Abstract
The response to ATP of peritoneal macrophages from wild-type (WT) and P2X7-invalidated (KO) mice was tested. Low concentrations (1–100 μM) of ATP transiently increased the intracellular concentration of calcium ([Ca2+]i) in cells from both mice. The inhibition of the polyphosphoinositide-specific phospholipase C with U73122 inhibited this response especially in WT mice suggesting that the responses coupled to P2Y receptors were potentiated by the expression of P2X7 receptors. One millimolar ATP provoked a sustained increase in the [Ca2+]i only in WT mice. The response to 10 μM ATP was potentiated and prolonged by ivermectin in both mice. One millimolar ATP increased the influx of extracellular calcium, decreased the intracellular concentration of potassium ([K+]i) and stimulated the secretion of interleukin-1β (IL-1β) only in cells from WT mice. Ten micromolar ATP in combination with 3 μM ivermectin reproduced these responses both in WT and KO mice. The secretion of IL-1β was also increased by nigericin in WT mice and the secretory effect of a combination of ivermectin with ATP in KO mice was suppressed in a medium containing a high concentration of potassium. In WT mice, 150 μM BzATP stimulated the uptake of YOPRO-1. Incubation of macrophages from WT and KO mice with 10 μM ATP resulted in a small increase of YOPRO-1 uptake, which was potentiated by addition of 3 μM ivermectin. The uptake of this dye was unaffected by pannexin-1 blockers. In conclusion, prolonged stimulation of P2X4 receptors by a combination of low concentrations of ATP plus ivermectin produced a sustained activation of the non-selective cation channel coupled to this receptor. The ensuing variations of the [K+]i triggered the secretion of IL-1β. Pore formation was also triggered by activation of P2X4 receptors. Higher concentrations of ATP elicited similar responses after binding to P2X7 receptors. The expression of the P2X7 receptors was also coupled to a better response to P2Y receptors.
Keywords: Macrophages, Inflammation, Cytokines
Introduction
Tissue damage triggers the activation of macrophages and an inflammatory reaction. ATP is a potential mediator of this response because damaged cells release high concentrations of this nucleotide. When exposed to ATP, macrophages previously primed with lipopolysaccharides (LPS) secrete mature IL-1β. This response to ATP is coupled to the expression of P2X7 receptors [1]. This receptor is an ionotropic receptor which, like all the P2X receptors, has two transmembrane domains and intracellular N- and C-terminal sequences. The binding of an agonist promotes the formation of a functional hetero- or homotrimer which is a non-selective cation channel permeant to calcium, sodium, potassium and protons [2]. The P2X7 receptor is the most peculiar P2X receptor: it has a much longer C-terminal tail which promotes its interaction with intracellular proteins [3]. This structural difference accounts for the unique ability of this receptor to form a pore permeant to charged molecules up to 800 Da after prolonged stimulation. This will ultimately provoke the death of the cell [4].
The pathway leading from receptor occupancy to IL-1β secretion by macrophages is not yet fully understood. The assembly of a multiprotein platform, the inflammasome, recruits procaspase-1, and triggers the proteolytic activation of this proenzyme. Caspase-1, in turn, converts the inactive proIL-1β to its active IL-1β form [5]. Several observations confirm the role of potassium in the activation of this process by purinergic agonists. First, the opening of the non-selective cation channel coupled to P2X7 receptors provokes a massive efflux of potassium [4]. Second, the secretion of IL-1β in response to extracellular ATP is blunted when primed macrophages are incubated in the presence of a high concentration of extracellular potassium [6]. Third, nigericin (a K+/H+ exchanger) or toxins like maitotoxin which decrease the [K+]i stimulate the secretion of IL-1β from primed macrophages [6]. Fourth, in vitro experiments have demonstrated that the assembly of the cryopyrin inflammasome is triggered by the decrease of the [K+]i [7].
There is a general consensus that P2X7 receptors are the only purinergic receptors of macrophages coupled to IL-1β secretion and that these receptors are potential targets for the development of anti-inflammatory drugs. Yet macrophages express other purinergic receptors like P2Y1, P2Y2, P2X1, or P2X4 receptors [8, 9]. Considering that all the P2X receptors can form a non-selective cation channel and that the release of potassium plays a central role in the response to P2X7 receptors, P2X4 receptors might activate caspase-1 and IL-1β secretion. The P2X4 receptors desensitize more rapidly than P2X7 receptors [2]. Ivermectin, a high-molecular weight lipophilic drug which is used in the treatment of parasitosis in human and veterinary medicine [10] reduces the desensitization process and increases the responses coupled to P2X4 receptors [11]. The purpose of our work was to test for a possible role of sustained activation of P2X4 receptors with a combination of ATP and ivermectin in the response of peritoneal macrophages to extracellular ATP. To avoid any P2X7 component which might complicate the interpretation of the results, most of these experiments were also performed on cells from P2X7-KO mice [1]. Our results show that P2X4 receptors can form a channel permeant to calcium and to potassium. The ensuing decrease of the [K+]i triggers the secretion of IL-1β from LPS-primed macrophages. We also present evidence that the P2X4 receptors can form a pore permeant to YOPRO-1 and which is not affected by antagonists of pannexin-1.
Materials and methods
Cell culture Adult male C57Bl/6J wild-type (WT) or P2X7 receptor KO mice (25 g) generated as reported by Solle [1], were used for these experiments. Peritoneal macrophages were prepared according to McCarron et al. [12]. Briefly, peritoneal exsudates were induced by injection of 1.5 mL thioglycollate solution (4% in sterile water) 3 or 4 days before harvesting the cells. The animals were fasted overnight and sacrificed by increasing the CO2 concentration in accordance with the procedures approved by the Belgian Ministry of Agriculture under the supervision of the institutional ethical committee (protocol n° 221 N). Ten milliliter phosphate-buffered saline (PBS) without calcium or magnesium, pH 7.3, containing 10 U/mL heparin were injected in the peritoneal cavity of the animal. The abdomen was gently massaged after its distension. The fluid was recovered and transferred to a sterile tube kept on ice. After centrifugation at 1,500×g for 10 min at 4°C, the cellular pellet was dispersed in RPMI 1640 medium supplemented with 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.4), 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (RPMI medium). Ten million cells were added to culture flasks (usually two flasks per mouse) and incubated at 37°C for at least 2 h in a humidified atmosphere containing 5% CO2. Nonadherent cells and the medium were aspirated and replaced with 10 mL fresh RPMI medium. The adherent cells were incubated overnight at 37°C. The next day, the cells were incubated for 4 h in 10 mL fresh RPMI medium containing 250 ng/mL LPS. At the end of the incubation, the cells were washed twice with 10 mL PBS before use.
