Abstract
Purpose
To review the mechanisms of sedative-hypnotic action with respect to the risk of delirium imparted by drugs that act on γ-amino-butyric-acid type A receptors or α2 adrenoceptors.
Source
MEDLINE was searched for relevant articles.
Principal findings
Development of the acute confusional state of delirium is associated with longer intensive care unit (ICU) and hospital lengths of stay, significantly higher risk of functional decline, and increased mortality. Disruption of sleep is a modifiable risk factor that may contribute to delirium and cognitive dysfunction in ICU patients. Among the functions of sleep are repair of defective processes and restoration of the brain to a state in which it is ready to acquire new knowledge. It is logical that disruption of these processes may produce acute confusion. Delirium develops through a complex interaction between the patient’s baseline vulnerability (patient’s predisposing risk factors before hospitalization) and precipitating factors or insults (modifiable events that occur during hospitalization). The latter factors include both sleep disruption and sedation. We present a hypothesis that these two factors are causally linked through effects on memory. Our hypothesis explains why patients randomized to receive an α2 adrenoceptor agonist are less likely to develop delirium (and the attendant cognitive dysfunction) than those randomized to receive benzodiazepines.
Conclusion
Herein we present our hypothesis that alternate mechanisms of hypnotic action may differentiate the deleriogenic properties of the two classes of sedatives. Future studies should focus on whether a causal relationship can be established between sedative administration, sleep disruption, and delirium.
Résumé
Objectif
Passer en revue les mécanismes d’action sédatifs-hypnotiques par rapport au risque de delirium imparti par les médicaments qui agissent sur les récepteurs de l’acide γ-amino-butyrique de type A (GABAA) et les adrénocepteurs α2.
Source
Une recherche a été effectuée dans la base de données MEDLINE pour en extraire les articles pertinents.
Constatations principales
L’apparition d’un état de confusion aigu de delirium est associée à des durées prolongées de séjour à l’unité des soins intensifs (USI) et à l’hôpital, à un risque significativement plus élevé de déclin fonctionnel et à une mortalité accrue. La perturbation du sommeil est un facteur de risque modifiable qui pourrait contribuer au delirium et à la dysfonction cognitive chez les patients de l’USI. La réparation des processus déficients et le rétablissement du cerveau à un état préparé à acquérir de nouvelles connaissances sont certaines des fonctions du sommeil. Il est logique que la perturbation de ces processus puisse provoquer une confusion aiguë. Le delirium survient suite à une interaction complexe entre la vulnérabilité fondamentale du patient (les facteurs de risque avant l’hospitalisation prédisposant le patient au delirium) et des facteurs ou lésions précipitants (événements modifiables survenant pendant l’hospitalisation). Ces seconds facteurs comprennent les perturbations du sommeil et la sédation. Nous présentons l’hypothèse que ces deux facteurs ont un lien de causalité par le biais d’effets sur la mémoire. Notre hypothèse explique pourquoi il est moins probable que des patients randomisés à recevoir un agoniste de l’adrénocepteur α2 manifestent un delirium (et la dysfonction cognitive concomitante) que des patients randomisés à recevoir des benzodiazépines.
Conclusion
Nous présentons ici notre hypothèse selon laquelle des mécanismes d’action hypnotique différents pourraient permettre de distinguer les propriétés délirogènes des deux classes de sédatifs. Les études devraient à l’avenir essayer de déterminer s’il existe une relation de causalité entre l’administration de sédatifs, les perturbations du sommeil et le delirium.
Attainment of the lighter stage of general anesthesia is practiced widely on intensive care patients to enable patients to tolerate invasive diagnostic and therapeutic (e.g., mechanical ventilation via an endotracheal tube) maneuvers. These general anesthetics go on for days, if not weeks, with escalating doses exposing the patient to cumulative benefits as well as to toxicities that are not encountered in the few hours of anesthesia typical for surgical patients in the operating room. The intensive care unit (ICU) is the most expensive clinical environment by far, consuming nearly 10% of all health care dollars in the USA – nearly 1% of the total GDP! The focus of attempts to curtail those costs has been on preventing complications in order to reduce the ICU length of stay. Intensivists have led this assault through widespread adoption of guidelines that have resulted in decreases in catheter-associated bloodstream infections, ventilator-associated pneumonias, and stress-induced ulcers. They have launched ambitious strategies to combat the ravages of sepsis with limited success. However, little has been done to reduce the incidence and prevalence of delirium, and the interplay between sleep and sedative-hypnotics in the genesis of this condition has not been explored. We first present a review of the restorative properties of sleep, particularly on cognitive function, the clinical evidence for sleep disruption and cognitive dysfunction in ICU patients, and how existing sedative-hypnotic agents may exacerbate or ameliorate this situation. We performed a wide-ranging MEDLINE search for relevant articles with keywords including “sleep”, “sedation”, “hypnotic”, “delirium”, “cognitive dysfunction,” and “memory”.
