Abstract
Rationale
Smoking cues are theorized to be conditioned stimuli (CSs) formed by repeated pairing with drug. Smoking paraphernalia can elicit subjective and physiological responses in smokers, indicative of positive affect and motivation to consume. Although these responses are probably the result of conditioning, direct evidence from human conditioning studies with physiological measures of motivational valence is rare.
Objective
The present study investigated the motivational properties of experimentally conditioned cues for smoking.
Methods
Thirty-nine smokers completed a differential conditioning protocol. Abstract pictures were used as CSs and single puffs on a cigarette as unconditioned stimulus (US). Skin conductance responses and facial electromyography of the zygomatic, corrugator, and orbicularis oris muscles were measured during conditioning.
Results
The conditioned cue for smoking (CS+) elicited stronger skin conductance responses and more activity of the zygomatic and orbicularis oris muscles than the CS−.
Conclusions
These results support the notion that through pairing with smoking, neutral stimuli acquire the ability to elicit preparatory physiological responses, which are assumed to play an important role in the maintenance of addiction and relapse in the natural environment.
Keywords: Addiction, Conditioning, Smoking, Nicotine, Incentive motivation, Emotion, Facial EMG, Cue reactivity, Human
Introduction
Important criteria of nicotine dependence, like craving or relapse, are highly situational specific. This emphasizes the role of learning processes. The animal literature indicates that through pairing with drug consumption, previously neutral stimuli acquire the ability to elicit a plethora of conditioned responses. These may include changes in physiological and motivational processes (e.g., Siegel et al. 2000; Stewart et al. 1984), changes in attention (e.g., Robinson and Berridge 1993), and overt drug-seeking or drug-taking behavior (e.g., Le Foll and Goldberg 2006).
According to incentive theories of addiction (e.g., Panksepp et al. 2002; Robinson and Berridge 1993; Stewart et al. 1984), drugs act on brain systems mediating incentive functions of natural rewards (e.g., Martin-Soelch et al. 2007; Stippekohl et al. 2010; Volkow et al. 2004). Primary incentives like food or water are assumed to generate an appetitive motivational state, resulting in a tendency to approach and contact these objects (e.g., Berridge 2004). Previously neutral stimuli predictive of incentives acquire the ability to activate this motivational state and thereby come to elicit appetitive reactions. According to incentive theories, stimuli accompanying drug-intake therefore become conditioned incentives and evoke drug-seeking and drug consumption.
In the dependent human, this issue primarily has been addressed by measuring subjective and physiological responses to naturalistic drug-related cues, e.g., cigarettes (e.g., Carter and Tiffany 1999). This research has shown that drug-related stimuli can evoke subjective craving and pleasure (e.g., Mucha et al. 2008; Stippekohl et al. 2010), as predicted by classic incentive theories (e.g., Bindra 1974; Stewart et al. 1984). However, cue-evoked pleasure can be dissociated from physiological measures of appetitive motivation under certain circumstances (e.g., Mucha et al. 2000). In some cases, this dissociation may reflect social demand effects, as drug-dependent individuals may not always honestly report their subjective experience. However, even in cases when individuals report cue-evoked subjective pleasure, this response could be dissociated from objectives measures of motivation for drug (e.g., Hogarth et al. 2010; Moeller et al. 2009). The functional role of subjective pleasure can be addressed from different theoretical perspectives (e.g., Berridge and Kringelbach 2008; Bindra 1974; Dickinson and Balleine 2010; Frijda 2010; Toates 1994). Physiological measures of motivational state may be fruitfully applied to advance our understanding of the emotional quality of cue-evoked responses and its relationship to phenomenological experience in humans.
There are at least two physiological measures of motivational valence which have been successfully applied in research on smoking cues. Previous animal and human studies on the modulation of the acoustic startle response have shown that the magnitude of the response increases in a linear fashion with the negativity of an emotional state (e.g., Fendt and Mucha 2001; Lang et al. 1990). Based on this approach, Geier et al. (2000) have shown that the acoustic startle response is attenuated in smokers during the presentation of pictorial smoking cues. These results were confirmed by later studies (Mucha et al. 2008) and other research groups (Dempsey et al. 2007), although there seems to be some variability in the results, probably due to methodological discrepancies (e.g., Elash et al. 1995; Mucha et al. 2006; Orain-Pelissolo et al. 2004).
Besides the affect modulation of the startle response, facial reactions can be used as a reliable index of affective state and as a valuable research tool in comparative psychology (e.g., Berridge and Robinson 2003). Facial electromyography (EMG) has been proven as a sensitive measure of affective responses (e.g., Lang et al. 1993). The most frequently measured muscles include the M. zygomaticus major (“smiling muscle”) and the M. corrugator supercilii (“frowning muscle”). Using facial EMG, Drobes and Tiffany (1997) have found that in smokers, smoking cue exposure increases activity of the zygomatic muscle and decreases activity of the corrugator muscle, indicative of enhanced positive affect and reduced negative affect, respectively. Geier et al. (2000) also used facial EMG to test the motivational valence of smoking cues. Overall, the results were more in line with an appetitive reaction to smoking cues. However, there are also reports in the literature of cue-induced ambivalence (e.g., Griffin and Sayette 2008).