Determination of the [Ca2+]i The cells were gently scraped off the flasks with a rubber policeman and dispersed in HEPES-buffered saline (HBS) medium containing (mM): 24.5 HEPES (pH 7.4), 96 NaCl, 6 KCl, 1 MgCl2, 2.5 NaH2PO4, 11.5 glucose, 5 sodium pyruvate, 5 sodium glutamate, 5 sodium fumarate supplemented with 1% (v/v) glutamine-free amino acids mixture, 0.1% (w/v) bovine serum albumin (BSA), and 1 mM CaCl2. They were incubated at 25°C for 45 min in the presence of 3 μM fura-2/AM. At the end of this incubation, the cells were washed and dispersed in HBS medium without magnesium but with 1 mM CaCl2. They were transferred in the cuvette of a fluorimeter and maintained at 25°C under constant stirring. The light emitted at 490 nm (slitwidth 16 nm) was measured after excitation at 345 and 380 nm (slitwidth 4 nm; SLM Aminco Bowman, Urbana, IL, USA). At the end of the measurement, the cells were permeabilized with 100 μM digitonin and EGTA (final concentration 40 mM, pH 8.5) was added to the cuvette. The autofluorescence of the samples was estimated by quenching fura-2 with 100 mM MnCl2 and this blank value was subtracted from the results. The ratio F345/F380 was used as an estimate of the variation of the [Ca2+]i as previously described [13].
Measurement of the Ca2+ influx Cells were loaded with fura-2/AM as described in the previous paragraph. The cellular pellet was dispersed in HBS medium in the absence of magnesium and calcium, in the absence or in the presence of ivermectin. Ninety seconds after the start of the measurement, 100 μM EGTA was added to the cuvette and 30 s later the cells were exposed to ATP. Calcium chloride (1 mM final concentration) was added 3 min after the addition of ATP. The traces were calibrated as described above.
Determination of the variation of the [K+]i Macrophages were dispersed in HBS medium with amino acids, BSA, and calcium. They were incubated at 25°C for at least 1 h in the presence of 3 μM PBFI/AM. At the end of this incubation, the cells were washed and dispersed in HBS medium without magnesium but with 1 mM CaCl2. They were transferred in the cuvette of a fluorimeter and maintained at 25°C under constant stirring. The light emitted at 490 nm (slitwidth 16 nm) was measured after excitation at 345 nm (slit width 16 nm; SLM Aminco Bowman, Urbana, IL, USA). Before the beginning of each measurement, the voltage was adjusted to get a signal corresponding to 10% of the maximal signal. The isosbestic wavelength of the probe was 390 nm and the signal at this wavelength was very weak precluding any use of the dye in a ratiometric way. Results have thus been plotted as % of the initial signal without any attempt to calibrate the traces.
Assay of the permeabilization of the cells The permeabilization of the plasma membrane was measured with the fluorescent dye YOPRO-1. Macrophages were dispersed in sodium-depleted HEPES-buffer containing (mM): 280 sucrose, 10 HEPES (pH 7.4), 10 N-methyl-D-glucamine (NMDG), 10 glucose, and 5 KCl [14]. YOPRO-1 was added at a final concentration of 5 μM. The cells were allowed to equilibrate for 5 min before the beginning of the measurement which was performed at 37°C under constant stirring. The samples were excited at 490 nm (slitwidth 10 nm) and the light emitted at 509 nm (slitwidth 10 nm) was measured every second (Photon Technology International, Birmingham, NJ, USA). At the end of the measurement, the maximal uptake was estimated by adding 100 μM digitonin and the results were calculated taking this value as the maximal uptake of YOPRO-1.
Assay of IL-1β secretion Peritoneal macrophages from one mouse were dispersed in RPMI medium at a 500,000 cells/mL dilution and 1 mL aliquots were transferred in a 12-well plate. After priming the cells for 4 h in RPMI medium containing 250 ng/mL LPS, they were incubated for 15 min at 37°C in 1 mL HBS medium without magnesium but containing 1 mM CaCl2, 0.1% BSA or in the same medium but with a high concentration of potassium (NaCl replaced by KCl, final concentration of potassium: 100 mM) and the tested agent. Each condition was performed in duplicates. Incubation medium was collected, centrifuged at 10,000×g for 5 min to remove detached cells and the supernatant sonicated for 15 s at a 15-μm amplitude (Soniprep 150, MSE, London, UK). The IL-1β content was assayed by sandwich enzyme-linked immunosorbent assay (ELISA) [15]. A 96-well plate was coated overnight with 1 μg/mL polyclonal anti-mouse IL-1β antibody and blocked with 4% BSA in PBS for 1 h at room temperature. The wells were washed twice with Tris-Tween buffer (Tris–HCl 50 mM, pH 7.5, and 0.2% Tween-20). Twenty-five microliter of medium samples or recombinant IL-1β standards were diluted to 150 μL with HBS medium containing 1 mM CaCl2 and 0.1% BSA and mixed with 150 μL biotinylated monoclonal anti-mouse IL-1β antibody (0.4 μg/mL solution). One hundred micoliter of this mix was added in duplicate to the wells of the coated plate. Within each experiment, each condition is tested in duplicates. The plate was incubated at 37°C for 2 h and then washed four times. The captured immune complexes were incubated for 30 min at room temperature with 100 μL streptavidin horseradish peroxidase (HRP; 0.2 μg/mL), washed four times and colorimetrically developed with 100 μL HRP substrate. After the addition of 100 μL stop solution (H2SO4 1.8 M), the absorbance was read at 450 nm in a Synergy HT plate reader (Bio-Tek, Winooski, VT, USA). Both antibodies could bind to IL-1β as well as to proIL-1β and a Western blot confirmed that both the cytokine and its precursor were released in the supernatant of LPS-primed macrophages from WT mice incubated in the presence of 1 mM ATP or 10 μM nigericin. As a consequence, results were expressed as pg IL-1β equivalents/mL supernatant.
Western Blot analysis of P2X7 and P2X4 receptors Macrophages from one mouse were dispersed in 1 mL ice-cold buffer containing 20 mM Tris (pH 8.1), 1 mM EGTA, 1 mM EDTA, and a cocktail of protease inhibitors (TEEI buffer). The cells were homogenized by aspiration through 19 and 29 G needles. The homogenate was centrifuged for 10 min at 4°C at 1,000×g and the supernatant transferred to a tube kept on ice. One milliliter TEEI buffer was added on the pellet which was aspirated through the needles and centrifuged. This step was performed four times and the combined supernatants were centrifuged for 45 min at 100,000×g. The supernatant was discarded and 300 μL TEEI buffer with 0.3% Triton X-100 was added to the pellet and kept on ice. After 30 min, the suspension was aspirated through the needles and centrifuged for 10 min at 10,000×g. The supernatant containing the proteins extracted from the membranes was kept at −20°C. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a NuPAGE® 4–12% Bis-Tris gel and the proteins were transferred to a nitrocellulose membrane. After incubation of the membrane with a polyclonal anti-P2X7 or anti-P2X4 antibody from rabbit, the two receptors were revealed using a secondary anti-rabbit antibody coupled to peroxidase and a chemiluminescent substrate (ECL Plus).
