Abstract
This review is motivated by the growing demand for low-cost, easy-to-use, compact-size yet powerful micro-nanofabrication technology to address emerging challenges of fundamental biology and translational medicine in regular laboratory settings. Recent advancements in the field benefit considerably from rapidly expanding material selections, ranging from inorganics to organics and from nanoparticles to self-assembled molecules. Meanwhile a great number of novel methodologies, employing off-the-shelf consumer electronics, intriguing interfacial phenomena, bottom-up self-assembly principles, etc., have been implemented to transit micro-nanofabrication from a cleanroom environment to a desktop setup. Furthermore, the latest application of micro-nanofabrication to emerging biomedical research will be presented in detail, which includes point-of-care diagnostics, on-chip cell culture as well as bio-manipulation. While significant progresses have been made in the rapidly growing field, both apparent and unrevealed roadblocks will need to be addressed in the future. We conclude this review by offering our perspectives on the current technical challenges and future research opportunities.
Keywords: Biomedical engineering, Lithography, Microfabrication, Nanofabrication, Out-of-cleanroom
Introduction
Development of micro-nanoscale devices and systems has been one of the most noticeable trends in many areas of biomedical research over the past decades. The momentum toward building smaller instruments and more compact analytical systems is driven by the ability to offer high-precision assessment of minute biological components (e.g., single cells, DNA strands, and viral particles), to facilitate biochemical reactions in a high-throughput fashion, to provide parallel multiplexed functionality, as well as to reduce consumption of expensive reagents. Micro-nanofabrication technology has played a central role in such an implementation. Benefiting from the rapid-expanding microelectronic industry, the cleanroom-based micro-nanofabrication has evolved at a remarkable pace as predicted by Moore’s law.132 The current industrial standard ensures highly reliable processing to produce trillions of basic electrical elements—transistors—with lithographic resolution of tens of nanometers within one square inch area. Using the established micro-nanofabrication techniques, microchips for various biological and clinical applications have been successfully demonstrated in the recent years, including implantable neural probes,202 microfluidics-based biological systems,21 physiological pressure sensors,4,97 and cochlear and retinal implants.69,203 One major advantage of using the conventional cleanroom-based fabrication technology is the capacity to directly integrate the biochips with powerful electronic processing units underneath, which eliminates extensive electrical wiring and further reduces overall system dimensions. However, insurmountable obstacles are presented when applying the conventional techniques to fast-growing biological and clinical applications. These include the limited access to cleanroom facilities, incompatible chemical and thermal treatments, complicated and inflexible process flows, and restricted material selection options, in addition to high-maintenance and operation costs of fabrication equipment.
A group of emerging lithography-based techniques, such as soft lithography, have partially addressed the aforementioned concerns by reducing the micro-nanofabrication complexities while offering flexible processing schemes and material selections. Since the original introduction, soft lithography techniques have been increasingly explored in a wide range of biomedical applications, including integrated cell sorters,54 in situ cellular biomechanical analysis,177 stationary/dynamic bio-molecular gradients,87 and on-chip genomic analysis.152 Recent trends lead to exploiting the powerful performance of high-precision consumer electronics, intriguing interfacial chemical/physical phenomena, or self-assembled micro-nanobuilding blocks to further extend micro-nanofabrication capacity to an out-of-cleanroom laboratory environment. For instance, off-the-shelf printers and digital projectors have been extensively employed as inexpensive and rapid micropattern generators in place of conventional photomask exposure systems.191,199,214 Micro-nanoscopic chemical and physical phenomena at interfaces, such as elastic deformation (wrinkling and collapsing), have been frequently used to fabricate specific micro-nanopatterns.27,146 Furthermore, the bottom-up self assembly techniques can be of particular use to construct integrated functional micro-nanosystems for biomedical application.125,147 In addition, the rapid development of novel functional materials, ranging from inorganics to organics and from nanoparticles to self-assembled molecules, offers emerging alternatives to conventional material processing.115,127,155,167,212 Overall, the latest research activities in micro-nanofabrication enlightens this consistent evolution from the conventional cleanroom-based techniques toward easy-to-use, low-cost, compact-size, rapid-prototyping and out-of-cleanroom processing for specific applications (e.g., biologically oriented use), which follows the very similar trend to microelectronics and internet booming over the past decades.
In this review, we will highlight the recent achievements in the rapid-expanding direction with a focus on out-of-cleanroom techniques for biological and medical applications, followed by featuring a few promising examples from active research, including point-of-care diagnostics, integrated cell culture as well as micro-nanoscopic bio-manipulation. Although tremendous efforts have been made in such an implementation with appreciable advantages over the conventional counterparts (e.g., low cost, fast turnout, custom configurability, easy operation and maintenance, etc.), current technical challenges with potential solutions and opportunities (e.g., feature resolution, process reliability, and system integration) will be further discussed.
Materials for Desktop Micro-Nanofabrication
With the remarkable advances of material sciences and technologies in recent years, rapidly growing number of new functional materials have been introduced to micro-nanofabrication society. In addition to the conventional silicon-based materials, those new materials, as listed in Table 1, facilitate the biomedical research in an easy, fast, low-cost, multifunctional, and more importantly, out-of-cleanroom manner.155 For instance, flexible polymeric materials have been extended to fabricate and/or functionalize biological micro-nanosystems.115 Nanoscale building elements, such as nanowires and nanoparticles, are widely used to provide specific biological cues as well as to form scaffolds for tissue engineering.167,212 Moreover, emerging environmentally responsive materials can find their applications in biosensing and bio-manipulation platforms.127
Table 1.
Materials used in the desktop micro-nanofabrication
| Material categories | Representative materials | Typical micro-nanofabrication methods | Biocompatibility and toxicity | Biomedical applications |
|---|---|---|---|---|
| Thermoset polymers | PDMS6,49,53,62,116,126,156,177,201,204,216,217 | Molding | Biocompatible | Used in almost all microfluidic and bio-/nanopatterning applications |
| Thermoplastic polymers | PMMA102,115 | Hot embossing | Biocompatible | Construct for microfluidics |
| COC172 | Hot embossing | Biocompatible | Used in optofluidic applications primarily | |
| Polystyrene/polyolefin28,59,137,171 | Heat-activated shrinkage | Biocompatible | Device packaging; pattern transfer; cell culture platform | |
| Photopatternable polymers |
KMPR170 |
Lithography | Toxic | Master for microfluidics and bio/nanopatterning |
| Dry film79,105,173,183,184,192,193,214 | Lithography | Biocompatible | Master for microfluidics and bio/nanopatterning | |
| PEG38,100,104,159,160 | Lithography | Biocompatible | Used in cellular and biomolecular investigations and implantations | |
| Thiolene60,70,135 | Lithography | Biocompatible | Solvent-resistant for biocompatible applications | |
| Photopatternable PDMS16,48,81 | Lithography, molding | Usually toxic due to the additive chemicals | Construct for microfluidics; device packaging | |
| Nanomaterials | Nanoparticles9,20,106,153,211 | Self-assembly | Under study | Nanofluidics, nanosensing, nanomanipulation |
| Nanofiber78,110,112,176,196,205,208 | Electrospinning | Depended on the used polymer, usually biocompatible | 2D/3D cell culture scaffold | |
| Nanocomposites37,50,58,64–66,139,198 | Molding | Depended on the functional components | Providing conductive, hydrophobic properties | |
| Biological materials | Silk5,96,109,140,141,197 | Electrospinning | Biocompatible after surface treatment | 2D/3D cell culture scaffold, implantation |
| DNA11,12,107,161,207 | Self assembly | Biocompatible | Nanomachinary, 3D nanostructures | |
| Virus36,119,187 | Self assembly | Biocompatible | Nanomachine, nanostructure synthesis | |
| Chitosan149,209 | Electrodeposition | Biocompatible | Bioactive coating |
Thermoset Polymers
Thermoset polymers refer to polymers that irreversibly cure through a thermal, chemical, or photochemical reaction. With fluidic properties and one-step curing, thermoset polymers have been frequently used as inexpensive and durable masters for hot embossing99 and replica molding.52 In particular, as the most commonly used thermoset polymer in micro-nanofabrication, polydimethylsiloxane (PDMS) can be processed by mixing two components, a base and a curing agent, at a given weight ratio (e.g., 10:1) and cross-linked at a room or slightly elevated temperature. The uncured pre-polymer of PDMS is fluidic and can flow into micro/nanostructures, which makes it an excellent material option for the molding process. Combining excellent mechanical (e.g., elastic and flexible), optical (e.g., transparent) and biological (e.g., biocompatible and non-toxic) properties, together with its low price, PDMS is a popular material selection for biomedical microdevices.6,49,53,126 Besides its dominant use for microfluidics and bio-patterning,201 the elasticity of PDMS has been particularly explored in biomechanical applications. Chen and coworkers have first utilized a PDMS substrate decorated with a densely packed micropost array to investigate cellular adhesion mechanics,177 on which different levels of bending of posts indicate the localized stress experienced by individual cells. More recently, a similar system has been adopted to quantitatively measure mechanical forces at cell–matrix and cell–cell contacts by the same group.116 Furthermore, molecular structure of PDMS allows for potential surface modification, another highly desired feature for biological applications where specific bio-functionality or bio-activation is often necessary. Though the long-term stability of PDMS surface chemistry still remains unclear,216 a group of physical and chemical modification methods have been reported on this topic, including corona treatment,62 chemical grafting,217 surface-initiated radical polymerization,204 etc. Chemically modified PDMS surfaces have found a variety of uses in bio-patterning, also known as micro-contact printing (μCP), in which micro-nanopatterns of a self-assembled biomolecular monolayer can be transferred from a PDMS stamp to a desired substrate.156 The easy processability, excellent adaptability, multiplexed functionality, and elasticity of PDMS ensure its popularity will continue in desktop bio-oriented micro-nanofabrication.
Themoplastic Polymers
Unlike the thermoset polymers, thermoplastics can be melted and shaped repetitively above their glass transition temperatures (T g). The reshaping capacity of thermoplastics serves as the basis for its primary use in highly efficient thermal molding processes in micro-nanofabrication at low cost. For instance, acrylics, such as polymethyl methacrylate (PMMA), are common choices in hot embossing applications for mass production.115 A microcapillary electrophoretic system has been devised for DNA separation and detection using hot-embossed PMMA microchannels.102 In addition, cyclic olefin copolymer (COC), another thermoplastic polymer, is primarily used in optofluidic devices for its excellent optical clarity.172 Moreover, thermoplastics can be manufactured with programmable shape memory. Pre-stressed thermoplastics, e.g., polystyrene and polyolefin, are capable of rapidly reducing the lateral dimensions triggered by heating above the glass transition temperature. This process has been applied to adhesive-free microfluidic packaging, where a thermally shrinkable piece of polyolefin tube is heated to seal an embedded funnel-shaped connector in a silicon nanofluidic chip to an external conduit with hermetic yet re-workable packaging (the leakage pressure is greater than 200 kPa).145 Furthermore, the size-reduction characteristics also enables pattern transfer with improved lithographic resolution, which will be described in the following section.28,59,137,171
Photopatternable Polymers
Photosensitive polymers represent another large family of polymers with light-triggered chemical reactions. The built-in photopatternability of these polymers enables processing micrometer or sub-micrometer structures in a single lithographic step. Among them, those sensitive to ultraviolet (UV) lights have been long employed as photoresist materials in micro-nanofabrication, to which the identical or complementary patterns of the photomask is transferred during exposure.117 In particular, the negative-tone photoresist, SU-8, becomes a popular choice as microfluidic molds as well as structural MEMS layers for its optical transparency, chemical inertness, and high-aspect ratio.1,18,23,45,113 Unlike SU-8, another resin-based negative-tone photoresist, KMPR, has been recently introduced featuring easy chemical removability.170 Derived from the conventional solvent-based photoresist formula, a new category of photoresists has been developed in solid form, known as dry-film resists, which eliminates both spin coating and soft bake steps from traditional photolithography. Various groups have utilized this solid-phase resist film to devise a number of biomedical microsystems.79,105,183,184,192,193 Unlike their solvent-based counterpart, the dry-film resists possess excellent layer-to-layer adhesion and can form suspended microstructures, both of which are crucial to building stereo MEMS structures and devising 3D microfluidics. Recent reports have demonstrated the application of multilayer dry-film constructs to the formation of 3D microfluidics (e.g., mixing and chemical gradient generation) for out-of-cleanroom rapid prototyping.173,214
Besides the photoresists, other photosensitive biopolymers have also been widely explored in the biomedical microsystems. As the most studied non-fouling biomaterial, polyethylene glycol (PEG) can be incorporated with photochemistry (e.g., acrylation combined with photo-initiator addition) to generate functional micropatterns for bio-applications. Revzin et al. have first illustrated the use of photo-definable PEG microstructures for in vitro cell culture,159 in which UV light selectively polymerizes PEG hydrogel microstructures through photo-initiators (such as 1-phenyl-2-hydroxy-2-methyl-1-propanone).100,104,160 Similar micro-nanostructured PEG surfaces have been applied to template-assisted nanoparticle assembly.38 In addition, photopatternable adhesives have been directly used as microfluidic constructs for its exceptional chemical resistance and packaging performance.46 Thiolene, a UV curable polymer, has recently attracted considerable attention to establish solvent-resistant, biocompatible, and hybrid micro-nanodevices.60,70,135
Furthermore, photopatternability has been incorporated in PDMS matrix for its wide acceptance in micro-nanofabrication. By introducing photo-initiators into the polymer base, either positive- or negative-tone patternability of PDMS can be achieved. For example, using 2,2-dimethoxy 2-phenylacetophenone as the photo-crosslinker, a negative photo-definable formula of PDMS has been reported.48 In another approach, benzophenone is added as a neutralizer to the curing agent, from which positive-tone partternability can be established for PDMS polymer.16,81 Complimentary to the traditional PDMS molding technique, photopatternable PDMS offers direct processability and additional flexibility to building microstructures, though it has limited feature resolution (≥10 μm) and undetermined biocompatibility.
