Abstract
Objective
More than half of pediatric deaths occur in the hospital inpatient setting. An analysis of pediatric deaths can potentially help with understanding why children die and provide opportunities to improve the care for this patient population. This study’s primary objective was to obtain a broad understanding of inpatient mortality across academic, children’s hospitals.
Patients and Methods
A non-concurrent cohort study of hospitalized children from 37 academic, children’s hospitals during calendar year 2005. The primary outcome measure was death. Associated patient level characteristics included age, gender, race, diagnostic grouping, and insurance status. Important epidemiologic measures included standardized mortality and standardized mortality ratios (SMRs).
Results
There were 427,615 patients discharged from participating institutions during the study period of which 4,529 (1.1%) died. Neonates had the highest mortality rate (4.03%; OR 8.66; p < .001) followed by those > 18 years (1.4%; OR 2.86; P < .001). The standardized mortality ranged from 0.46 (APR-DRG 663: Other Anemia and Disorders of Blood) to 30.0 (APR-DRG 383: Cellulitis and Other Bacterial Skin Infection). When deaths were compared by institution, there was considerable variability in both the number of children who died and the SMRs at those institutions.
Conclusions
Patient characteristics, like age, severity, and diagnosis, were all substantive factors associated with the death of children. Opportunities to improve the environment of care by reducing variability within and between hospitals may improve mortality for hospitalized children.
Keywords: Pediatrics, Mortality, Death, Standardized Mortality, Children
Introduction
According to the National Center for Health Statistics, children < 19 years of age represent 30% of the United States population and account for approximately 60,000 deaths annually.1 More than half of these deaths occur in the inpatient setting.2 Studies of pediatric inpatient deaths fall into three major categories: those investigating all-cause mortality 3–9, those investigating specific-cause mortality 3, and those investigating occurrences at the end of life.10,11
The Institute for Healthcare Improvement has helped to provide a focus on improving healthcare quality by providing methods for measuring, evaluating and reducing mortality rates for hospitalized patients.12, 13 These and similar approaches have helped to inform healthcare decisions in adult patients admitted to intensive care units (ICUs).14–16 These approaches may also provide opportunities to understand the causes of death in children and the variability of childhood death rates across institutions and, consequently, allow for benchmarking and targeted interventions to improve hospital care for this children. To date, no studies have investigated diagnosis specific death rates or the demographic characteristics associated with pediatric deaths as a measure of healthcare quality in a multi-institutional sample of hospitalized children. Furthermore, no study has investigated the differences in mortality by institution after controlling for diagnosis and severity.
This study, using a multi-institutional data source of academic, children’s hospitals, was designed with three objectives. First, to compare the demographics of children who died during hospitalization versus those who survived. Second, was to understand opportunities for identifying patients by diagnosis who died, but were not predicted to die. Third, was to understand mortality rates across similar institutions. When taken together, achieving these objectives would help to inform efforts at tertiary care, childrens hospitals to improve mortality for hospitalized children.
Methods
Database
Data for this retrospective cohort study were obtained from the Pediatric Health Information System (PHIS), a national administrative database containing resource utilization data from 37 freestanding, tertiary care children’s hospitals. They are located in 23 different states plus the District of Columbia.. These hospitals are affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS), a business alliance of children’s hospitals. Data quality and reliability are assured through a joint effort between CHCA and participating hospitals. Systematic monitoring occurs on an ongoing basis to ensure data quality. The processes for monitoring include bimonthly coding consensus meetings, coding consistency reviews, and quarterly data quality reports. For the purposes of external benchmarking, participating hospitals provide discharge data including patient demographics, diagnoses, and procedures. Billing data is also available that details all of the drugs, radiologic imaging studies, laboratory tests, and supplies charged to each patient. Data are de-identified prior to inclusion in the database.
Study Participants
Children ≤ 21 years of age were eligible for this study if their admission date to a participating hospital occurred during calendar year 2005.
Variables
Demographic Variables
Patient demographic variables included age, sex, participant-reported race, primary payer, and the priority of admission. Patients between the ages of 1–30 days at admission were classified as neonates, and those 31–365 days were classified as infants. A race indicator of “other” included all individuals who were not White, Black, Asian, or American Indian and individuals for whom race was not indicated. Government insurance included individuals with Medicare, Medicaid, Title V, or other Government insurance. Private insurance included individuals who were commercially insured, self-pay included uninsured individuals and individuals who paid directly for their services and a primary payer indication of “other” included no charge patients.