Western blot analysis of IL-1β secretion Peritoneal macrophages were plated in 12-well plates at a density of 1.5 × 106 cells per well. After incubation overnight, they were primed for 4 h in RPMI medium containing 250 ng/mL LPS. They were preincubated for 30 min at 37°C in HBS medium without magnesium but with amino acids, 0.05% BSA, and 1 mM CaCl2, either in control condition or in the presence of carbenoxolone or of the pannexin-1 mimetic blocking decapeptide 10panx1 (WRQAAFVDSY). The cells were then stimulated for 30 min with the tested agents. At the end of the incubation, the supernatant was collected and the secreted proteins were concentrated using 3-kDa cut-off filters according to the instructions of the manufacturer (Pall Life Sciences, Port Washington, NY, USA). After SDS-PAGE on a NuPAGE® 4–12% Bis-Tris gel, the proteins were transferred to a PVDF membrane. The membrane was incubated overnight with a polyclonal anti-mouse IL-1β antibody from goat. A secondary anti-goat antibody coupled to peroxidase and chemiluminescent substrate (ECL Plus) were used for revelation.
Protein determination Proteins were measured with the method using bicinchoninic acid (BCA kit from Pierce). Bovine serum albumin was used as a standard.
Statistical analysis All the results were compared with the Mann–Whitney non-parametric test.
Reagents Fura-2/AM, PBFI/AM, YOPRO-1, nitrocellulose and PVDF membranes, NuPage® 4–12% Bis-Tris gels, NuPage® Transfer buffer, NuPage® MOPS SDS Running buffer, NuPage® MES SDS Running buffer, and NuPage® LDS sample buffer were from Invitrogen (Groningen, Netherlands). ATP, BzATP, EGTA, EDTA, NMDG, HEPES, ivermectin, nigericin, LPS from Escherichia coli 055-B5, carbenoxolone, digitonin, sodium pyruvate, Tween 20, U73122, fetal bovine serum, Triton X-100 and the proteases inhibitor cocktail were purchased from Sigma (St. Louis, MO, USA). BSA fraction V was from Roche Diagnostics (Mannheim, Germany). The glutamine-free amino acids mixture, l-glutamine, PBS, RPMI 1640, penicillin and streptomycin were obtained from Gibco BRL (Paisley, Scotland). The chemiluminescent ECL Plus reagent and the peroxidase-linked anti-rabbit IgG were from GE Healthcare (Buckinghamshire, England). The anti-P2X7 and anti-P2X4 polyclonal antibodies were supplied by Alomone (Jerusalem, Israël). Thioglycollate was from Becton, Dickinson and Company (Franklin Lakes, NJ, USA). The BCA protein assay reagent, the HRP substrate 1 Step Ultra TMB-ELISA and the peroxidase-linked streptavidin were obtained from Pierce (Perbio Science, Erembodegem, Belgium). The biotinylated monoclonal anti-mouse IL-1β antibody and the mouse IL-1β were from Endogen (Perbio Science, Erembodegem, Belgium). The polyclonal anti-mouse IL-1β antibody was from R&D Systems (Minneapolis, MN, USA). The pannexin-1 mimetic blocking peptide 10panx1 (WRQAAFVDSY) was supplied by GL Biochem (Shanghai, China). The caspase-1 inhibitor AC-YVAD-CHO was from Calbiochem (Merck Chemicals, Nottingham, UK). The peroxidase-linked anti-goat antibody was purchased from Santa Cruz (Santa Cruz, CA, USA).
Results
Response to ATP of macrophages from WT or P2X7-KO mice
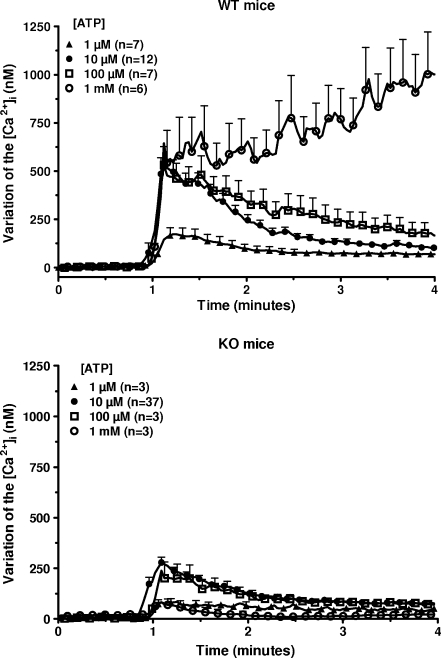
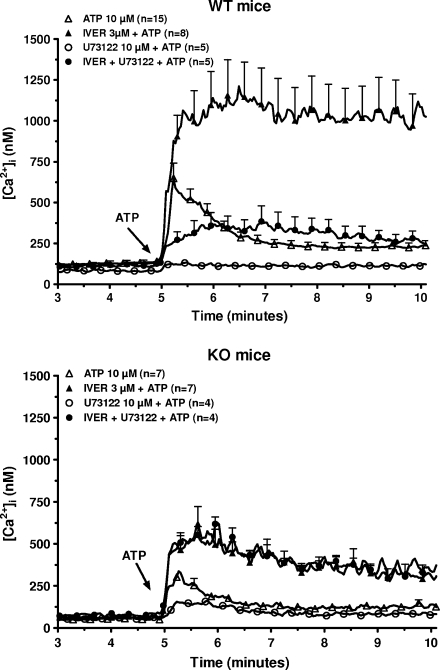
Macrophages from WT and P2X7-KO mice were incubated in the presence of 1 mM CaCl2 and of various concentrations of ATP (from 1 μM to 1 mM) and the [Ca2+]i was estimated. In cells from WT mice, low concentrations of ATP (1–100 μM) sharply increased the [Ca2+]i (10 s after the addition of 10 μM ATP, increase of 583 ± 90 nM, n = 12; Fig. 1, upper panel). This peak increase was followed by a drop of the [Ca2+]i (3 min after the addition of 10 μM ATP, increase of 105 ± 15 nM, n = 12) The time-course was completely different at 1 mM ATP: the initial peak (10 s after the addition of 1 mM ATP, increase of 543 ± 67 nM, n = 6) was followed by a steady increase (3 min after the addition of 1 mM ATP, increase of 1005 ± 152 nM, n = 6). In cells from P2X7-KO mice, 10 μM ATP transiently increased the [Ca2+]i (10 s after the addition of ATP, increase of 278 ± 26 nM, n = 37; Fig. 1, lower panel). This response was significantly lower than in WT mice (P < 0.01). At a 1-mM concentration, ATP only evoked a marginal and transient stimulation (increase of 82 ± 25 nM after 10 s, n = 3). To determine the contribution of a metabotropic purinergic receptor in the response to 10 μM ATP, the macrophages from both mice were exposed to 10 μM U73122, an inhibitor of the polyphosphoinositide-specific phospholipase C (PPI-PLC) [16], before the addition of ATP to the medium (Fig. 2). In macrophages from WT mice the drug nearly fully blocked the variation of the [Ca2+]i in response to 10 μM ATP (increase of 41 ± 11 nM, n = 5) suggesting that most of the response of macrophages to 10 μM ATP was mediated by P2Y receptors. In macrophages from P2X7-KO mice, the increase in the [Ca2+]i in response to 10 μM ATP remained important in the presence of U73122 in the medium and averaged 102 ± 21 nM (n = 4). This result confirmed that these cells expressed a P2X receptor distinct from P2X7 receptor. This receptor was probably a P2X4 receptor since it had apparently a much higher affinity for ATP and desensitized much faster than the P2X7 receptor [8, 9].