In general, the results of these studies using physiological measures of motivational valence are in line with the prediction of incentive theories. However, they are silent about whether the reactivity evoked by naturalistic cues is indeed the result of conditioning (Robbins and Ehrman 1992). Direct evidence for this assumption comes from studies in which conditioning of previously neutral stimuli actually took place. In human studies, the learning process underlying tobacco dependence often is modeled by pairing artificial stimuli with the opportunity to smoke. Lazev et al. (1999) were one of the first who used a differential conditioning protocol with smoking of a single cigarette as reward and found that the smoke-paired stimulus (CS+) increased self-reported craving, positive affect and pulse rate. A study conducted by Mucha et al. (1998) used a behavioral measure of preference and found that smokers listened more to an auditory CS+ previously paired with smoking a cigarette. In addition, subjects drew more on a cigarette in presence of the CS+ compared to the non-paired stimulus (CS−). It was also seen that presentation of the CS+ under extinction elicited an increase in activity of the trapezius muscle at the time after CS+ onset when smoking previously occurred during conditioning. Finally, Field and Duka (2001) used salivation as possible index of appetitive responses. However, although the CS+ increased subjective craving, the results were inconsistent regarding cue-evoked salivation.
All conditioning studies reported above used the smoking of an entire cigarette as unconditioned stimulus (US). That single puffs on a cigarette may condition physiological responses was first reported by Lewin et al. (1986) using EMG of the frontalis muscle as dependent variable. Later on, Carter and Tiffany (2001) found that the availability of single puffs on a cigarette increased the reactivity to smoking cues. Participants in their study showed enhanced cue-evoked craving and positive affect, an increase in skin conductance, and a decrease in response latency when smoking was available. These results were further supported by Hogarth et al. (2003) who reported conditioning of selective attention and elevated skin conductance to a discriminative CS+ for single puffs on a cigarette (see also Hogarth and Duka (2006) for a review of studies on human smoke conditioning). These results support the notion that previously neutral stimuli paired with smoking become conditioned cues and evoke similar responses as naturalistic smoking cues. However, human conditioning studies which assessed objective measures only delivered hints that smoking cues elicit appetitive reactions in smokers. To our knowledge, no conditioning study has assessed physiological measures of motivational valence. Therefore, we conducted a study with smokers using a differential conditioning protocol with single puffs on a cigarette as reward. We used facial EMG of the zygomatic and corrugator muscle as dependent variable. Facial EMG has been proven to be both a sensitive index of motivational valence (see above) and a sensitive measure of learning (e.g., Dimberg 1990). In addition, due to reports that cues for smoking resulted in both anticipatory muscular activity and increased puffing on a cigarette (Mucha et al. 1998), we recorded activity of the orbicularis oris muscle (lip muscle) as a sensitive measure of motor activity characterizing puffing on a cigarette (Mueller et al. 2003). Skin conductance responses were assessed as an indicator of autonomic arousal and orienting.
Materials and methods
Participants
Forty-five participants were recruited from the student population at the University of Würzburg. They provided written informed consent prior to the study, which was approved by the ethical committee of the German Psychological Association and was carried out in accordance with the ethical standards of the fifth revision of the Declaration of Helsinki.
Participants were included if they smoked an average of at least ten cigarettes per day for at least 1 year and agreed to abstain from smoking 2 h prior to the experiment. Exclusion criteria were an age under 18 or over 40 years, a major somatic or psychiatric illness, and self-reported consumption of alcohol or illicit drugs before the experiment. Subjects were paid 20 euros (approximately US $25) for participation and, in addition, received the monetary equivalent of the cigarettes smoked during the study. Three recruited subjects had to be excluded from the study because of a high number of artifacts in the psychophysiological recording. Three participants were dropped because of the absence of contingency awareness (see Results). Therefore, the results are based on the data of 39 subjects (15 males and 24 females). The mean age of the sample was 24.36 years (SD = 3.73). Subjects reported regular smoking for 6.63 years (SD = 3.07) and consumed 13.39 (SD = 3.28) cigarettes per day on average. The mean Fagerström Test for Nicotine Dependence (FTND) score was 3.19 (SD = 1.53), and the mean Questionnaire on Smoking Urges (QSU-G) score was 3.39 (SD = 0.91).
Stimulus material
Unconditioned stimulus
One or two puffs on the subjects preferred brand of cigarettes served as unconditioned stimulus. We allowed the subjects to choose by themselves how often (once or twice) and how deep they liked to inhale because of two reasons. First, the application of the maximum puff number during the experiment may result in high smoke uptake (e.g., Morris and Gale 1994; Schupp et al. 1999), which might render smoking aversive. Second, motivational differences between self-administered and yoked delivery of drug have been reported previously (e.g., Twining et al. 2009). The cigarettes were provided by the participants. They were put in a bowl and were placed in a green plastic box, together with an ashtray and a lighter. The box was 23 cm high, 22.5 cm broad and 27.5 cm deep. It could be opened on the front. The box was placed on the side of the dominant hand of the subject, approximately 30 cm besides the monitor on which the CSs were presented (see next paragraph).