Clinical Variables
The PHIS database uses All Patient-Refined, Diagnosis-Related Groups (3M Health Information Systems; APR-DRG) and the International Classification of Diseases, 9th Revision, (ICD-9) to classify patients. For each hospitalization, the primary diagnosis, medical or surgical complication, nosocomial infections, the APR-DRG severity level, and the disposition were determined. Complications of medical care (medical, surgical, and nosocomial infections) were identified using an algorithm based upon diagnostic coding.17, 18 The APR-DRG severity variable represents a relative severity score (1=Minor, 2=Moderate, 3=Major, 4=Extreme) and risk of mortality score that is derived using a computerized algorithm by encoding age, diagnostic, and procedural codes using commercially available software.20
Ratio Computations
Mortality ratios are epidemiologic tools that allow comparisons across institutions or geographic regions. Generally, standardized mortality measures compare observed mortality to expected mortality within APR-DRG categories (observed mortality divided by expected mortality) where the expected mortality is a measure that is assigned by Thomson Healthcare to each discharge and results from a severity adjustment process accounting for diagnosis and patient specific attributes.18, 20, 21 The Standardized Mortality Ratio (SMR) is the ratio of observed-to-expected mortality for a given APR-DRG divided by the ratio of observed-to-expected mortality for all patients.18, 20, 21 We also computed the Hospital SMRs (HSMRs) as the observed-to-expected mortality ratio for each institution divided by the observed-to-expected mortality ratio for all institutions. The SMR can be used to compare providers or institutions over time on their performance; it can also be used to determine whether a particular provider or institution’s performance is above or below that of comparable institutions overall and within APR-DRG categories.18, 21–24 The SMR is now a standard for benchmarking ICU and hospital performance and quality.18
Analytic Sequence
Statistical analyses were performed using the SAS® (SAS Institute, Cary, NC) software package.25 First, at the patient level, the bi-variate analyses contrasted the demographic and care characteristics for patients who died to those who survived. The chi-square test was used for the comparison of nominal data. After investigating the demographic variables associated with death in the bi-variate analyses, a multivariable logistic regression model was performed to identify the characteristics independently associated with death.
Second, because diagnosis is a particularly important marker for both outcome and resource use in the pediatric inpatient setting, comparisons based upon the frequency of diagnosis and frequency of deaths in a particular diagnosis were performed to allow the calculation of standardized mortality, to prepare for the institution level analyses, and to improve generalizability. In all instances, a p value of 0.05 was used as the significance level. Finally, HSMRs calculated across APR-DRGs for each institution were compared across the participating hospitals. Regression analyses were performed at the hospital level to identify trends. The research protocol was approved by the Children’s National Medical Center Institutional Review Board.
Results
There were 427,615 patients discharged during the study period of which 4,529 (1.1%) died. Table 1 compares the demographic characteristics of patients who died versus those who survived. Important characteristics associated with death included patient age, gender, race, severity level, payer. Neonates had the highest unadjusted mortality rate (4.03%; odds ratio [OR] 8.66; p < .001) followed by those > 18 years (1.4%; OR 2.86; P < .001). Children ages 6–12 years had the lowest mortality rate during hospitalization (0.48%). Male patients died more than females (1.1% versus 1%; p = .02). There were no significant mortality differences among racial groups except for the category of “other” (1.95%; OR 2.05; p < .001). Patients hospitalized with a severity level of “minor” died less often than those with a severity level of “extreme” (0.07 vs. 27.98%; OR 540.97; p < .001) and a linear trend existed across severity levels from minor to extreme (Cochran-Armitage test; p < 0.001) (Table 1). Deaths in patients with commercial insurance were less frequent than deaths in patients with government insurance (OR 1.39; p < .001). Self-pay patients had the highest mortality rate of 1.4% (OR 1.60; p < .001).
Table 1.