Fig. 1.
Effect of ATP on the [Ca2+]i in murine peritoneal macrophages. Macrophages from WT (upper panel) or P2X7-KO (lower panel) mice primed for 4 h with 250 ng/mL LPS were loaded with fura-2/AM. After washing, they were incubated in a medium containing 1 mM CaCl2 but no magnesium and they were transferred in a temperature-controlled cuvette of a fluorimeter for calcium measurement. One minute after the start of the experiment, the cells were exposed to various concentrations of ATP. At the end of the measurement, the traces were calibrated as described in “Materials and methods” section. Results are expressed as the variation of the [Ca2+]i and are the means + SEM of n experiments
Fig. 2.
Effect of U73122 and ivermectin on the response to ATP of murine peritoneal macrophages. Macrophages from WT (upper panel) or P2X7-KO (lower panel) mice primed for 4 h with 250 ng/mL LPS were loaded with fura-2/AM. After washing, they were incubated in a medium without magnesium but with 1 mM CaCl2 in control conditions or in the presence of 10 μM U73122 or 3 μM ivermectin (IVER) or U73122 plus ivermectin. Five minutes after the start of the measurement, the cells were stimulated with 10 μM ATP. Results are the means + SEM of n experiments
Effect of ivermectin on the response of mouse peritoneal macrophages to ATP
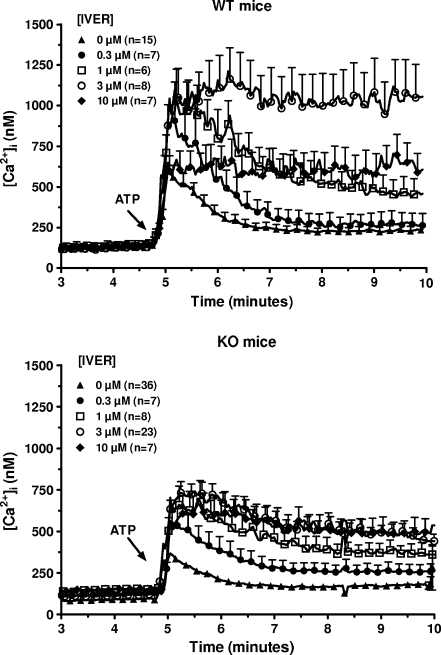
Ivermectin is an anti-parasite drug which potentiates the responses coupled to P2X4 receptors [17]. Macrophages from WT and P2X7-KO mice were stimulated with 10 μM ATP in the absence or in the presence of various concentrations of ivermectin and the [Ca2+]i was estimated. As shown in Fig. 3, this drug increased and prolonged the response to ATP in both mice. The effect of ivermectin was already observed at 0.3 μM and reached a maximum at 3 μM. In macrophages from WT mice incubated in the presence of this concentration of the drug, the [Ca2+]i reached 1158 ± 218 nM (n = 8) 1 min after the addition of 10 μM ATP to the medium and remained stable for the next 4 min. In macrophages from P2X7-KO mice, the [Ca2+]i reached 688 ± 65 nM (n = 23) within 1 min and slightly decreased for the next 4 min. Ten micrometer U73122 inhibited the response to ivermectin plus ATP in WT mice (down to 346 ± 45 nM, n = 5 after 1 min) but not in P2X7-KO mice (541 ± 54 nM, n = 4 after 1 min; Fig. 2). In these conditions (in the presence of ivermectin and U73122), ATP provoked a significantly higher increase in the [Ca2+]i in macrophages from P2X7-KO mice than from WT mice (P < 0.05).
Fig. 3.
Effect of various concentrations of ivermectin on the response to ATP of murine peritoneal macrophages. Macrophages from WT (upper panel) or P2X7-KO (lower panel) mice primed for 4 h with 250 ng/mL LPS were loaded with fura-2/AM. After washing, they were incubated in a medium without magnesium but with 1 mM CaCl2 and in the presence of various concentrations of ivermectin ([IVER]). Five minutes after the start of the measurement, the cells were stimulated with 10 μM ATP. Results are the means + SEM of n experiments
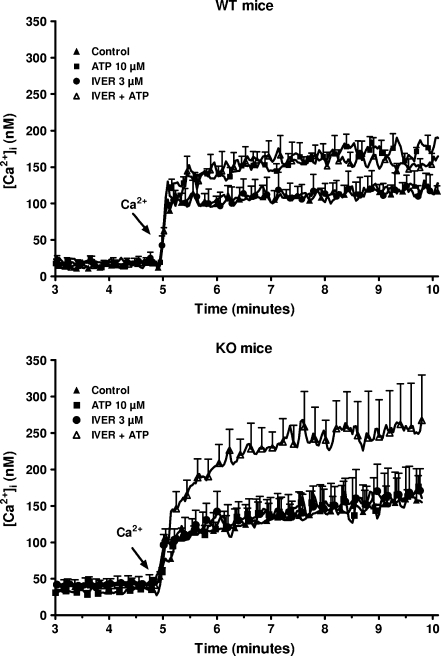
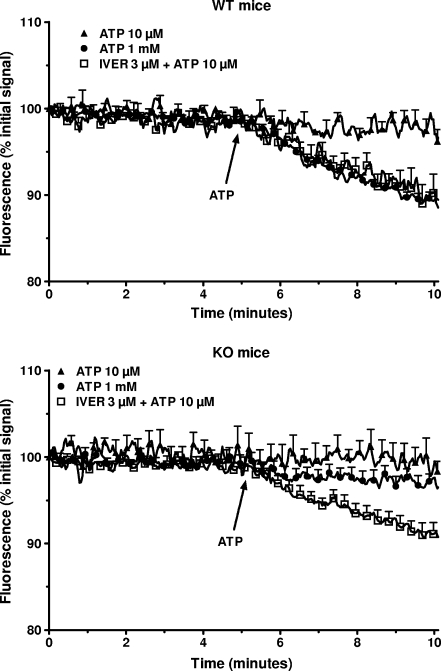
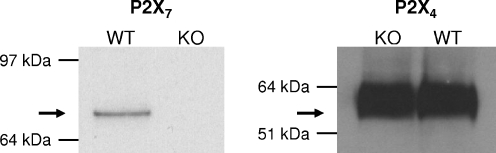
Considering that all the P2X receptors are coupled to a non-selective cation channel, the effect of ivermectin on the uptake of calcium was tested next. As shown in Fig. 4 (upper panel), the addition of 1 mM calcium to a calcium-free medium provoked a rapid (within 10 s) increase in the [Ca2+]i in macrophages from WT mice incubated in control condition. This initial increase was followed by a slight increase for the next 5 min from 101 ± 12 nM to 133 ± 11 nM (n = 3). In cells preincubated for 3 min with 10 μM ATP before the addition of calcium to the medium, the [Ca2+]i reached 181 ± 22 nM (n = 3) after 5 min. Three micromolar ivermectin had no effect by itself (121 ± 17 nM, n = 3) or in the presence of 10 μM ATP (154 ± 17 nM, n = 3). In macrophages from P2X7-KO mice, 10 μM ATP did not increase the basal uptake of calcium (Fig. 4, lower panel). In the presence of 3 μM ivermectin, the nucleotide increased the [Ca2+]i from 