Summary
Background
Severe malaria is a major cause of childhood death and often the main reason for paediatric hospital admission in sub-Saharan Africa. Quinine is still the established treatment of choice, although evidence from Asia suggests that artesunate is associated with a lower mortality. We compared parenteral treatment with either artesunate or quinine in African children with severe malaria.
Methods
This open-label, randomised trial was undertaken in 11 centres in nine African countries. Children (<15 years) with severe falciparum malaria were randomly assigned to parenteral artesunate or parenteral quinine. Randomisation was in blocks of 20, with study numbers corresponding to treatment allocations kept inside opaque sealed paper envelopes. The trial was open label at each site, and none of the investigators or trialists, apart from for the trial statistician, had access to the summaries of treatment allocations. The primary outcome measure was in-hospital mortality, analysed by intention to treat. This trial is registered, number ISRCTN50258054.
Findings
5425 children were enrolled; 2712 were assigned to artesunate and 2713 to quinine. All patients were analysed for the primary outcome. 230 (8·5%) patients assigned to artesunate treatment died compared with 297 (10·9%) assigned to quinine treatment (odds ratio [OR] stratified for study site 0·75, 95% CI 0·63–0·90; relative reduction 22·5%, 95% CI 8·1–36·9; p=0·0022). Incidence of neurological sequelae did not differ significantly between groups, but the development of coma (65/1832 [3·5%] with artesunate vs 91/1768 [5·1%] with quinine; OR 0·69 95% CI 0·49–0·95; p=0·0231), convulsions (224/2712 [8·3%] vs 273/2713 [10·1%]; OR 0·80, 0·66–0·97; p=0·0199), and deterioration of the coma score (166/2712 [6·1%] vs 208/2713 [7·7%]; OR 0·78, 0·64–0·97; p=0·0245) were all significantly less frequent in artesunate recipients than in quinine recipients. Post-treatment hypoglycaemia was also less frequent in patients assigned to artesunate than in those assigned to quinine (48/2712 [1·8%] vs 75/2713 [2·8%]; OR 0·63, 0·43–0·91; p=0·0134). Artesunate was well tolerated, with no serious drug-related adverse effects.
Interpretation
Artesunate substantially reduces mortality in African children with severe malaria. These data, together with a meta-analysis of all trials comparing artesunate and quinine, strongly suggest that parenteral artesunate should replace quinine as the treatment of choice for severe falciparum malaria worldwide.
Funding
The Wellcome Trust.
Introduction
Falciparum malaria is a major contributor to child mortality in Africa and one of the main causes of paediatric hospital admission across sub-Saharan Africa. Many deaths occur in or near the home, but for children who are admitted to hospital with severe malaria and receive parenteral antimalarial treatment, about one in six will die.1,2 From the time of its introduction to European medicine in the 1630s until the deployment of parenteral chloroquine in the 1950s, quinine was the mainstay of severe malaria treatment. Resistance to chloroquine emerged in southeast Asia and then spread to Africa at the end of the 1970s. Quinine then resumed its primary role in the treatment of severe malaria. Parenteral quinine has a narrow therapeutic ratio.1–3 Intravenous quinine administration needs a constant rate infusion with dosing three times a day. Intramuscular administration is painful, and can cause sterile abscesses and predispose to lethal tetanus.4 Although blindness and deafness may follow self poisoning, these side-effects are rare in severe malaria; however, quinine-induced hyperinsulinaemic hypoglycaemia is a particular problem in patient management, especially in pregnant women.1,2,5
The primacy of quinine in the treatment of severe malaria has been challenged by the introduction of artemisinin derivatives. The first comparative clinical trials were done with intramuscular artemether—a lipophilic derivative of dihydroartemisinin. Artemether proved safer and easier to use than quinine, but did not improve overall survival in an individual patient data meta-analysis6 of 1919 randomised patients. In a prospectively defined subgroup analysis, artemether reduced mortality significantly in southeast Asian adults, but not in African children. A large multicentre randomised trial (South East Asian Quinine Artesunate Malaria Trial [SEAQUAMAT]),7 which compared intravenous artesunate with quinine in Asian patients (mainly adults) with severe malaria was then undertaken. It was stopped after enrolment of 1461 patients because of a substantial survival benefit in favour of artesunate. In a meta-analysis of the SEAQUAMAT study and earlier, smaller randomised trials,7 artesunate reduced the mortality of severe malaria in Asian patients from 23·1% to 14·2%, a relative reduction of 38·6%. The treatment was also highly cost effective.8 In 2006, WHO changed its guidelines to recommend artesunate for severe malaria in adults.9
The treatment effect in the SEAQUAMAT trial was similar in adults and the 202 children enrolled, but perceived differences in the natural history and drug susceptibility of severe falciparum malaria in African children compared with Asian patients left uncertainty about the optimum treatment for this important patient group. Expert opinion regarded parenteral quinine as a satisfactory treatment, and research priorities focused on improving other aspects of the care of the sick child. Nowadays quinine remains by far the most widely used treatment of severe malaria in Africa. We undertook a large multicentre randomised trial (African Quinine Artesunate Malaria Trial [AQUAMAT]) that compared parenteral treatment with either artesunate or quinine in African children with severe malaria.
Methods
Study design
This was a multicentre, open-label trial in children admitted to hospital with severe malaria, undertaken between Oct 3, 2005, and July 14, 2010. 11 centres in nine countries (Mozambique, The Gambia, Ghana, Kenya, Tanzania, Nigeria, Uganda, Rwanda, and Democratic Republic of the Congo) in Africa participated. The study was coordinated by the Mahidol-Oxford Tropical Medicine Research Unit in Bangkok, Thailand, which provided logistic support and data management. All clinicians were familiarised with the severe malaria criteria for enrolment (panel 1). Most children were managed on general paediatric wards. Children younger than 15 years were included if they had a positive rapid diagnostic test for Plasmodium falciparum lactate dehydrogenase (Optimal, Diamed, Cressier, Switzerland) and, in the admitting physician's clinical opinion, they had severe malaria, and they or their attendant relative or guardian gave fully informed written consent. Patients were not included if there was a convincing history of full treatment with parenteral quinine or an artemisinin derivative for more than 24 h before admission. Age criteria varied slightly between sites at the request of the respective ethics review boards (webappendix p 13). In Mozambique, adults were also studied but were analysed separately and will be reported elsewhere.
Panel 1. Modified criteria for severe falciparum malaria.
At least one of:
-
•
Plasma base excess less than −3·3 mmol/L
-
•
Glasgow coma scale less than 11 of 15, or Blantyre coma scale less than 3 of 5 in preverbal children
-
•
Haemoglobin less than 50 g/L and parasitaemia greater than 100 000 parasites per μL
-
•
Blood urea greater than 10 mmol/L
-
•
Compensated shock (capillary refill ≥3 s or temperature gradient on legs, but no hypotension)
-
•
Decompensated shock; systolic blood pressure less than 70 mm Hg and cool peripheries
-
•
Asexual parasitaemia more than 10%
-
•
Visible jaundice and more than 100 000 parasites per μL
-
•
Plasma glucose less than 3 mmol/L
-
•
Respiratory distress, defined as costal indrawing, use of accessory muscles, nasal alar flaring, deep breathing, or severe tachypnoea
The trial protocol was reviewed and approved by each site's ethics review board, and by the Oxford Tropical Research Ethics Committee. Permission to investigate HIV status was obtained only from some ethics review boards.