Conditioned stimuli
Conditioned stimuli (see Fig. 1) were modified versions of pictures (picture B and C) used in previous studies (e.g., Hogarth et al. 2006). The pictures used as CS+ and CS− were counterbalanced over the subjects. They were displayed on a white background in the center of a 17-in. color screen (1024 × 768 pixels) placed about 70 cm in front of the subjects.
Fig. 1.
Stimuli counterbalanced between subjects in the role of CS+ and CS− during the conditioning phase (not to scale)
Questionnaires
The FTND (Heatherton et al. 1991) was used as self-report measure of nicotine dependence. The QSU-G (Mueller et al. 2001) was used to assess baseline craving. It consists of several questions on desire to smoke and anticipated pleasure as well as anticipated relief from withdrawal. Self-Assessment Manikins (SAM; Lang 1980) were used to document changes in momentary pleasure and arousal during the experiment. The manikins are graphic figures visualizing different values of emotional reactions on the dimensions of pleasure and arousal. For pleasure, the poles are visualized by a smiling happy figure and a frowning unhappy figure, respectively. For arousal, the poles are visualized by a wide-eyed aroused figure and a sleepy relaxed figure, respectively. Changes in momentary desire to smoke, eat, and drink water or alcohol (not at all–high) were assessed using nine-point scales (see Mucha et al. 1999).
Procedure
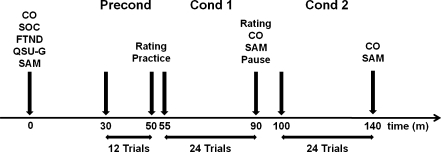
After arrival at the laboratory, participants completed a socio-demographic questionnaire, including questions on smoking-related activities and filled in the QSU-G. Next, the motivational state of the subjects was assessed using the questionnaires described above and an alveolar carbon monoxide (CO) sample was taken using a Bedfont Micro Smokerlyzer. After preparation for psychophysiological recording, subjects were seated comfortably in a chair, and the computer-assisted part of the experiment was started. The experimental protocol was controlled by Presentation software (Neurobehavioral Systems, Inc.). During a preconditioning phase, each CS was presented six times, to habituate putative unconditioned responses to the CSs. The trial sequence was pseudorandomized, with the constraint of no more than two successive trials of the same CS. Stimuli were presented for 28 s, preceded by a fixation cross for 1.5 s. CS presentation was followed by a 60 s intertrial interval.
At the end of the preconditioning phase, each CS was presented again under free-viewing conditions and subjects rated their evoked craving (not at all–high), pleasure (unpleasant–pleasant), and arousal (relaxed–aroused) on nine-point scales appearing on the screen after picture presentation. Next, subjects were informed that in the following part of the experiment sometimes during stimulus presentation a sentence would appear on the screen which would ask them to smoke. In this case, they should open the box, light a cigarette, and take one or two puffs. After that, they should butt out the cigarette, put the smoking paraphernalia back into the box, and finally close the box. To ensure that the subjects understood the procedure one supervised CS+ trial as well as one supervised CS− trial followed. If the participants had no further questions, the first block of the conditioning phase started, containing 12 CS+ and 12 CS− trials. Stimulus presentation parameters were the same as during the preconditioning phase, with the exception that on CS+ trials a text appeared on the screen above the CS+ 8 s after stimulus onset and asked subjects to smoke. The first conditioning block ended with the assessment of CS-evoked craving, pleasure, and arousal as described above. In addition, after presentation of the CSs subjects had to state if they were allowed to smoke during presentation of this picture during the last block (yes/no). Next, the second measurement of breath CO and self-reported motivational state followed. After a short break of 5 min, a second identical conditioning block was run. The experiment ended with the third assessment of CO and self-reported motivational state. Overall, the study lasted about 2.5 h (see Fig. 2 for a scheme of the experimental protocol).
Fig. 2.
Scheme of the experimental protocol. Precond preconditioning, Cond 1 conditioning 1, Cond 2 conditioning 2, Rating CS−rating, Practice practice trials, CO carbon monoxide test, FTND Fagerström Test for Nicotine Dependence, QSU-G Questionnaire on Smoking Urges-German version, SAM modified version of the Self-Assessment Manikins
Data recording
Psychophysiological activity was recorded continuously by a Vitaport II system (Becker Engineering, Karlsruhe, Germany). Facial EMG was recorded over the left corrugator supercilli and zygomaticus major muscle (according to Fridlund and Cacioppo 1986) and over the orbicularis oris muscle (according to Mueller et al. 2003) using Ag/AgCl miniature electrodes. Impedance was kept below 10 kΩ. Sampling was at 512 Hz with online high- and low-pass filter settings of 0.015 and 2,190 Hz, respectively. The signals were rectified, integrated, and stored at 16 Hz (corrugator and zygomaticus) and 256 Hz (orbicularis oris), respectively. EMG of the orbicularis oris was smoothed offline (using a time window of 150 ms).
