Abstract
Most hosts, including humans, are simultaneously or sequentially infected with several parasites. A key question is whether patterns of coinfection arise because infection by one parasite species affects susceptibility to others or because of inherent differences between hosts. We used time-series data from individual hosts in natural populations to analyze patterns of infection risk for a microparasite community, detecting large positive and negative effects of other infections. Patterns remain once variations in host susceptibility and exposure are accounted for. Indeed, effects are typically of greater magnitude, and explain more variation in infection risk, than the effects associated with host and environmental factors more commonly considered in disease studies. We highlight the danger of mistaken inference when considering parasite species in isolation rather than parasite communities.
Macroparasites (helminths and arthropods) and microparasites (viruses, bacteria, and protozoa) are integral components of the ecological communities that include their hosts (1), and it is likely that most hosts, most of the time, are infected with more than one parasite species (2). Interactions between parasites in natural populations, however, have been studied only rarely. A community ecology perspective is particularly relevant for studies of coinfection, as parasites may interact directly by competing for resources or indirectly via the host immune system (3). Interactions may be antagonistic to at least one of the parasites, either as a result of resource shortage or where there are cross-effective immune responses, or they may be beneficial to one or both parasites, as a result of parasite-induced immunosuppression or down-regulation of all or part of the immune system (1).
Experiments in laboratory model systems have demonstrated effects of coinfection on host susceptibility, infection length, and intensity and clinical symptoms (3–5), whereas studies in wild-life populations and humans have established firmly that positive and negative associations can occur between parasites (6–8). Such associations may reflect interactions between parasites. Indeed, interactions between parasites can have substantial effects. For example, human immunodeficiency virus infections are thought to be driving resurgent tuberculosis epidemics in Africa (9). Hence, single parasite studies may yield incorrect or incomplete conclusions. Nonetheless, most epidemiological studies, in animals and humans, still focus on single species. Studies that do consider multiple infections are typically cross-sectional, with each host providing infection data at one time point. The time of initial infection is unknown in such studies. There is, therefore, limited scope for determining whether patterns reflect inherent differences between hosts in either susceptibility or exposure to infection, rather than interactions (10), or for exploring the impact of infection sequence (11). Consequently, in natural populations, the relative importance of interspecific interactions, compared with other factors, in determining the dynamics and structure of parasite communities remains underexplored. Studies that monitor infection status through time are required.
Here, we report an analysis of infection risk using unprecedented time-series data on individual infection histories from four replicate natural populations of field voles, Microtus agrestis, comprising 14,075 captures of 5981 individual voles. Populations were sampled every four weeks (May to November 2001; March to November 2002 to 2006), with animals tagged individually and blood samples taken to allow infection status to be monitored over time (12). We investigated individual infection risks for a community of microparasites consisting of cowpox virus, Babesia microti, Bartonella spp. and Anaplasma phagocytophilum (see Table 1 for details of their biology). Infection risk will depend on both the probability of encountering an infectious dose and the probability of infection given exposure (host susceptibility). We aimed to determine whether susceptibility to infection by a microparasite was influenced by other microparasites. Therefore, for each microparasite, we investigated whether the other microparasites influenced the probability that a susceptible animal became infected at a given time point (t0), adding infection status for these other microparasites as explanatory variables to baseline statistical models that accounted for environmental and individual variables (e.g., sex and season) (12) (table S1). Thus, we guard against detecting spurious associations, which, in reality, reflect correlated exposure risk (e.g., a positive association simply because both parasites are most prevalent in late summer). For parasites causing self-limiting infections, infection status at both t0 and/or the previous month (t−1) were considered as explanatory variables, whereas for B. microti infections, which are chronic, a three-level covariate was used: uninfected, newly infected (infected at t0 but not before), and chronically infected (first infected prior to t0).
Table 1.
Summary information on the microparasite community, based on best available knowledge for infections in rodents.