168 ± 16 nM to 252 ± 26 nM (n = 3). This result confirmed that, at least in macrophages from P2X7-KO mice, the combination of ivermectin plus ATP opened a channel permeant to calcium. The channel coupled to P2X receptors permeates not only to calcium but also to other cations like potassium or sodium [2]. This was confirmed in the data presented in Fig. 5. Exposure of macrophages from WT mice but not from P2X7-KO mice to 1 mM ATP decreased by about 10% the fluorescence of PBFI, a dye sensitive to the concentration of potassium. At 10 μM, the nucleotide alone had no effect but in the presence of 3 μM ivermectin, a 10% drop of the fluorescence of the potassium-sensitive probe was observed both in WT and P2X7-KO mice. These results were consistent with the contribution of a P2X receptor different from the P2X7 receptor (probably the P2X4 receptor) to the response of peritoneal macrophages to extracellular ATP. A Western blot analysis of the macrophages confirmed that cells from WT mice expressed both P2X7 and P2X4 receptors while cells from KO mice expressed only P2X4 receptors (Fig. 6).
Fig. 4.
Effect of the combination of ATP and ivermectin on calcium uptake by murine peritoneal macrophages. Macrophages from WT (upper panel) or P2X7-KO (lower panel) mice primed for 4 h with 250 ng/mL LPS were loaded with fura-2/AM. After washing, they were incubated in a medium without calcium or magnesium, in the absence or in the presence of 3 μM ivermectin (IVER). Ninety seconds, 2 and 5 min after the start of the measurement, 100 μM EGTA, 10 μM ATP, and 1 mM CaCl2 were successively added to the cuvette. Results are the means + SEM of three experiments
Fig. 5.
Effect of the combination of ATP and ivermectin on the decrease of the [K+]i in murine peritoneal macrophages. Macrophages from WT (upper panel) or P2X7-KO (lower panel) mice primed for 4 h with 250 ng/mL LPS were loaded with PBFI/AM. After washing, they were incubated in a medium containing 1 mM CaCl2 but no magnesium, in control conditions or in the presence of 3 μM ivermectin (IVER). Five minutes after the start of the measurement, 10 μM or 1 mM ATP was added to the cuvette. Results are expressed as % of the initial signal and are the means + SEM of four experiments
Fig. 6.
Western blot analysis of P2X4 and P2X7 receptors in murine peritoneal macrophages. The membranes of macrophages from WT or P2X7-KO mice were isolated and after solubilization tested for P2X7 (left panel) and P2X4 (right panel) receptors expression. The results are representative of four experiments
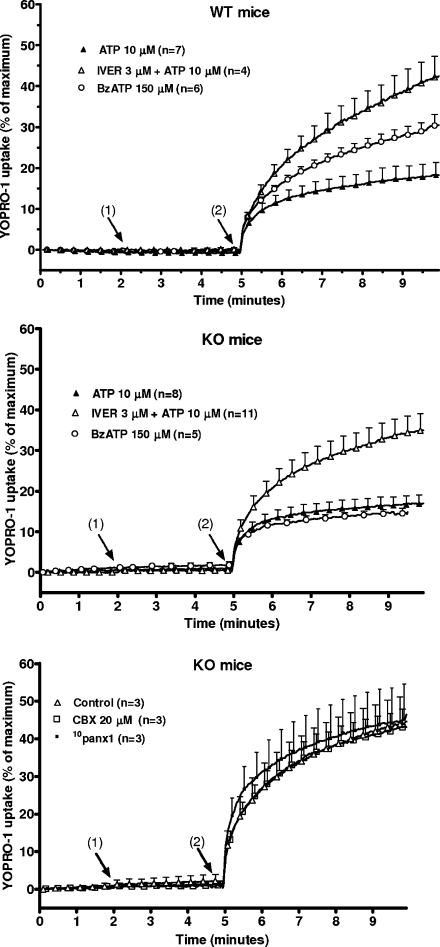
P2X7 receptors not only open a non-selective cation channel but after a sustained activation they can also form a pore permeant to the fluorescent dye YOPRO-1 [18]. The uptake of the dye was measured in the presence of a low concentration of sodium and after exposure of the macrophages to BzATP which is a more potent agonist than ATP to stimulate P2X7 receptor-mediated YOPRO-1 uptake [19]. As shown in Fig. 7 (upper panel), the addition of 150 μM BzATP to the incubation medium increased the fluorescence of macrophages from WT mice exposed to YOPRO-1 (30.5 ± 2.7% of maximal signal after 5 min, n = 6). Ten micrometer ATP slightly increased the fluorescence (18.2 ± 3.2%, after 5 min, n = 7). Ivermectin (3 μM) doubled the uptake of the dye in the presence of 10 μM ATP (from 18.2 ± 3.2% to 42.1 ± 5.1%, n = 4, 5 min after the addition of ATP). In macrophages from P2X7-KO mice, 10 μM ATP and 150 μM BzATP slightly increased the uptake of YOPRO-1 (Fig. 7, middle panel, 16.9 ± 2.1% after 5 min, n = 8, and 14.5 ± 1.3%, n = 5, respectively). When 3 μM ivermectin was added to the medium, 10 μM ATP increased the uptake of YOPRO-1 after 5 min from 16.9 ± 2.1% (n = 8) to 34.9 ± 4.2% (n = 11). The involvement of pannexin-1 in the P2X4 associated dye uptake was next assessed by using the hemichannel inhibitor carbenoxolone and the pannexin-1 mimetic blocking peptide 10panx1. As illustrated in Fig. 7 (lower panel), these antagonists did not affect the uptake of YOPRO-1 in response to a combination of ivermectin and ATP.