Skin conductance was measured with two Ag/AgCl electrodes, filled with a 0.05 M sodium chloride electrolyte paste. Electrodes were placed on the thenar and hypothenar eminences of the non-dominant hand. The Vitaport II system constantly delivered 0.5 V across the two electrodes and sampled skin conductance at a rate of 16 Hz.
Data reduction and statistical analysis
EMG activity is expressed as the difference between the mean activity during the 8 s after CS onset and the mean activity during the second before each CS presentation. The skin conductance response (SCR) was scored as the largest increase between 1.0 and 6.5 s after CS onset compared to the 1 s of baseline mean activity. Responses less than 0.01 μMho were scored as zero. Before statistical analysis, the logarithms of the SCR values (SCR + 1) were calculated to normalize the distribution (Venables and Christie 1980). Scores for each CS were generated by computing the mean of all trials during the preconditioning phase as well as during the first and the second block of the conditioning phase, respectively.
Subjective and physiological data of the preconditioning and conditioning phase were analyzed separately. Paired t tests were used to test for differences between the two CSs before training. Data of the conditioning phase were analyzed with two-way repeated measures ANOVAs with CS and conditioning block as factors.1 Alpha level was set at p = 0.05 (two tailed).
Results
Manipulation check
An analysis of the alveolar carbon monoxide levels (see Table 1) confirmed that subjects followed the instruction and really inhaled smoke from the cigarette [F(2,76) = 77.17, p < 0.001]. Compared to the beginning of the experiment, carbon monoxide levels were elevated after the end of the first conditioning block [t(38) = 7.29, p < 0.001]. After the end of the second conditioning block, carbon monoxide levels were higher compared to both the beginning of the experiment and the end of the first conditioning block [t(38) = 9.61, p < 0.001 vs. t(38) = 8.62, p < 0.001, respectively].
Table 1.
Breath CO (parts per million) and ratings of pleasure, arousal, and craving for cigarettes, alcohol, food, or water (scale-range: 1–9) before preconditioning and after the first and second block of conditioning (M ± SD)
| Preconditioning | Conditioning 1 | Conditioning 2 | |
|---|---|---|---|
| CO | 6.23 ± 8.32 | 12.44 ± 8.41 | 16.77 ± 8.67 |
| Pleasure | 6.46 ± 1.23 | 5.95 ± 1.86 | 6.36 ± 2.13 |
| Arousal | 3.20 ± 1.54 | 3.08 ± 1.90 | 3.05 ± 1.78 |
| Cigarettes | 5.50 ± 1.92 | 3.06 ± 1.56 | 2.81 ± 1.83 |
| Alcohol | 1.36 ± 0.38 | 1.46 ± 0.65 | 1.37 ± 0.52 |
| Food | 3.45 ± 2.17 | 4.45 ± 2.53 | 5.01 ± 2.55 |
| Water | 5.47 ± 1.74 | 6.62 ± 2.19 | 6.78 ± 1.50 |
Changes in motivational state during the study
There were no significant changes in self-reported pleasure, arousal, or desire to drink alcohol (see Table 1). Overall, subjects felt rather pleasant and relaxed during the experiment. As expected, cigarette craving decreased during the study [F(2,76) = 42.01, p < 0.001]. Compared to the beginning of the experiment, cigarette craving was lower after the first [t(38) = 6.94, p < 0.001] and second block of the conditioning phase [t(38) = 7.00, p < 0.001]. Instead, desire to eat showed an increase [F(2,76) = 17.03, p < 0.001]. Compared to the beginning of the experiment, desire to eat was higher after the first conditioning block [t(38) = 3.61, p = 0.001]. After the second conditioning block, desire to eat was increased compared to both the beginning of the experiment and the first block of the conditioning phase [t(38) = 4.76, p < 0.001 vs. t(38) = 2.95, p = 0.005, respectively]. Desire to drink water also increased during the experiment [F(2,76) = 11.97, p < 0.001]. Compared to the beginning of the experiment, desire to drink water was higher after the first [t(38) = 3.58, p = 0.001] and after the second conditioning block [t(38) = 4.57, p < 0.001].
Contingency awareness
Contingency awareness was assessed because human studies on aversive (e.g., Lovibond and Shanks 2002) and smoke conditioning (e.g., Hogarth and Duka 2006) suggest that awareness may be necessary for conditioning (but see also Hamm and Vaitl 1996). Subjects were defined as aware of the experimental contingency if they were able to correctly report the CS+ and CS− at least at the end of the second conditioning block. Three subjects lacked contingency awareness and were excluded from further analyses. The remaining subjects (74.4%; i.e., ten participants) showed contingency awareness after the first conditioning block and 100% after the second conditioning block.