Demographic and Clinical Characteristics of Patients who Died and Survived at 37 Academic, Children’s Hospitals.
| Groups |
Died (N=4529) |
Survived (N= 423086) | Odds Ratio | P-value | ||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | |||
| Age | ||||||
| 1–30 days | 1919 | 4.03 | 45655 | 95.97 | 8.66 | 0.01 |
| 31–365 days | 811 | 1.07 | 75200 | 98.93 | 2.22 | 0.01 |
| 1–5 years | 705 | 0.57 | 122038 | 99.43 | 1.18 | 0.01 |
| 6–12 years | 461 | 0.48 | 94947 | 99.52 | ||
| 13–18 years | 546 | 0.69 | 78971 | 99.31 | 1.42 | 0.01 |
| 19–21 years | 87 | 1.37 | 6273 | 98.63 | 2.86 | 0.01 |
| Sex | ||||||
| Male | 2587 | 1.10 | 232028 | 98.90 | 1.10 | 0.01 |
| Female | 1939 | 1.00 | 191050 | 99.00 | ||
| Race | ||||||
| White | 2626 | 0.96 | 270963 | 99.04 | ||
| Black | 902 | 0.96 | 92604 | 99.04 | 1.01 | 0.90 |
| Asian | 98 | 1.12 | 8634 | 98.88 | 1.17 | 0.13 |
| American Indian | 16 | 1.33 | 1187 | 98.67 | 1.39 | 0.19 |
| Other | 553 | 1.95 | 27867 | 98.05 | 2.05 | 0.01 |
| Severity Level | ||||||
| Minor | 250 | 0.07 | 348039 | 99.93 | ||
| Moderate | 503 | 0.98 | 50663 | 99.02 | 13.82 | 0.01 |
| Major | 1318 | 7.03 | 17429 | 92.97 | 105.28 | 0.01 |
| Extreme | 2417 | 27.98 | 6220 | 72.02 | 540.97 | 0.01 |
| Primary Payer | ||||||
| Private commercial | 1233 | 0.91 | 134902 | 99.09 | ||
| Government | 2444 | 1.25 | 192479 | 98.75 | 1.39 | 0.01 |
| Self Pay | 165 | 1.44 | 11266 | 98.56 | 1.60 | 0.01 |
| Other | 682 | 0.81 | 84036 | 99.19 | 0.89 | 0.01 |
| Nosocomial Infection | ||||||
| No | 2172 | 0.84 | 255803 | 99.16 | ||
| Yes | 2357 | 1.39 | 167283 | 98.61 | 1.66 | 0.01 |
| Medical Complication | ||||||
| No | 4479 | 1.05 | 421485 | 98.95 | ||
| Yes | 50 | 3.03 | 1601 | 96.97 | 2.94 | 0.01 |
| Surgical Complication | ||||||
| No | 3319 | 0.85 | 386910 | 99.15 | ||
| Yes | 1210 | 3.24 | 36176 | 96.76 | 3.90 | 0.01 |
Patients with complications of medical care died more often than those without complications (Table 1). The highest unadjusted mortality rate was in those with surgical complications (3.24%; OR 3.90). Patients who acquired a nosocomial infection had a mortality rate of 1.39 and a 66% higher risk of mortality than those who did not (p < .001).
In the multivariable logistic regression, gender, medical and surgical complications were no longer significant (p=0.5330, 0.642 and 0.783, respectively), but the remaining variables including age (p<.001), race (p<.001), payer (p<.039), severity level (p<.001), hospital-associated infection (p<.001) and the institution in which the child was hospitalized (p< .001) remained significantly associated with mortality.
Table 2 summarizes the analysis of patient deaths for the 30 most frequent APR-DRG diagnosis categories. These 30 categories accounted for 52.5% of all diagnoses and 8.4% of the total deaths. Each of the top 30 APR-DRGs there had a significantly lower risk of dying as compared to surviving with that DRG (all OR < 1; all p < 0.05) except for diagnoses #21 and #19 (Table 2). The standardized mortality ranged from 0.46 for diagnosis #17 to 30.0 for diagnosis #7.
Table 2.