Randomisation and masking
Eligible patients were randomly assigned to treatment with either intravenous or intramuscular artesunate or quinine. Each centre had a policy of using one route of administration. Randomisation was done by people unrelated to the study and provided to the study sites in blocks of 20. Study numbers were kept inside opaque sealed paper envelopes. After full informed written consent was obtained, the next envelope, which contained a unique study box number, was opened by the study physician or nurse. Then the corresponding numbered sealed box was opened. This box contained the study drug, case record form (labelled with the unique study number), and all disposables needed for drug administration and blood sampling. Treatment was started immediately. All other aspects of supportive treatment, based on WHO guidelines, were unaffected by the trial.1,2,9
Although the trial was open label at each site, none of the investigators or trialists, apart from for the trial statistician (TEP), had access to the summaries of treatment allocations. When notes or case record forms were reviewed, all study drug details were removed to preserve masking. All case reviews and laboratory assessments were done blinded to treatment allocation, which was not revealed until the database was locked at the end of the trial.
Procedures
Artesunate (Guilin Pharmaceutical Factory, Guangxi, China) was given in a dose of 2·4 mg/kg on admission, at 12 h, at 24 h, and thereafter once daily until oral medication could be taken reliably. The contents of each 60 mg vial were dissolved initially in 1 mL 5% sodium bicarbonate (provided with the drug) and then diluted with 5% dextrose before injection either as a bolus into an indwelling intravenous cannula, or administration by deep intramuscular injection to the anterior thigh. Quinine dihydrochloride (Indus Pharma, Karachi, Pakistan) was given in a 20 mg salt per kg loading dose infused over 4 h (in 5–10 mL/kg of 5% dextrose), followed by a 10 mg salt per kg infusion over 2–8 h three times daily until starting oral therapy. For intramuscular treatment the doses were the same as for intravenous treatment; quinine was diluted in normal saline to a concentration of 60 mg/mL, and injected into the anterior thigh. The loading dose was given as a split dose into each thigh.
When the patient was able to take tablets, but after a minimum of 24 h of parenteral treatment, oral artemether-lumefantrine (Coartem, Novartis, Basel, Switzerland) in a full standard dose (1·5/9 mg/kg twice daily for 3 days with milk or fat) was given to complete the treatment.
A 1 mL blood sample was taken for immediate haematocrit and biochemical analyses with the EC8+ card for a handheld blood analyser (i-STAT, Abbott Laboratories, Abbott Park, IL, USA). This device produced an immediate printed paper report with time of day, which was kept with the case record form. Haemoglobin was reported with the i-STAT result or, when not available, calculated from the measured haematocrit (n=146).10 Thick and thin blood smears were prepared for later malaria parasite counting at the Bangkok reference laboratory. In 109 cases no count was available from the reference laboratory, so the parasitaemia reported by the study site was used. Children were discharged from hospital at the discretion of the physician, and a discharge assessment was completed. Children who had not made a full neurological recovery by discharge were followed up at regular intervals for 12 months or until full recovery. Full neurological assessments were completed at every visit. Training in neurological assessment was provided to all sites by a specialist paediatric neurologist (MO) to ensure uniformity.
Trial sites were monitored regularly by Family Health International, Nairobi, Kenya. Investigators provided reports every 2 weeks to the coordinating centre in Bangkok and met every year to review study progress. Drug content and quality were checked in ampoules taken randomly from the purchase lots (webappendix p 7). The data and safety monitoring committee made three interim analyses during the trial. Adequacy of randomisation was assessed by checking by centre that randomisation was balanced according to baseline variables, and by allocation time to assess whether the expected random variations were observed.
Study outcomes
The analysis was undertaken according to a prespecified analytical plan. The primary outcome measure was in-hospital mortality compared between treatments on an intention-to-treat basis. Secondary outcome measures were the incidence of severe neurological complications (assessed at 28 days, range 3–8 weeks) and a combined outcome measure of death and severe persistent neurological sequelae. Initially, neurological outcomes were assessed only at discharge from hospital, but this procedure led to substantial overestimation of neurological deficit, especially in young children. The protocol was therefore changed in April, 2007, (after 11% of patients had been enrolled) so that children who had not yet fully recovered at discharge were assessed 28 days after enrolment, and active follow-up was instituted. Outcome measures were also assessed per protocol, excluding patients not fulfilling the entry criteria, those with a negative or missing admission blood slide for P falciparum, those dying before receiving the study drug, and those missing a study drug dose on the first day of treatment. Subgroup analyses included stratification into two predefined groups: those who fulfilled the criteria for severe malaria1,2,11 and those who did not. In sites at which testing was approved, the HIV status of participants was assessed after obtaining a separate informed consent, and the outcomes in HIV-1 infected patients with malaria were compared according to treatment allocation.
Two committees were formed to provide independent expert assessment and classification of outcomes before study unblinding. A neurological outcome committee, comprising two experienced paediatricians (both with neurological expertise and experience of working in Africa) and one clinical malariologist, graded neurological sequelae. These sequelae were assessed in four domains: motor function, visual function, hearing, and speech, and also whether the child had developed epilepsy. Sequelae persisting at follow-up were graded as mild, moderate, or severe in each domain according to the extent of functional impairment (webappendix p 5). The overall grade was combined. If there were sequelae in more than one functional domain the grade was increased. The relation of sequelae to severe malaria was assessed taking into account any pre-existing comorbidity. A separate endpoint review committee, comprising one paediatrician with malaria experience and one clinical malariologist, identified cases in which a pathological change other than malaria or its acute complications was likely to be the main cause of death.
Statistical analysis
Inclusion of more than 5306 children with severe malaria was needed to show, with 80% power and 95% confidence, a 25% reduction in mortality from 8% to 6%. This calculation was based on an estimated severe malaria mortality of 16% with about half the enrolled patients anticipated to fulfil the criteria for severe malaria. Patient data and outcomes were provided to an independent data and safety monitoring committee, who reviewed the trial yearly.
For binary outcomes, the odds ratios (ORs) between treatment groups, stratified by study site, were estimated by the Mantel-Haenszel method. Heterogeneity between sites was examined with the Breslow-Day test. In Rwanda, the two small sites run by the same investigators were pooled. Time to event outcomes were compared with the log-rank test, and hazard ratios (HRs) were estimated by a Cox proportional hazard model, stratified by site. Statistical analyses were done with Stata (version 11.1).