Sleep and restoration of cognitive function
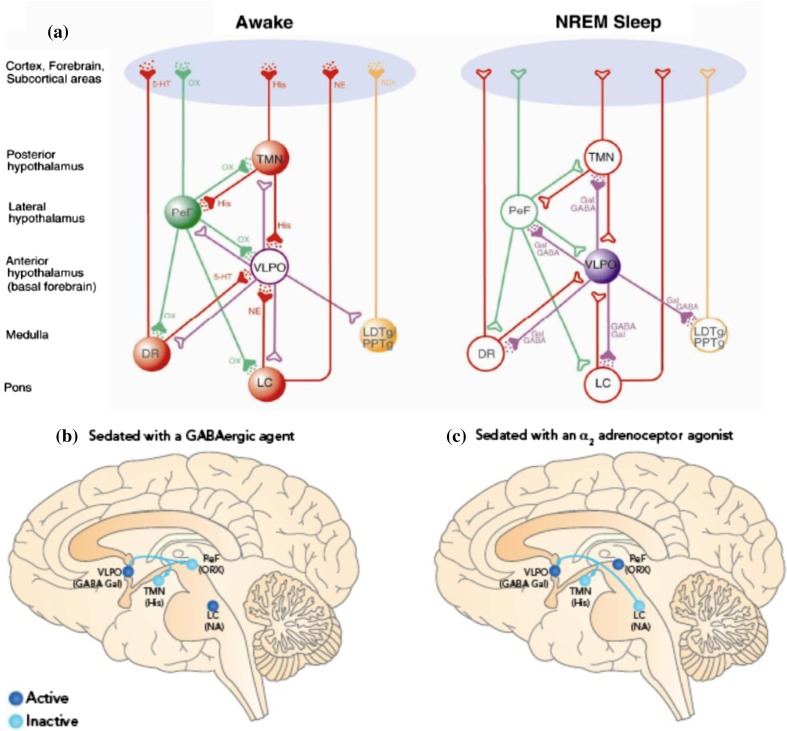
Sleep is under control of two processes, a circadian clock that regulates the appropriate timing of sleep and wakefulness across the 24-hr day and a homeostatic process (“sleep homeostasis”) that regulates sleep need and intensity according to the time spent awake or asleep.1 Sleep is a non-homogenous state that can be divided into non-rapid eye movement (NREM) sleep and rapid eye movement (REM; “paradoxical”) sleep. Neurochemical changes accompany these different types of sleep, with cholinergic (in brain stem and forebrain), noradrenergic (locus ceruleus), and serotonergic (dorsal raphe) activity all becoming less active in NREM sleep and cholinergic activity increasing in REM sleep2 (Fig. 1a). Activity in the ventrolateral pre-optic nucleus (VLPO) is increased in NREM sleep, and the γ-aminobutyric acid (GABA)ergic/galanin input from VLPO inhibits the histaminergic tuberomammillary nucleus3 (Fig. 1a). Orexinergic pathways from the perifornical nucleus are inactive during NREM sleep (Fig. 1a).3
Fig. 1.
Neural mechanisms of γ-aminobutyric acid (GABA)ergic and α2 adrenoceptor agonist sedation. a Wakefulness is promoted by the release of the arousal-promoting monoamine (red) neurotransmitters, norepinephrine (NE), serotonin (5-HT), and histamine (His), from the locus ceruleus (LC), dorsal raphe nucleus (DR), and tuberomammillary nucleus (TMN), respectively, as well as acetylcholine (ACh; orange) from the pedunculopontine and laterodorsal tegmental nuclei (PPTg and LDTg) and orexin (OX; green) into the cortex, forebrain, and subcortical areas. Conversely, during the deeper stages of NREM sleep, the activity is reversed by the inhibitory action of GABA and galanin (gal; purple) released from the ventrolateral pre-optic nucleus (VLPO). b, c Activity in brain nuclei involved in sleep pathways under sedation with a (b) GABAergic agent and c α2 adrenoceptor agonist. Abbreviations: GABA gal = neurons containing GABA and galanin; His = histamine; NA = noradrenergic; ORX = orexin; LC = locus ceruleus; PeF = perifornical nucleus; TMN = tuberomammillary nucleus; VLPO = ventrolateral pre-optic nucleus. Reproduced with permission from Nelson et al. (a)27 and modified with permission (intensetimes issue 9; available at www.intensetimes.eu) from Sanders et al. (b, c)1
Applying electroencephalogram (EEG) criteria, NREM sleep is composed of four distinct stages. Stages 1 and 2 reflect light sleep and are followed by stages 3 and 4 of a deep sleep plane. Stages 3 and 4 are often paired together as slow-wave sleep (SWS), as the EEG in these stages is dominated by a delta rhythm (frequency 0.5-4 Hz). Slow-wave sleep may be the mechanism that drives sleep homeostasis, as it peaks early on during sleep and decreases with the decline in sleep pressure.1 Physiologic repair of the organism is accelerated during SWS, as evidenced by the increase in the rate of anabolism.4 Within the brain, the slow wave activity (the “power” in the delta rhythm range) diminishes strengthening of synapses that have occurred during wakefulness5,6 and restores the brain to a state that is subsequently capable of appropriately processing new sensory input in the succeeding period of wakefulness.7 These synaptic homeostatic processes6 accommodate the brain’s energy8 and space9 requirements and allow the brain to acquire new information, which would not be possible in the absence of downscaling synaptic strength.