Number of Deaths for Top 30 All Patient-Refined, Diagnosis-Related Groups (APR-DRGs) (ranked by the total number of discharges)
| Rank | APR-DRG (APR-DRG Number) | Died (N=4,529) |
Survived (N=423,086) |
OR | P-value | Standardized Mortality | ||
|---|---|---|---|---|---|---|---|---|
| n | % | N | % | |||||
| 1 | Asthma (141) | 6 | 0.03 | 22920 | 99.97 | 0.02 | < 0.001 | 0.72 |
| 2 | Seizure (53) | 35 | 0.22 | 15649 | 99.78 | 0.20 | < 0.001 | 1.28 |
| 3 | Bronchiolitis & RSV Pneumonia (138) | 8 | 0.05 | 15532 | 99.95 | 0.05 | < 0.001 | 0.95 |
| 4 | Pneumonia NEC (139) | 31 | 0.22 | 14053 | 99.78 | 0.20 | < 0.001 | 0.57 |
| 5 | Chemotherapy (693) | 12 | 0.09 | 13856 | 99.91 | 0.08 | < 0.001 | 1.12 |
| 6 | Non-bacterial Gastroenteritis, Nausea & Vomiting (249) | 6 | 0.04 | 13401 | 99.96 | 0.04 | < 0.001 | 0.55 |
| 7 | Cellulitis & Other Bacterial Skin Infection (383) | 3 | 0.03 | 10414 | 99.97 | 0.00 | < 0.001 | 30.00 |
| 8 | Appendectomy (225) | 2 | 0.02 | 8846 | 99.98 | 0.02 | < 0.001 | 1.36 |
| 9 | Upper Respiratory Tract Infection (113) | 6 | 0.07 | 8681 | 99.93 | 0.06 | < 0.001 | 2.11 |
| 10 | NB BWT > 2499g, Normal Newborn, or w/Other Problem (640) | 3 | 0.04 | 8517 | 99.96 | 0.03 | < 0.001 | 0.78 |
| 11 | Kidney & Urinary Infection (463) | 8 | 0.11 | 7436 | 99.89 | 0.10 | < 0.001 | 3.15 |
| 12 | Diabetes (420) | 13 | 0.18 | 7283 | 99.82 | 0.16 | < 0.001 | 2.04 |
| 13 | Fever (722) | 2 | 0.03 | 6172 | 99.97 | 0.03 | < 0.001 | 0.85 |
| 14 | Major Hematologic/Immunologic Diagnosis (660) | 41 | 0.73 | 5551 | 99.27 | 0.69 | 0.016 | 1.21 |
| 15 | Digestive System Diagnosis NEC (254) | 10 | 0.18 | 5409 | 99.82 | 0.17 | < 0.001 | 1.28 |
| 16 | Sickle Cell Anemia Crisis (662) | 3 | 0.06 | 5407 | 99.94 | 0.05 | < 0.001 | 0.65 |
| 17 | Other Anemia & Disorders of Blood (663) | 3 | 0.06 | 4973 | 99.94 | 0.06 | < 0.001 | 0.46 |
| 18 | Viral Illness (723) | 5 | 0.1 | 4883 | 99.9 | 0.09 | < 0.001 | 3.30 |
| 19 | Craniotomy Except for Trauma (21) | 78 | 1.7 | 4514 | 98.3 | 1.62 | < 0.001 | 0.74 |
| 20 | Ventricular Shunt Procedure (22) | 23 | 0.53 | 4357 | 99.47 | 0.49 | < 0.001 | 0.51 |
| 21 | Other Resp. Diagnosis Except Signs, Symp. & Minor Diagnoses (143) | 32 | 0.75 | 4213 | 99.25 | 0.71 | 0.051 | 0.63 |
| 22 | Kidney & Urinary Tract Procedures for Nonmalignancy (443) | 4 | 0.1 | 4132 | 99.9 | 0.09 | < 0.001 | 0.58 |
| 23 | Shoulder, Upper Arm & Forearm Procedure (315) | 1 | 0.03 | 3919 | 99.97 | 0.02 | < 0.001 | 10.00 |