This trial is registered, number ISRCTN50258054.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
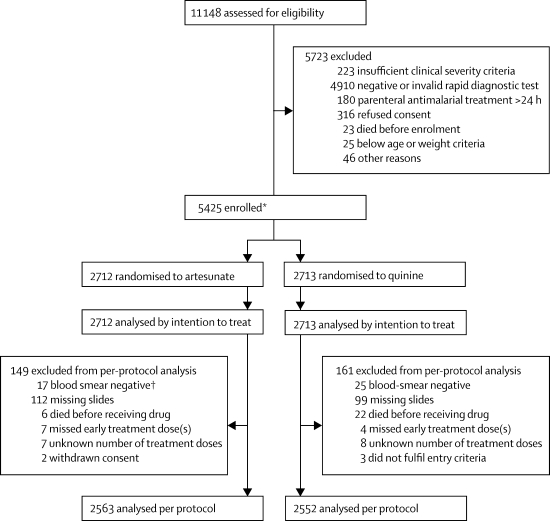
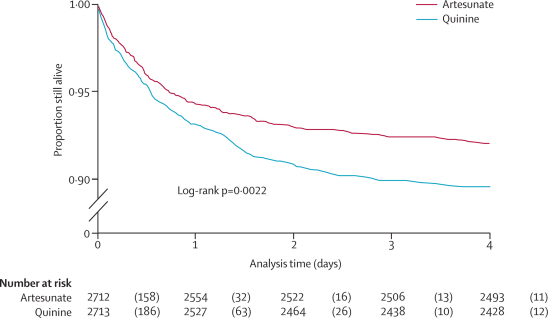
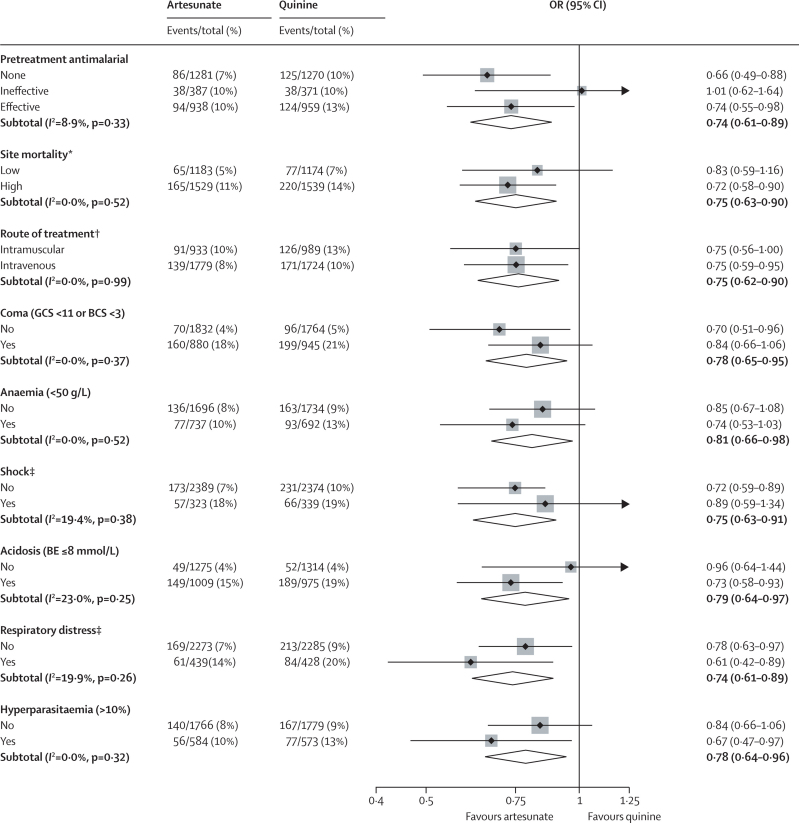
Figure 1 shows the trial profile. We recorded no major differences in baseline characteristics between the two treatment groups (table 1). 5425 patients were recruited (figure 1), of whom 527 (9·7%) died. 230 of 2712 (8·5%) patients given artesunate died versus 297 of 2713 (10·9%) given quinine (relative risk 0·78, 95% CI 0·66–0·91; OR 0·75, 0·63–0·90, in favour of artesunate; p=0·0022). This represents a relative reduction in mortality of 22·5% (95% CI 8·1–36·9%) and corresponds to an overall number needed to treat to prevent one death of 41 (95% CI 25–112). We recorded no heterogeneity between study sites (p=0·99). Eight patients (five artesunate, three quinine) were taken from hospital against advice and could not be followed up further. They were censored in the survival analysis at the time of discharge. The survival analysis (figure 2) for overall mortality during admission by antimalarial treatment gives the same result as the Mantel-Haenszel analysis (HR stratified by study site 0·76, 95% CI 0·64–0·91; p=0·0022). Mantel-Haenszel analysis of the predefined subgroups showed no evidence of any differences in odds ratios between subgroups (figure 3), and these results were confirmed by Cox regression (data not shown).
Figure 1.
Trial profile
*663 in Beira, 442 in Kilifi, 436 in Kumasi, 921 in Muheza, 540 in Korogwe, 502 in Banjul, 450 in Ilorin, 386 in Rwanda, 663 in Mbarara, and 422 in Kinshasa. †Two patients also had other criteria.
Table 1.
Baseline characteristics in the two treatment groups
| Quinine (N=2713) | Artesunate (N=2712) | |||
|---|---|---|---|---|
| Female sex | 1295 (48%) | 1315 (48%) | ||
| Age (years) | 2·9 (1·7–4·3) | 2·8 (1·6–4·2) | ||
| Fever before enrolment (days) | 3 (2–4) | 3 (2–4) | ||
| Coma before enrolment (h) | 5·0 (2·5–10) | 5·0 (2·0–9·5) | ||
| Pretreatment with antimalarials | ||||
| None | 1270 (47%) | 1281 (47%) | ||
| Ineffective* | 371 (14%) | 387 (15%) | ||
| Effective* | 959 (37%) | 938 (36%) | ||
| Complications on admission | ||||
| Coma† | 945 (35%) | 880 (32%) | ||
| Convulsions | 879 (32%) | 811 (30%) | ||
| Jaundice | 59 (2%) | 55 (2%) | ||
| Severe anaemia (haemoglobin <50 g/L) | 692 (29%) | 737 (30%) | ||
| Shock | 339 (12%) | 323 (12%) | ||
| Decompensated shock | 88 (35%) | 90 (39%) | ||
| Severe acidosis (BE <−8 mmol/L) | 975 (43%) | 1009 (44%) | ||
| Hypoglycaemia (<3 mmol/L) | 278 (10%) | 277 (10%) | ||
| Respiratory distress‡ | 428 (16%) | 439 (16%) | ||
| Severe prostration§ | 1668 (61%) | 1683 (62%) | ||
| Blackwater fever | 116 (4%) | 121 (4%) | ||
| Hyperparasitaemia (>10%) | 573 (24%) | 584 (25%) | ||
| Clinical examination | ||||
| Weight (kg) | 12·6 (4·6) | 12·4 (4·8) | ||
| Temperature (°C) | 38·0 (1·1) | 38·0 (1·1) | ||
| Blood pressure (mm Hg) | ||||
| Systolic | 95 (16) | 95 (16) | ||
| Diastolic | 56 (14) | 56 (14) | ||
| Coma depth (total N, median [range]) | ||||
| Blantyre coma score | 1704, 4 (2–5) | 1713, 4 (2–5) | ||
| Glasgow coma score | 1005, 11 (8–15) | 999, 11 (8–15) | ||
| Comorbidity | ||||
| Immune compromised (from history) | 49 (2%) | 45 (2%) | ||
| Severe malnutrition | 43 (2%) | 54 (2%) | ||
| Suspected pneumonia | 226 (8%) | 227 (8%) | ||
| Confirmed by radiograph | 29 (13%) | 29 (13%) | ||
| Clinical sepsis | 355 (13%) | 302 (11%) | ||
| Confirmed by culture | 33 (9%) | 32 (11%) | ||
| Suspected meningitis | 166 (6%) | 169 (6%) | ||
| Confirmed meningitis | 3 (2%) | 6 (4%) | ||
| Other significant comorbidities | 71 (3%) | 80 (3%) | ||
| Laboratory assessments | ||||
| Parasitaemia (parasites per μL; geometric mean, range) | 49 110 (0–1 858 880) | 47 922 (0–1 494 640) | ||
| Sodium (mmol/L) | 132 (6·5) | 131 (6·5) | ||
| Potassium (mmol/L) | 4·1 (0·9) | 4·1 (0·9) | ||
| Chloride (mmol/L) | 105 (10) | 105 (10) | ||
| Blood urea nitrogen (mmol/L) | 6·1 (4·9) | 6·1 (4·6) | ||
| Haemoglobin (g/L) | 70 (31) | 68 (29) | ||
| pH | 7·36 (0·14) | 7·36 (0·14) | ||
| PaCO2 (mm Hg) | 28·2 (10·1) | 27·9 (9·1) | ||
| HCO3 (mmol/L) | 16·6 (5·7) | 16·6 (5·6) | ||
| Plasma BE (mmol/L) | −8·6 (7·3) | −8·5 (7·3) | ||
| Anion gap (mmol/L) | 17·2 (5·0) | 17·0 (4·9) | ||
Data are number (%), median (IQR), or mean (SD), unless otherwise indicated. BE=base excess. PaCO2=partial pressure of carbon dioxide. HCO3=bicarbonate.