The different forms of memory, referred to as declarative or explicit (consciously accessible memories of fact-based information – knowing “what”) and non-declarative or implicit (procedural memory – knowing “how”) develop over time through several unique stages (acquisition, translocation, consolidation [comprising stabilization and enhancement], and reconsolidation). Deeper stages of NREM as well as REM sleep are required for some of these stages of learning and memory.2,10-12 Truncating stages of sleep can result in development of cognitive dysfunction,2,13 the most severe of which occurs following total sleep deprivation.10
We consider that impairment in the formation and retrieval of memories may account for much of the cognitive dysfunction associated with delirium. It seems intuitive that memory deficits combined with a fluctuating level of arousal (both symptoms of sleep deprivation) could produce a disorientated patient with reduced attention (Box) – a description of the delirious patient. Nonetheless, sedative medication also directly affects memory (independently from effects on sleep), and this likely also contributes to the delirium phenotype.
Box.
| Delirium is defined by the presence of disturbed consciousness (reduced clarity of awareness of the environment with reduced ability to focus, to sustain, or to shift attention) and a change in cognition (such as memory deficit, disorientation, or language disturbance) or the development of a perceptual disturbance that is not better accounted for by a pre-existing, established or evolving dementia. The disturbance develops acutely (usually hours to days) and tends to fluctuate during the course of the day. Delirium may exhibit hyperactive or hypoactive features. Key risk factors for delirium include age, co-morbidity, an acute inflammatory precipitant, sleep deprivation and sedative medication that targets γ-aminobutyric acid (GABA)A receptors. |
Reference: American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: APA, 1994
Sleep disruption and cognitive dysfunction in ICU patients
Early polysomnographic studies had revealed extreme sleep disruption in ICU patients, with decreases in total sleep time, altered sleep architecture (predominance of stages 1 and 2 sleep, decreased or absent stages 3 and 4 and REM sleep, and shortened REM periods), sleep fragmentation,14,15 and up to 50% of total sleep time occurring during the light phase. Among the causes contributing to sleep disruption in the ICU are those related to the patient’s acute illness and co-morbidities, environmental factors (including noise and inappropriate light), and iatrogenic factors, including frequent care-related interruptions and medications prescribed for analgesia and sedation. Among the causes potentially amenable to modification, excessive noise appears not to contribute as much as was anticipated,16 and attention has focused on sedative practices.17,18 Several studies have now demonstrated the association between benzodiazepine use and both greater incidence19 and duration20 of delirium in medical ICU patients, although the relationship of the development and duration of delirium to sleep disruption was not ascertained. Acute withdrawal from long-term sedation with benzodiazepines and opiate narcotics results in profound sleep disruption.21 Interestingly, although the α2 agonists can be used to treat symptoms of acute withdrawal from psychoactive drugs, the effect they have on withdrawal-induced sleep disturbances has not been reported.