| 24 | Cystic Fibrosis - Pulmonary Disease (131) | 15 | 0.43 | 3495 | 99.57 | 0.40 | < 0.001 | 0.66 |
| 25 | Other Esophageal Disorder (243) | 1 | 0.03 | 3654 | 99.97 | 0.02 | < 0.001 | 10.00 |
| 26 | Hypovolemia & Related Electrolyte Disorder (422) | 8 | 0.22 | 3622 | 99.78 | 0.20 | < 0.001 | 0.98 |
| 27 | Malnutrition, Failure To Thrive & Other Nutritional Disorder (421) | 10 | 0.28 | 3586 | 99.72 | 0.26 | < 0.001 | 7.82 |
| 28 | Tonsil & Adenoid Procedure (97) | 2 | 0.06 | 3371 | 99.94 | 0.06 | < 0.001 | 20.00 |
| 29 | Hip & Femur Procedures Non-Trauma Except Joint Replacement (309) | 1 | 0.03 | 3117 | 99.97 | 0.03 | < 0.001 | 1.47 |
| 30 | Connective Tissue Disorder (346) | 7 | 0.23 | 3102 | 99.77 | 0.21 | < 0.001 | 0.96 |
RSV = Respiratory Syncytial Virus
NEC = Not Elsewhere Classified
NB = Newborn (Neonate)
BWT = Birth Weight
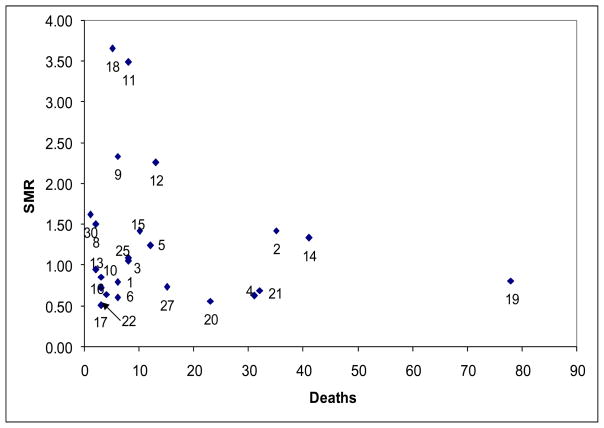
Figure 1 contrasts the standardized mortality with the actual number of deaths for the 30 most frequent APR-DRGs ranked by the most frequent number of discharges in Table 2. Diagnosis #18 (APR-DRG 723: Viral Illness) had the highest SMR, but relatively few deaths associated with it. In contrast, Diagnosis #19 (APR-DRG 21: Craniotomy Except for Trauma) had a high number of deaths with an SMR < 1, which implies that the mortality for this diagnosis is better than expected.
Figure 1.
Standardized Mortality Ratio (SMR) vs. Actual Number of Deaths for Top 30 All Patient-Refined, Diagnosis-Related Groups (APR-DRGs) ranked by the number of patient admissions. (Values 1–30, refer to Table 2)
Table 3 summarizes the analysis of patient deaths for the 30 most frequent APR-DRG diagnosis categories ranked by the number of inpatient hospital deaths. These 30 categories accounted for 62.7% of all the deaths in these institutions. Furthermore, 13 of the most frequent diagnostic categories involved newborn APR-DRGs. The highest risk of mortality was Diagnosis #17, which had a mortality rate of 79.4% and an OR of 364.78. Diagnosis #14 (APR-DRG 21: Craniotomy Except for Trauma), which had a 62% higher risk of death and had the highest risk of death when diagnoses were ranked by frequency, had the lowest risk of dying when the diagnoses were ranked by the number of deaths. The standardized mortality ranged from 0.59 for Diagnosis # 26 to 1.76 for Diagnosis #21.
Table 3.