See webappendix p 12 for classification of categories.
Depth of coma was assessed either by Blantyre coma score (for preverbal children, n=3417) or Glasgow coma scale (n=2004).
Respiratory distress was defined as costal indrawing, use of accessory muscles, nasal alar flaring, deep breathing, or severe tachypnoea.
Severe prostration was defined as inability to breastfeed for children younger than 6 months or inability to sit for older children.
Figure 2.
Kaplan-Meier curves comparing survival in African children with severe falciparum malaria treated with either parenteral artesunate or quinine
The numbers in parentheses are the deaths during the indicated time. In eight patients the exact time of death during the night was missing and was estimated as 2359 h.
Figure 3.
Treatment effect in protocol-specified subgroups
The forest plot shows odds ratios and 95% CIs. The size of the squares is proportional to the size, and therefore weight, of the subgroup. The diamonds show the combined differences. The efficacy of antimalarial pretreatment was classified before study unblinding (webappendix p 12). Hyperparasitaemia means greater than 10% of red cells parasitised. OR=odds ratio. GCS=Glasgow coma scale. BCS=Blantyre coma scale. BE=base excess. *Site mortality classified as low if the site mortality rate was lower than the overall study mortality rate, and high if the site mortality rate was higher than the overall study mortality rate. †Classified according to centre policy (ten sites); classified according to individual data (one site). ‡Decompensated or compensated shock. I2 denotes the percentage of total variation across sites resulting from heterogeneity rather than chance, with the p value of significance.
The per-protocol analysis excluded patients who died rapidly before receiving antimalarial treatment (six artesunate, 22 quinine; p=0·0023), patients with incomplete initial antimalarial treatment with the study drug, and those with negative or missing blood slides for P falciparum (figure 1), but these omissions did not substantially affect the result (table 2). The endpoint review committee identified 16 children (seven artesunate, nine quinine) in whom death was unlikely to be related to severe malaria. Omission of these cases from the analysis also had no effect on the magnitude of the survival benefit with artesunate (table 2 and webappendix p 3). In 4618 children fulfilling strict criteria for severe malaria (panel 1), mortality was 9·9% (226/2280) with artesunate and 12·4% (291/2338) with quinine (OR 0·77, 95% CI 0·64–0·93; p=0·0055). Eight fatal cases (five quinine, three artesunate) did not have an accurate time of death recorded. In the remainder, 345 of 519 deaths occurred within the first 24 h after admission, of which 158 of 2709 (5·8%) were assigned to artesunate treatment and 187 of 2708 (6·9%) to quinine treatment (OR 0·84, 95% CI 0·67–1·04, p=0·109). Of the 174 of 519 deaths that occurred more than 24 h after admission, 69 of 2551 (2·7%) patients were treated with artesunate and 105 of 2521 (4·2%) with quinine (OR 0·63, 0·47–0·87, p=0·004). HIV serology was assessed routinely in Beira, Muheza, and Kilifi. Of 2095 patients tested, 125 (6%) were positive (64 artesunate, 61 quinine). Mortality in these patients was high (table 2).
Table 2.
Mortality and complications according to treatment group
| Quinine (n/N, %) | Artesunate (n/N, %) | OR (95% CI) | p value | |
|---|---|---|---|---|
| Mortality, ITT analysis | 297/2713 (10·9%) | 230/2712 (8·5%) | 0·75 (0·63–0·90) | 0·0022 |
| Mortality, per-protocol analysis | 260/2552 (10·2%) | 208/2563 (8·1%) | 0·78 (0·64–0·94) | 0·0099 |
| Death or sequelae at 28 days | 2316/2695 (11·7%) | 253/2689 (9·4%) | 0·78 (0·65–0·93) | 0·0056 |
| Malaria-attributable mortality* | 288/2704 (10·7%) | 223/2705 (8·2%) | 0·75 (0·63–0·91) | 0·0025 |
| Mortality in strictly defined severe malaria† | 291/2338 (12·4%) | 226/2280 (9·9%) | 0·77 (0·64–0·93) | 0·0055 |
| Case fatality in HIV-positive children‡ | 19/61 (31%) | 16/64 (25%) | 0·74 (0·33–1·62) | 0·45 |
| Development of coma§ | 91/1768 (5·1%) | 65/1832 (3·5%) | 0·69 (0·49–0·95) | 0·0231 |
| Deterioration of coma score | 208/2713 (7·7%) | 166/2712 (6·1%) | 0·78 (0·64–0·97) | 0·0245 |
| Convulsions developing or persisting >6 h after admission | 273/2713 (10·1%) | 224/2712 (8·3%) | 0·80 (0·66–0·97) | 0·0199 |
| Hypoglycaemia | 75/2713 (2·8%) | 48/2712 (1·8%) | 0·63 (0·43–0·91) | 0·0134 |
| Severe anaemia (<50 g/L) after admission§ | 98/1734 (5·7%) | 78/1696 (4·6%) | 0·81 (0·59–1·11) | 0·18 |
| Blackwater fever§ | 18/2597 (0·7%) | 30/2591 (1·2%) | 1·69 (0·94–3·05) | 0·076 |
ITT=intention to treat.
The likelihood that malaria contributed to or directly caused the death was assessed by an independent endpoint review committee blinded to the treatment allocation.
As defined in panel 1.
HIV status was assessed only in Beira, Muheza, and Kilifi (n=2095).
Development of coma, anaemia, and blackwater fever was assessed only in patients without these disorders on admission.