Mechanisms and use of sedative-hypnotic agents in the ICU
Benzodiazepines enhance fast inhibitory neurotransmission by modulating the activity of GABAA receptors in postsynaptic membranes. The GABAA receptor is a heteropentamer, and most GABAA receptors have a binding site for benzodiazepines (formed by α and γ2 subunits) in addition to binding sites for the physiological neurotransmitter GABA (formed by α and ß subunits).22 Knock-in studies perturbing the GABAA receptors in mice have revealed that the α1 subunit in glutamatergic forebrain neurons is necessary for changes in locomotion (sedation), while the α2 subunit in hypothalamic nuclei is required for the transduction of the hypnotic properties (and its attendant EEG properties) of benzodiazepines.23,24 Benzodiazepine hypnotics depress slow-wave activity in NREM sleep, not only during the night when subjects receive the drugs but also during the subsequent night.25 Neither sleep homeostasis nor circadian rhythm is altered by acute benzodiazepine administration. Recently, the dopaminergic action of benzodiazepines has been revealed, which is mediated through the α1 subunit containing GABAA receptors in the reward centre (nucleus accumbens). The GABAA receptors likely contribute to the addictive features of benzodiazepines.26
In a series of studies involving GABAergic agents, we reported that, unlike NREM sleep, these hypnotic agents did not alter noradrenergic activity in the locus ceruleus (Fig. 1b).27,28 Instead, these agents converged on the NREM sleep pathway at the level of the hypothalamus.28 Nonetheless, short-term administration of the GABAergic agent, propofol, permits normal recovery after a period of sleep deprivation, indicating similarities between propofol-induced hypnosis and sleep.29
Benzodiazepines also exert significant memory-modulating effects, though the extent to which they impair explicit and implicit memory appears paradigm dependent.30 While some have suggested that both implicit and explicit memory are impaired following administration of midazolam,31 only explicit memory is affected in children.32
We have shown that α2 agonists transduce their hypnotic response after binding to the α2A receptor subtype33 through effects in the locus ceruleus (LC).34,35 The noradrenergic neurons become hyperpolarized and are less likely to achieve an action potential due to signalling processes that involve both pertussis toxin-sensitive G proteins36 and effector mechanisms, including inhibition of adenylyl cyclase34 and ligand-gated calcium channels as well as activation of inwardly-rectifying potassium channels.37
The relatively quiescent LC facilitates a series of changes, including activation of the galanin/GABA-containing neurons of the VLPO nucleus that terminate on and inhibit aminergic neurons within the tuberomammillary nucleus (Fig. 1c).38 Thus, α2 agonists are associated with similar changes in neuronal activity as is seen in NREM sleep,3,39 apart from the absence of an inhibitory effect on the orexinergic neurons in the perifornical nucleus.28 In a functional magnetic resonance imaging (fMRI) study comparing sedation with α2 agonists and benzodiazepines, we showed that a thalamic nucleus receiving afferent input from orexinergic neurons is activated during an arousal stimulus in α2 agonist-sedated subjects but not in benzodiazepine-sedated subjects.40 The preservation of orexin signalling may account for the patient rousability noted with dexmedetomidine sedation. In turn, this clinical effect may be important in permitting weaning from mechanical ventilation and patient examination.