Number of Deaths for Top 30 All Patient-Refined, Diagnosis-Related Groups (APR-DRGs) (ranked by the number of deaths)
| Rank | APR-DRG (APR-DRG Number) | Died (N= 4,529) |
Survived (N=423,086) |
OR | P-value | Standardized Mortality | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| 1 | NB BWT 500–749g w/o MP (591) | 210 | 50.85 | 203 | 49.15 | 101.29 | < 0.001 | 1.09 |
| 2 | NB w/ECMO (583) | 199 | 46.93 | 225 | 53.07 | 86.37 | < 0.001 | 1.00 |
| 3 | NB BWT < 1500g w/MP (588) | 196 | 17.21 | 943 | 82.79 | 20.25 | < 0.001 | 0.78 |
| 4 | NB BWT > 2499g w/MA (633) | 192 | 7.65 | 2317 | 92.35 | 8.04 | < 0.001 | 0.81 |
| 5 | Respiratory System Diagnosis w/Ventilator Support 96+ Hrs (130) | 182 | 8.59 | 1937 | 91.41 | 9.1 | < 0.001 | 1.17 |
| 6 | Septicemia & Disseminated Infection (720) | 145 | 6.86 | 1969 | 93.14 | 7.07 | < 0.001 | 0.80 |
| 7 | NB BWT > 2499g w/Major Cardiovascular Procedure (630) | 115 | 6.23 | 1731 | 93.77 | 6.34 | < 0.001 | 0.61 |
| 8 | Pulmonary Edema & Respiratory Failure (133) | 109 | 8.83 | 1126 | 91.17 | 9.24 | < 0.001 | 0.94 |
| 9 | NB BWT 1500–2499g w/MP (609) | 104 | 9.67 | 971 | 90.33 | 10.22 | < 0.001 | 0.81 |
| 10 | NB BWT 750–999g w/o MP (593) | 98 | 16.58 | 493 | 83.42 | 18.96 | < 0.001 | 0.88 |
| 11 | Other Injury, Poisoning & Toxic Effect Diagnosis (815) | 86 | 12.39 | 608 | 87.61 | 13.45 | < 0.001 | 1.14 |
| 12 | Cardiac Arrest (196) | 83 | 70.94 | 34 | 29.06 | 232.29 | < 0.001 | 1.00 |
| 13 | NB BWT 2000–2499g w/MA (621) | 79 | 15.05 | 446 | 84.95 | 16.82 | < 0.001 | 0.90 |
| 14 | Craniotomy Except for Trauma (21) | 78 | 1.7 | 4514 | 98.3 | 1.63 | < 0.001 | 0.74 |
| 15 | Bone Marrow Transplant (3) | 75 | 8.22 | 837 | 91.78 | 8.49 | < 0.001 | 0.91 |
| 16 | Acute Leukemia (690) | 74 | 3.95 | 1799 | 96.05 | 3.89 | < 0.001 | 1.19 |
| 17 | NB BWT < 500g (589) | 73 | 79.35 | 19 | 20.65 | 364.78 | < 0.001 | 1.61 |
| 18 | NB BWT > 2499g w/Other MP (631) | 72 | 3.56 | 1950 | 96.44 | 3.49 | < 0.001 | 0.95 |
| 19 | NB BWT 1500–1999g w/MA (611) | 67 | 17.31 | 320 | 82.69 | 19.84 | < 0.001 | 0.81 |
| 20 | Major Small & Large Bowel Procedure (221) | 66 | 3.04 | 2105 | 96.96 | 2.96 | < 0.001 | 1.31 |
| 21 | Other Mental Health Disorder (760) | 62 | 5.76 | 1014 | 94.24 | 5.78 | < 0.001 | 1.76 |
| 22 | NB BWT 1000–1249g w/RDS, Other MRC, or MA (602) | 60 | 10.2 | 528 | 89.8 | 10.74 | < 0.001 | 0.90 |
| 23 | Major Cardiothoracic Repair of Heart Anomaly (160) | 58 | 2.2 | 2583 | 97.8 | 2.11 | < 0.001 | 0.71 |