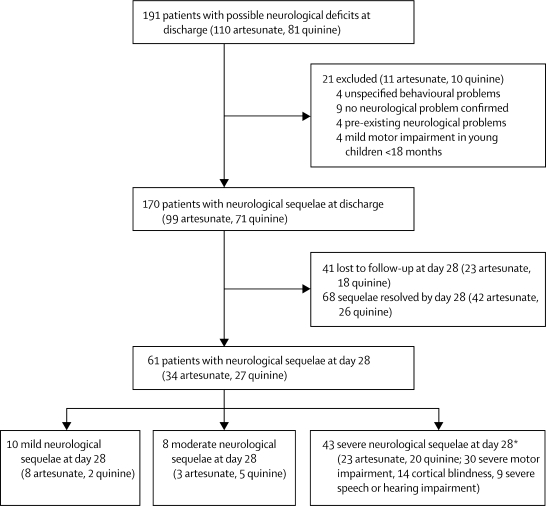
497 children (224 artesunate, 273 quinine) had convulsions that either developed after admission, irrespective of duration, or were present on admission and persisted for more than 6 h, and 156 patients (65 artesunate, 91 quinine) developed coma or had a deterioration of their coma score after starting antimalarial treatment (table 2). The development of coma, deterioration in coma score, and convulsions all occurred more frequently in patients who received quinine than in those who received artesunate (table 2). In the 4898 survivors, 170 (99 artesunate, 71 quinine) had not yet made full neurological recoveries at discharge (figure 4). Of these patients, 129 (76 artesunate, 53 quinine) were followed up between 3 and 8 weeks after enrolment. At this first follow-up assessment 68 (53%) had recovered fully, 18 (14%; 11 artesunate, seven quinine) were mildly or moderately impaired, and 43 (33%; 20 quinine, 23 artesunate) had severe neurological deficits. The overall incidence of any persistent neurological sequelae in assessed survivors at 28 days after cerebral malaria was 3·2% (24/706 artesunate, 23/737 quinine) and of severe neurological sequelae was 2·3% (17/706 artesunate, 17/737 quinine). We recorded no difference between the treatment groups. Of the 14 patients with any neurological sequelae who did not have cerebral malaria initially (ten artesunate, four quinine), seven had multiple convulsions (three quinine, four artesunate) and all had severe prostration on admission.
Figure 4.
Neurological sequelae at discharge and after 28 days (range 3–8 weeks) in children with severe falciparum malaria
*Some patients had severe impairment in more than one domain.
We detected no severe adverse effects that could be attributed directly to drug toxicity. Although one patient treated with artesunate developed a mild urticarial rash, no severe type 1 hypersensitivity reactions were recorded. Another patient treated with artesunate developed peripheral gangrene of toes and fingers, which was attributed to the disease and not the drug. One patient given quinine developed severe stridor after administration of ampicillin, and died. This death was attributed to ampicillin rather than quinine. Hypoglycaemia after starting antimalarial treatment was significantly less frequent in patients who received artesunate than in those who received quinine (table 2). Blackwater fever was rare in both groups (table 2).
Blood transfusions were given to 1487/2712 (55%) patients assigned to artesunate and 1495/2713 (55%) assigned to quinine. A fluid bolus at the start of treatment was given to 589/2712 (22%) patients assigned to artesunate and 596/2713 (22%) assigned to quinine. 3259 (60%) children received antibiotics (1606 artesunate, 1653 quinine; p=0·20).
In cerebral malaria survivors, the time from randomisation until the child was able to localise a painful stimulus or was able to speak was slightly longer overall in patients treated with artesunate than in those given quinine when compared by survival analysis (table 3). The times to eat or to sit unsupported did not differ between treatment groups (table 3).
Table 3.
Recovery times in surviving patients according to treatment group
| Quinine (median, IQR) | N | Artesunate (median, IQR) | N | HR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Time to discharge (days) | 3·0 (2·0–5·0) | 2412 | 3·0 (2·0–5·0) | 2478 | 1·04 (0·99–1·10) | 0·059 |
| Time to eat (h) | 12 (2–24) | 2269 | 9 (0–24) | 2358 | 0·99 (0·93–1·06) | 0·74 |
| Time to sit unsupported (h) | 22 (6–44) | 2312 | 18 (6–42) | 2373 | 1·02 (0·95–1·08) | 0·60 |
| Time to localise pain (h)* | 12 (6–24) | 726 | 12 (6–24) | 698 | 0·87 (0·78–0·98) | 0·0093 |
| Time to speak (h)* | 18 (11–36) | 695 | 20 (8–42) | 664 | 0·88 (0·79–0·99) | 0·016 |
Time to localise pain and time to speak was assessed only for surviving patients with coma on admission (Blantyre coma scale <3 or Glasgow coma scale <11).
The content of artesunate in all ampoules tested was within the pharmaceutical content limits of 10% of the stated active ingredient. All tested vials of quinine contained between 105% and 112% of the stated content (webappendix p 7).
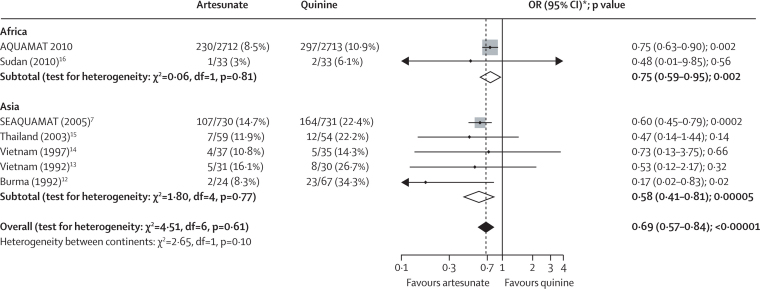
In a meta-analysis of all severe malaria trials that have compared survival after parenteral artesunate with that after parenteral quinine, the overall OR was 0·69 (0·57–0·84; p<0·00001) in favour of artesunate (figure 5). We noted no significant heterogeneity between the results generated in Africa and Asia (figure 5).
Figure 5.
Meta-analysis of all randomised controlled trials that have compared parenteral artesunate and parenteral quinine in severe malaria12–16
The solid vertical line represents equality of the two groups; the dashed line is the overall treatment difference. The horizontal lines and the width of the diamonds show the CIs for the odds ratios. The size of the squares is proportional to the size, and therefore weight, of the trial. OR=odds ratio. *99% CIs for totals.
Discussion
This large multicentre trial shows that artesunate substantially reduces the overall mortality of African children diagnosed with severe malaria. We recorded little heterogeneity between treatment centres in the benefit associated with artesunate, suggesting that the findings are robust. Before this trial, concerns had been raised that artesunate might not be better than quinine in African children, whereas it clearly was in Asian patients. The concerns arose because of perceived differences in pathology and the prevalence of more quinine-susceptible malaria parasites, and because in the earlier SEAQUAMAT trial undertaken in Asia,7 the survival curves did not separate clearly until 48 h after starting treatment, whereas most deaths in African children occurred before this time. Fortunately these concerns were not substantiated. More than 7000 severely ill patients have now been included in randomised comparisons of parenteral artesunate and quinine (figure 5). Compared with parenteral quinine, artesunate reduced the mortality of severe malaria in African children by 22·5%, and in Asian patients by 38·6%.7 These studies together comprise nearly 80% of all patients ever enrolled in randomised controlled trials of patients admitted with severe malaria, and they provide definitive evidence that artesunate is the most effective available treatment for severe malaria, and that it is safe (panel 2).
Panel 2. Research in context.