Nanomaterials
Nanomaterials represent an exceedingly broad category of materials with one or more dimensions falling into the nanometer range, and usually exhibit unique physical and structural properties in addition to the intrinsic material properties as scaling down (e.g., metamaterials).3 Herein, we confine the discussion to the nanomaterials explicitly used in out-of-cleanroom fabrication for biomedical applications. Although the bottom-up self-assembly-based approaches have been primarily utilized, the nanoengineered building blocks (e.g., mono-dispersed nanobeads with a wide range of diameters, nanowires with different hierarchies, and quantum dots with various excitation wavelengths) are incorporated into a variety of structures and surfaces with dedicated fabrication schemes, which inherit their intrinsic functionality while gaining structural advantages (e.g., large surface-to-volume ratio and intriguing optical properties).41,42,144 Moreover, since their dimensions are comparable to biomolecules, such as proteins and polynucleic acids, these nanoscale components can be applied broadly to many areas of medicine from diagnosis to treatment.2,44,150 For instance, self-assembled silver/gold nanoparticles have worked as effective optical tags for surface enhanced Raman spectroscopy (SERS), from which DNA molecules and tumor ligands have been detected.9,20,153 In addition, organizing nanoparticles into closely packed hexagonal structures can form a nanoporous matrix for biomolecular separation and detection.106,211
Moreover, incorporating functional nanoscopic components into a continuous matrix (usually polymer) forms a subgroup of nanomaterials, called nanocomposites. One major advantage of these binary composite materials is that they typically derive combined properties from both the supporting matrix and embedded nanofiller, sometimes even from the structural formation (e.g., metamaterials).83 For example, conductive and elastomeric materials have been long pursued for wearable biosensing and bioelectronics, to which nanocomposite materials offer a simple yet effective solution. In one approach, PDMS pre-polymer has been mixed with conductive nanopowders (e.g., silver particles and carbon nanotubes) to create a conductive elastomeric composite, which can be used in micro-heaters and temperature sensors in polymerase chain reaction (PCR) chip as well as pressure sensors in microfluidics.50,58,139,198 PDMS-based nanocomposites, combined with the aforementioned photopatternability, have proved to be both positively and negatively photo-definable with conductive properties, being useful for bioelectrical sensing and actuation.37 Furthermore, by mixing chemically inert polytetrafluoroethylene (PTFE) nanoparticles with a high-aspect ratio photoresist matrix (SU-8), unique surface microfluidics can be built on any substrate permitting SU-8 chemistry, where the flow path is confined by the boundary of the superhydrophobic nanocomposite patterns (known as triple lines of gas/liquid/solid interphase).64–66 Benefiting from the interfacial wetting control, the automatic flow on the open microfluidic platform allows building inexpensive and rapid biological detection schemes for point-of-care diagnostics.
Biological Materials
Organizing biological materials into desired 2D/3D formation can provide biophysical cues for regeneration of functional tissues or even organ. Restoration of tissues has always been an ultimate goal for tissue engineers and attracted wide interests from micro-nanofabrication society recently. Kaplan and coworkers have led the effort to investigate and re-engineer the micro-nanotopology of one of the historic biomaterials, silk fibroin fibers (the structural protein of natural silk).5,141 Produced by the electrospinning technique, silk fibroin nanofibers have been constructed as 3D scaffolds for tissue regeneration and targeted biomolecule delivery.109,140,197 In the latest use, a silk-based biodegradable film has been employed as a biocompatible and flexible substrate for implantable neural mapping.96 In another paradigm, DNA has been actively researched in building nanoarchitectures by designing molecular sequences and/or programmed hybridizations properly.12,107 For example, arrays of protein patterns and conductive nanowires are fabricated on programmable DNA nanostructural templates.207 Furthermore, DNA origami can be created by folding long, single-stranded DNA molecules, from which planar nanostructures with high-spatial resolution of 6 nm are achieved.161 Moreover, utilizing the sequence-specific interactions of complementary oligonucleotides, 3D nanomachinery has been devised for molecular sensing, controlled drug delivery, as well as nanomotors (using catalytic bioreactions).11 Besides DNA, viruses have been deployed to construct specific nanostructures autonomously. A virus-based toolkit is reported to direct the synthesis of magnetic and semiconducting nanowires.119 Single enzymatic reactions can be incorporated inside the cowpea chlorotic mottle virus used as natural nanoreactors.36 Interestingly, embedded into platinum nanoparticles, the tobacco mosaic virus (TMV) functions as a novel electronic memory device with bistable states enabled by charge trapping and releasing inside the hybrid bio-inorganic nanostructures.187 In addition, the aminopolysaccharide chitosan has been widely explored to assemble nanoscale biological substances, including proteins, vesicles, and viral particles, which can serve as a generic nanomanufacturing platform for various biomaterials.149 An intriguing bio-fabrication scheme has been recently introduced to achieve selective biomolecular deposition on different substrates, in which its solubility in buffered solutions at varying pH values is exploited to control biomaterial deposition conformally. In one report, chitosan molecules are assembled under an electrochemical gradient on top of a cathode.209
Desktop Micro-Nanofabrication Techniques
Micro-nanofabrication has been generally considered to comprise three essential elements: lithography, pattern transfer, and packaging. Lithography outlines desired features on a dedicated imaging layer, through which the micro-nanopatterns are transferred onto the target substrate.114,117 Packaging, the final step, becomes necessary when the microsystem needs to be operated independently from environmental influence, e.g., preventing particular contamination in sensors or eliminating interfacial instability of microfluidics.133 In conventional fabrication setup, the dedicated cleanroom environment, high-maintenance equipment, and inflexible and cumbersome operation procedures are all indispensible. However, present rapid-evolving biomedical research poses new challenges, where simple, fast, inexpensive, and environmentally friendly approaches are called for. In the following sections, we will review the latest advances of emerging out-of-cleanroom micro-nanofabrication techniques that meet the aforementioned requirements for biomedical applications.
Direct Lithography Processes
Introduced to microelectronics manufacturing in the late 1950, photolithography (latin: light-stone-writing) has been employed as the primary lithographic step in micro-nanofabrication. The high resolution (sub-micrometers to tens of nanometers) and reliability of the process are achieved at the expense of complicated exposure systems and toxic photo-reactive chemicals, which limits its application to the cleanroom environment.117 Various direct lithographic approaches developed over the past decade have attempted to fulfill desktop microfabrication requirements for biomedical uses. Among those, off-the-shelf microelectronics has been closely investigated to simplify and expedite the lithography process. Digital projectors become a natural alternative to the photo-exposure units. By replacing the projection lens with reduction optics, miniature digital images can be produced using any commercial graphic software, which enables direct lithography in an out-of-cleanroom setting.73–76,98,164,213,214 Using the maskless projection process, micropatterns of various photosensitive polymers, including polyethylene glycol75 and polyacrylamide,74,76,164 have been successfully established for selective cell adhesion control and biofluidic manipulations.98 By modifying the exposure system with an interchangeable lens, variable focal distances and magnifications can be obtained with feature resolutions down to a few micrometers. Moreover, combining synchronized image control and an XY traveling stage, maskless exposures over a large surface area (over 50 × 50 mm2) have been realized, as shown in Fig. 1a.73 More recently, an integrated direct projection lithography process has been introduced to photopattern multilayer microstructures of the dry-film resists in a desktop setup, which is capable of producing 3D micro-topology, achieving multilayer alignment and forming a seamless enclosure. Feature resolutions of 10 μm and an alignment precision of less than 10 μm, using a unique software alignment strategy, have been reliably achieved in this process.214 Furthermore, 3D shadow masks fabricated by the maskless projection process can be directly employed to deposit multiple living or functional biological objects with micrometer precisions in sequence, referred to as Stereomask Lithography (Fig. 1b).213 As a promising candidate for the next-generation desktop lithography platform, projection-based lithography systems considerably lower the technical threshold and significantly facilitate the prototyping process for bio-oriented microfabrication.
Figure 1.
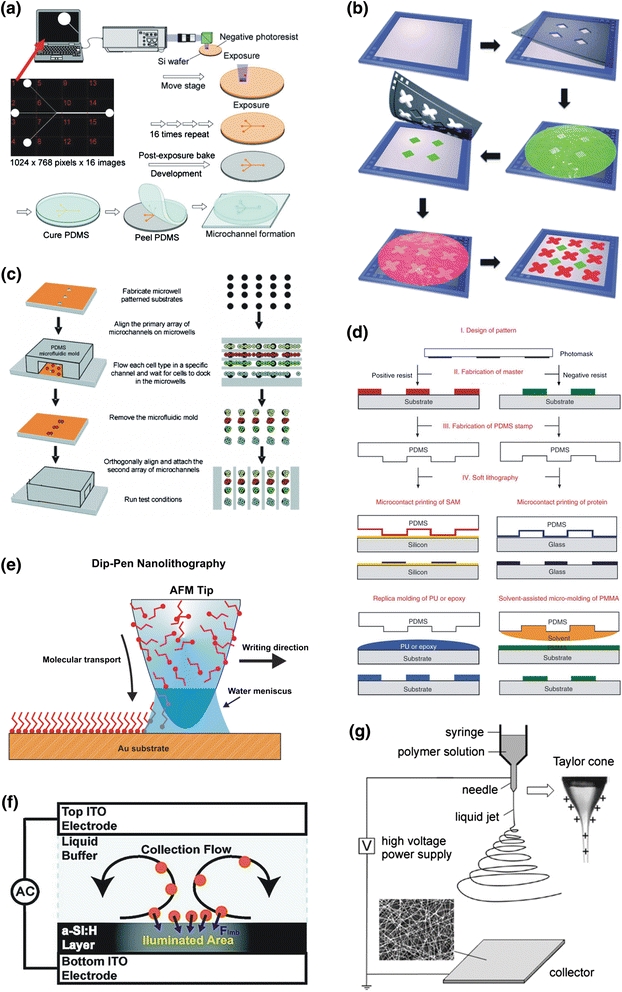
Direct lithography processes. Schematic illustrations of (a) large-area maskless exposure system. Reprinted with permission from Ref 73. Copyright 2008 American Chemical Society; (b) Stereomask lithography,213 Reproduced by permission of The Royal Society of Chemistry; (c) Microfluidic patterning.90 Reproduced by permission of The Royal Society of Chemistry; (d) Soft lithography.154 Reprinted by permission from Macmillan Publishers Ltd: [Nature Protocol] Ref 154, copyright (2010); (e) Dip-pen nanolithography. From Ref 151, reprinted with permission from AAAS; (f) nanoPen lithography; Reprinted with permission from Ref 77. Copyright 2009 American Chemical Society; (g) Electrospinning.110 Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission
Another promising approach utilizes commercially available printers to create the imaging layer, in place of photo-exposure and toxic photoresists.63,84,179,191,199 By completely eliminating photochemical reactions, environmentally friendly inks are used to generate a replica mold or a pattern transfer mask. PDMS molding from a printed substrate, named as Print-and-Peel, is first introduced by Vullev et al., and has been applied to an array of biological applications since, such as bacterial spore detection,191 capillary electrophoresis,84 and capacitance-based cell detection.63 In an alternative route, we have demonstrated that printed wax images from a solid-ink printer (Xerox Phaser™) can act as an excellent chemical barrier for subsequent wet etch and chemical modification.199 In addition, a novel folding strategy has been presented to form multilayer microstructures from one double-side printed substrate. A recent review from Vullev’s group covers more details on printer-based microfluidic fabrication techniques.179
Moreover, microfluidics has been implemented to establish micropatterns of specific biological objects. For example, as shown in Fig. 1c, PDMS microchannels are reversibly bonded onto a bioactive substrate, through which multi-phenotype cell array is formed. After peeling the cell-patterning channels, another set of microfluidic channels is positioned orthogonally to deliver different chemical stimuli to the cell array for high-throughput investigations of cell–biomolecule interactions.90,111 In addition, a similar technique has been applied to form hyaluronic acid (HA) patterns on tissue scaffold templates, which induces regeneration of cardiac tissues in a 3D fashion.88 Moreover, micropatterns of ordered monolayer nanoparticles can be formed by tuning microflow profiles and interfacial interactions, which can also be employed to biological patterning.31
Soft lithography is another important category of the out-of-cleanroom lithography, introduced by George Whitesides’ group in the late 1990s (Fig. 1d).6,49,51,53,126,154,162 Since then, the replica molding-based micro-nanofabrication technique has been widely employed to produce a large variety of chemical and biological microdevices. Detailed technical reviews on soft lithography can be found elsewhere.51,53,154,162
In addition to desktop micropatterning, a number of powerful yet inexpensive lithographic techniques have evolved and targeted toward patterning nanoscopic objects (e.g., nanoparticles and biomolecules). Converted from a regular desktop atomic force microscope, a nanolithography system—dip-pen nanolithography—has been developed to print biomolecules with nanometer precision in a serial writing manner, as schematically illustrated in Fig. 1e, and has found a group of biomedical implications.151 Among those, homogenous nanopatterns obtained by the dip-pen nanolithography have shown the capacity for directing mesenchymal stem cell differentiation with high reproducibility.43 Recently, another light-actuated patterning technique for nanoscopic objects, referred to as nanopen, has been recently reported by Wu et al.,77 which allows low power actuation, dynamic optical manipulation as well as high adaptability, as shown in Fig. 1f. Using the combined effect of various light-induced electrokinetic phenomena (including light-actuated AC electroosmosis, electrothermal flow, and dielectrophresis), the nanopen is capable of organizing various nanoobjects, including metallic nanoparticles and carbon nanotubes, into arbitrary shapes over an area of thousands of square micrometers, being highly applicable to biochemical analysis and sub-cellular manipulation. Moreover, Jack Judy and his colleagues use micropatterned ferromagnetic substrates to achieve intracellular nanoparticle positioning with sub-micrometer precision, which can be potentially applied to generate localized chemical or mechanical signaling for high-precision cellular analysis.185
Electrospinning represents an increasingly important nanolithography technique to construct versatile nanofibrous scaffolds.110 Driven by the complex electro-fluid–mechanical interactions, an ultrathin polymer stream ejects from a cone-shaped droplet (known as Taylor cone) at the tip of a conductive needle charged to a high electrical potential (thousands to tens of thousands volts) toward to a grounded collector, as illustrated in Fig. 1g. By incorporating conducting filler into the polymer solution, for example, multiwall carbon nanotubes in PMMA, conductive nanofiber patterns can be electrospun.176 Furthermore, coaxial electrospinning has been established to generate nanochannels, employing motor oil as a dissolvable core and a silica sol–gel solution as an exterior shell, for single molecule detection.196 In addition, nanofibrous membranes fabricated by the electrospinning technique closely mimics in vivo extracellular matrices and exhibits excellent biocompatibility, which makes it an ideal substrate for tissue regeneration as well as bio-sensing.78,112,205 Recently, electrospun PDMS/PMMA nanofibrous membranes have served as a functional substrate for protein microarrays with an ultralow detection threshold (i.e., more than 30 times more sensitive) in comparison with the current standard.208
Facile Pattern Transfer
The pattern transfer process aims to construct the desired features on the target substrate from the lithographically defined imaging layer. A variety of techniques, dry or wet, subtractive or additive, have served for this purpose in the conventional micro-nanofabrication.114,117 Differing from the precise yet expensive fabrication techniques, pattern transfer processes for desktop micro/nanofabrication usually relay on simple chemical reactions, unique material properties or interfacial interactions, and can potentially combine with the aforementioned direct lithography techniques. Single-step chemical reactions have been primarily employed in rapid prototyping processes. For instance, KOH-based chemicals have been reported to wet etch microchannels into polymer substrates,199 while copper layers are electroplated as electrodes to monitor impedance variations in a fluid.63 Direct photochemistry represents another powerful patterning technique. Unlike the lithographic use, photo-crosslinked polymers, which include dry-film photoresists,214 polyethylene glycol,104 and UV-curable adhesives,46,60 can form microstructures by themselves.