| 24 | Nonextensive Procedure Unrelated to Principal Diagnosis (952) | 56 | 5.74 | 919 | 94.26 | 5.75 | < 0.001 | 0.83 |
| 25 | Multiple Significant Trauma w/o OR Procedure (930) | 54 | 7.66 | 651 | 92.34 | 7.83 | < 0.001 | 1.16 |
| 26 | Nontraumatic Stupor & Coma (52) | 54 | 7.1 | 707 | 92.9 | 7.21 | < 0.001 | 0.59 |
| 27 | NB BWT > 2499g w/RDS or Other MRC (634) | 54 | 2.52 | 2091 | 97.48 | 2.43 | < 0.001 | 1.14 |
| 28 | Tracheostomy w/Long Term Mechanical Ventilation w/EP (4) | 46 | 12.01 | 337 | 87.99 | 12.87 | < 0.001 | 1.87 |
| 29 | Other Digestive System & Abdominal Procedure (229) | 46 | 7.19 | 594 | 92.81 | 7.3 | < 0.001 | 1.99 |
| 30 | Craniotomy for Trauma (20) | 45 | 6.59 | 638 | 93.41 | 6.65 | < 0.001 | 0.77 |
NB = Newborn (Neonate)
BWT = Birth Weight
MP = Major Procedure
ECMO = Extracorporeal Membraneous Oxygenation
MA = Major Anomaly
RDS = Respiratory Distress Syndrome
MRC = Major Respiratory Condition
OR = Operating Room
EP = Extensive Procedure
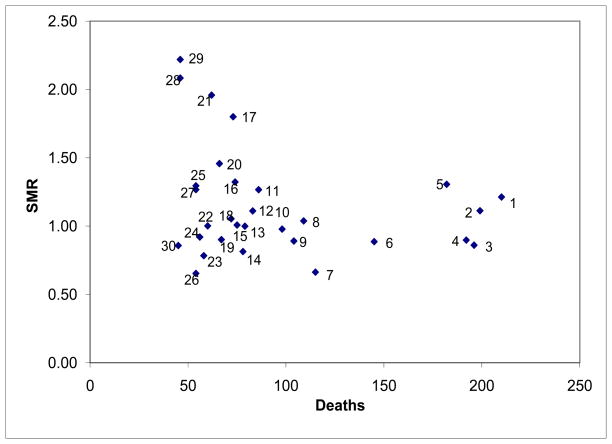
Figure 2 compares the standardized mortality to the actual number of deaths for the 30 most frequent APR-DRGs ranked by the highest number of patient deaths in Table 3. A total of 9 of the 10 APR-DRGs with the highest death rates had > 100 deaths and 70% of them had SMRs ≤ 1. However, Figure 2 also demonstrates considerable dispersion for SMR in the diagnoses with < 100 deaths. Diagnoses #1, #2, and #5 had both high numbers of patients dying and patients dying at a rate that was higher than expected given their diagnosis and condition. In contrast, Diagnoses #3 and #4 also had high numbers of patients dying, but at a rate that was lower than expected given their diagnosis and patient attributes.
Figure 2.
Standardized Mortality Ratio (SMR) vs. Actual Number of Deaths for Top 30 All Patient-Refined, Diagnosis-Related Groups (APR-DRGs) ranked by the number of patient deaths. (Values 1–30, refer to Table 3)
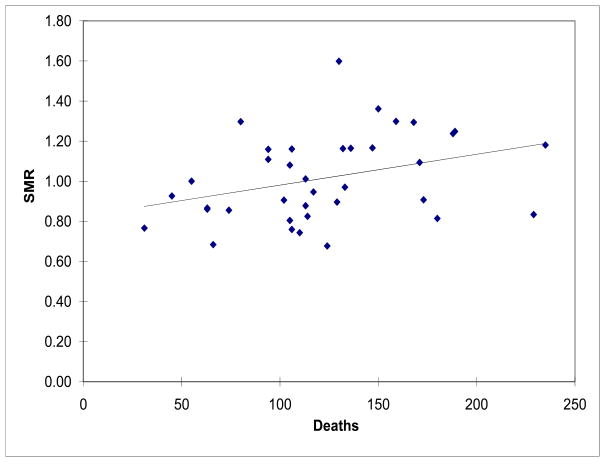
Figure 3 provides a comparison of the variation of HSMR and number of deaths at 37 PHIS hospitals for all the APR-DRGs. Of importance, there is considerable variability in both the number of children who died and the HSMR. The number of deaths ranged from 31 to 235, the crude mortality rate ranged from 0.5 to 2.1% and the standardized mortality rate ranged from 0.7 to 1.6 (Figure 3). There was a 4-fold difference in the crude and 2-fold difference in the adjusted mortality rates across the institutions.
Figure 3.