Fig. 7.
Effect of the combination of ATP plus ivermectin on the formation of a pore in murine peritoneal macrophages. Macrophages from WT (upper panel) or P2X7-KO (middle and lower panel) mice, primed for 4 h with 250 ng/mL LPS, were incubated in a low-sodium medium without calcium or magnesium. The macrophages were preincubated for 5 min at 25°C in the presence of 5 μM YOPRO-1, in control condition or in the presence of 20 μM carbenoxolone (CBX) or 10panx1 (lower panel). They were then transferred to the cuvette of a fluorimeter and incubated at 37°C. Two minutes (1) after the start of the measurement, 3 μM ivermectin (IVER) was added to some samples. Three minutes later (2), 10 μM ATP or 150 μM BzATP were added to the cuvette. Maximal uptake was estimated by addition of 100 μM digitonin to the medium. Results are expressed as percent maximal uptake and are the means + SEM of n experiments
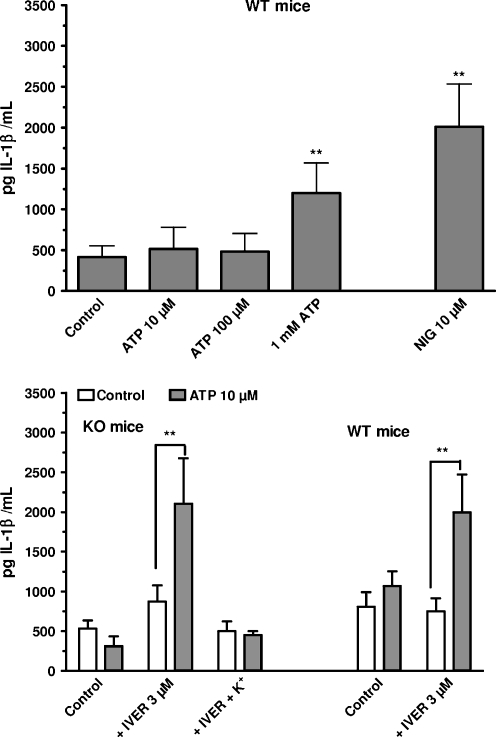
In the last run of experiments, the stimulation of IL-1β release in response to ATP was examined. In macrophages from WT mice, ATP, at concentrations higher than 100 μM, promoted the release of IL-1β (Fig. 8, upper panel). By itself, 3 μM ivermectin had no effect but in the presence of 10 μM ATP, it provoked a secretion of IL-1β (Fig. 8, lower panel) comparable to the release observed with 1 mM ATP. In macrophages from P2X7-KO mice, 10 μM ATP had no effect on the release of IL-1β. In the presence of 3 μM ivermectin, the nucleotide provoked the secretion of IL-1β (Fig. 8, lower panel). When the extracellular concentration of potassium was raised from 6 to 100 mM the release of IL-1β in response to 10 μM nigericin or to 1 mM ATP alone (WT mice, data not shown) or to the combination of ivermectin plus 10 μM ATP (WT and P2X7-KO mice, Fig. 8, lower panel) was abolished.
Fig. 8.
Effect of the combination of ATP plus ivermectin on the release of IL-1β from murine peritoneal macrophages. Upper panel Macrophages from WT mice primed for 4 h with 250 ng/mL LPS were incubated for 15 min at 37°C in the presence of 1 mM CaCl2 and in the absence of magnesium, in the presence of increasing concentrations of ATP or of 10 μM nigericin (NIG). Lower panel Macrophages from WT or P2X7-KO mice primed for 4 h with 250 ng/mL LPS were incubated for 15 min at 37°C in the presence of 1 mM CaCl2 and in the absence of magnesium, in regular HBS medium or in a medium with high potassium (100 mEq/L). The cells were incubated in the absence (open bars) or in the presence (squared bars) of 10 μM ATP and in the absence or in the presence of 3 μM ivermectin (IVER). The IL-1β content of the supernatant was assayed by ELISA after centrifugation and sonication. Results are the means + SEM of six experiments. **p < 0.01 when compared to control
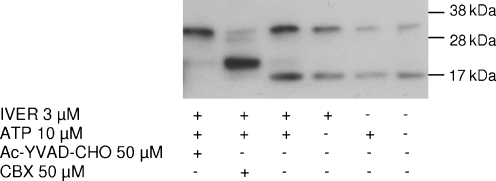
Caspase-1 and pannexin-1 are two proteins which are essential for P2X7 associated IL-1β secretion [20]. Immunoblotting of secreted IL-1β was performed after incubation of macrophages from P2X7-KO mice in the presence of ivermectin and 10 μM ATP, in the absence or in the presence of inhibitors of these two proteins. In control conditions, two immunoreactive bands were observed, corresponding to pro-IL-1β (~35 kDa) and to the mature form of the cytokine (~17 kDa; Fig. 9). In the presence of carbenoxolone, these two bands were either fully suppressed (17 kDa) or greatly attenuated (35 kDa). They were replaced by a major band corresponding to a protein of about 20 kDa. The caspase-1 inhibitor Ac-YVAD-CHO prevented the appearance of the mature 17 kDa form of IL-1β without affecting the 35-kDa band.
Fig. 9.
Immunoblot analysis of the release of IL-1β. Peritoneal macrophages from P2X7-KO mice primed for 4 h with 250 ng/mL LPS were incubated for 30 min at 37°C in control condition or in the presence of 50 μM carbenoxolone (CBX) or 50 μM Ac-YVAD-CHO. They were then stimulated for 30 min with 10 μM ATP, 3 μM ivermectin (IVER) or a combination of ivermectin and ATP. The supernatant was collected and IL-1β secretion was assessed by immunoblotting
Discussion
In this work, we showed that low concentrations of ATP increased the [Ca2+]i in peritoneal macrophages from WT and P2X7-KO mice. U73122, an inhibitor of the PPI-PLC fully blocked the response of WT mice but only partially inhibited the response of P2X7-KO mice. These results suggested that the stimulation of P2Y receptors accounted for most of the response of macrophages to low concentrations of ATP and that this response was much bigger in WT than in P2X7-KO mice. This conclusion was reinforced by the measurement of calcium uptake in response to ATP. The activation of P2Y receptors and the depletion of intracellular calcium pools open calcium channels and promote the uptake of extracellular calcium. In agreement with this model, 10 μM ATP increased the uptake of calcium in WT mice but had no effect by itself in P2X7-KO mice. Considering that the activation of P2X7 receptor inhibits the PPI-PLC signaling pathway [21] it might look paradoxical that the response coupled to P2Y receptors was larger in cells expressing the P2X7 receptor. The group of Di Virgilio has very recently reported that transfection of HEK293 cells with P2X7 receptors increased their capacity to accumulate calcium in the endoplasmic reticulum [22]. As a consequence, the agonists mobilizing this intracellular pool of calcium (carbachol or bradykinin in their hands) provoked a larger increase in the cytosolic concentration of calcium in the cells expressing the P2X7 receptor than in cells which did not express it. But this explanation cannot account for the increased uptake of calcium in response to low concentrations of ATP in macrophages from WT mice. The group of Turner demonstrated that the expression of P2Y1 and P2Y2 receptors and/or the response coupled to these receptors were dynamically regulated [23]. Our results might be better explained by a reduction of expression or function of the P2Y receptors in P2X7-KO mice.