| Microparasite | Type | Mode of transmission |
Infection length |
Site of infection | Clinical signs/effects on fitness |
|---|---|---|---|---|---|
| Cowpox virus (CPXV) (19–21) |
Virus | Direct | Self-limiting (4 weeks) |
Respiratory tract and lymphoid tissues, mainly macrophages and monocytes |
No obvious clinical signs but reductions in survival and fecundity |
|
Babesia microti (22) |
Protozoan | Ticks | Chronic (lifelong) |
Erythrocytes, then sequestered in spleen and liver, periodic release into wider circulation |
Hemolytic anemia but usually subclinical |
|
Anaplasma phagocytophilum (23) |
Bacterium | Ticks | Self-limiting (4 to 8 weeks) |
Granulocytes, primarily neutrophils |
Transient cytopenias |
|
Bartonella spp. (24, 25) |
Bacterium | Fleas | Self-limiting (4 to 8 weeks) |
Vascular endothelium, followed by release and invasion of erythrocytes |
None described |
We found that this community of parasites represents not four independent infections but an interconnected web of interactions: Effects of other infections on infection risk were both strong and widespread, and connectance within the parasite community was exceptionally high, with evidence detected for all possible pair-wise interactions (Fig. 1 and table S2). Both positive and negative associations were detected, and their magnitude was frequently considerable: increases up to a factor of 5.5 in risk and reductions in the odds of becoming infected on the order of 15% compared with uninfected individuals (Figs. 1 and 2). Indeed, perhaps most strikingly, in all cases, except for cowpox, infection with other parasite species explained more variation in infection risk than factors related to exposure risk and host condition, such as age and season (see relative changes in Akaike Criterion Information index (AIC) (13) in table S3). Moreover, the sizes of the effects of other parasites on infection risk were also similar to, and frequently greater than, other factors. For example, of all the noninfection variables, season generally had the largest effect on infection risk (table S3 and fig. S1), with seasonal increases in infection probability ranging from ~3 times as high (A. phagocytophilum) to 15 times as high (B. microti), but these were broadly matched by the magnitude of infection effects (Figs. 1 and 2). Effect sizes for noninfection variables are shown in fig.S1.These results are not explicable by simple co-occurrence of infections in hosts in poor condition, because for a subset of the data we explicitly account for variations in individual host condition indices at the time of infection (body condition and haemotological condition (12), and there was no evidence of any reduction in the strength of between-parasite interactions (table S4). Thus, the most likely explanation for these effects is that interactions between these microparasites within individual hosts have a large impact on host susceptibility.
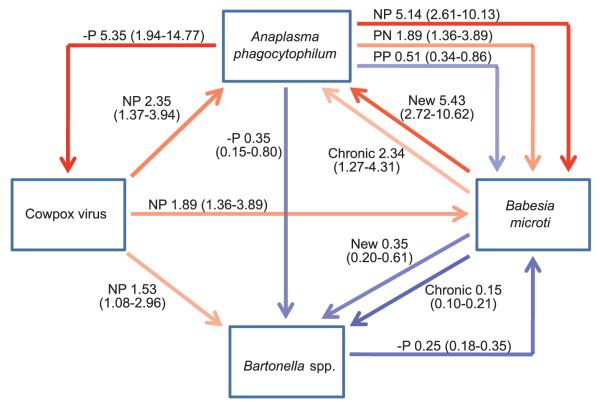
Fig. 1.
The web of interactions between microparasite species within this community, showing the magnitude of effects. All associations shown obtained overwhelming support (accumulative weight in models >0.9) (see table S3). Positive associations are shown in red [odds ratio (OR) >1] and negative associations (OR < 1) in blue, with the strength of the line color reflecting the magnitude of the effect. ORs (exp β) (see table S3) relative to uninfected individuals, with 95% confidence intervals in brackets. Thus an individual with a new B. microti infection is ~5 times as likely to become infected with A. phagocytophilum than an uninfected individual (OR = 5.43). Infection history associated with each effect is also indicated: N, negative, P, positive. Thus, NP indicates no infection at t−1, infection at t0. −P is used to signify that NP and PP show similar effects. Because B. microti induces a chronic infection, there are three infection status histories (uninfected, chronic, and new infections). For cowpox virus, a probability of infection was used in analyses, and results are for individuals that sero-converted at t0 (i.e., NP infection history, probability of infection at t0 = 0.5) (12).
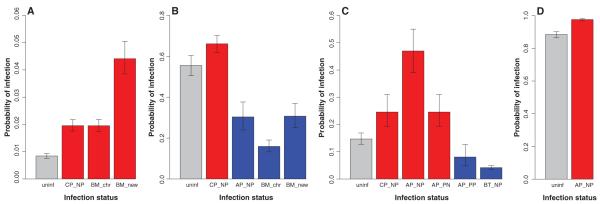
Fig. 2.
Predicted probabilities of acquiring an infection depending on infection status by other parasites for (A) A. phagocytophilum (AP), (B) Bartonella spp. (BT), (C) B. microti (BM), and (D) cowpox virus (CP). Predictions are based on the models in table S3. Gray bars represent individuals without other infections (uninf); red and blue bars highlight positive and negative effects on susceptibility, respectively. Each bar is labeled with the infection (e.g., CP) and the infection history (see Fig. 1 for explanations) associated with each effect. Error bars represent 95% confidence intervals, averaged over random effects. Predicted probabilities are defined with reference to an 18-g male in July at one specific site. In cases where NP and PP show similar effects, predicted probabilities are based on an NP infection history.