Systematic review
We searched Medline (from January, 1966, to September, 2010) and Embase (from January, 1980, to September, 2010) for randomised controlled trials. Search terms used were: “malaria”, “cerebral malaria”, “severe malaria”, “complicated malaria”, “malaria falciparum”, “quinine”, and “cinchona alkaloids” (MeSH/EMTREE terms); and “artemisinin” and “artesunate”. We searched for randomised controlled trials that compared parenteral artesunate with quinine for treatment of severe malaria. We identified six randomised controlled trials,7,12–16 which we have included in the meta-analysis presented in figure 5.
Interpretation
Together these trials provide substantial evidence of the life-saving benefit of artesunate compared with quinine in the treatment of severe falciparum malaria in all age groups worldwide.
This multicentre trial was open label because the substantial differences in the parenteral formulations of the two drugs prevented adequate concealment. Although individual trial site investigators knew the individual treatment allocations, all the other clinical and laboratory investigators were masked to the parenteral treatments given. The similarity of results across the different trial sites suggests that significant bias was unlikely. The great strength of this trial is its unprecedented size, its consistency both internally and with previous studies, and therefore the confidence that health-care workers throughout the tropical world can have in its result.
Artesunate prevented death from severe malaria, but not at the expense of an increase in neurological sequelae. Indeed, the overall incidence of confirmed persistent severe sequelae after severe malaria was low.17 Initially the assessment of neurological deficit was made at discharge from hospital, but many children, especially in the younger age group, had not made a full neurological recovery at discharge. Subsequently, assessments were made 4 weeks later, by which time about half the apparent deficits had resolved fully.
This life-saving benefit of artesunate compared with quinine in severe malaria has to derive from its greater intrinsic parasiticidal activity. The principal pharmacodynamic advantage of artesunate is that it has a much broader stage-specificity of action than does quinine.18 The artemisinins kill circulating ring-stage parasites before they can mature,19,20 which reduces sequestration of infected erythrocytes in the venules and capillaries of vital organs and thereby prevents potentially lethal microvascular obstruction.21–23 The large and consistent reduction in mortality associated with artesunate, and the consistent finding that mortality reduction is greatest in hyperparasitaemia,7 lends support to the central quantitative role of parasitised erythrocyte sequestration in the pathology of malaria.
The benefits of parenteral artesunate compared with quinine were greater than the benefits of intramuscular artemether reported in previous trials.6,7 In a double-blind trial24 comparing artesunate and artemether in 370 Vietnamese adults with severe falciparum malaria, 13 patients died in the artesunate group (7%) and 24 in the artemether group (13%). Taken together, these different study results suggest that the live-saving benefit compared with quinine provided by artesunate is roughly twice that provided by artemether. Artesunate and artemether have similar pharmacodynamic properties in vitro, so the most likely explanation for their different efficacies in vivo is the substantial difference in their pharmacokinetic properties. Parenteral artemether is an oil-based formulation given only by intramuscular injection. Absorption is slow and erratic, whereas the water-soluble hemisuccinate artesunate is absorbed rapidly and reliably after intramuscular injection and can be given intravenously.25–30 Thus the pharmacodynamic advantage of artemether over quinine might have been offset by its poor absorption kinetics. In summary, in the treatment of severe falciparum malaria, it seems that artesunate is better than artemether, which in turn is better than quinine.
The benefits of artesunate (and artemether) compared with quinine were greater in patients from southeast Asia than in African children,6,7 although the studies were not large enough to show this difference reliably. There are several possible explanations for this finding. African children usually have some background immunity that assists the therapeutic response, and in particular accelerates circulating parasite clearance.31 This effect hastens recovery and could reduce the therapeutic advantage of artesunate. The greater quinine susceptibility of P falciparum in Africa is often proposed as contributing to improved therapeutic responses, but the differences are not large, and are unlikely to have a major effect in vivo. Incorrect diagnosis is likely to be a major contributory factor; severe malaria is overdiagnosed in African children, and sepsis is underdiagnosed.32 Febrile sick children with positive malaria blood smears are usually diagnosed initially as having severe malaria, but the specificity of a positive blood smear is poor in settings in which a high proportion of all children are parasitaemic. Septicaemia and pneumonia are especially difficult to differentiate clinically from severe malaria. Sepsis and severe malaria also commonly coexist in African children.32 Prompt and appropriate treatment of sepsis should further reduce the mortality of severely ill children with malaria parasitaemia.
Parenteral artesunate is simple to administer, is safe, and reduces mortality substantially compared with quinine. No serious adverse effects were identified in this large study or in previous large studies.7,24,33 By contrast, intramuscular quinine is locally toxic4 (because of its acidity), and intravenous quinine needs a carefully rate-controlled infusion and continuous or three times daily administration to avoid dangerous hypotension.9 Importantly, quinine is associated with potentially serious hypoglycaemia.1,2,5 In this trial, 22 children died before receiving quinine compared with only six who died before receiving artesunate. This finding is probably indicative of the difficulties in administering parenteral quinine promptly and safely. Any delay in treating severe infection will increase mortality.33 The ease and safety of parenteral artesunate are important practical advantages. Artesunate is more expensive to buy, but quinine is more expensive to administer. A major factor restricting the deployment of artesunate has been unavailability of a product satisfying international good manufacturing standards. The most widely used product, assessed in this study, does not yet have this certification, which has prevented deployment in some countries.34–39 This barrier must be overcome speedily so that parenteral artesunate can be deployed in malaria-endemic areas to save lives.
Artesunate should now become the treatment of choice for severe malaria for children and adults worldwide. Malaria causes an estimated 800 000 deaths every year in African children.40 Severe malaria is often the most common admission diagnosis in febrile children, so a change in treatment policy from quinine to artesunate has the potential to save thousands of children's lives every year. If 4 million African children with severe malaria every year were to receive prompt treatment with parenteral artesunate instead of quinine, and the benefits were similar to those recorded in this trial, then approximately 100 000 lives might be saved per year.
Acknowledgments
Acknowledgments
TEP is funded by the National Institute for Health Research Oxford Biomedical Research Centre. This trial was supported by grants 076908 and 082541 from the Wellcome Trust, and was coordinated as part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain. We thank all the patients and their families, the directors and staff of the trial hospitals and the Bangkok coordinating centre, and the many doctors, research nurses, and research assistants for their help and support; the ministries of health and institutional authorities for their support; Loice Magaria and Ghiorghis Belai for their assistance and support; Novartis for providing artemether-lumefantrine for several of the study sites; Marja Schilstra for help with database construction; Epicentre (Philippe Guerin and Elizabeth Ashley) for assistance in setting up the study in Uganda; and Richard Peto for advice.
Contributors
The coordinating committee designed the study. All investigators in the trial sites undertook the trial, with support from the team in Bangkok. All investigators and the coordinating committee reviewed and discussed the trial results. The writing committee did the data analysis and prepared the report.
The AQUAMAT trial group
Writing committee: Arjen M Dondorp, Caterina I Fanello, Ilse C E Hendriksen, Tim E Peto, Lorenz von Seidlein, Nicholas P J Day, Nicholas J White.