Recently, unique interfacial phenomena have been explored to produce special micro-nanoscale patterns directly. It is known that after exposure to oxygen plasma, an ultrathin brittle SiO2-like layer emerges on top of the treated PDMS substrate.19 Using this phenomenon, a tensile strain is applied onto the PDMS substrate during the plasma treatment, from which nanowrinkles with periodic sinusoidal waveforms are resulted in the perpendicular direction to the strain after release, as indicated in Fig. 2a.35,93 The wrinkle-based nanofabrication technique has been used to rapidly prototype nanofluidic channels for biological applications. For instance, nanochannels have been devised to pre-concentrate target proteins using overlapped electrical double layers in the highly confined space.35 Further investigation shows that stretching an oxidized PDMS substrate can directly lead to wrinkled nanometer-size features, which has been employed as a replica mold in PDMS nanofabrication.129 In addition, the interfacial nanoconduits can form different cross-sectional profiles by simply tuning the strains loaded onto the substrate, and can be utilized in various bio-applications, such as tunable sieving and trapping, dynamic manipulating and in situ fabricating nanoobjects.68 However, the major limitation of the interfacial nanofabrication is that the interface-induced nanostructure formation is only limited to certain periodic patterns. Another intriguing pattern transfer strategy employs pre-defined microstructures to achieve tunable feature sizes. In one recent report, shown in Fig. 2b, structural collapse of elastomeric PDMS microchannels has been engineered to produce nanofluidic features as small as 60 nm under controlled surface chemistry, which illustrates its potential for DNA manipulation and surface-enhanced optical detection.146 In addition, the elastic deformation has been utilized to achieve tunable patterns of particle separation in a microfluidic chip using the deterministic lateral displacement principle, where the inter-obstacle distances among microposts are altered by the tensile stress applied externally.13 Furthermore, the heat-induced shrinkage of the aforementioned shape-memory thermoplastics has been exploited to proportionally reduce the size of patterned features. Polymers with high shrinkage ratio have been studied for optimal performance of the down-scaled patterning, including biaxially oriented polystyrene films (e.g., Shrinky-Dinks®) and pre-stressed polyolefin sheets. Moreover, thermal shrinkage is capable of inducing linear or biaxial nanopatterns with tunable wavelengths at a polymer–metal interface which can be controlled by the shrinkage ratio (through the time duration or temperature of the shrinking process), similar to the interfacial wrinkle formation on PDMS surfaces. As illustrated in Fig. 2c, it can be utilized as an alternative nanomolding template.55,59,137
Figure 2.
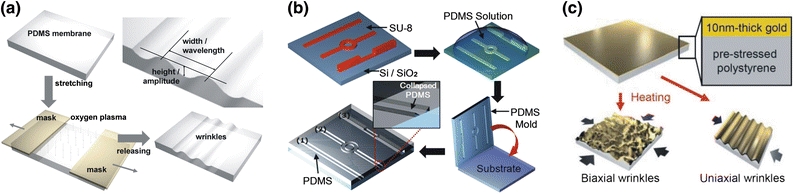
Interfacial nanometer pattern transfer. (a) Nanowrinkle generation on top of PDMS film under mechanical stretch coupled with oxygen plasma treatment;35 Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. (b) Structural collapse of elastomeric PDMS microchannels for nanopatterning;146 (c) Biaxial and uniaxial-wrinkle generation at polymer–metal interface on a heat-shrinkable thermoplastic sheet.55 Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission
Self-assembly processes provide another facile yet powerful technical platform for bottom-up nanostructure formation in a bench setup. Organized nanoscale building blocks, e.g., functional nanoparticles and biomolecules, are of broad interest to emerging biomedical applications. Highly organized 2D nanoparticle patterns have been assembled into microwells relying on the differential interfacial interactions38 or adhere to surfaces with pre-defined geometries utilizing a peel-off method.213 Monodispersed nanoparticles can be closely packed into microchannels, functioning as 3D nanofluidic sieves for biomolecule separation.211 With tunable pore sizes, this methodology enables separation of proteins based on molecular sizes ranging from 20 to 200 kDa and separation of double-strand DNAs based on lengths varied from 50 bp to 50 kbp, which is directly applicable to, for example, high-throughput continuous DNA fractionation.210 A similar strategy is proposed to realize reverse-phase liquid chromatography with sub-micrometer plate height for improved separation speed and resolution.118 More recently, the closely packed nanoparticle matrix has shown amplified electrokinetic activities in comparison with those in a single nanochannel configuration.30 As electrical double layers overlap inside the interstitial spaces among the packed nanoparticles, the electrical conductance, proportional to the surface charge density, provides a simple and rapid detection scheme for bio-sensing.106 Furthermore, the self-assembly principle can be extended to micrometer-sized objects. Considering joint effects from geometry, capillarity, fluidic, electrokinetic and gravitational forces, a large number of microscopic components ranging from sub-micrometer to millimeter scales can be self-organized with highly ordered architecture in a high-yield and programmable way.125 This approach has been proposed to build multifunctional bionic contact lens with integrated electronic circuitry and bio-sensing capacity.147
Packaging
Packaging usually comes as the last step in the entire fabrication process. Conventional MEMS packaging techniques (e.g., anodic, eutectic, or thermal compression bonding) usually experience high temperature (a few hundred to more than a thousand degrees), heavy mechanical load (greater than 1 atm), and/or strong electric field (a few hundred to a few thousand volts across the interface), through which hermetic seal of the device forms as necessary. However, packaging for biomedical devices confronts completely different challenges in which bio-compatibility, bio-functionality, reversibility, as well as macro-to-micro interfaces preclude many of the traditional packaging processes from being used in traditional microelectronics.
Surface chemical modification serves as the primary route to join two bio-functional surfaces. It relies on the formation of strong intermolecular interactions, e.g., covalent, electrostatic, or hydrogen bonds, between two sides.215 As aforementioned, PDMS-based microdevices have been widely employed in chemical and biological applications. The standard packaging technique to enclose PDMS microfluidic channels relies on plasma activation of the surface. When exposed to oxygen plasma, silanol groups (Si–OH) are introduced to the PDMS surface in place of methyl groups, which lead to the formation of covalent bonds with the substrates presenting hydroxyl groups.49,80 In addition, an irreversible chemical bond forms between the oxygen plasma-treated PDMS and a large number of surfaces, including glass, silicon oxide, quartz, silicon nitride, and various polymers (e.g., polyethylene, polystyrene, and glassy carbon).17,49 Recently, an alternative low cost, handheld corona system has shown to be able to activate PDMS surface, which achieves comparable bonding strength to that attained through the use of a plasma reactor, although the mechanism of this surface activation approach remains unconclusive.62 Besides the dry activation schemes, wet chemical treatment of surfaces is another group of packaging methods used for polymer microdevices. For example, organofunctional silanes can be used to adhere various plastic substrates, such as PMMA, polycarbonate (PC), polyimide (PI), and polyethylene terephthalate (PET), to PDMS, which offers an expanded material portfolio to the desktop fabrication.103,178 Furthermore, solvent-assisted bonding, which swells the surfaces prior to the contact, can also join two polymer substrates seamlessly. In another study, PMMA has been reported to seal by using ethylene glycol dimethacrylate as the functional solvent, in which microchannels with an aspect ratio of 0.03 and bonding strength of 3.5 MPa can be realized.188 As alternative bonding strategy, step control of the curing/polymerization process (e.g., altering crosslink ratio or curing time) has also been explored.57,189 As a common practice, incompletely cured PDMS presents appreciable adhesion to various biological substrates. Similarly, completely curing partially crosslinked PEG films led to the hermetic seal of micro- and nanochannels (with feature sizes as small as 50 nm).95 In addition, multilayer microfluidic structures of thiolene can be also fabricated using such a process.135
Moreover, physical forces, such as magnetic or vacuum forces, are also utilized to establish reversible packaging for microfluidic systems. Magnetic forces can be used to establish reversible seal of microfluidic devices with reasonable bonding strength (up to 145 kPa), and highly adaptive to integrate a broad category of bio-functionalized substrates.157,180 Alternatively, vacuum forces have been utilized to achieve on demand packaging for a group of lab-on-a-chip devices.14,165 Reversible vacuum-assisted bonding can be employed by including a network of vacuum microchannels to seal around the microfluidic structures, which is directly linked to a negative pressure source (e.g., a facility vacuum line or a vacuum pump). This concept has been successfully applied to study the interactions between leukocytes and biochemically/biologically functionalized surfaces under various shear flow conditions, in which seal of microfluidic channels to bio-active surfaces is usually challenging.165,166
Another important topic in the biomedical packaging is to establish the world-to-chip connections in a simple and effective way. Inserting tubes into through-holes of PDMS layer can directly function as universal interconnects in microfluidic packaging.108 Leakage of this type of connectors is found at the working pressure of 137 kPa. Tuning the connector structures as well as applying suitable glues (Fig. 3a), further improvement on packaging performance has been reported, i.e., the maximal non-leaking pressure over 2.2 MPa at an elevated temperature (up to 275 °C).148 Importantly, a “press-fit” strategy as illustrated in Fig. 3b has been introduced as an inexpensive, epoxy-free and reusable packaging route for rapid prototyping.34 A trimmed needle is used to punch through-holes in the PDMS substrate, and subsequently, a blunt one with the same outer diameter can be inserted to create a compressed seal under the elastic deformation of the bulk PDMS surrounding the needle. The leakage pressures as high as 700 kPa has been reported for this method, satisfying most biofluidic applications. Similar to the aforementioned magnetic bonding, Fig. 3c shows disc and ring magnets with microfluidic tubing that are paired up to form reversible fluidic interconnects in the standard microfluidic devices.7 Lately, simple strategies have been reported to achieve standardized adhesive-free reversible microfluidic interfaces with high packaging reliability and high-tubing density as well as re-workability, referred to as fit-to-flow interconnects. Analogous to the concept of universal serial bus in computer peripherals, molded PDMS blocks integrated with micro-tubes form the female socket (Fig. 3d), while male plug-in components with complementary shapes can slide into the opening and establish microfluidic passage under a mechanical seal reversibly (press-fit or vacuum-fit), which hold promise for low-cost, easy-to-use, plug-and-play fluidic connections in rapid growing biofluidic applications.29
Figure 3.