The effects of self-limiting infections were generally short-lived. Thus, although cowpox virus infection at t0 consistently increased susceptibility to other parasites (by a factor of ~2) (Fig. 1), there was little evidence of effects of cowpox infection at t−1, despite a correlation between infection status at t−1 and t0. Likewise, animals with ongoing A. phagocytophilum infections (infected at t−1 and t0) were less likely to become infected with B. microti, but risk was not reduced in animals that had recently cleared an infection (infected at t−1 but not t0) (Fig. 1). These observations highlight the dynamic nature of the immunological environment within hosts, and we speculate that susceptibility frequently returns to normal levels soon after clearance of the first infection.
Several infections increased susceptibility to other microparasite species. In such cases, release from effective control by the immune system is the most likely explanation, especially when supported by experimental studies. For example, laboratory studies have indicated the importance of immunomodulation for host exploitation by pox viruses (14), which may explain the positive effect of cowpox virus on susceptibility to other parasites. The same immune-mediated mechanisms might also account for our earlier demonstration that cowpox virus increases the length of Bartonella taylorii infections (10). Thus, mechanisms responsible for increasing susceptibility may also prolong infections in those that do succumb.
Strong decreases in susceptibility caused by other infections were also observed. The largest effect overall was reduced susceptibility to Bartonella spp. in individuals infected with B. microti and was especially apparent in chronically infected animals, where the odds of infection were 15% of those of uninfected animals (Figs. 1 and 2B). B. microti also decreases the length of Bartonella taylorii infections (10). Reciprocally, current Bartonella spp. infections were associated with reduced susceptibility to B. microti (Figs. 1 and 2C). Resource depletion may play a role here because both species target erythrocytes (Table 1). Reduced infection intensity of haemoparasites has been observed in mice coinfected with anemia-causing helminths (3). Alternatively, negative effects may reflect up-regulation of mediators of a cross-effective Th1 response and therefore could represent an example of immunologically driven ecological interference (7, 15).
Our data also highlight the contrasting effects that one parasite may have on susceptibility to other parasites. A. phagocytophilum infection at t0 increased susceptibility to cowpox by a factor of 5 (Figs. 1 and 2D) but substantially decreased susceptibility to Bartonella spp. (Figs. 1 and 2B). A. phagocytophilum infections are known to have immunomodulatory effects, provoking cytopenias and reducing numbers of both red and white blood cells (Table 1). Consequently, positive effects may be immune-mediated (down-regulation of immune response), whereas the reduced susceptibility to Bartonella spp. may also be immunemediated (cross-effective response) and/or due to resource depletion.
We found, further, that whereas new and recently cleared A. phagocytophilum infections increased susceptibility to B. microti, ongoing A. phagocytophilum infections, as previously noted, decreased susceptibility to B. microti (Figs. 1 and 2C). There was also evidence in this case of reciprocal positive effects: Chronic and new B. microti infections increased the risk of A. phagocytophilum infection factors of 2 and 5, respectively (Figs. 1 and 2A). Both of these microparasite species are tick transmitted, and the co-occurrence of tick-borne pathogens is common (16). Despite the inclusion of tick abundance in the models, the reciprocal positive interactions could be a case of unaccounted-for correlations in exposure risk such as coinfected ticks (17), whereas the negative effect of ongoing A. phagocytophilum infection could reflect an antagonistic interaction within hosts . For example, the suppression of Babesia divergens by simultaneous A. phagocytophilum infections has been observed in cattle (18).
Finally, our results emphasize that the standard practice of classifying individuals in natural populations as infected or uninfected by one parasite alone fails to recognize that much more may be implied by the categorization “infected.” For example, cowpox virus infection has been associated with major reductions in survival and fecundity (19, 20). However, in our results, 39% of those infected with cowpox virus were also infected with B. microti (n = 651), 65% of the remainder had Bartonella spp. infections (n = 396), and overall 79% were coinfected with at least one of the three microparasites considered. Clearly, even when substantial associations between a given infection and host fitness are detected in a wildlife host, attributing the effect to that parasite alone may be unjustified.
This study demonstrates that communities of microparasites may be structured by strong interactions between species, consistent with past cross-sectional studies that have highlighted associations between parasite species (7, 8). In particular, by using time-series data, we provide the first evidence for microparasites in natural populations that such interactions can be driven by effects on susceptibility and can have as much impact on infection risk as more commonly considered factors such as host age and season. Because field voles are also infected by macroparasites, as well as other microparasites, our study only considers a portion of possible interactions in the parasite community. For instance, macroparasites and microparasites apparently interact negatively in natural populations of African buffalo through accelerated mortality and immune-driven effects on susceptibility (15). Thus, parasite community interactions in voles may well be even richer than those we have measured. Such strong effects on individual host susceptibility are likely to directly influence population-level parasite dynamics and may have profound consequences for disease management programs. Specifically, where interactions are antagonistic, control that targets one parasite species may result in unexpected increases in a second parasite species. To predict and control parasites and disease in natural populations, we need to understand community interactions among parasites and not just single host-parasite interactions.