Compatible with overlapping neural substrates, dexmedetomidine induces a very similar EEG pattern in human volunteers as that seen in stages 2-4 of NREM sleep.41 Children sedated with dexmedetomidine exhibited an EEG pattern that was similar to that seen in stage 2 NREM sleep.42 Dexmedetomidine and sleep also share similarities in hypercarbic ventilator,43 hormonal,44 and auditory evoked response.45
Recently, Veselis et al. addressed the effects of dexmedetomidine in a particular memory paradigm (continuous recognition task) and reported less memory perturbation (if any) than was seen with GABAergic agents.46 In animal studies, acutely-administered dexmedetomidine was noted to have variable effects on learning and memory depending on the dose.47 Recently reported rat studies showed that dexmedetomidine interferred only with memory formation if perception of sensory input was decreased during very deep levels.48
Relevant clinical investigations
Two randomized controlled trials (RCTs) investigated whether dexmedetomidine could provide superior sedation to benzodiazepine sedation. The Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) RCT compared dexmedetomidine and lorazepam sedation in 106 mechanically ventilated patients (three patients were withdrawn).17 Sedation with dexmedetomidine resulted in more days thriving without delirium or coma, a lower prevalence of coma, and more on-target sedation than lorazepam administration.17 The follow-up Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) trial randomized 375 patients to dexmedetomidine or midazolam sedation.18 Though no difference in time at target sedation was observed, patients sedated with dexmedetomidine had a reduced prevalence of delirium with a reduced duration of mechanical ventilation.18
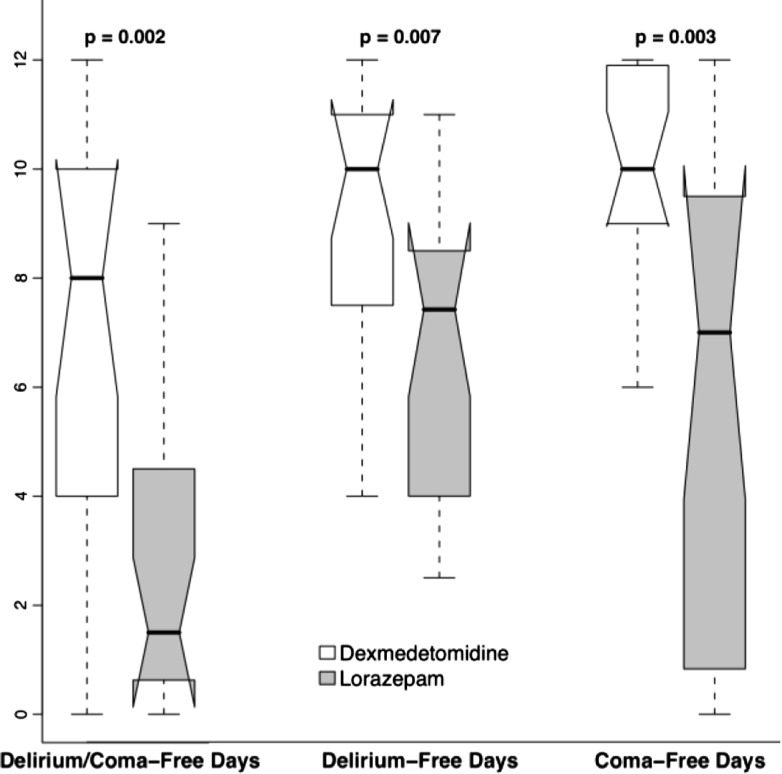
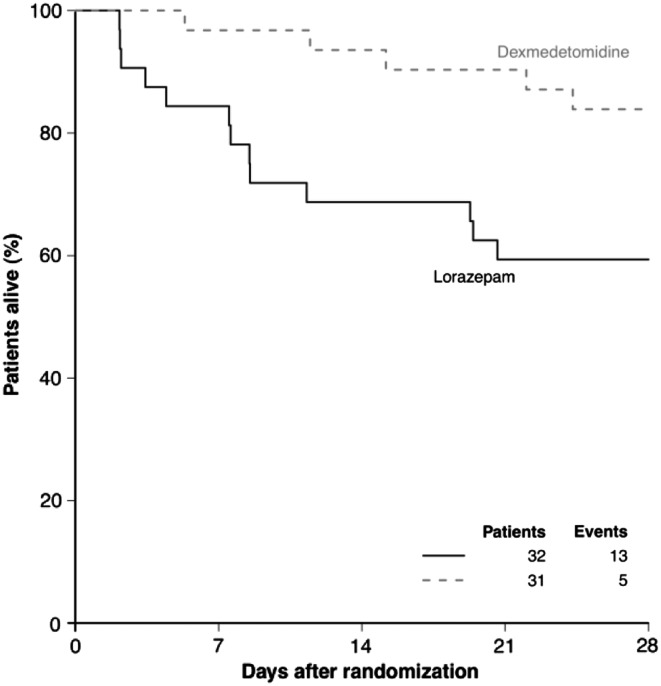
While we did not randomize patients according to whether they were septic on admission to our MENDS RCT,17 we had decided a priori to perform a post-hoc analysis of septic vs non-septic subgroups of patients who received dexmedetomidine-based or lorazepam-based sedation for up to five days.49 More than half of the 103 patients included (63 patients; 31 dexmedetomidine, 32 lorazepam) were admitted with sepsis. Demographic and severity data were balanced between the two cohorts. Compared with septic patients who received lorazepam, the septic patients who received dexmedetomidine had 3.2 more delirium/coma-free days and more ventilator-free days on average (95% confidence intervals for difference, 1.1-4.9 and 0.3-11.1, respectively) (Fig. 2). The risk of dying at 28 days was reduced by 70% (hazard ratio 0.3: 0.1-0.9) in dexmedetomidine patients with sepsis compared with the lorazepam patients (Fig. 3). In addition to alternate effects on innate immunity and physiological response to the infection,49,50 we speculate that the immune dysfunctional effects of sleep deprivation in the lorazepam group may have contributed to the higher death rate from infection. Our speculation is supported by data from the SEDCOM trial in which the rate of infection was 50% lower in the DEX group.18
Fig. 2.
Days free from complications associated with acute brain failure in septic intensive care unit patients sedated with the α2 adrenoceptor agonist, dexmedetomidine, or the benzodiazepine, lorazepam. Data are represented in a box and whisker plot reflecting the median, lower, and upper quartiles and the lower and upper extremes of days/patient. Reproduced with permission from Pandharipande et al. 49
Fig. 3.