Subjective data
Besides marginally significant higher pleasantness ratings of the CS+ after the preconditioning phase [t(38) = 1.92, p = 0.062], there were no further reliable effects [all Fs < 2.13, all ps > 0.152]. In contrast to our assumptions, there were no reliable effects of cue-evoked subjective craving, pleasure, or arousal during the conditioning phase (see Table 2).
Table 2.
Ratings of craving, pleasure, and arousal (scale-range: 1–9) in response to CS+ and CS− after preconditioning and after the first and second block of conditioning (M ± SD)
| Preconditioning | Conditioning 1 | Conditioning 2 | ||
|---|---|---|---|---|
| Craving | CS+ | 5.74 ± 2.17 | 3.10 ± 2.14 | 2.74 ± 2.20 |
| CS− | 5.74 ± 2.25 | 2.85 ± 2.03 | 2.92 ± 2.28 | |
| Pleasure | CS+ | 5.97 ± 1.91 | 6.23 ± 2.32 | 6.15 ± 2.25 |
| CS− | 5.46 ± 2.29 | 5.56 ± 2.26 | 6.15 ± 2.03 | |
| Arousal | CS+ | 3.44 ± 1.97 | 3.10 ± 1.85 | 3.05 ± 1.99 |
| CS− | 3.56 ± 2.00 | 3.36 ± 2.02 | 3.15 ± 1.86 | |
Physiological data
Skin conductance responses
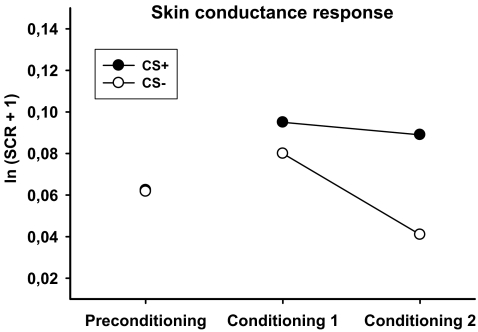
Skin conductance responses to the CSs did not differ prior to conditioning [t(38) = 0.05, p = 0.961]. An analysis of the data of the conditioning phase revealed a significant main effect of CS [F(1,38) = 7.49, p = 0.009]. As expected, SCRs to the CS+ were stronger than those to the CS− (see Fig. 3). The interaction between CS and phase did not reach significance [F(1,38) = 2.60, p = 0.115].
Fig. 3.
Mean skin conductance responses (ln (SCR + 1)) in response to CS + and CS− during preconditioning and during the first and second block of conditioning
M. corrugator supercilii
Neither the analysis of the preconditioning phase [t(38) = 1.74; p = 0.090] nor the analysis of the conditioning phase revealed a significant effect [all Fs < 0.97, all ps > 0.332].
M. zygomaticus major
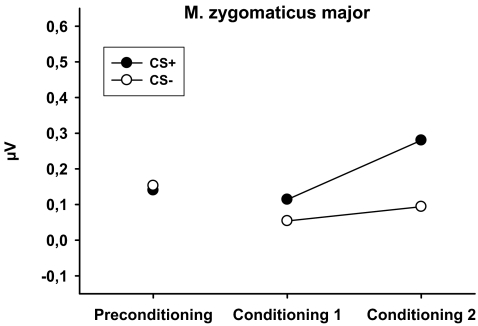
There was no difference between the CSs during the preconditioning phase [t(38) = 0.15, p = 0.879]. The analysis of the conditioning phase revealed a significant main effect of CS [F(1,38) = 6.66, p = 0.014]. As expected, the CS+ elicited stronger activity than the CS− during conditioning (see Fig. 4). In addition, there was a significant main effect of block [F(1,38) = 5.76, p = 0.021]. Overall, zygomatic activity was increased during the second conditioning block. The interaction between CS and phase was not reliable [F(1,38) = 1.43, p = 0.240].
Fig. 4.
Mean change in EMG activity (microvolt) of the M. zygomaticus major in response to CS+ and CS− during preconditioning and during the first and second block of conditioning
M. orbicularis oris
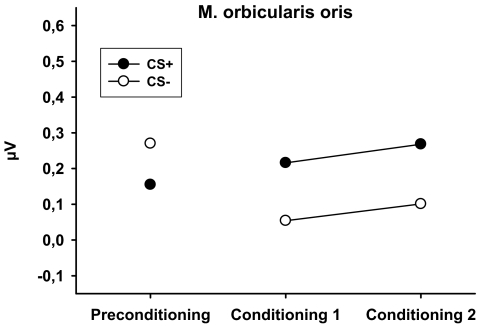
EMG activity did not differ between the CSs during the preconditioning phase [t(38) = 1.02, p = 0.317]. An analysis of the conditioning phase revealed a significant main effect of CS [F(1,38) = 4.13, p = 0.049], which was due to higher activity during presentation of the CS+ (see Fig. 5). The interaction between CS and phase was not significant [F(1,38) < 1.0, p = 0.982].