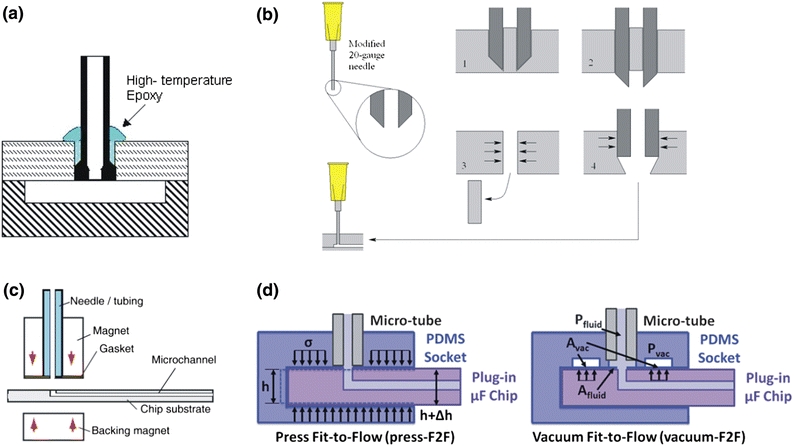
Interconnection strategies for desktop micro-nanofabrication: (a) Glue-sealing assisted interconnection,148 Reproduced by permission of Institute of Physics; (b) “press-fit” strategy,34 Reproduced by permission of Institute of Physics; (c) magnet-assisted interconnection,7 Reproduced by permission of The Royal Society of Chemistry; and (d) fit-to-flow (F2F) interconnection,29 Reproduced by permission of The Royal Society of Chemistry
Out-of-Cleanroom Micro-Nanofabrication for Biomedical Applications
As above illustrated, the rapid development of out-of-cleanroom micro-nanofabrication techniques equips bioengineers, biologists, and medical researchers with an array of inexpensive, functional, adaptive, and customized gadgets aimed at specific miniature bio-objects with micro- to nanometer precisions, which opens enormous opportunities and possibilities in the new biomedical era. Here, we highlight a few emerging areas of active research employing the desktop micro-nanofabrication platforms.
Point-of-Care Diagnostics
Recent studies aim at development of diagnostic devices for point-of-care testing. Potential low cost, rapid outcome, and easy-to-use features of point-of-care diagnostics make it highly attractive to clinical analysis, and can be widely employed at the side of patients (e.g., outside of the hospital, in the field, and in the developing world).101 Enabled by microfabrication and microfluidics, point-of-care diagnostics has been explored in a wide variety of medical situations, including monitoring disease markers, assessing therapies, and detecting chemical and biological hazards, and identifying infectious population during pandemics.
Among those, microfluidics-based immunoassays have been studied for use in public health monitoring over the past decade, originally proposed by Yager et al.206 T-shaped microchannels, fabricated by single-step laser micromachining in a PET film (Mylar®), have been employed for high-sensitivity immunodiagnostics based on rapid diffusion of biomolecules (within a minute).61 For instance, integrated with SPR detection, the T-sensor is capable of quantitative analysis of phenytoin with concentrations varying from 75 to 1000 nM within 10 min.136 In addition, reagents preserved by filtrating through microfluidic membranes from urine samples can be applied to multiplexed quantitative immunoassays.174
More recently, building multiplexed diagnostics on inexpensive substrates serves as another appealing direction. Paper-based microfluidic analytical devices introduced by George Whitesides and his team have been extensively studied as a disposable low-cost diagnostic platform.26,123 Soaking chromatography papers in photoresist followed by lithographic patterning, chemical reagents can be loaded and confined in desired regions on the biofunctional papers, known as lab-on-a-paper.26,124 3D microfluidic devices have been developed on paper devices with the help of adhesive tapes, as shown in Fig. 4a.121,122 Alternatively, solid ink printing followed by thermal reflow treatment can be exploited to replace the photolithography step, leading to a completely cleanroom-independent process.25 By cutting nitrocellulose membranes with a CO2 laser, two-dimensional paper networks (2DPNs) have been developed by Yager’s group for self-propelled microfluidics142 and chemical signal amplification.56 Methods for flow visualization, analytical measurement,86 and biomolecule patterning134 based on the paper networks are also developed to facilitate the implementation of point-of-care diagnostics. Compatible with a commercially available glucometer, the latest paper-based microfluidic device can quantitatively analyze a variety of biochemical compounds, such as glucose, cholesterol, lactate and alcohol electrochemically.138 As an emerging technology alternative, the paper-based analytical systems have demonstrated their potential as inexpensive, easy-to-use, disposable bioassays for multiple analytes, which can be widely deployed in developing countries in the foreseeable future.120
Figure 4.
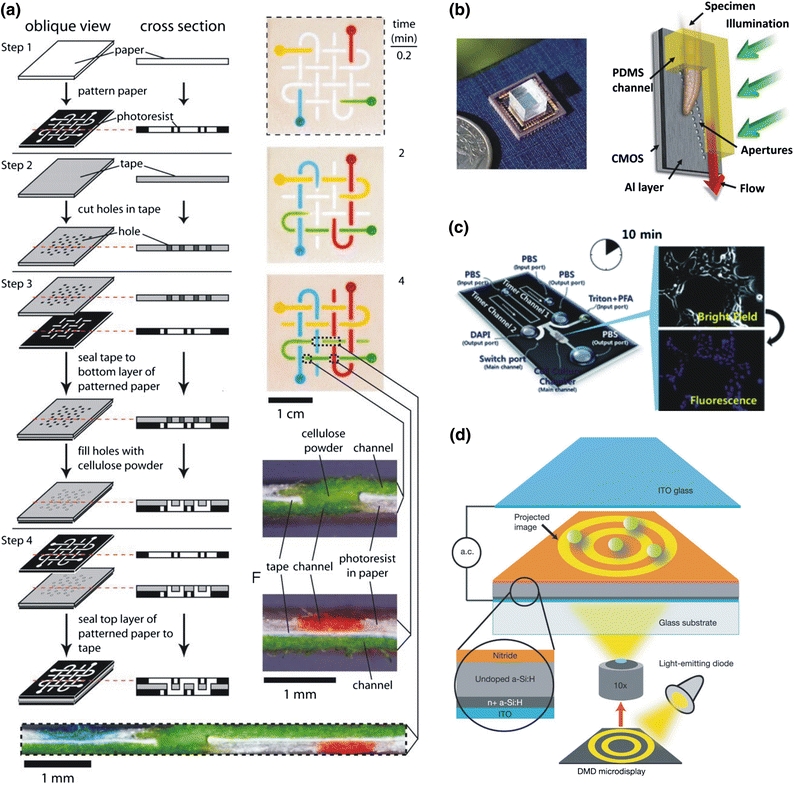
Applications of out-of-cleanroom micro-nanofabrication in biomedical research. (a) 3D microfluidics on the paper-based substrate;122 Copyright (2008) National Academy of Sciences, U.S.A. (b) Biological imaging on an optofluidic scanning chip;40 Copyright (2008) National Academy of Sciences, U.S.A. (c) Passive microfluidics for timed multi-step cell culture;82 Reproduced by permission of The Royal Society of Chemistry; and (d) Optoelectronic tweezers for biological manipulation.32 Reprinted by permission from Macmillan Publishers Ltd: [Nature], copyright (2005)
Meanwhile, several groups have extended the power of high-definition digital imaging chips of consumer cameras (e.g., CMOS imaging chips) to lens-free microscopic detection units integrated with microfluidic platforms. Yang and coworkers have achieved submicrometer resolution of biological imaging using an optofluidic scanning technique, as indicated in Fig. 4b.40 Recently, groups in UC Berkeley and UCLA have independently reported integrated versions of the lens-free microscopy on cell phone platforms, which can be of immediate use to screen blood cells or detect water-borne parasites using an optically modified cell phone. Moreover, the digital diagnostic information can be transmitted to and processed in remote medical centers through wireless networks, from which the follow-on instruction and treatment plan are to be provided. Due to the dominant popularity of cellphones worldwide, cell phone-based diagnostics could be a vital tool to improve health conditions in the developing countries or remote villages.22,92,143,168,186 In brief, the emerging concepts for point-of-care diagnostics, quickly evolving with customized enabling micro-nanotechnology, may play a leading role in future global health care.
On-Chip Cell Culture
Integrated cell culture, permitting on-chip screening and diagnostics, quantitative biophysical and biochemical analyses, high-throughput bioreactions, and controlled cell interaction assays, has been extensively investigated in both fundamental and translational biomedical researches. A wide range of out-of-cleanroom fabrication techniques have been developed due to the demand of highly customized designs. In particular, single or multiple cell arrays have been placed and aligned in integrated culture platforms, which are microfabricated by various biomaterials as aforementioned (e.g., PEG and PDMS).24,39,158,182 Recently, the on-chip cell microarray is extensively used to create stem cell aggregates (such as an embryoid body or neurosphere) with defined sizes and shapes in regenerative medicine, leading to better control of stem cell differentiation and proliferation.39,85,89,130,131 Moreover, Beebe and coworkers have built a simple self-operated microfluidic platform for controlled cell culture and high-throughput screening using the standard soft lithography technique. Utilizing the Laplace pressure gradient generated by surface tension under geometric variation, the lab-on-a-chip device eliminates both complicated control units and external energy sources.15,194,195 Further investigations show successful incorporation of control logics and timing units into the passive networks, which is capable of conducting multi-step programmable biological protocols. A series of operations, including culturing, fixing, and staining cells, have been successfully performed on a culture containing embryonic kidney cells, as shown in Fig. 4c.82,94,181 Digital microfluidics based on electrowetting-on-dielectrics (EWOD) has also been implemented to perform mammalian cell cultures in sub-microliter droplets. The multiplexed digital microfluidic platform is capable of automatic seeding, growing, and removing cells on an array of individual addressable electrodes.10 Recently, a similar system has been extended to analyze microorganism growth (e.g., bacteria, algae, and yeast cells).8 Moreover, the paper substrates have also been introduced to mammalian cell culture. 3D cell constructs can be formed by simply stacking paper substrates layer-by-layer to study molecular and genetic responses at tissue- and organ-levels under the simulated oxygen and nutrient gradients.47 Behaviors of breast cancer cells in 3D tumor spheroids in vitro and tumors in vivo are successfully recapitulated by this approach.
Co-culture of various cell types represents another important aspect in engineered cell culture. Khademhosseini and coworkers have docked cells within the defined locations by combining PEG-micropatterned surface with reversibly packaged microfluidic channels.88–91,130 Multiple cell types, including hepatocytes, fibroblasts, and embryonic stem cells, can be deposited onto a single substrate to realize multi-phenotype cellular arrays. Furthermore, by introducing orthogonal microflow through another set of microchannels on the cell co-culture platform, a high-throughput strategy for drug screening has been described.90 In addition, temporal control of cell–substrate adhesion leads to an alternative co-culture approach. Electrochemical switching has been applied to activate chemically inert molecules, which are self-assembled in a monolayer configuration. For instance, PEG-thiol patterns can be controllably stripped off from conductive substrates by applying electrochemical potentials. Using this method, different cell types can be patterned on the substrates, following sequential stripping and seeding steps of pre-patterned electrodes.111 Patterning fibronectin and HA using this method, cardiomyocytes adhere, elongate, and align with the electrochemically defined patterns and form organoids on the conductive leads of a glass substrate, which can be collected with synchronized contractility after 3-day culture.88 Moreover, Revzin and coworkers employs a similar strategy to passivate optically transparent ITO electrodes on a glass surface with PEG-silane molecules.169 Micropatterned co-cultures of primary hepatocytes and fibroblasts are created, which remain functional after 9 days as verified by the analysis of hepatic albumin. Integrating cell culture with micro-nanofabricated substrates empowers modern biological research with quantitative and multiplexed investigational platforms of high precision, high reproducibility, and novel functionality.