Kaplan-Meier curve showing the probability of survival according to sedation group during the first 28 days following admission to the intensive care unit for sepsis. Avoidance of lorazepam sedation using dexmedetomidine decreased the probability of dying within 28 days by 70%. Reproduced with permission from Pandharipande et al. 49
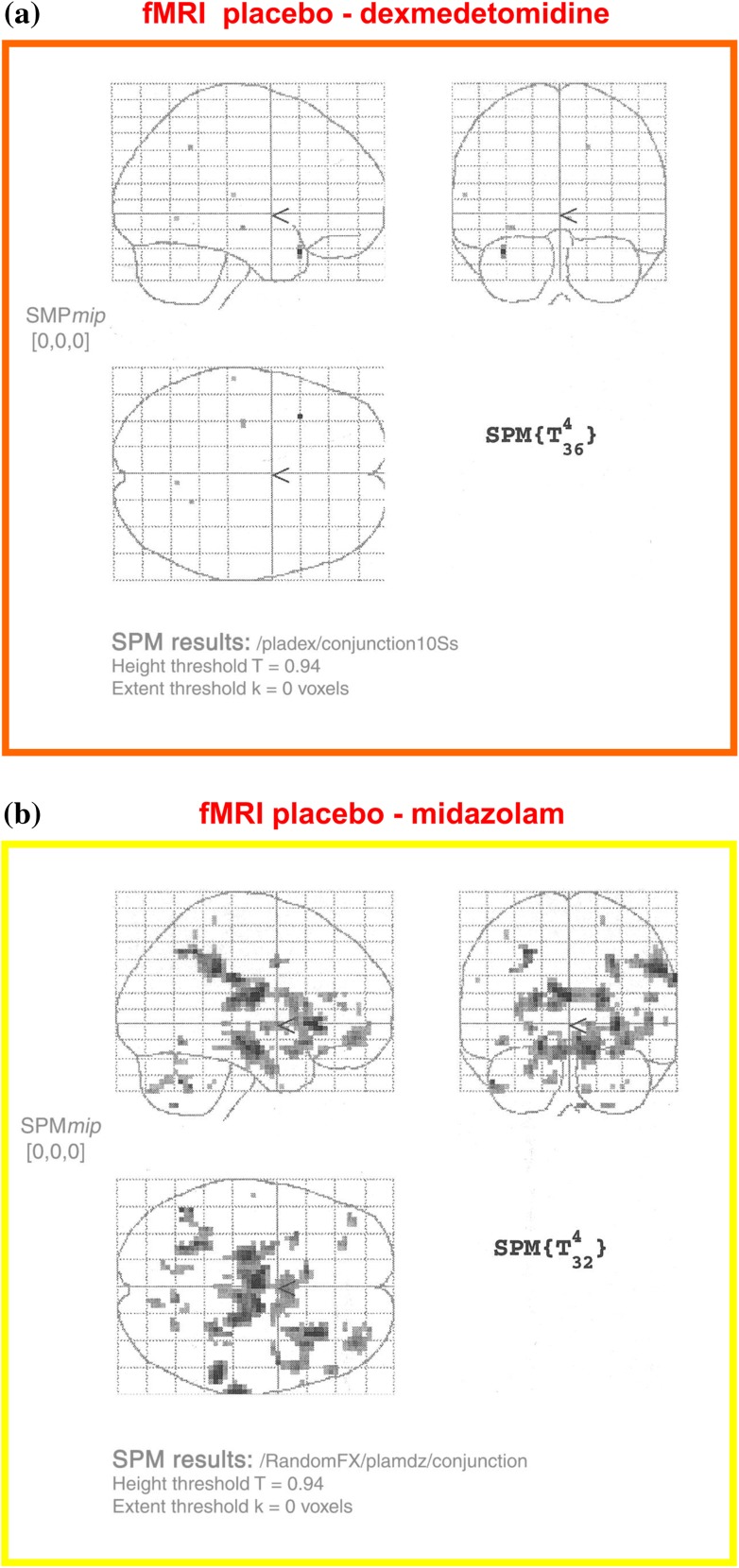
Functional MRI was performed in volunteers tested on three separate occasions during which they received saline, dexmedetomidine, or midazolam.40 Subjects were infused to achieve a target concentration that produced equivalent sedation, as assessed by electroencephalography (bispectral index [BIS]) and observer rating (Observer Assessment of Alertness/Sedation [OAA/S]). In a single subject, sleep occurred during saline infusion while undergoing fMRI. Subtraction scans were performed to yield the difference in blood oxygen level-dependent (BOLD) activity between natural sleep and the sedated states provided by either dexmedetomidine or midazolam. There were fewer voxels of BOLD activity seen in the subtraction scan between dexmedetomidine-sedation and natural sleep (Fig. 4a) than between midazolam-sedation and natural sleep (Fig. 4b).
Fig. 4.