Fig. 5.
Mean change in EMG activity (microvolt) of the M. orbicularis oris in response to CS+ and CS− during preconditioning and during the first and second block of conditioning
Discussion
The present study investigated the development of conditioned responses evoked by an experimentally produced cue for smoking. The study was conducted on healthy smokers and used a differential conditioning protocol which allowed for control of sensitization and pseudoconditioning. Abstract pictorial stimuli served as CSs and single puffs on a cigarette as US (see also Hogarth et al. 2003). An important aspect of the present study was the use of physiological measures of motivational valence which were not included in previous studies.
The study revealed clear evidence for conditioned physiological responses to the smoking cue. During conditioning, the CS+ evoked larger skin conductance responses than the CS−. This is suggestive of increased autonomic arousal and attentional orienting and in line with previous data of Hogarth et al. (2003). These authors demonstrated that a discriminative CS+ for smoke reward came to evoke larger skin conductance responses than the CS− at the end of the training. In addition, the magnitude of the skin conductance response was correlated with an attentional bias for the CS+ as assessed with a dot-probe task. Furthermore, these results are in line with studies showing that naturalistic smoking cues evoke increases in skin conductance (e.g., Carter and Tiffany 1999), which could be further modulated by drug availability (e.g., Carter and Tiffany 2001).
In line with assumptions of incentive theories of addiction, the CS+ for smoke reward evoked a significant increase in zygomatic activity, indicative of positive affect. In contrast, activity of the corrugator muscle did not decrease during presentation of the CS+. It is not entirely clear why the activity of the zygomatic muscle was sensitive to the experimental contingency whereas the activity of the corrugator muscle was not. Interestingly, in the study conducted by Geier et al. (2000), activity of the corrugator muscle did not differentiate between positive, neutral, and smoking pictures and was sensitive to the presentation of negative pictures only. In a recent study conducted by Waters et al. (2009), corrugator activity was also insensitive to the presentation of smoking cues. In this study, attentional bias to smoking cues was assessed using an addiction Stroop task. Whereas stroop interference to smoking stimuli was positively associated with zygomatic activity, there was no association with corrugator activity.
Finally, we found increased activity of the orbicularis oris muscle (lip muscle) during CS+ trials. Although this effect was predicted, additional analysis suggested that it may be slightly less reliable than the effect of the other measures. Increased activity of the lip muscle very likely reflects preparation for smoking since smoke uptake from a cigarette is accomplished by sucking smoke into the mouth followed by a deep inhalation (Mueller et al. 2003). In line with this notion are results reported by Mucha et al. (1998) which demonstrated that an experimentally produced cue for smoking increased puffing on a cigarette. These results are further supported by animal studies demonstrating the importance of pavlovian CSs in the self-administration of drug. For example, a study by Corbit and Janak (2007) demonstrated that a separately trained pavlovian CS+ for ethanol increased operant responding for ethanol. Furthermore, previous studies demonstrated that nicotine-paired stimuli have a crucial influence on nicotine self-administration (e.g., Caggiula et al. 2002), may retard extinction of self-administration (e.g., Cohen et al. 2005), and reinstate nicotine-seeking after completed extinction (e.g., LeSage et al. 2004). In sum, the results of the present study are in line with notions of incentive theories that conditioned incentive stimuli evoke appetitive and consummatory responses directed to the incentive (e.g., Berridge 2004). They may provide a fruitful basis for further research which may benefit from the application of other objective measures of motivational valence and systematic manipulations of the test conditions.
In the present study, the clear demonstration of conditioning using physiological measures stands in contrast to the lack of conditioned subjective responses. Neither self-reported craving nor subjective pleasure differentiated between CS+ and CS−. To explain this observation, one could ask if single puffs on a cigarette were indeed consciously experienced as rewarding by smokers. According to Berridge and Kringelbach (2008), reward can be divided into several psychological components and the hedonic impact of rewarding stimuli can be dissociated under certain conditions from their motivational effect. Furthermore, objective hedonic reactions (“liking”, with quotation marks)—measured in the form of facial expressions—do not necessarily have to be accompanied by conscious subjective pleasure (liking, without quotation marks). The same distinction could be made in the case of wanting (e.g., Berridge and Kringelbach 2008; Berridge and Robinson 2003) and indeed, there are empirical data which support the assumption that emotional reactions may sometimes be too subtle to overcome the threshold of subjective experience, but still may have an influence on behavior (e.g., Childress et al. 2008; Winkielman et al. 2005). Therefore, our results might be interpreted accordingly and may point to impaired insight into the motivational processes underlying drug addiction in dependent individuals (e.g., Goldstein et al. 2009). However, such a conclusion could be challenged by studies which demonstrated convincingly that the availability of single puffs on a cigarette increases subjective craving and pleasure in smokers (e.g., Carter and Tiffany 2001).