Ivermectin potentiated the response to ATP of macrophages from WT and P2X7-KO mice. This macrocyclic lactone has pleiotropic effects. Its antiparasitic property is probably secondary to its action on a glutamate-gated chloride channel receptor expressed by invertebrates. In vertebrates, this drug regulates the channel coupled to glycinergic or GABAergic receptors [10]. When tested on the P2X receptors, ivermectin proved to selectively and exclusively potentiate the responses coupled to P2X4 receptors increasing the amplitude and the duration of the responses [17]. It was later shown that ivermectin interfered with the desensitization process [11]. In our hands, very low concentrations of ivermectin not only potentiated the maximal increase in the [Ca2+]i in response to ATP but also prolonged the response to the nucleotide in both mice. Brône and colleagues showed that ivermectin potentiated a current elicited by extracellular ATP in macrophages from WT mice but not from P2X4-KO mice [8]. Stokes and Surprenant [24] reported that ivermectin potentiated the current evoked by low concentrations of ATP in human alveolar macrophages. Our results are also fully consistent with recent results [25] who reported that ivermectin potentiated the increase in the [Ca2+]i in murine macrophages in response to low concentrations of ATP but not to UDP, an agonist of P2Y receptors. Considering that the potentiation by ivermectin was observed even in the presence of U73122 (i.e., when the P2Y signaling route was inhibited) and in P2X7-KO mice (i.e., in cells devoid of P2X7 receptors), and that P2X4 receptors are expressed by murine peritoneal macrophages, we can conclude that the combination of low concentrations of ATP and ivermectin stimulated these receptors. Macrophages also express P2X1 receptors, which may form heteromeric channels with P2X4 receptors [26]. Thus, the increase of the [Ca2+]i in response to ATP and ivermectin was secondary to the formation of a homotrimer of P2X4 subunits or of a hypothetical heterotrimer formed by P2X1 and P2X4 receptors.
When compared to the other P2X receptors, the P2X7 receptor has some unique properties which have been attributed to its extended intracellular C-terminal tail through which the receptor interacts with intracellular proteins [3]. The P2X7 receptor has different permeability states from a non-selective cation channel to a pore permeant to molecules up to 800 Da. A sustained activation of the P2X7 receptor can ultimately lead to cell death by apoptosis or necrosis [4]. But more relevant to our work, it has been claimed that the P2X7 receptor is the only purinergic receptor triggering IL-1β secretion and contributing to inflammation [27]. The ability of P2X4 receptors to reproduce some of these responses usually associated with P2X7 receptors was tested with the combination of ATP plus ivermectin. To avoid the formation of P2X4-P2X7 hybrid structures [28] which would make the interpretation of the results impossible, only P2X7-KO mice were tested for the P2X4 responses.
The combination of 10 μM ATP plus ivermectin increased the transport of cations through the channel coupled to P2X4 receptors provoking an increase in the [Ca2+]i, a drop of the [K+]i and the release of IL-1β. As previously reported, the release of IL-1β was also triggered by nigericin, a K+/H+ antiport ionophore that provokes a decrease in the [K+]i [15]. The secretion in response to ATP plus ivermectin and to nigericin was blocked when the decrease of the [K+]i was prevented by raising the concentration of the ion in the incubation medium. Immunoblotting showed an increase of pro-IL-1β and of the mature 17 kDa form in the supernatant of macrophages stimulated with ivermectin plus ATP. Inhibition of caspase-1 prevented the appearance of the 17 kDa form, suggesting that caspase-1 was involved in P2X4 receptor-mediated IL-1β maturation and secretion. In the presence of carbenoxolone, the 17 kDa form was replaced by a ~20 kDa form. This form is not generated by caspase-1: it is observed after hypotonic lysis of macrophages [29]. It is also generated by incubating pro-IL-1β with various serine proteases such as proteinase 3, cathepsin G or elastase [30]. These results contradict the general consensus and establish that providing it is activated for a long time, the P2X4 receptor, like the P2X7 receptor, can decrease the [K+]i to levels low enough to trigger IL-1β secretion after activation of caspase-1.
The activation of the P2X4 receptor with ATP plus ivermectin stimulated the uptake of the cationic dye YOPRO-1, an index of the pore formation. This suggested that the permeability of the channel coupled to P2X4 receptors could increase, allowing the passage of molecules of up to 375 Da, which corresponds to the value of the ionized form of YOPRO-1 in solution. This is in agreement with the results of Khakh et al. [31] who reported that P2X4 receptors expressed in oocytes could eventually form a pore after prolonged stimulation. The mechanism involved in this increased permeability remains to be determined. Shinozaki et al. [32] observed a P2X4 receptor associated pore dilation in the absence of calcium. It has been claimed that pannexin-1 is an accessory protein responsible for the uptake of fluorescent dyes and the secretion of IL-1β linked to the activation of the P2X7 receptor [20]. Pannexin-1 can form a hemichannel-like non-selective pore and co-immunoprecipitates with the P2X7 receptor. Recent data suggest that the C-terminal extension of the P2X7 receptor interacts with src tyrosine kinase which mediates the activation of pannexin-1 [33]. Considering that not only P2X4 but also P2X2 receptors, two receptors without the C-terminal extension of P2X7 receptors, can form a pore permeant to YOPRO-1 it is unlikely that pannexin-1 might contribute to the increased permeability of these two receptors. This is in agreement with our results showing a lack of effect of carbenoxolone and 10panx1 on the P2X4-receptor associated uptake of YOPRO-1, and with the results of Chaumont and Kahkh [34] who reported that pannexin-1 channels did not contribute to P2X2 receptor permeability increase. Khakh et al. suggested that the changes in permeability of P2X2 and P2X4 receptors could be controlled at least in part by the second transmembrane domain or by conformational changes in the cytosolic domain, remote from the selectivity filter itself [31, 34].