Projection-Based Bio-Micromanipulation
The concept to utilize dynamically projected images to manipulate miniature biological objects becomes an attractive alternative for microparticle sorting, cell manipulation, and localized concentration. Ming Wu and his groups have first demonstrated the capability of parallel single-particle manipulation using dynamic digital images from an off-the-shelf projector. The projected images create high-resolution dielectrophoresis (DEP) forces on a photoconductive surface, referred to as optoelectronic tweezers (OET) (Fig. 4d),32 which permits highly localized electric fields for single-particle manipulation and requires substantially less power intensity than conventional optical tweezers. Importantly, the parallel processability under a large active surface area (~1 mm2) enables high-throughput operation, as well as complex multi-step manipulation. This technique has shown applicable to both polystyrene microbeads and live blood cells. Later development introduces new phototransistor consisting of single-crystalline bipolar junction transistors with high photoconductivity in place of amorphous silicon, which allows the OET devices operate in integrated cell culture environments. As a paradigm, efficient cell trapping of live HeLa and Jurkat cells has been realized in standard cell culture media (e.g., phosphate buffered saline and Dulbecco’s modified eagle’s medium).67 In a similar route, a group in KAIST has turned a regular liquid crystal display (LCD) into the OET configuration. Without incorporating any optical component, the LCD image directly forms virtual particle traps on a photoconductive layer using the DEP forces.33 Further improvement focuses on integration of the LCD-based OET system and a condenser lens with a conventional microscope platform, from which both stronger DEP forces and higher virtual electrode resolution are achieved. The integrated system has been successfully applied to the parallel and interactive manipulation of blood cells.71 Moreover, applying the frequency-dependent DEP forces to various sizes of micro-particles, rapid selection, and concentration of micro-objects on the custom OET platform have also been accomplished.72 Overall, with the simple inexpensive setup and high-resolution dynamic performance, the projection-based bio-manipulation systems show great potential for biological screening and cellular manipulation.
Future Prospects
The past decade has seen a significant growth of research publications relating to the development of novel out-of-cleanroom micro-nanofabrication techniques toward biomedical applications. Although most of them are still in the early stages of development, the new implementations, offering distinct advantages (e.g., simplicity, low cost, compatible size, and quick turnover) over the conventional counterparts, hold the promise for modern biology studies and translational clinical researches. For instance, the nanoengineered physiologically relevant chemical, fluidic, or mechanical environments can be of extreme use to discover signal pathways in cell biology, for the investigation of disease mechanisms, and testing of drug efficacy and toxicity.190 On-chip integration of quantitative analysis and detection considerably extends the capacity to understand fundamental biological processes in a high-precision and high-throughput manner. Moreover, development of point-of-care diagnostics by flexible fabrication schemes provides fast, efficient, and inexpensive responses to a wide variety of pandemics worldwide. In addition to the current area of active research, nanoengineered personal medicine, general public nanofabrication education, and home-use nanofabrication stations would be long-term hopeful directions for bio-nanoengineers to pursue in the future. Furthermore, the multifunctional or multiplex techniques to study complex microenvironment would be highly attractive, e.g., combining construction of various biochemical and biophysical cues in the same cell culture, which can be of particular use to investigate cascaded bio-signaling and compare significance among cues.163
Besides all aforementioned complimentary aspects where the out-of-cleanroom bio-oriented micro-nanofabrication has shown significant potential, there are many technical challenges and open scientific questions in the transition process from traditional cleanroom fabrication to the desktop setup.
First of all, scalability is always one of the most crucial issues involved in any fabrication process. In principle, the aforementioned desktop fabrication techniques are directly associated with the precision of specific electronic components (e.g., printers, projectors, and cameras),179,213,214 and therefore, the advances in electronics manufacturing in turn push the fabrication precision forward. Furthermore, novel interfacial phenomena (physical or chemical) facilitate the development of inexpensive nanofabrication techniques, among which electrospinning and electrokinetic manipulation have illustrated their potential capacities.110,175
Although it offers great flexibility in material selection for biological applications, the drastic expanded material portfolio causes potential problems in system integration and process compatibility. For example, bonding between two different materials could be a very challenging task (e.g., inorganic to organic substrates, or high-energy to low-energy surfaces). Furthermore, outcome of the non-standard fabrication and material processing could be inconsistent and incompatible with large-scale system integration and industrial production.
In addition, metallization process, as a necessary step in many bio-sensing and actuation applications, is neither well established in a regular laboratory setting nor fully compatible with the typical soft lithography process. Inexpensive yet reliable metallization methods for non-conductive polymeric surfaces are highly in demand for the out-of-cleanroom applications. A recent report on the electroless plating on elastomer reflects an effort in this direction.128
Moreover, establishing reliable interfaces between the micro-nanoengineered systems and mesoscale operations are always problematic and challenging, such as electrical wiring, mechanical packaging, and fluidic connection.200 Individual considerations with the dedicated design and special processing usually need to be placed. More universally applicable techniques and approaches are still under further investigation.
Last but not the least, this type of research requires not only in-depth knowledge of micro-nanofabrication and material science, but also essential understanding of the underlying biological or medical problems. Bridging the multidisciplinary fields could facilitate and strengthen the collaboration between micro-nanoengineers and biologists/clinicians, which can further unleash the actual potential of micro-nanofabrication to investigate biological matters as well as to implement clinical translation in the coming decades.
Acknowledgments
TP would like to acknowledge financial support from National Science Foundation CAREER (award no. ECCS-0846502) and EFRI Programs (award no. EFRI-0937997) and WW would like to acknowledge the financial support from National Natural Science Foundation of China (award nos. 60976086 and 91023045) and National Basic Research Program of China (award no. 2009CB320300).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Abgrall P, Conedera V, Camon H, Gue AM, Nguyen NT. SU-8 as a structural material for labs-on-chips and microelectromechanical systems. Electrophoresis. 2007;28(24):4539–4551. doi: 10.1002/elps.200700333. [DOI] [PubMed] [Google Scholar]
- 2.Agasti SS, Rana S, Park MH, Kim CK, You CC, Rotello VM. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010;62:316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup. Med. 2006;56:300–306. doi: 10.1093/occmed/kql051. [DOI] [PubMed] [Google Scholar]
- 4.Allen, M. G. Micromachined endovascularly-implantable wireless aneurysm pressure sensor: from concept to clinic. In: Proc. Transducer’05, 2005, pp. 275–278.
- 5.Altman G, Diaz HF, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JR, Chiu DT, Jackman RJ, Cherniavskaya O, Mcdonald JC, Wu H, Whitesides SH, Whitesides GM. Fabrication of topologically complex three-dimensional microfluidic systems in PDMS by rapid prototyping. Anal. Chem. 2000;72:3158–3164. doi: 10.1021/ac9912294. [DOI] [PubMed] [Google Scholar]
- 7.Atencia J, Cooksey GA, Jahn A, Zook JM, Vreeland WN, Locascio LE. Magnetic connectors for microfluidic applications. Lab Chip. 2010;10:246–249. doi: 10.1039/b913331c. [DOI] [PubMed] [Google Scholar]
- 8.Au S. H., S. C. C. Shih, and A. R. Wheeler. Integrated microbioreactor for culture and analysis of bacteria, algae and yeast. Biomed. Microdevices. doi:10.1007/s10544-010-9469-3. [DOI] [PubMed]
- 9.Banholzer MJ, Millstone JE, Qin L, Mirkin CA. Rationally designed nanostructures for surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2008;37:885–897. doi: 10.1039/b710915f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbulovic-Nad I, Yang H, Park PS, Wheeler AR. Digital microfluidics for cell-based assays. Lab Chip. 2008;8:519–526. doi: 10.1039/b717759c. [DOI] [PubMed] [Google Scholar]
- 11.Bath J, Turberfield AJ. DNA nanomachines. Nat. Nanotechnol. 2007;2:275–284. doi: 10.1038/nnano.2007.104. [DOI] [PubMed] [Google Scholar]
- 12.Becerril HA, Woolley AT. DNA-templated nanofabrication. Chem. Soc. Rev. 2009;38:329–337. doi: 10.1039/b718440a. [DOI] [PubMed] [Google Scholar]
- 13.Beech JP, Tegenfeldt JO. Tunable separation in elastomeric microfluidics devices. Lab Chip. 2008;8:657–659. doi: 10.1039/b719449h. [DOI] [PubMed] [Google Scholar]
- 14.Berre ML, Crozatier C, Casquillas GV, Chen Y. Reversible assembling of microfluidic devices by aspiration. Microelectron. Eng. 2006;83:1284–1287. [Google Scholar]
- 15.Berthier E, Beebe DJ. Flow rate analysis of a surface tension driven passive micropump. Lab Chip. 2007;7:1475–1478. doi: 10.1039/b707637a. [DOI] [PubMed] [Google Scholar]
- 16.Bhagat AAS, Jothimuthu P, Papautsky I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on-a-chip prototyping. Lab Chip. 2007;7:1192–1197. doi: 10.1039/b704946c. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya S, Datta A, Berg JM, Gangopadhyay S. Studies on surface wettability of poly(dimethyl)siloxane (PDMS) and glass under oxygen plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005;14(3):590–597. [Google Scholar]
- 18.Bohl B, Steger R, Zengerle R, Koltay P. Multi-layer SU-8 lift-off technology for microfluidic devices. J. Micromech. Microeng. 2005;15(6):1125–1135. [Google Scholar]
- 19.Bowden N, Huck WT, Paul KE, Whitesides GM. The controlled formation of ordered, sinusoidal structures by plasma oxidation of an elastomeric polymer. Appl. Phys. Lett. 2009;75(17):2557–2559. [Google Scholar]
- 20.Braun G, Lee SJ, Dante M, Nguyen TQ, Moskovits M, Reich N. Surface-enhanced Raman spectroscopy for DNA detection by nanoparticle assembly onto smooth metal films. J. Am. Chem. Soc. 2007;129:6378–6379. doi: 10.1021/ja070514z. [DOI] [PubMed] [Google Scholar]
- 21.Breslauer DN, Lee PJ, Lee LP. Microfluidics-based systems biology. Mol. Biosyst. 2006;2:97–112. doi: 10.1039/b515632g. [DOI] [PubMed] [Google Scholar]
- 22.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLoS One. 2009;4(7):e6320-1-7. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlier J, Arscott S, Thomy V, Fourrier JC, Caron F, Camart JC, Druon C, Tabourier P. Integrated microfluidics based on multi-layered SU-8 for mass spectrometry analysis. J. Micromech. Microeng. 2004;14:619–624. [Google Scholar]
- 24.Carlo DD, Wu LY, Lee LP. Dynamic single cell culture array. Lab Chip. 2006;6:1445–1449. doi: 10.1039/b605937f. [DOI] [PubMed] [Google Scholar]
- 25.Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009;81:7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 26.Carrilho E, Philips ST, Vella SJ, Martinez AW, Whitesides GM. Paper microzone plates. Anal. Chem. 2009;81:5990–5998. doi: 10.1021/ac900847g. [DOI] [PubMed] [Google Scholar]
- 27.Chan EP, Smith EJ, Hayward RC, Crosby AJ. Surface wrinkles for smart adhesion. Adv. Mater. 2008;20(4):711–716. [Google Scholar]
- 28.Chen CS, Breslauer DN, Luna JL, Grimes A, Chin WC, Lee LP, Khine M. Shrinky-dink microfluidics: 3D polystyrene chips. Lab Chip. 2008;8:622–624. doi: 10.1039/b719029h. [DOI] [PubMed] [Google Scholar]
- 29.Chen, A., and T. Pan. Fit-to-flow (F2F) interconnects: universal integrated microfluidic connections for lab-on-a-chip systems. Lab Chip, 2010. doi:10.1039/c0lc00384k. [DOI] [PubMed]
- 30.Chen Z, Wang YS, Wang W, Li ZH. Nanofluidic electrokinetics in nanoparticle crystal. Appl. Phys. Lett. 2009;95:102105. [Google Scholar]
- 31.Chen Z, Zhao Y, Wang W, Li Z. Microfluidic patterning of nanoparticle monolayers. Microfluid. Nanofluid. 2009;7:585–591. [Google Scholar]
- 32.Chiou PY, Ohta AT, Wu MC. Massively parallel manipulation of single cells and microparticles using optical images. Nature. 2005;436:370–372. doi: 10.1038/nature03831. [DOI] [PubMed] [Google Scholar]
- 33.Choi W, Kim SH, Jang J, Park JK. Lab-on-a-display: a new microparticle manipulation platform using a liquid crystal display(LCD) Microfluid. Nanofluid. 2007;3:217–225. [Google Scholar]
- 34.Christensen AM, Yen DAC, Gale BK. Characterization of interconnects used in PDMS microfluidic systems. J. Micromech. Microeng. 2005;15:928–934. [Google Scholar]
- 35.Chung S, Lee JJ, Moon M, Han J, Kamm RD. Non-lithographic wrinkle nanochannels for protein preconcentration. Adv. Mater. 2008;20:3011–3016. [Google Scholar]
- 36.Comellas-Aragones M, Engelkamp H, Claessen VI, Sommerdijk NAJM, Rowan AE, Christianen PCM, Maan JC, Verduin BJM, Cornelissen JJLM, Nolte RJM. A virus-based single enzyme nanoreactor. Nat. Nanotechnol. 2007;2:635–639. doi: 10.1038/nnano.2007.299. [DOI] [PubMed] [Google Scholar]
- 37.Cong H, Pan T. Photopatternable conductive PDMS materials for microfabrication. Adv. Funct. Mater. 2008;18:1912–1921. [Google Scholar]
- 38.Cong H, Revzin A, Pan T. Non-adhesive PEG hydrogel nanostructures for self-assembly of highly ordered colloids. Nanotechnology. 2009;20:075307. doi: 10.1088/0957-4484/20/7/075307. [DOI] [PubMed] [Google Scholar]
- 39.Cordey M, Limacher M, Kobel S, Taylor V, Lutolf MP. Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays. Stem Cells. 2008;26(10):2586–2594. doi: 10.1634/stemcells.2008-0498. [DOI] [PubMed] [Google Scholar]
- 40.Cui X, Lee LM, Heng X, Zhong W, Sternberg PW, Psaltis D, Yang C. Lensless high-resolution on-chip optofluidic microscopes for Caenorhabditis elegans and cell imaging. Proc. Natl. Acad. Sci. USA. 2008;105(31):10670–10675. doi: 10.1073/pnas.0804612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui Y, Lieber CM. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science. 2001;291:851–853. doi: 10.1126/science.291.5505.851. [DOI] [PubMed] [Google Scholar]
- 42.Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293:1289–1292. doi: 10.1126/science.1062711. [DOI] [PubMed] [Google Scholar]
- 43.Curran JM, Stokes R, Irvine E, Graham D, Amro NA, Sanedrin RG, Jamil H, Hunt JA. Introducing dip pen nanolithography as a tool for controlling stem cell behaviour: unlocking the potential of the next generation of smart materials in regenerative medicine. Lab Chip. 2010;10:1662–1670. doi: 10.1039/c004149a. [DOI] [PubMed] [Google Scholar]
- 44.De M, Chosh PS, Rotello VM. Applications of nanoparticles in biology. Adv. Mater. 2008;20:4225–4241. [Google Scholar]
- 45.del Campo A, Greiner C. SU-8: a photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007;17(6):R81–R88. [Google Scholar]
- 46.Delille R, Urdaneta MG, Moseley SJ, Smela E. Benchtop polymer MEMS. J. Microelectromech. Syst. 2006;15(5):1108–1120. [Google Scholar]
- 47.Derda R, Laromaine A, Mammoto A, Tang SKY, Mammoto T, Ingber DE, Whitesides GM. Paper-supported 3D cell culture for tissue-based bioassays. Proc. Natl. Acad. Sci. USA. 2009;106(44):18457–18462. doi: 10.1073/pnas.0910666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desai SP, Taff BM, Voldman J. A photopatternable silicone for biological applications. Langmuir. 2008;24(2):575–581. doi: 10.1021/la702827v. [DOI] [PubMed] [Google Scholar]
- 49.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane) Anal. Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 50.Engel, J. M., N. Chen, K. Ryu, S. Pandya, C. Tucker, Y. Yang, and C. Liu. Multi-layer embedment of conductive and non-conductive PDMS for all-elastomer MEMS. In: Hilton Head’06, Hilton Head Island, SC, June 4–8, 2006.