To account for the different results of our study, it may be important to note that in the study of Carter and Tiffany (2001), subjective reactivity was assessed in anticipation of smoke reward. In contrast, in the present study, subjective responses to the CSs were assessed after the preconditioning phase and after each block of the conditioning phase. Therefore, it might have become clear to the participants that smoking was no longer available. This reduced expectancy to smoke might have the same effect as extinction learning. In line with this assumption, there are reports indicating that a cognitive representation of fear can induce anxious feelings and activation of the amygdala (e.g., Phelps et al. 2001). Similarly, an instructed expectancy to smoke increased cue-evoked craving (e.g., Droungas et al. 1995). Finally, Field and Duka (2001) have shown that the removal of smoke expectancy after conditioning by instruction eliminated the subjective craving response to the CS+. Further studies could therefore benefit from assessing subjective responses to the CSs during conditioning in anticipation of smoking.
In sum, recent studies have demonstrated that experimentally produced cues for smoking elicit subjective craving, physiological drug-related responses, and overt drug-seeking behavior in humans. The present study further extended those data by including physiological measures of motivational valence and demonstrated that an experimentally produced cue for smoking elicits facial reactions, which may be indicative of appetitive and consummatory motivation. The implications of these findings are that stimulus-evoked motivational tendencies to seek out and consume a drug may at least partly play a significant role in the maintenance of addiction and relapse in the natural environment (e.g., O’Brien et al. 1998).
Acknowledgement
This work was supported by the German Research Foundation (DFG): research group “Emotion and Behavior” (FOR 605, PA 566/9-1). The authors are grateful to Anita Kraiß and Elena Flohr for collecting the data and A.B.M. Gerdes for valuable comments on a previous draft of this paper.
Conflict of interest
The present study was conceived and implemented without any conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Additional analyses of covariance were conducted including the three contingency-unaware participants. The difference between CS-evoked responses during preconditioning was used as covariate. For the skin conductance data this analysis revealed a significant main effect of CS (p = 0.003). The main effect of phase (p = 0.095) and the interaction between CS and phase marginally reached significance (p = 0.051). For the lip EMG, the main effect of CS was marginally significant (p = 0.089). Regarding subjective pleasure, there was a significant main effect of phase (p = 0.037). The other effects remained as reported.
References
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bindra D. A motivational view of learning, performance, and behavior modification. Psychol Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi: 10.1046/j.1360-0443.1999.9433273.x. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol. 2001;9:183–190. doi: 10.1037/1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcohol Clin Exp Res. 2007;31:766–774. doi: 10.1111/j.1530-0277.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- Dempsey JP, Cohen LM, Hobson VL, Randall PK. Appetitive nature of drug cues re-confirmed with physiological measures and the potential role of stage of change. Psychopharmacology. 2007;194:253–260. doi: 10.1007/s00213-007-0839-3. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Hedonics: the cognitive-motivational interface. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. New York, NY: Oxford University Press; US; 2010. pp. 74–84. [Google Scholar]
- Dimberg U. Facial electromyography and emotional reactions. Psychophysiology. 1990;27:481–494. doi: 10.1111/j.1469-8986.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997;106:15–25. doi: 10.1037/0021-843X.106.1.15. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-C. [DOI] [PubMed] [Google Scholar]
- Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure: self-report, psychophysiological, and startle probe responses. Exp Clin Psychopharmacol. 1995;3:156–162. doi: 10.1037/1064-1297.3.2.156. [DOI] [Google Scholar]
- Fendt M, Mucha RF. Anxiogenic-like effects of opiate withdrawal seen in the fear-potentiated startle test, an interdisciplinary probe for drug-related motivational states. Psychopharmacology. 2001;155:242–250. doi: 10.1007/s002130100709. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Smoking expectancy mediates the conditioned responses to arbitrary smoking cues. Behav Pharmacol. 2001;12:183–194. doi: 10.1097/00008877-200105000-00004. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Frijda NH. On the nature and function of pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. New York, NY: Oxford University Press; US; 2010. pp. 99–112. [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology. 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KM, Sayette MA. Facial reactions to smoking cues relate to ambivalence about smoking. Psychol Addict Behav. 2008;22:551–556. doi: 10.1037/0893-164X.22.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D. Affective learning: awareness and aversion. Psychophysiology. 1996;33:698–710. doi: 10.1111/j.1469-8986.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Duka T. Human nicotine conditioning requires explicit contingency knowledge: is addictive behaviour cognitively mediated? Psychopharmacology. 2006;184:553–566. doi: 10.1007/s00213-005-0150-0. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Duka T. Discriminative stimuli that control instrumental tobacco-seeking by human smokers also command selective attention. Psychopharmacology. 