The responses mediated by the P2X7 receptors are observed at low concentrations of cations and require extracellular concentrations of ATP of the same order of magnitude as the intracellular concentration of the nucleotide. The role of this receptor in cell physiology has thus been questioned. Indeed it is very unlikely that the P2X7 receptors might be stimulated via a paracrine mechanism which would dilute ATP in the extracellular space when it is not released in the cleft of a purinergic synapse. But it is conceivable that the release of ATP by a cell involved in exocytosis and the accumulation of the nucleotide in the vicinity of its plasma membrane might produce an autocrine stimulation mediated by the low affinity P2X7 receptors [35]. The responses elicited by P2X4 receptors require much lower concentrations of ATP (two to three orders of magnitude lower than the intracellular concentration of the nucleotide) which can be found in the extracellular medium. The P2X4 receptor is constantly transferred from the plasma membrane to an intracellular pool [36]. In our hands, to be effective, the activation of the plasma membrane receptors by the nucleotide had to be potentiated by ivermectin raising some doubts on their physiological role [37]. Stokes and Surprenant [24] have recently proposed a model to explain the contribution in vivo of the P2X4 receptors to the physiology of macrophages. According to these authors, the activation of macrophages by a phagocytic stimulus promotes the translocation of P2X4 receptors from the lysosomal compartment to the plasma membrane. These receptors which are very sensitive to ATP could trigger an initial and transient step of activation and the release of IL-1β. A sustained activation of the macrophages would eventually activate the P2X7 receptors which are always located in the membrane. The activation of these receptors would prolong the response of the macrophages and even trigger their death.
In conclusion, our results clearly establish that the secretion of IL-1β and the formation of a pore are not properties exclusive to P2X7 receptors but can also be triggered by P2X4 receptors either alone or in complexes with P2X1 receptors. Our results also show that the lack of expression of P2X7 receptors in mice affects the responses coupled to P2Y receptors.
Acknowledgment
This work was supported by grant n° 3.4.528.07 from the Fonds National de la Recherche Scientifique Médicale to JPD and by grant n° BFU/2007-62728 BMC from the Ministry of Education to AM. SP was a postdoctoral fellow from the Fonds National de la Recherche Scientifique. The authors thank Ms. S Dulanto for her skillful technical help.
List of abbreviations
- WT
wild-type
- KO
knockout
- PPI-PLC
polyphosphoinositide-specific phospholipase C
- HBS
HEPES-buffered saline
- TEEI buffer
Tris/EGTA/EDTA/protease inhibitors buffer
- LPS
lipopolysaccharide
- IL-1β
interleukin-1β
- NMDG
N-methyl-D-glucamine
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- PAGE
polyacrylamide gel electrophoresis
References
- 1.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 2.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 3.Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor P2X7. Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 5.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 6.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 7.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 8.Brône B, Moechars D, Marrannes R, Mercken M, Meert T. P2X currents in peritoneal macrophages of wild type and P2X4−/− mice. Immunol Lett. 2007;113:83–89. doi: 10.1016/j.imlet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Sim JA, Park C-K, Oh SB, Evans RJ, North RA. P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharm. 2007;152:1283–1290. doi: 10.1038/sj.bjp.0707504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor HR, Pacqué M, Muñoz B, Greene BM. Impact of mass treatment of onchocerciasis with ivermectin on the transmission of infection. Science. 1990;250:116–118. doi: 10.1126/science.2218502. [DOI] [PubMed] [Google Scholar]
- 11.Toulmé E, Soto F, Garret M, Boué-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69:576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- 12.McCarron RM, Goroff DK, Luhr JE, Murphy MA, Herscowitz HB. Methods for the collection of peritoneal and alveolar macrophages. Methods Enzymol. 1984;108:274–284. doi: 10.1016/S0076-6879(84)08092-7. [DOI] [PubMed] [Google Scholar]
- 13.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 14.Michel AD, Chessell IP, Humphrey PP. Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:102–109. doi: 10.1007/PL00005328. [DOI] [PubMed] [Google Scholar]
- 15.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 16.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- 17.Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickman SE, Khoury J, Greenberg S, Schieren I, Silverstein SC. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84:2452–2456. [PubMed] [Google Scholar]
- 19.Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Métioui M, Amsallem H, Alzola E, Chaïb N, Elyamani A, Moran A, Marino A, Dehaye JP. Low affinity purinergic receptor modulates the response of rat submandibular glands to carbachol and substance P. J Cell Physiol. 1996;188:462–475. doi: 10.1002/(SICI)1097-4652(199608)168:2<462::AID-JCP25>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Adinolfi E, Callegari MG, Cirillo M, Pinton P, Giorgi C, Cavagna D, Rizzuto R, Virgilio F. Expression of the P2X7 receptor increases the Ca2+ content of the endoplasmic reticulum, activates NFATc1 and protects from apoptosis. J Biol Chem. 2009;284:10120–10128. doi: 10.1074/jbc.M805805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner JT, Park M, Camden JM, Weisman GA. Salivary gland nucleotide receptors. Changes in expression and activity related to development and tissue damage. Ann NY Acad Sci. 1998;842:70–75. doi: 10.1111/j.1749-6632.1998.tb09633.x. [DOI] [PubMed] [Google Scholar]
- 24.Stokes L, Surprenant A. Dynamic regulation of the P2X(4) receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol. 2009;39:986–995. doi: 10.1002/eji.200838818. [DOI] [PubMed] [Google Scholar]
- 25.Ito M, Matsuoka I. Regulation of purinergic signaling by prostaglandin E2 in murine macrophages. J Pharmacol Sci. 2008;107:443–450. doi: 10.1254/jphs.08087FP. [DOI] [PubMed] [Google Scholar]
- 26.Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 28.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 29.Perregaux D, Barberia J, Lanzetti AJ, Geoghegan KF, Carty TJ, Gabel CA. IL-1 beta maturation: evidence that mature cytokine formation can be induced specifically by nigericin. J Immunol. 1992;149:1294–1303. [PubMed] [Google Scholar]
- 30.Netea MG, Simon A, Veerdonk F, Kullberg BJ, Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 32.Shinozaki Y, Sumitomo K, Tsuda M, Koizumi S, Inoue K, et al. Direct observation of ATP-induced conformational changes in single P2X4 receptors. PLoS Biol. 2009;7(5):e1000103. doi: 10.1371/journal.pbio.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iglesias R, Locovei S, Roque AP, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaumont S, Khakh BS. Patch-clamp coordinated spectroscopy shows P2X2 receptor permeability dynamics require cytosolic domain rearrangements but not Panx-1 channels. Proc Natl Acad Sci USA. 2008;105:12063–12068. doi: 10.1073/pnas.0803008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–4824. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boumechache M, Masin M, Edwardson JM, Górecki DC, Murrell-Lagnado R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284:13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]