- 51.Englert DL, Manson MD, Jayaraman A. Investigation of bacterial chemotaxis in flow-based microfluidic devices. Nat. Protoc. 2010;5(5):864–872. doi: 10.1038/nprot.2010.18. [DOI] [PubMed] [Google Scholar]
- 52.Estevez-Torres, A., A. Yamada, and L. Wang. An inexpensive and surable epoxy mold for PDMS. Lab Chip, Chips and Tips. http://www.rsc.org/Publishing/Journals/lc/Chips_and_Tips/epoxy_mould.asp, 2009.
- 53.Friend J, Yeo L. Fabrication of microfluidic devices using poly(dimethylsiloxane) Biomicrofluidics. 2010;4:0265502. doi: 10.1063/1.3259624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu AY, Chou HP, Spence C, Arnold FH, Quake SR. An integrated microfabricated cell sorter. Anal. Chem. 2002;74:2451–2457. doi: 10.1021/ac0255330. [DOI] [PubMed] [Google Scholar]
- 55.Fu CC, Grimes A, Long M, Ferri CGL, Rich BD, Ghosh S, Ghosh S, Lee LP, Gopinathan A, Khine M. Tunable nanowrinkles on shape memory polymer sheets. Adv. Mater. 2009;21:4472–4476. [Google Scholar]
- 56.Fu E, Kauffman P, Lutz B, Yager P. Chemical signal amplification in two-dimensional paper networks. Sens. Actuator A Chem. 2010;149:325–328. doi: 10.1016/j.snb.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Go JS, Shoji S. A disposable, dead volume-free and leak-free in-plane PDMS microvalve. Sens. Actuator A Phys. 2004;114:438–444. [Google Scholar]
- 58.Gong X, Wen WJ. Polydimethylsiloxane-based conducting composites and their applications in microfluidic chip fabrication. Biomicrofluidics. 2009;3:012007. doi: 10.1063/1.3098963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grimes A, Breslauer DN, Long M, Pegan J, Lee LP, Khine M. Shrinky-dink microfluidics: rapid generation of deep and rounded patterns. Lab Chip. 2008;8:170–172. doi: 10.1039/b711622e. [DOI] [PubMed] [Google Scholar]
- 60.Gu H, Duits MHG, Mugele F. A hybrid microfluidic chip with electrowetting functionality using ultraviolet(UV)-curable polymer. Lab Chip. 2010;10:1550–1556. doi: 10.1039/c001524e. [DOI] [PubMed] [Google Scholar]
- 61.Hatch A, Kamholz AE, Hawkins KR, Munson MS, Schilling EA, Weigl BH, Yager P. A rapid diffusion immunoassay in a T-sensor. Nat. Biotechnol. 2001;19:461–465. doi: 10.1038/88135. [DOI] [PubMed] [Google Scholar]
- 62.Haubert K, Drier T, Beebe D. PDMS bonding by means of a portable, low-cost corona system. Lab Chip. 2006;6:1548–1549. doi: 10.1039/b610567j. [DOI] [PubMed] [Google Scholar]
- 63.Hong C, Bao D, Thomas MS, Clift JM, Vullev VI. Print-and-peel fabrication of microelectrodes. Langmuir. 2008;24:8439–8442. doi: 10.1021/la801752k. [DOI] [PubMed] [Google Scholar]
- 64.Hong L, Pan T. Photopatternable superhydrophobic nanocomposites for microfabrication. J. Microelectromech. Syst. 2010;19(2):246–253. [Google Scholar]
- 65.Hong, L., and T. Pan. Novel three-dimensional surface microfluidics enabled by spatiotemporal control of unconventional fluidic interface. Lab Chip. doi:10.1039/c0lc00173b. [DOI] [PubMed]
- 66.Hong, L., and T. Pan. Surface microfluidics fabricated by photopatternable superhydrophobic nanocomposite. Microfluid. Nanofluid. doi:10.1007/s10404-010-0728-7.
- 67.Hsu HY, Ohta AT, Chiou PY, Jamshidi A, Neale SL, Wu MC. Phototransistor-based optoelectronics tweezers for dynamic cell manipulation in cell culture media. Lab Chip. 2010;10:165–172. doi: 10.1039/b906593h. [DOI] [PubMed] [Google Scholar]
- 68.Huh D, Mills KL, Zhu X, Burns MA, Thouless MD, Takayama S. Tunable elastomeric nanochannels for nanofluidic manipulation. Nat. Mater. 2007;6:424–428. doi: 10.1038/nmat1907. [DOI] [PubMed] [Google Scholar]
- 69.Humayun MS, de Juan E, Jr, Weiland JD, Dagnelie G, Katona S, Greenberg R, Suzuki S. Pattern electrical stimulation of the human retina. Vis. Res. 1999;39:2569–2576. doi: 10.1016/s0042-6989(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 70.Hung LH, Lin R, Lee AP. Rapid microfabrication of solvent-resistant biocompatible microfluidic devices. Lab Chip. 2008;8:983–987. doi: 10.1039/b717710k. [DOI] [PubMed] [Google Scholar]
- 71.Hwang H, Choi YJ, Choi W, Kim SH, Jang J, Park JK. Interactive manipulation of blood cells using a lens-integrated liquid crystal display based optoelectronic tweezers system. Electrophoresis. 2008;29:1203–1212. doi: 10.1002/elps.200700415. [DOI] [PubMed] [Google Scholar]
- 72.Hwang H, Park JK. Rapid and selective concentration of microparticles in an optoelectrofluidic platform. Lab Chip. 2009;9:199–206. doi: 10.1039/b811740c. [DOI] [PubMed] [Google Scholar]
- 73.Itoga K, Kobayashi J, Tsuda Y, Yamato M, Okano T. Second generation maskless photolithography device for surface micropatterning and microfluidic channel fabrication. Anal. Chem. 2008;80:1323–1327. doi: 10.1021/ac702208d. [DOI] [PubMed] [Google Scholar]
- 74.Itoga K, Kobayashi J, Yamato M, Kikuchi A, Okano T. Maskless liquid crystal display projection photolithography for improved design flexibility of cellular micropatterns. Biomaterials. 2006;27:3005–3009. doi: 10.1016/j.biomaterials.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 75.Itoga K, Yamato M, Kobayashi J, Kikuchi A, Okano T. Cell micropatterning using photopolymerization with a liquid crystal device commercial projector. Biomaterials. 2004;25:2047–2053. doi: 10.1016/j.biomaterials.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 76.Itoga K, Yamato M, Kobayashi J, Kikuchi A, Okano T. Micropatterned surfaces prepared using a liquid crystal projector-modified photopolymerization device and microfluidics. J. Biomed. Mater. Res. A. 2004;69A(3):391–397. doi: 10.1002/jbm.a.30010. [DOI] [PubMed] [Google Scholar]
- 77.Jamshidi A, Neale SL, Yu K, Pauzauskie PJ, Schuck PJ, Valley JK, Hsu HY, Ohta AT, Wu MC. NanoPen: dynamic, low-power, and light-actuated patterning of nanoparticles. Nano Lett. 2009;9(8):2921–2925. doi: 10.1021/nl901239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jayakumar R, Prabaharan M, Nair SV, Tamura H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010;28(1):142–150. doi: 10.1016/j.biotechadv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Jiang LT, Huang TC, Chang CY, Chiou JR, Yang SY, Huang PH. Direct fabrication of rigid microstructures on a metallic roller using a dry film resist. J. Micromech. Microeng. 2008;18(1):015004. [Google Scholar]
- 80.Jo BH, Lerberghe LM, Motsegood KM, Beebe DJ. Three dimensional microchannel fabrication in poly(dimethylsiloxane) (PDMS) elastomer. J. Microelectromech. Syst. 2000;9(1):76–81. [Google Scholar]
- 81.Jothimuthu P, Carroll A, Bhagat AAS, Lin G, Mark JE, Papautsky I. Photodefinable PDMS thin films for microfabrication applications. J. Micromech. Microeng. 2009;19(4):045024. [Google Scholar]
- 82.Ju J, Warrick J, Beebe DJ. A cell programmable assay (CPA) chip. Lab Chip. 2010;10:2071–2076. doi: 10.1039/c005103a. [DOI] [PubMed] [Google Scholar]
- 83.Kabashin AV, Evans P, Pastkovsky S, Hendren W, Wurtz GA, Atkinson R, Pollard R, Podolskiy VA, Zayats AV. Plasmonic nanorod metamaterials for biosensing. Nat. Mater. 2009;8:867–871. doi: 10.1038/nmat2546. [DOI] [PubMed] [Google Scholar]
- 84.Kaigala GV, Ho S, Penterman R, Backhouse CJ. Rapid prototyping of microfluidic devices with a wax printer. Lab Chip. 2007;7:384–387. doi: 10.1039/b617764f. [DOI] [PubMed] [Google Scholar]
- 85.Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryonic bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–794. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 86.Kauffman P, Fu E, Lutz B, Yager P. Visualization and measurement of flow in two-dimensional paper networks. Lab Chip. 2010;10:2614–2617. doi: 10.1039/c004766j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keenan TM, Floch A. Biomolecular gradients in cell culture systems. Lab Chip. 2008;8:34–57. doi: 10.1039/b711887b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M. Microfluidic patterning for fabrication of contractile cardic organoids. Biomed. Microdevices. 2007;9:149–157. doi: 10.1007/s10544-006-9013-7. [DOI] [PubMed] [Google Scholar]
- 89.Khademhosseini A, Ferreira L, Blumling J, III, Yeh J, Karp JM, Fukuda J, Langer R. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrate. Biomaterials. 2006;27:5968–5977. doi: 10.1016/j.biomaterials.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 90.Khademhosseini A, Yeh J, Eng G, Karp J, Kaji H, Borenstein J, Farokhzad OC, Langer R. Cell docking inside microwells within reversibly sealed microfluidic channels for fabricating multiphenotype cell arrays. Lab Chip. 2005;5:1380–1386. doi: 10.1039/b508096g. [DOI] [PubMed] [Google Scholar]
- 91.Khademhosseini A, Yeh J, Jon S, Eng G, Suh KY, Burdick JA, Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4:425–430. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 92.Khademhosseinieh B, Sencan I, Biener G, Su TW, Coskun AF, Tseng D, Ozcan A. Lens free on-chip imaging using nanostructured surfaces. Appl. Phys. Lett. 2010;96:171106. doi: 10.1063/1.3405719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khare K, Zhou J, Yang S. Tunable open channel microfluidics on soft poly(dimethylsiloxane) (PDMS) substrates with sinusoidal grooves. Langmuir. 2009;25(21):12794–12799. doi: 10.1021/la901736n. [DOI] [PubMed] [Google Scholar]
- 94.Khnouf R, Beebe DJ, Fan ZH. Cell-free protein expression in a microchannel array with passive pumping. Lab Chip. 2009;9:56–61. doi: 10.1039/b808034h. [DOI] [PubMed] [Google Scholar]
- 95.Kim P, Jeong HW, Khademhosseini A, Suh KY. Fabrication of non-biofouling polyethylene glycol micro- and nanochannels by ultraviolet-assisted irreversible sealing. Lab Chip. 2006;6:1432–1437. doi: 10.1039/b610503c. [DOI] [PubMed] [Google Scholar]
- 96.Kim DK, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim YS, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang KC, Zakin MR, Litt B, Rogers JA. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010;9:511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko WH. Solid-state physical transducers for biomedical research. IEEE Trans. Biomed. Eng. 1986;2:153–162. doi: 10.1109/TBME.1986.325848. [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi J, Yamato M, Itoga K, Kikuchi A, Okano T. Preparation of microfluidic devices using micropatterning of a photosensitive material by a maskless, liquid-crystal-display projection method. Adv. Mater. 2004;16(22):1997–2001. [Google Scholar]
- 99.Koerner T, Brown L, Xie R, Oleschuk RD. Epoxy resins as stamps for hot embossing of microstructures and microfluidic channels. Sens. Actuators B Chem. 2005;107(2):632–639. [Google Scholar]
- 100.Koh WG, Revzin A, Pishko MV. Poly(ethylene glycol) hydrogel microstructures encapsulating living cells. Langmuir. 2002;18:2459–2462. doi: 10.1021/la0115740. [DOI] [PubMed] [Google Scholar]
- 101.Kost GJ, Ehrmeyer SS, Chernow B, Winkelman JW, Zaloga GP, Dellinger RP, Shirey T. The laboratory–clinical interface: point-of-care testing. Chest. 1999;115:1140–1154. doi: 10.1378/chest.115.4.1140. [DOI] [PubMed] [Google Scholar]