Sedation with α2 adrenoceptor agonist produces a more “natural sleep” response than sedation with benzodiazepine. Functional magnetic resonance imaging (fMRI) was performed in volunteers tested on three separate occasions, during which they received saline, dexmedetomidine, or midazolam.40 Subjects were infused to achieve a target concentration that produced equivalent sedation as assessed by electroencephalography (bispectral index, BIS) and observer rating (Observer Assessment of Alertness/Sedation, OAA/S). In a single subject, sleep occurred during saline infusion while undergoing fMRI. Subtraction scans were performed yielding the difference in blood oxygen level-dependent (BOLD) activity between natural sleep and the sedated states provided by either dexmedetomidine or midazolam. There were fewer voxels of BOLD activity seen in the subtraction scan between dexmedetomidine-sedation and natural sleep (a) than seen between midazolam-sedation and natural sleep (b)
The changes in neuronal activity that benzodiazepines induce are inconsistent with the deeper stages of NREM sleep. Consequently, the restorative properties of natural sleep are lacking in patients on prolonged benzodiazepine infusions, resulting in acute brain and immune system dysfunction that may complicate the recovery of critically ill patients. The addictive properties of benzodiazepines result in a rapid escalation in dose requirements. When benzodiazepines are part of the sedative regimen, it is difficult to perform “interruption of sedation” standard of nursing care because of the likelihood of the supervention of withdrawal phenomena, including a hypernoradrenergic state and anxiogenesis.
Conclusions
Sedative-hypnotic agents contribute to the development of delirium in critically ill patients.17-19 We hypothesize that α2 adrenoceptor agonists are beneficial relative to benzodiazepines due to subtle differences in their mechanisms of action. In particular, we suggest that dexmedetomidine sedation may provide a more restorative – perhaps “natural sleep-like” - state than GABAergic sedatives, such as the benzodiazepines. Our proposition centres on the discovery that α2 adrenoceptor agonists act on the sleep pathway at the brainstem level, while GABAergic agents act at the level of the hypothalamus. The dissimilar actions produce different sedative profiles for the two classes of agents, and we suspect they contribute to the risk of delirium in the intensive care unit through alternate effects on the restorative nature of the sedation. This may also explain why outcomes from sepsis49 and infection18 are very different for α2 adrenoceptor agonists than they are for GABAergic agents (though we suspect ongoing studies will identify the importance of the immune actions of the two drug classes). Furthermore, α2 adrenoceptor agonists may provide a state from which patients are rousable, possibly through preserved orexinergic signalling. Rousability is important to facilitate weaning from mechanical ventilation and neurological examination. Finally, in addition to preventing the cognitive consequences of sleep deprivation, the drugs alternately affect memory formation. We suggest that the impairment of memory formation by GABAergic drugs contributes to the acute confusion in delirium, while α2 adrenoceptor agonists produce little in the way of memory impairment and thus reduce the burden of patient disorientation.
Clinical studies continue to reveal the benefits of understanding the differences in sedative-hypnotic mechanisms, and as further mechanistic understanding can drive advances in clinical medicine, we urge clinicians and scientists alike to continue this fruitful path of discovery to aid patient care at the bedside. The adoption of “sedation holidays”51 and “spontaneous breathing trials”52 have shown that sedation in our most vulnerable patients is an important determinant of outcome. We now have the opportunity to define the agents for best sedating our patients, and we suggest that α2 adrenoceptor agonists may offer particular advantages in the critically ill.17,18,49,50
Competing interests
Dr. Maze discovered and patented the anesthetic properties of dexmedetomidine in 1987. He reverted back his patent rights to Orion Farmos for $250,000 in support of laboratory activities. Dr. Maze has received grant support, speaker fees, and honoraria from Orion, Abbott Labs (which registered dexmedetomidine for its sedative use) and Hospira (which market dexmedetomidine).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Sanders RD, Hussell T, Maze M. Sedation & immunity: optimisation for critically ill patients. Intense Times 2010; 9: 2-5
This article is accompanied by an editorial. Please see Can J Anesth 2011; 58(2).