2003;168:435–445. doi: 10.1007/s00213-003-1456-4. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Hutton SB, Elbers N, Duka T. Drug expectancy is necessary for stimulus control of human attention, instrumental drug-seeking behaviour and subjective pleasure. Psychopharmacology. 2006;185:495–504. doi: 10.1007/s00213-005-0287-x. [DOI] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Duka T. The associative basis of cue-elicited drug taking in humans. Psychopharmacology. 2010;208:337–351. doi: 10.1007/s00213-009-1735-9. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in mental health care delivery systems. Norwood: Ablex; 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–395. doi: 10.1037/0033-295X.97.3.377. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lazev AB, Herzog TA, Brandon TH. Classical conditioning of environmental cues to cigarette smoking. Exp Clin Psychopharmacol. 1999;7:56–63. doi: 10.1037/1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lewin LM, Biglan A, Inman D. Operant conditioning of EMG activity using cigarette puffs as a reinforcer. Addict Behav. 1986;11:197–200. doi: 10.1016/0306-4603(86)90046-8. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. J Exp Psychol Anim Behav Process. 2002;28:3–26. doi: 10.1037/0097-7403.28.1.3. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Linthicum J, Ernst M. Appetitive conditioning: neural bases and implications for psychopathology. Neurosci Biobehav Rev. 2007;31:426–440. doi: 10.1016/j.neubiorev.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang G-J, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PH, Gale A. Is sham smoking an adequate control condition for the motoric component of smoking? Addict Behav. 1994;19:393–400. doi: 10.1016/0306-4603(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Pauli P, Angrilli A. Conditioned responses elicited by experimentally produced cues for smoking. Can J Physiol Pharmacol. 1998;76:259–268. doi: 10.1139/cjpp-76-3-259. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Pauli P. Modulation of craving by cues having differential overlap with pharmacological effect: evidence for cue approach in smokers and social drinkers. Psychopharmacology. 1999;147:306–313. doi: 10.1007/s002130051172. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Stuhlinger M, Mundle G. Appetitive effects of drug cues modelled by pictures of the intake ritual: generality of cue-modulated startle examined with inpatient alcoholics. Psychopharmacology. 2000;151:428–432. doi: 10.1007/s002130000508. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Pauli P, Weyers P. Psychophysiology and implicit cognition in drug use: significance and measurement of motivation for drug use with emphasis on startle tests. In: Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks: Sage Publications; 2006. pp. 201–214. [Google Scholar]
- Mucha RF, Pauli P, Weber M, Winkler M. Smoking stimuli from the terminal phase of cigarette consumption may not be cues for smoking in healthy smokers. Psychopharmacology. 2008;201:81–95. doi: 10.1007/s00213-008-1249-x. [DOI] [PubMed] [Google Scholar]
- Mueller V, Mucha RF, Ackermann K, Pauli P. Die Erfassung des Cravings bei Rauchern mit einer deutschen Version des “Ouestionnaire on Smoking Urges” (OSU-G) [The assessment of craving in smokers with a German version of the “Questionnaire on Smoking Urges” (QSU-G)] Z Klin Psychol Psychother Forsch Prax. 2001;30:164–171. doi: 10.1026//1616-3443.30.3.164. [DOI] [Google Scholar]
- Mueller V, Mucha RF, Pauli P. Electromyographic activity of the lip muscle as a measure of puffing on a cigarette. Physiol Behav. 2003;78:741–749. doi: 10.1016/S0031-9384(03)00059-3. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Orain Pelissolo S, Grillon C, Perez Diaz F, Jouvent R. Lack of startle modulation by smoking cues in smokers. Psychopharmacology. 2004;173:160–166. doi: 10.1007/s00213-003-1715-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new “self-report” animal model. Addiction. 2002;97:459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. Designing studies of drug conditioning in humans. Psychopharmacology. 1992;106:143–153. doi: 10.1007/BF02801965. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Schupp P, Mucha RF, Pauli P. Topography of sham and real puffing examined using a paced smoking regimen. Addict Behav. 1999;24:695–699. doi: 10.1016/S0306-4603(98)00094-X. [DOI] [PubMed] [Google Scholar]
- Siegel S, Baptista MAS, Kim JA, McDonald RV, Weise Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol. 2000;8:276–293. doi: 10.1037/1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. doi: 10.1037/0033-295X.91.2.251. [DOI] [PubMed] [Google Scholar]
- Stippekohl B, Winkler M, Mucha RF, Pauli P, Walter B, Vaitl D, Stark R. Neural responses to BEGIN- and END-stimuli of the smoking ritual in nonsmokers, nondeprived smokers, and deprived smokers. Neuropsychopharmacology. 2010;35:1209–1225. doi: 10.1038/npp.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toates F. Comparing motivational systems—an incentive motivation perspective. In: Booth, David A, Legg, Charles R, editors. Appetite: neural and behavioural bases. London: Oxford University Press; 1994. pp. 305–327. [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci. 2009;123:913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques in psychophysiology. New York: Wiley; 1980. pp. 4–67. [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Carter BL, Robinson JD, Wetter DW, Lam CY, Kerst W, Cinciripini PM. Attentional bias is associated with incentive-related physiological and subjective measures. Exp Clin Psychopharmacol. 2009;17:247–257. doi: 10.1037/a0016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull. 2005;31:121–135. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]