- 102.Lee GB, Chen SH, Huang GR, Sung WC, Lin YH. Microfabricated plastic chips by hot embossing methods and their applications for DNA separation and detection. Sens. Actuators B Chem. 2001;75:142–148. [Google Scholar]
- 103.Lee KS, Ram RJ. Plastic PDMS bonding for high pressure hydrolytically stable active microfluidics. Lab Chip. 2009;9:1618–1624. doi: 10.1039/b820924c. [DOI] [PubMed] [Google Scholar]
- 104.Lee JY, Shah SS, Yan J, Howland MC, Parikh AN, Pan T, Revzin A. Integrating sensing hydrogel microstructures into micropatterned hepatocellular cocultures. Langmuir. 2009;25(6):3880–3886. doi: 10.1021/la803635r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leech PW, Wu N, Zhu Y. Application of dry film resist in the fabrication of microfluidic chips for roplet generation. J. Micromech. Microeng. 2009;19(6):065019. [Google Scholar]
- 106.Lei Y, Xie HF, Wang W, Wu WG, Li ZH. Suspended nanoparticle crystal (S-NPC): a nanofluidics-based, electrical read-out biosensor. Lab Chip. 2010;10:2338–2340. doi: 10.1039/c004758a. [DOI] [PubMed] [Google Scholar]
- 107.Li H, Carter JD, LaBean TH. Nanofabrication by DNA self-assembly. Mater. Today. 2009;12(5):24–32. [Google Scholar]
- 108.Li S, Chen S. Polydimethylsioxane fluidic interconnects for microfluidic systems. IEEE Trans. Adv. Packag. 2003;26(3):242–247. [Google Scholar]
- 109.Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 110.Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel. Adv. Mater. 2004;16(14):1151–1170. [Google Scholar]
- 111.Li Y, Yuan B, Ji H, Han D, Chen S, Tian F, Jiang X. A method for patterning multiple types of cells by using electrochemical desorption of self-assembled monolayers within microfluidic channels. Angew. Chem. Int. Ed. 2007;119:1112–1114. doi: 10.1002/anie.200603844. [DOI] [PubMed] [Google Scholar]
- 112.Liang D, Hsiao BS, Chu B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliev. Rev. 2007;59(14):1392–1412. doi: 10.1016/j.addr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin CH, Lee GB, Chang BW, Chang GL. A new fabrication process for ultra-thick microfluidic microstructures utilizing SU-8 photoresist. J. Micromech. Microeng. 2002;12(5):590–600. [Google Scholar]
- 114.Liu C. Foundations of MEMS. New Jersey: Prentice Hall Press; 2005. [Google Scholar]
- 115.Liu C. Recent developments in polymer MEMS. Adv. Mater. 2007;19:3783–3790. [Google Scholar]
- 116.Liu, Z., J. L. Tan, D. M. Cohen, M. T. Yang, N. J. Sniadecki, S. A. Ruiz, C. M. Nelson, and C. S. Chen. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl. Acad. Sci. USA, 2010. doi:10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed]
- 117.Madou MJ. Fundamentals of Microfabrication: The Science of Miniaturization. 2. Boca Raton: CRC Press; 2002. [Google Scholar]
- 118.Malkin DS, Wei B, Fogiel AJ, Staats SL, Wirth MJ. Submicrometer plate heights for capillaries packed with silica colloidal crystals. Anal. Chem. 2010;82:2175–2177. doi: 10.1021/ac100062t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mao C, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, Georgiou G, Iverson B, Belcher AM. Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires. Science. 2004;303(5655):213–217. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- 120.Martinez AW, Philips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007;46(8):1318–1320. doi: 10.1002/anie.200603817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinez, A. W., S. T. Philips, Z. Nie, C. M. Cheng, E. Carrilho, B. J. Wiley, and G. M. Whitesides. Programmable diagnostic devices made from paper and tape. Lab Chip, 2010. doi:10.1039/c01c00021c. [DOI] [PubMed]
- 122.Martinez AW, Philips ST, Whitesides GM. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA. 2008;105(50):19606–19611. doi: 10.1073/pnas.0810903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martinez AW, Philips ST, Whitesides GM. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal. Chem. 2010;82:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 124.Martinez AW, Philips ST, Wiley BJ, Gupta M, Whitesides GM. FLASH: a rapid method for prototyping paper-based microfluidic devices. Lab Chip. 2008;8:2146–2150. doi: 10.1039/b811135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mastrangeli M, Abbasi S, Varel C, van Hoof C, Celis JP, Bohringer KF. Self-assembly from milli- to nanoscale: methods and applications. J. Micromech. Microeng. 2009;19(8):083001. doi: 10.1088/0960-1317/19/8/083001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McDonald JC, Whitesides GM. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002;35(7):491–499. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 127.Mendes PM. Stimuli-responsive surfaces for bio-applications. Chem. Soc. Rev. 2009;37:2512–2529. doi: 10.1039/b714635n. [DOI] [PubMed] [Google Scholar]
- 128.Miller MS, Davidson GJE, Sahli BJ, Mailloux CM, Carmichael TB. Fabrication of elastomeric wires by selective electroless metallization of poly(dimethylsiloxane) Adv. Mater. 2008;20:59–64. [Google Scholar]
- 129.Mills KL, Huh D, Takayama S, Thouless MD. Instantaneous fabrication of arrays of normally closed, adjustable, and reversible nanochannels by tunnel cracking. Lab Chip. 2010;10:1627–1630. doi: 10.1039/c000863j. [DOI] [PubMed] [Google Scholar]
- 130.Moeller HC, Mian MK, Shrivastava S, Chung BG, Khadaemhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29:752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 132.Moore GE. Lithography and the future of Moore’s law. Proc. SPIE. 1995;2438:2–17. [Google Scholar]
- 133.Najafi K. Micropackaging technologies for integrated microsystems: applications to MEMS and MOEMS. Proc. SPIE. 2003;4979:1–19. [Google Scholar]
- 134.Nash MA, Hoffman JM, Stevens DY, Hoffman AS, Stayton PS, Yager P. Laboratory-scale protein striping system for patterning biomolecules onto paper-based immunochromatographic test strips. Lab Chip. 2010;10:2279–2282. doi: 10.1039/c004991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Natali M, Begolo S, Carofiglio T, Mistura G. Rapid prototyping of multilayer thiolene microfluidic chips by photopolymerization and transfer lamination. Lab Chip. 2008;8:492–494. doi: 10.1039/b716594c. [DOI] [PubMed] [Google Scholar]
- 136.Nelson KE, Foley JO, Yager P. Concentration gradient immunoassay. 1. An immunoassay based on interdiffusion and surface binding in a microchannel. Anal. Chem. 2007;79:3542–3548. doi: 10.1021/ac062349w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nguyen D, Taylor D, Qian K, Norouzi N, Rasmussen J, Botzet S, Lehmann M, Halverson K, Khine M. Better shrinkage than Shrinky-Dinks. Lab Chip. 2010;10:1623–1626. doi: 10.1039/c001082k. [DOI] [PubMed] [Google Scholar]
- 138.Nie Z., F. Deiss, X. Liu, O. Akbulut, and G. M. Whitesides. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip. doi:10.1039/c0lc00237b. [DOI] [PMC free article] [PubMed]
- 139.Niu XZ, Peng SL, Liu LY, Wen WJ, Sheng P. Characterizing and patterning of PDMS-based conducting composites. Adv. Mater. 2007;19(18):2682–2686. [Google Scholar]
- 140.Numata, K., and D. L. Kaplan. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev., 2010. doi:10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed]
- 141.Omenetto FG, Kaplan DL. New opportunities for an ancient material. Science. 2010;329:528–531. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Osborn JL, Lutz B, Fu E, Kauffman P, Stevens DY, Yager P. Microfluidics without pumps: reinventing the T-sensor and H-filter in paper networks. Lab Chip. 2010;10:2659–2665. doi: 10.1039/c004821f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip. 2008;8:98–106. doi: 10.1039/b713695a. [DOI] [PubMed] [Google Scholar]
- 144.Ozin GA, Hou K, Lotsch BV, Cademartiri L, Puzzo DP, Scotognella F, Ghadimi A, Thomson J. Nanofabrication by self-assembly. Mater. Today. 2009;12(5):12–23. [Google Scholar]
- 145.Pan T, Baldi A, Ziaie B. A reworkable adhesive-free interconnection technology for microfluidic systems. J. Microelectromech. Syst. 2006;15(1):267–272. [Google Scholar]
- 146.Park S, Huh YS, Craighead HG, Erickson D. A method for nanofluidic device prototyping using elastomeric collapse. Proc. Natl. Acad. Sci. USA. 2009;106(37):15549–15554. doi: 10.1073/pnas.0904004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parviz BA. For your eye only. IEEE Spectr. 2009;September:36–41. [Google Scholar]
- 148.Pattekar AV, Kothare MV. Novel microfluidic interconnectors for high temperature and pressure applications. J. Micromech. Microeng. 2003;13:337–345. [Google Scholar]
- 149.Payne GP, Raghavan SR. Chitosan: a soft interconnect for hierarchical assembly of nano-scale components. Soft Matter. 2007;3:521–527. doi: 10.1039/b613872a. [DOI] [PubMed] [Google Scholar]
- 150.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 151.Piner RD, Zhu J, Xu F, Hong S, Mirkin CA. “Dip-Pen” nanolithography. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 152.Pushkarev D, Neff NF, Quake SR. Single-molecule sequencing of an individual human genome. Nat. Biotechnol. 2009;27(9):847–850. doi: 10.1038/nbt.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qian X, Peng XH, Ansari DO, Goen QY, Chen GZ, Shin DM, Yang L, Toung AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008;26:83–91. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 154.Qin D, Xia Y, Whitesides GM. Soft lithography for micro and nanoscale patterning. Nat. Protoc. 2010;5(3):491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 155.Quake SR, Scherer A. From micro to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- 156.Quist AP, Pavlovic E, Oscarsson S. Recent advances in microcontact printing. Anal. Bioanal. Chem. 2005;381(3):591–600. doi: 10.1007/s00216-004-2847-z. [DOI] [PubMed] [Google Scholar]
- 157.Rafat M, Raad DR, Rowat AC, Augueste DT. Fabrication of reversibly adhesive fluidic devices using magnetism. Lab Chip. 2009;9:3016–3019. doi: 10.1039/b907957b. [DOI] [PubMed] [Google Scholar]
- 158.Rettig JR, Folch A. Large-scale single-cell trapping and imaging using microwell arrays. Anal. Chem. 2005;77:5628–5634. doi: 10.1021/ac0505977. [DOI] [PubMed] [Google Scholar]
- 159.Revzin A, Russell RJ, Yadavalli VK, Koh WG, Deister C, Hile DD, Mellott MB, Pishko MV. Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir. 2001;17:5440–5447. doi: 10.1021/la010075w. [DOI] [PubMed] [Google Scholar]
- 160.Revzin A, Tompkins RG, Toner M. Surface engineering with poly(ethylene glycol) photolithography to create high-density cell arrays on glass. Langmuir. 2003;19(23):9855–9862. [Google Scholar]
- 161.Rothemund PWK. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440:297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 162.Ruiz SA, Chen CS. Microcontact printing: a tool to pattern. Soft Matter. 2007;3:168–177. doi: 10.1039/b613349e. [DOI] [PubMed] [Google Scholar]