Supplementary Material
Acknowledgments
We thank the following for data collection in the field and laboratory and for discussion and comments: R. Anderson, M. Bennett, K. Bown, S. Gebert, C. Glover, A. Goodsall, G. Hutcheson, D. Jones, L. Lukomski, J. Rogers, A. Smith, G. Telford, and D. Tidhar. The work was supported by funding from the Natural Environment Research Council (GR3/13051) and The Wellcome Trust (075202/Z/04/Z; 070675/Z/03/Z). P.B. was supported by a Dorothy Hodgkin Studentship and is currently a career investigator of the Argentine Council for Science and Technology (CONICET). Metadata are available at the Knowledge Network for Biocomplexity (KNB) data repository (http://knb.ecoinformatics.org). After discussion of specific requirements, more detailed data will be made available on request from mbegon@liv.ac.uk or s.telfer@abdn.ac.uk.
Footnotes
Supporting Online Material www.sciencemag.org/cgi/content/full/330/6001/243/DC1
Materials and Methods
Fig. S1
Tables S1 to S5
References
References and Notes
- 1.Pedersen AB, Fenton A. Trends Ecol. Evol. 2007;22:133. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Cox FEG. Parasitology. 2001;122(suppl.):S23. doi: 10.1017/s003118200001698x. [DOI] [PubMed] [Google Scholar]
- 3.Graham AL. Proc. Natl. Acad. Sci. U.S.A. 2008;105:566. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez M, Terrazas LI, Márquez R, Bojalil R. Parasite Immunol. 1999;21:177. doi: 10.1046/j.1365-3024.1999.00218.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham AL, Lamb TJ, Read AF, Allen JE. J. Infect. Dis. 2005;191:410. doi: 10.1086/426871. [DOI] [PubMed] [Google Scholar]
- 6.Booth M. Trends Parasitol. 2006;22:359. doi: 10.1016/j.pt.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ. Nature. 2004;428:840. doi: 10.1038/nature02490. [DOI] [PubMed] [Google Scholar]
- 8.Behnke JM. Parasitology. 2008;135:751. doi: 10.1017/S0031182008000334. [DOI] [PubMed] [Google Scholar]
- 9.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Clin. Infect. Dis. 2006;42:1040. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 10.Telfer S, et al. Parasitology. 2008;135:767. doi: 10.1017/S0031182008000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson JA, Pleass RJ, Cable J, Bradley JE, Tinsley RC. Int. J. Parasitol. 2006;36:1341. doi: 10.1016/j.ijpara.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Materials and methods are available as supporting material on Science Online.
- 13.Akaike H. In: Petran BN, Csaki F, editors. International Symposium on Information Theory; Budapest: Akademiai Kiadi; 1973. pp. 267–281. [Google Scholar]
- 14.Seet BT, et al. Annu. Rev. Immunol. 2003;21:377. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 15.Jolles AE, Ezenwa VO, Etienne RS, Turner WC, Olff H. Ecology. 2008;89:2239. doi: 10.1890/07-0995.1. [DOI] [PubMed] [Google Scholar]
- 16.Belongia EA. Vector Borne Zoonotic Dis. 2002;2:265. doi: 10.1089/153036602321653851. [DOI] [PubMed] [Google Scholar]
- 17.Swanson SJ, Neitzel D, Reed KD, Belongia EA. Clin. Microbiol. Rev. 2006;19:708. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purnell RE, Young ER, Brocklesby DW, Hendry DJ. Vet. Rec. 1977;100:4. doi: 10.1136/vr.100.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Burthe S, et al. J. Anim. Ecol. 2008;77:110. doi: 10.1111/j.1365-2656.2007.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telfer S, et al. Oikos. 2005;109:317. [Google Scholar]
- 21.Chantrey J, et al. Epidemiol. Infect. 1999;122:455. doi: 10.1017/s0950268899002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin P. In: Arthropod-borne Infectious Diseases of the Dog and Cat. Shaw SE, Day MJ, editors. Manson Publishing; London: 2005. pp. 63–77. [Google Scholar]
- 23.Johns JL, Macnamara KC, Walker NJ, Winslow GM, Borjesson DL. Infect. Immun. 2009;77:4070. doi: 10.1128/IAI.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birtles RJ. In: Rickettsioses: From Genome to Proteome, Pathobiology, and Rickettsiae as an International Threat. Oteo JA, Hechemy KE, Raoult DA, Silverman DJ, Blanco JR, editors. vol. 1063. 2005. pp. 270–279. [Google Scholar]
- 25.Telfer S, et al. Parasitology. 2007;134:413. doi: 10.1017/S0031182006001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.