibit human malaria parasites (Plasmodium falciparum): implications for drug design. Proc Natl Acad Sci USA. 1987;84:7310–7314. doi: 10.1073/pnas.84.20.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billker O, Lourido S, Sibley LD. Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe. 2009;5:612–622. doi: 10.1016/j.chom.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beraldo FH, Mikoshiba K, Garcia CRS. Human malarial parasite, Plasmodium falciparum, displays capacitative calcium entry: 2-aminoethyl diphenylborinate blocks the signal transduction pathway of melatonin action on the P. falciparum cell cycle. J Pineal Res. 2007;43:360–364. doi: 10.1111/j.1600-079X.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 37.Budu A, Peres R, Bueno VB, Catalani LH, Garcia CR. N1-acetyl-N2-formyl-5-methoxykynuramine modulates the cell cycle of malaria parasites. J Pineal Res. 2007;42:261–266. doi: 10.1111/j.1600-079X.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 38.Garcia CR, Markus RP, Madeira L. Tertian and quartan fevers: temporal regulation in malarial infection. J Biol Rhythms. 2001;16:436–443. doi: 10.1177/074873001129002114. [DOI] [PubMed] [Google Scholar]
- 39.Beraldo FH, Almeida FM, Silva AM, Garcia CR. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J Cell Biol. 2005;170:551–557. doi: 10.1083/jcb.200505117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 41.Farias SL, Gazarini ML, Melo RL, Hirata IY, Juliano MA, Juliano L, Garcia CR. Cysteine-protease activity elicited by Ca2+ stimulus in Plasmodium. Mol Biochem Parasitol. 2005;141:71–79. doi: 10.1016/j.molbiopara.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Wasserman M. The role of calcium ions in the invasion of Plasmodium falciparum. Blood Cells. 1990;16:450–451. [PubMed] [Google Scholar]
- 43.Vaid A, Thomas DC, Sharma P. Role of Ca2+/calmodulin-PfPKB signaling pathway in erythrocyte invasion by Plasmodium falciparum. J Biol Chem. 2008;283:5589–5597. doi: 10.1074/jbc.M708465200. [DOI] [PubMed] [Google Scholar]
- 44.Ayi K, Liles WC, Gros P, Kain KC. Adenosine Triphosphate Depletion of Erythrocytes Simulates the Phenotype Associated with Pyruvate Kinase Deficiency and Confers Protection against Plasmodium falciparum In Vitro. Journal of Infectious Diseases. 2009;200:1289–1299. doi: 10.1086/605843. [DOI] [PubMed] [Google Scholar]
- 45.Tanneur V, Duranton C, Brand VB, Sandu CD, Akkaya C, Kasinathan RS, Gachet C, Sluyter R, Barden JA, Wiley JS, et al. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J. 2006;20:133–135. doi: 10.1096/fj.04-3371fje. [DOI] [PubMed] [Google Scholar]
- 46.Gati WP, Lin AN, Wang TI, Young JD, Paterson AR. Parasite-induced processes for adenosine permeation in mouse erythrocytes infected with the malarial parasite Plasmodium yoelii. Biochem J. 1990;272:277–280. doi: 10.1042/bj2720277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagamune K, Sibley LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol Biol Evol. 2006;23:1613–1627. doi: 10.1093/molbev/msl026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Addition of nucleosides CTP, GTP and ADP has no effect on calcium rise in trophozoites of P. falciparum. However, UTP (10 μM) is able to increase [Ca2+]c. Each experiment was carried out in triplicates at least three times. Student’s t test was applied, *p = 0.0322 (DOC 231 kb)
a ATP does not lead to a Ca2+ rise in absence of external calcium (adding 3 mM EGTA) in schizont parasites. Where indicated, ATP (100 μM) was added. b Addition of UTP (10 μM) does not induce an increase [Ca2+]c in trophozoite parasites in the absence extracellular of calcium. Thapsigargin addition as control is able to raise [Ca2+]c (DOC 95 kb)
Cultures of Plasmodium 1 h post-invasion were incubated in the presence of ATP (50 μM), KN-62 (1 μM) and apyrase (10 nM) for 17 h at 37°C. Percentage of parasitemia presented as mean ± SEM are based on three independent experiments in triplicate (DOC 262 kb)
Development of parasites after in vitro treatment with KN62, Ip5I or apyrase during 24 or 48 h. P. falciparum-infected RBCs do not change morphology after treatment for 24 or 48 h, showing that these inhibitors act on parasite invasion but do not have any action on parasite development inside the RBC. Slides were fixed with methanol and stained with Giemsa (DOC 1833 kb)