- 163.Sands RW, Mooney DJ. Polymers to direct cell fate by controlling the microenvironment. Curr. Opin. Biotechnol. 2007;18:448–453. doi: 10.1016/j.copbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sasaki D, Shimizu T, Masuda S, Kobayashi J, Itoga K, Tsuda Y, Yamashita JK, Yamato M, Okano T. Mass preparation of size-controlled mouse embryonic stem cell aggregates and induction of cardiac differentiation by cell patterning method. Biomaterials. 2009;30:4384–4389. doi: 10.1016/j.biomaterials.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 165.Schaff UY, Xing MMQ, Lin KK, Pan N, Jeon NL, Simon SI. Vascular mimetics based on microfluidics for imaging the leukocyte-endothelial inflammatory response. Lab Chip. 2007;7:448–456. doi: 10.1039/b617915k. [DOI] [PubMed] [Google Scholar]
- 166.Schaff UY, Yamayoshi I, Tse T, Griffin D, Kibathi L, Simon SI. Calcium flux in neutrophils synchronizes β2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann. Biomed. Eng. 2008;36(4):632–646. doi: 10.1007/s10439-008-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Seidlits SK, Lee JY, Schmidt CE. Nanostructured scaffolds for neural applications. Nanomedicine. 2008;3(2):183–199. doi: 10.2217/17435889.3.2.183. [DOI] [PubMed] [Google Scholar]
- 168.Seo S, Isikman SO, Sencan I, Mudanyali O, Su TW, Bishara W, Erlinger A, Ozcan A. High-throughput lens-free blood analysis on a chip. Anal. Chem. 2010;82:4621–4627. doi: 10.1021/ac1007915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shah SS, Howland MC, Chen LJ, Silangcruz J, Verkhoturov SV, Schweikert EA, Parikh AN, Revzin A. Micropatterning of proteins and mammalian cells on indium tin oxide. ACS Appl. Mater. Interfaces. 2009;1(11):2592–2601. doi: 10.1021/am900508m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shin YM, Gamzina D, Barnett LR, Yaghmaie F, Luhmann NC., Jr UV lithography and molding fabrication of ultrathick micrometallic structures using a KMPR photoresist. J. Microelectromech. Syst. 2010;19(3):683–689. [Google Scholar]
- 171.Sollier K, Mandon CA, Heyries KA, Blum LJ, Marquette CA. Print-n-shrink technology for the rapid production of microfluidic chips and protein microarrays. Lab Chip. 2009;9:3489–3494. doi: 10.1039/b913253h. [DOI] [PubMed] [Google Scholar]
- 172.Steigert J, Haeberle S, Brenner T, Muller C, Steinert CP, Koltay P, Gottschlich N, Reinecke H, Ruhe J, Zengerle R, Ducree J. Rapid prototyping of microfluidic chips in COC. J. Micromech. Microeng. 2007;17(2):333–343. [Google Scholar]
- 173.Stephan K, Pittet P, Renaud L, Kleimann P, Morin P, Ouaini N, Ferrigno R. Fast prototyping using a dry film photoresist: microfabrication of soft-lithography masters for microfluidic structures. J. Micromech. Microeng. 2007;17(10):N69–N79. [Google Scholar]
- 174.Stevens DY, Petri CR, Osborn JL, Spicar-Mihalic P, McKenzie KG, Yager P. Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab Chip. 2008;8:2038–2045. doi: 10.1039/b811158h. [DOI] [PubMed] [Google Scholar]
- 175.Sun D, Chang C, Li S, Lin L. Near-field electrospinning. Nano Lett. 2006;6(4):839–842. doi: 10.1021/nl0602701. [DOI] [PubMed] [Google Scholar]
- 176.Sundaray B, Subramanian V, Natarajan TS, Krishnamurthy K. Electrical conductivity of a single electrospun fiber of poly(methyl methacrylate) and multiwalled carbon nanotube nanocomposite. Appl. Phys. Lett. 2006;88:143114. [Google Scholar]
- 177.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 2003;200(4):1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tang L, Lee NY. A facile route for irreversible bonding of plastic PDMS hybrid microdevices at room temperature. Lab Chip. 2010;10:1274–1280. doi: 10.1039/b924753j. [DOI] [PubMed] [Google Scholar]
- 179.Thomas MS, Millare B, Clift JM, Bao D, Hong C, Vullev VI. Print-and-peel fabrication for microfluidics: what’s in it for biomedical applications. Ann. Biomed. Eng. 2010;38(1):21–32. doi: 10.1007/s10439-009-9831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tkachenko E, Gutierrez E, Ginsberg MH, Groisman A. An easy to assemble microfluidic perfusion device with a magnetic clamp. Lab Chip. 2009;9(8):1085–1095. doi: 10.1039/b812184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Toepke MW, Abhyankar VV, Beebe DJ. Microfluidic logic gates and timers. Lab Chip. 2007;7:1449–1453. doi: 10.1039/b708764k. [DOI] [PubMed] [Google Scholar]
- 182.Tourovskaia A, Figueroa-Masot X, Folch A. Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip. 2005;5:14–19. doi: 10.1039/b405719h. [DOI] [PubMed] [Google Scholar]
- 183.Tsai YC, Jen HP, Lin KW, Hsieh YZ. Fabrication of microfabrication devices using dry film photoresist for microchip capillary electrophoresis. J. Chromatogr. A. 2006;1111(2):267–271. doi: 10.1016/j.chroma.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 184.Tsai YC, Yang SJ, Lee HT, Jen HP, Hsieh YZ. Fabrication of a flexible and disposable microreactor using a dry film photoresist. J. Chin. Chem. Soc. 2006;53:683–688. [Google Scholar]
- 185.Tseng P, Carlo DD, Judy JW. Rapid and dynamic intracellular patterning of cell-internalized magnetic fluorescent nanoparticles. Nano Lett. 2009;9(8):3053–3059. doi: 10.1021/nl901535m. [DOI] [PubMed] [Google Scholar]
- 186.Tseng D, Mudanyali O, Oztoprak C, Isikman SO, Sencan I, Yaglidere O, Ozcan A. Lensfree microscopy on a cellphone. Lab Chip. 2010;10:1787–1792. doi: 10.1039/c003477k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tseng RJ, Tsai C, Ma L, Ouyang J, Ozkan CS, Yang Y. Digital memory device based on Tobacco Mosaic Virus conjugated with nanoparticles. Nat. Nanotechnol. 2006;1:72–77. doi: 10.1038/nnano.2006.55. [DOI] [PubMed] [Google Scholar]
- 188.Umbrecht F, Muller D, Gattiker F, Boutry CM, Neuenschwander J, Sennhauser U, Hierold C. Solvent assisted bonding of polymethylmethacrylate: characterization using the response surface methodology. Sens. Actuator A Phys. 2009;156:121–128. [Google Scholar]
- 189.Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
- 190.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr. Opin. Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vullev VI, Wan J, Heinrich V, Landsman P, Bower PE, Xia B, Millare B, Jones G. Nonlithographic fabrication of microfluidic devices. J. Am. Chem. Soc. 2006;128:16062–16072. doi: 10.1021/ja061776o. [DOI] [PubMed] [Google Scholar]
- 192.Vulto P, Glade N, Altomare L, Bablet J, Tin LD, Medoro G, Chartier I, Manaresi N, Tartgni M, Guerrieri R. Microfluidic channel fabrication in dry film resist for production and prototyping of hybrid chips. Lab Chip. 2005;5:158–162. doi: 10.1039/b411885e. [DOI] [PubMed] [Google Scholar]
- 193.Vulto P, Huesgen T, Albrecht B, Urban GA. A full wafer fabrication process for glass microfluidic chips with integrated electroplated electrodes by direct bonding of dry film resist. J. Micromech. Microeng. 2009;19(7):077001. [Google Scholar]
- 194.Walker GM, Beebe DJ. An evaporation-based microfluidic sample concentration method. Lab Chip. 2002;2:57–61. doi: 10.1039/b202473j. [DOI] [PubMed] [Google Scholar]
- 195.Walker GM, Beebe DJ. A passive pumping method for microfluidic devices. Lab Chip. 2002;2:131–134. doi: 10.1039/b204381e. [DOI] [PubMed] [Google Scholar]
- 196.Wang M, Jing N, Su CB, Kameoka J, Chou CK, Huang MC, Chang KA. Electrospinning of silica nanochannels for single molecule detection. Appl. Phys. Lett. 2006;88:033106. [Google Scholar]
- 197.Wang YZ, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27(36):6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 198.Wang L, Zhang MY, Yang M, Zhu WM, Wu JB, Gong XQ, Wen WJ. Polydimethylsiloxane-integratable micropressure sensor for microfluidic chips. Biomicrofluidics. 2009;3:034105. doi: 10.1063/1.3230500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang W, Zhao S, Pan T. Lab-on-a-print: from a single polymer film to three-dimensional integrated microfluidics. Lab Chip. 2009;9:1133–1137. doi: 10.1039/b816287e. [DOI] [PubMed] [Google Scholar]
- 200.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 201.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 202.Wise KD. Silicon microsystems for neuroscience and neural prostheses. IEEE Eng. Med. Biol. Mag. 2005;September/October:22–29. doi: 10.1109/memb.2005.1511497. [DOI] [PubMed] [Google Scholar]
- 203.Wise KD, Bhatti PT, Wang J, Friedrich CR. High-density cochlear implants with position sensing and control. Hearing Res. 2008;242:22–30. doi: 10.1016/j.heares.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 204.Wu YZ, Huang Y, Ma H. A facile method for permanent and functional surface modification of poly(dimethylsiloxane) J. Am. Chem. Soc. 2007;129:7226–7227. doi: 10.1021/ja071384x. [DOI] [PubMed] [Google Scholar]
- 205.Xie J, Li X, Xia Y. Putting electrospun nanofibers to work for biomedical research. Macromol. Rapid Commun. 2008;29(22):1775–1792. doi: 10.1002/marc.200800381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 207.Yan H, Park SH, Finkelstein G, Reif JH, LaBean TH. DNA-templated self-assembly of protein arrays and highly conductive nanowire. Science. 2003;301:1882–1884. doi: 10.1126/science.1089389. [DOI] [PubMed] [Google Scholar]
- 208.Yang D, Liu X, Jin Y, Zhu Y, Zeng D, Jiang X, Ma H. Electrospinning of poly(dimethylsiloxane)/poly(methylmethacrylate) nanofibrous membrane: fabrication and application in protein microarray. Biomacromolecules. 2009;10:3335–3340. doi: 10.1021/bm900955p. [DOI] [PubMed] [Google Scholar]
- 209.Yi H, Wu LQ, Bentley WE, Ghodssi R, Rubloff GW, Culver JN, Payne GF. Biofabrication with chitosan. Biomacromolecules. 2005;6(6):2881–2894. doi: 10.1021/bm050410l. [DOI] [PubMed] [Google Scholar]
- 210.Zeng Y, He M, Harrison DJ. Microfluidic self patterning of large scale crystalline nanoarrays for high throughput continuous DNA fractionation. Angew. Chem. Int. Ed. 2008;47:6388–6391. doi: 10.1002/anie.200800816. [DOI] [PubMed] [Google Scholar]
- 211.Zeng Y, Harrison DJ. Self-assembled colloidal arrays as three-dimensional nanofluidic sieves for separation of biomolecules on microchips. Anal. Chem. 2007;79:2289–2295. doi: 10.1021/ac061931h. [DOI] [PubMed] [Google Scholar]
- 212.Zhang LJ, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 213.Zhao, S., A. Chen, A. Revzin, and T. Pan. Stereomask lithography (SML): a universal multi-object micro-patterning technique for biological applications. Lab Chip, 2010. doi:10.1039/c0lc00275e. [DOI] [PubMed]
- 214.Zhao S, Cong H, Pan T. Direct projection on dry film photoresist (DP2): do-it-yourself three-dimensional polymer microfluidics. Lab Chip. 2009;9:1128–1132. doi: 10.1039/b817925e. [DOI] [PubMed] [Google Scholar]
- 215.Zhao YP, Wang LS, Yu TX. Mechanics of adhesion in MEMS—a review. J. Adhes. Sci. Technol. 2003;17(4):519–546. [Google Scholar]
- 216.Zhou J, Ellis AV, Voelcker NH. Recent developments in PDMS surface modification for microfluidics devices. Electrophoresis. 2010;31:2–16. doi: 10.1002/elps.200900475. [DOI] [PubMed] [Google Scholar]
- 217.Zhou J, Yan H, Ren K, Dai W, Wu H. Convenient method for modifying poly(dimethylsiloxane) with poly(ethylene glycol) in microfluidics. Anal. Chem. 2009;81:6627–6632. doi: 10.1021/ac900551m. [DOI] [PubMed] [Google Scholar]