Clinical trialists: Hospital Central da Beira, Beira, Mozambique Ermelinda Gomes (principal investigator [PI]), Amir Seni, Kajal D Chhaganlal, Ilse C E Hendriksen, Alínia José Pedro, César D R Jarach, Olivier Wingi, Ester I Fernando, Mara Zambruni, Zacarias Raimundo, Carlos J de Oliveira, Arjen Dondorp, Francisco Songane, Avertino Barreto, Marcelino Lucas; MRC laboratories and Royal Victoria Teaching Hospital, Banjul, The Gambia Kalifa Bojang PI, Rasaq Olaosebikan, Nkechinyere Anunobi, Tumani Corrah, David Conway, Sarah Rowland-Jones, Francis Akor, Joseph Okebe, Muminatou Jallow, Osaretin Chimah, Abubakar Ismaela, Mamkumba Sanneh, Saidykhan Mamodou, Fatou Jammeh, Janet Fullah, Abdou Bah, Horija Saine; Komfo Anokye Hospital, Kumasi, Ghana Tsiri Agbenyega PI, Samuel Blay Nguah, Jennifer Evans, Daniel Ansong, Justice Sylverken, Alex O Akoto, Larko D Owusu, David Sambian, Michael Ntim; Kilifi District General Hospital, Kilifi, Kenya Kathryn Maitland PI (and Wellcome Trust Centre for Clinical Tropical Medicine, Faculty of Medicine, Imperial College, London, UK), Esther Kivaya, Mohammed Abubakar, Ayub Mpoya, Charles Newton (and Neurosciences Unit, The Wolfson Centre, Institute of Child Health, University College of London, UK), Samson Gwer, Samuel Akech, Michael Kazungu, Michael Kihara, Molline Timbwa, Mwanamvua Boga; Magunga District Hospital, NIMR-Korogwe Research Laboratory, Tanga, Tanzania Samwel Gesase PI, Catherine Kahabuka, Martha Lemnge, Lorenz von Seidlein, Aileen Barongo, Anangisye Malabeja, Omary Abdul, Zacharia Savael, Gustav Tilaus, Laura Chuwa, Stella Kabela; Teule Designated District Hospital, Muheza, Tanzania Behzad Nadjm, Jacqueline Deen, Hugh Reyburn, Christopher Whitty, Ilse C E Hendriksen, Amani Shao, Selemani Mtunguja, Marwa Mwikwabe, Walli Msuya, Christina Kiemi, Edward Mtili, Lorenz von Seidlein, Emmanuel Swai, Ben Amos, Rajabu Malahiyo, Regina Malugu, Michael Mnongea, Stella Emmanuel, Nzitu J Msengi, Faraja M Mtimbo, Juma Kimera; NIMR-Amani Centre, Tanga, Tanzania George Mtove; Rwamagana Hospital and Nyanza Hospital, Rwanda and Malaria Control Program MoH Kigali Corine Karema PI, Noella Umulisa, Aline Uwimana, Josephine Ngalula Mulumba, Aimee Uwase, Devotha Rugandura, Claude Kawite, Deogratias Ruhangaza, Jean Claude Ndagijimana, Josephine Mukeshiyaremye, Antoinette Kayitesi, Violette Mukamugenzi, Ntwali Ndizeye, Tharcisse Munyaneza; University of Ilorin Teaching Hospital, Ilorin, Nigeria Olugbenga A Mokuolu PI, Olanrewaju T Adedoyin, Wahab B R Johnson, Ayodele Ojuawo, Samuel K Ernest, Omotayo O Adesiyun, Aishatu A Abdulkarim, Abdulrasheed O Adegboye, Ashau O Timi-Oladipo, Bukola R Kelani, Oyekunle Ogunkanbi, Abdulrahreem Yusuf, Oluyemisi Adegboye, Olabode T Oyewale, Olusegun Oladele, Alice Jacob, Oluseun Faniyi; Epicentre Research Base Mbarara and Mbarara University Teaching Hospital, Mbarara, Uganda Juliet Mwanga-Amumpaire PI, Margaret Nansumba, Patrice Piola, Pierre Debeaudrap, Yap Boum II, Mehul Dhorda, Eleanor Turyakira, Elias Kumbakumba, Eunice Nyesigire, Peters Kalubi, Santorino Data, Naome Natukunda, Antoney Ssegane, Nyehangane Dan, Benon Tumwebaze, Benson Twinomujuni, James Kinyatta, Suzan Esther Asaasira, Jacqueline Tumuhairwe, Sanyu Scovia, Primativa Kyohairwe, Shiela Mbabazi, Harriet Adrama, Thaddeus Turuho, Sulaiman Muwanga, John Bosco Ariyo, Peace Magezi, Agatha Twebaze; Kinshasa School of Public Health, Kingasani Research Centre, Kinshasa, Democratic Republic of the Congo Antoinette K Tshefu PI, Marie A Onyamboko, Jacques K Zandibeni, Elyse K Katokale, Charlie N Kabedi, Adolphine B Nkuadiolandu, Raoul N Mpoyi, Marcelline M Sakina, Crispin K Fela, Jacqueline A Omari, Odile K Muniaka, Betty D Omba, Antoinette I Boyanga, Elisee K Biyela, Benjamin B Badjanga, Yvonne B Kambashi.
Clinical, laboratory, statistical and logistic support: Mahidol Oxford Tropical Medicine Research Unit (MORU), Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand Arjen M Dondorp, Tharisara Sakulthaew, Caterina I Fanello, Wirichada Pan Ngum, Kasia Stepniewska, Kamolrat Silamut, Ilse C E Hendriksen, Nuttapol Panachuenwongsakul, Montri Rijaibun, Niklas Lindegardh, Warunee Hanpithakpong, Forradee Nuchsongsin, Benjamas Intarabut, Ketsanee Srinamon, Somporn Saiwaew, Kesinee Chotivanich, Nicholas J White, Nicholas P J Day.
Coordinating committee: Caterina I Fanello (study coordinator), Arjen M Dondorp, Ilse C E Hendriksen, Nicholas P J Day, Lorenz von Seidlein, Nicholas J White (chair).
Trial steering committee: Kalifa Bojang, Kathryn Maitland, Arjen M Dondorp, Nicholas P J Day, Nicholas J White, Brian Greenwood (chair).
Neurological outcome review committee: Martina Oneko, Bridget Wills, Nicholas White.
Endpoint review committee: Charles J Woodrow, Delia Bethell.
Trial statistician: Tim E Peto.
Data and Safety Monitoring Committee: David Lalloo (chair), Sarah Walker, Kojo Koram, Malcolm Molyneux, Grace Malenga.
Conflicts of interest
NJW is co-chairman and OAM is a member of the WHO antimalarial treatment guidelines committee. JD was Temporary Adviser for WHO Model List of Essential Medicines for Children in 2007–08. All other authors declare that they have no conflicts of interest.