References
- 1.Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–s693. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- 2.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest. 1968;47:2079–2090. doi: 10.1172/JCI105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 7.Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- 8.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 9.Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/S0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 10.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 11.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 12.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S29–S34. doi: 10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- 14.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290:1029–1032. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilton BA. Quantity and quality of patients’ sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976;1:453–468. doi: 10.1111/j.1365-2648.1976.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 16.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 17.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 18.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 19.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37:177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cammarano WB, Pittet JF, Weitz S, Schlobohm RM, Marks JD. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit Care Med. 1998;26:676–684. doi: 10.1097/00003246-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph U, Crestani F, Benke D, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 23.Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc Natl Acad Sci U S A. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp C, Rudolph U, Low K, Tobler I. Modulation of rhythmic brain activity by diazepam: GABA(A) receptor subtype and state specificity. Proc Natl Acad Sci U S A. 2004;101:3674–3679. doi: 10.1073/pnas.0306975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borbely AA, Achermann P. Ultradian dynamics of sleep after a single dose of benzodiazepine hypnotics. Eur J Pharmacol. 1991;195:11–18. doi: 10.1016/0014-2999(91)90376-2. [DOI] [PubMed] [Google Scholar]
- 26.Tan KR, Brown M, Labouebe G, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 28.Zecharia AY, Nelson LE, Gent TC, et al. The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265 M knock-in mouse. J Neurosci. 2009;29:2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004;100:1419–1426. doi: 10.1097/00000542-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Savic MM, Obradovic DI, Ugresic ND, Bokonjic DR. Memory effects of benzodiazepines: memory stages and types versus binding-site subtypes. Neural Plast. 2005;12:289–298. doi: 10.1155/NP.2005.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H, Quinlan J, Thornton E, Reder LM. The effect of midazolam on visual search: implications for understanding amnesia. Proc Natl Acad Sci U S A. 2004;101:17879–17883. doi: 10.1073/pnas.0408075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart SH, Buffett-Jerrott SE, Finley GA, Wright KD, Valois Gomez T. Effects of midazolam on explicit vs implicit memory in a pediatric surgery setting. Psychopharmacology (Berl) 2006;188:489–497. doi: 10.1007/s00213-006-0402-7. [DOI] [PubMed] [Google Scholar]
- 33.Lakhlani PP, MacMillan LB, Guo TZ, et al. Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correa-Sales C, Nacif-Coelho C, Reid K, Maze M. Inhibition of adenylate cyclase in the locus coeruleus mediates the hypnotic response to an alpha 2 agonist in the rat. J Pharmacol Exp Ther. 1992;263:1046–1049. [PubMed] [Google Scholar]
- 35.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Correa-Sales C, Reid K, Maze M. Pertussis toxin-mediated ribosylation of G proteins blocks the hypnotic response to an alpha 2-agonist in the locus coeruleus of the rat. Pharmacol Biochem Behav. 1992;43:723–727. doi: 10.1016/0091-3057(92)90400-A. [DOI] [PubMed] [Google Scholar]
- 37.Nacif-Coelho C, Correa-Sales C, Chang LL, Maze M. Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology. 1994;81:1527–1534. doi: 10.1097/00000542-199412000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 39.Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABAA drugs. J Clin Sleep Med. 2006;2:S19–S26. [PubMed] [Google Scholar]
- 40.Coull JT, Jones ME, Egan TD, Frith CD, Maze M. Attentional effects of noradrenaline vary with arousal level: selective activation of thalamic pulvinar in humans. Neuroimage. 2004;22:315–322. doi: 10.1016/j.neuroimage.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Huupponen E, Maksimow A, Lapinlampi P, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–294. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 42.Mason KP, O’Mahony E, Zurakowski D, Libenson MH. Effects of dexmedetomidine sedation on the EEG in children. Paediatr Anaesth. 2009;19:1175–1183. doi: 10.1111/j.1460-9592.2009.03160.x. [DOI] [PubMed] [Google Scholar]
- 43.Hsu YW, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–1076. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Kallio A, Scheinin M, Koulu M, et al. Effects of dexmedetomidine, a selective alpha 2-adrenoceptor agonist, on hemodynamic control mechanisms. Clin Pharmacol Ther. 1989;46:33–42. doi: 10.1038/clpt.1989.103. [DOI] [PubMed] [Google Scholar]
- 45.Thornton C, Lucas MA, Newton DE, Dore CJ, Jones RM. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 2: Auditory and somatosensory evoked responses. Br J Anaesth. 1999;83:381–386. doi: 10.1093/bja/83.3.381. [DOI] [PubMed] [Google Scholar]
- 46.Veselis RA, Pryor KO, Reinsel RA, Li Y, Mehta M, Johnson R., Jr Propofol and midazolam inhibit conscious memory processes very soon after encoding: an event-related potential study of familiarity and recollection in volunteers. Anesthesiology. 2009;110:295–312. doi: 10.1097/ALN.0b013e3181942ef0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirvio J, Riekkinen P, Jr, Ekonsalo T, Lammintausta R, Riekkinen PJ. The effects of dexmedetomidine, an alpha 2 agonist, on learning and memory, assessed using passive avoidance and water maze tasks in rats. Neuropharmacology. 1992;31:163–168. doi: 10.1016/0028-3908(92)90027-M. [DOI] [PubMed] [Google Scholar]
- 48.van Oostrom H, Stienen PJ, Doornenbal A, Hellebrekers LJ. The alpha(2)-adrenoceptor agonist dexmedetomidine suppresses memory formation only at doses attenuating the perception of sensory input. Eur J Pharmacol. 2010;629:58–62. doi: 10.1016/j.ejphar.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 49.Pandharipande PP, Sanders RD, Girard TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders RD, Hussell T, Maze M. Sedation & immunomodulation. Crit Care Clin 2009; 25: 551-70, ix. [DOI] [PubMed]
- 51.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 52.Girard TD, Kress JP, Fuchs BD, Thomason JW, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]