Web Extra Material
References
- 1.WHO Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(suppl 2):1–65. [PubMed] [Google Scholar]
- 2.WHO Severe and complicated malaria. Trans R Soc Trop Med Hyg. 2000;94(suppl 1):1–90. [Google Scholar]
- 3.White NJ. The treatment of malaria. N Engl J Med. 1996;335:800–806. doi: 10.1056/NEJM199609123351107. [DOI] [PubMed] [Google Scholar]
- 4.Yen LM, Dao LM, Day NP. Role of quinine in the high mortality of intramuscular injection tetanus. Lancet. 1994;344:786–877. doi: 10.1016/s0140-6736(94)92342-6. [DOI] [PubMed] [Google Scholar]
- 5.White NJ, Warrell DA, Chanthavanich P. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–66. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]
- 6.Artemether-Quinine Meta-analysis Study Group A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:637–650. doi: 10.1016/s0035-9203(01)90104-x. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp A, Nosten F, Stepniewska K, Day N, White NJ, for the South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–725. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 8.Lubell Y, Yeung S, Dondorp AM. Cost-effectiveness of artesunate for the treatment of severe malaria. Trop Med Int Health. 2009;14:332–337. doi: 10.1111/j.1365-3156.2009.02227.x. [DOI] [PubMed] [Google Scholar]
- 9.WHO . The treatment of malaria. 2nd edn. World Health Organization; Geneva: 2010. [Google Scholar]
- 10.Lee SJ, Stepniewska K, Anstey N. The relationship between the haemoglobin concentration and the haematocrit in Plasmodium falciparum malaria. Malar J. 2008;7:149. doi: 10.1186/1475-2875-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hien TT, Day NPJ, Phu NH. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 12.Win K, Than M, Thwe Y. Comparison of combinations of parenteral artemisinin derivatives plus oral mefloquine with intravenous quinine plus oral tetracycline for treating cerebral malaria. Bull World Health Organ. 1992;70:777–782. [PMC free article] [PubMed] [Google Scholar]
- 13.Hien TT, Arnold K, Vinh H. Comparison of artemisinin suppositories with intravenous artesunate and intravenous quinine in the treatment of cerebral malaria. Trans R Soc Trop Med Hyg. 1992;86:582–583. doi: 10.1016/0035-9203(92)90137-2. [DOI] [PubMed] [Google Scholar]
- 14.Cao XT, Bethell DB, Pham TP. Comparison of artemisinin suppositories, intramuscular artesunate and intravenous quinine for the treatment of severe childhood malaria. Trans R Soc Trop Med Hyg. 1997;91:335–342. doi: 10.1016/s0035-9203(97)90099-7. [DOI] [PubMed] [Google Scholar]
- 15.Newton P, Angus BJ, Chierakul W. A randomised comparison of intravenous artesunate or quinine in the treatment of severe falciparum malaria. Clin Infect Dis. 2003;37:7–16. doi: 10.1086/375059. [DOI] [PubMed] [Google Scholar]
- 16.Eltahir HG, Omer AA, Mohamed AA, Adam I. Comparison of artesunate and quinine in the treatment of Sudanese children with severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2010;104:684–686. doi: 10.1016/j.trstmh.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–840. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 18.ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in-vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76:86–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 19.Gravenor MB, van Hensbroek MB, Kwiatkowski D. Estimating sequestered parasite population dynamics in cerebral malaria. Proc Natl Acad Sci USA. 1998;95:7620–7624. doi: 10.1073/pnas.95.13.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy S, Watkins WM, Bray PG. Parasite viability during treatment of severe falciparum malaria: differential effects of artemether and quinine. Am J Trop Med Hyg. 1995;53:303–305. doi: 10.4269/ajtmh.1995.53.303. [DOI] [PubMed] [Google Scholar]
- 21.Chotivanich K, Udomsangpetch R, McGready R. The central role of the spleen in malaria parasite clearance. J Infect Dis. 2002;185:1538–1541. doi: 10.1086/340213. [DOI] [PubMed] [Google Scholar]
- 22.Udomsangpetch R, Pipitaporn B, Krishna S. Antimalarial drugs reduce cytoadherence and rosetting of Plasmodium falciparum. J Infect Dis. 1996;173:691–698. doi: 10.1093/infdis/173.3.691. [DOI] [PubMed] [Google Scholar]
- 23.Dondorp AM, Ince C, Charunwatthana P. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 24.Phu NH, Tuan PQ, Day NPJ. Randomized controlled trial of artesunate or artemether in Vietnamese adults with severe falciparum malaria. Malar J. 2010;9:e97. doi: 10.1186/1475-2875-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy SA, Mberu E, Muhia D. The disposition of intramuscular artemether in children with cerebral malaria; a preliminary study. Trans R Soc Trop Med Hyg. 1997;91:331–334. doi: 10.1016/s0035-9203(97)90097-3. [DOI] [PubMed] [Google Scholar]
- 26.Silamut K, Newton PN, Teja-Isavadharm P. Artemether bioavailability after oral or intramuscular administration in uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2003;47:3795–3798. doi: 10.1128/AAC.47.12.3795-3798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mithwani S, Aarons L, Kokwaro GO. Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with severe falciparum malaria. Br J Clin Pharmacol. 2004;57:146–152. doi: 10.1046/j.1365-2125.2003.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hien TT, Davis TM, Chuong LV. Comparative pharmacokinetics of intramuscular artesunate and artemether in patients with severe falciparum malaria. Antimicrob Agents Chemother. 2004;48:4234–4249. doi: 10.1128/AAC.48.11.4234-4239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilett KF, Batty KT, Powell SM. The pharmacokinetic properties of intramuscular artesunate and rectal dihydroartemisinin in uncomplicated falciparum malaria. Br J Clin Pharmacol. 2002;53:23–30. doi: 10.1046/j.0306-5251.2001.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nealon C, Dzeing A, Muller-Romer U. Intramuscular bioavailability and clinical efficacy of artesunate in Gabonese children with severe malaria. Antimicrob Agents Chemother. 2002;6:933–999. doi: 10.1128/AAC.46.12.3933-3939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepniewska K, Ashley E, Lee SJ. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2010;201:570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gwer S, Newton CR, Berkley JA. Over-diagnosis and co-morbidity of severe malaria in African children: a guide for clinicians. Am J Trop Med Hyg. 2007;77(suppl):6–13. [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes MF, Faiz MA, Gyapong JO, for the Study 13 Research Group Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial. Lancet. 2009;373:557–566. doi: 10.1016/S0140-6736(08)61734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartoloni A, Tomasoni L, Bartalesi F. Combined intravenous treatment with artesunate and quinine for severe malaria in Italy. Am J Trop Med Hyg. 2010;83:274–276. doi: 10.4269/ajtmh.2010.10-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal PJ. Artesunate for the treatment of severe falciparum malaria. N Engl J Med. 2008;358:1829–1836. doi: 10.1056/NEJMct0709050. [DOI] [PubMed] [Google Scholar]
- 36.White NJ, Day NP, Dondorp A, Anstey N. UK recommendations for severe malaria are worrying. BMJ. 2007;334:490. doi: 10.1136/bmj.39143.014005.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalloo DG, Shingadia D, Pasvol G, for the HPA Advisory Committee on Malaria Prevention in UK Travellers UK malaria treatment guidelines. J Infect. 2007;54:111–121. doi: 10.1016/j.jinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Jelinek T. Intravenous artesunate recommended for patients with severe malaria: position statement from TropNetEurop. Euro Surveill. 2005;10:2841. doi: 10.2807/esw.10.47.02841-en. [DOI] [PubMed] [Google Scholar]
- 39.Anstey NM, Price RN, White NJ. Improving the availability of artesunate for treatment of severe malaria. Med J Aust. 2006;184:3–4. doi: 10.5694/j.1326-5377.2006.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 40.Black RE, Cousens S, Johnson HL. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.