Hospital Standardized Mortality Ratios (HSMR) vs. Actual Number of Deaths among 37 PHIS Hospitals
Discussion
The hospital setting is worthy of study to help inform conditions and circumstances where mortality may be improved since more than half of all childhood deaths occur there2. Much of the work in this area has been limited to small series and single institution studies. We know, however, that many pediatric inpatients die in ICUs, 4–8 and > 50% fulfill a diagnostic classification for one or more complex chronic conditions.26 An improved understanding of pediatric inpatient deaths may be obtained through the analysis of patients from a multi-institutional cohort of academic, children’s hospitals. Therefore, we investigated the characteristics of patients who died in these centers to attempt to better understand the characteristics associated with mortality for these hospitalized children. We found that 1.1% of the pediatric inpatients ≤ 21 years died and that patient characteristics like age, severity level and diagnostic classifications proved to be important determinants of mortality.20 We also found that pediatric academic institutions demonstrate considerable heterogeneity when compared by both death rates and HSMR.
We hypothesized that an analysis and improved understanding of the association between mortality, diagnostic classes, and severity could help to identify patients at high risk of dying and perhaps focus efforts and resources to improve the outcomes of these patients. A diagnosis-specific mortality investigation revealed that for the 30 most common APR-DRG diagnostic categories, which accounted for 52.5% of all discharges in these hospitals, the mortality rate was 8.4% (Table 2). When the population was sorted by the 30 most common APR-DRG diagnostic categories ranked by frequency of deaths, the list changed considerably (Table 3). This approach represented only 8.75% of the population, but 62.7% of the deaths. Together, these diagnostic categories represented 71.1% of the deaths in pediatric institutions, which provides an important starting point for attempting to improve care. The majority of diagnoses, when ranked by frequency of deaths, dealt with neonatal care, which represented only a single diagnosis when ranked by frequency of discharges (APR-DRG 640: Newborn Birthweight > 2499g, Normal Newborn, or w/Other Problem). Only one diagnosis, APR-DRG 21: Craniotomy Except for Trauma, was on both the frequency-by-diagnosis and the frequency-by-deaths lists. This has important implications for the performance improvement aspects of this work. While the more frequent deaths are associated with many unmodifiable factors (eg. Birthweight < 500 grams), there are other important clinical interventions that can assist in preventing unnecessary death like the focus on assuring technique with central venous catheter and pneumonia care. When focusing on particular diagnoses to improve mortality, it is important to consider not only why children are dying, but also to extent that they are dying beyond what is predicted.
The standardized mortality is an important epidemiologic tool that assists with disentangling who was admitted and died more than they were expected to die based upon national estimates of survival in that diagnostic group and with that severity. Recent national efforts have focused on the opportunity for hospitals to improve the fundamental outcome measure of mortality,27 which is an important measure of the quality of care delivered in a hospital. By identifying unexpected deaths, process problems like delays in care and system issues like a lack of coordination may become apparent. Furthermore, identifying the diagnostic categories affected by complications like nosocomial infections and medical complications may allow providers to improve patient care. This becomes especially important for those deaths in low mortality diagnostic groups who die when they were not expected to die.
Performing an investigation of children with diagnoses in which the SMR > 1 provides institutions with a starting point for addressing mortality in hospitalized children. However, since the methods upon which the SMRs are calculated rely upon a ‘predicted’ mortality, hospitals that care for patients with complex healthcare needs who die more often may be disadvantaged since the predicted mortality inadequately accounts for patient level case mix. In our analyses, we attempted to further adjust for these institution level differences by normalizing the standardized SMRs to free standing childrens hospitals thereby creating the HSMR.
We also found considerable variability in the HSMR by institution highlighting opportunities to improve the way care is delivered in these settings. There is little doubt that inpatient pediatric care at these academic institutions is complex and that the patients may be sicker than can be appreciated by the severity score or that there may be a referral bias with sicker patients who were more likely to die being admitted to these centers. We have previously noted similar findings with a higher number of patient safety indicator (PSI) events being experienced as compared to non-academic hospitals.20, 28 Our current study, applying a consistent and well-validated approach that controls for severity, has highlighted differences in mortality across these institutions. A study done at the Bradford Teaching Hospitals used mortality data to better investigate the processes of clinical care. The hospital developed a programmatic approach for changing practice patterns aimed at reducing the hospital’s SMRs and similar approaches may be helpful here since these SMRs are publicly reported for adult hospitals and it is likely that childrens hospitals will be facing increased public scrutiny on these important outcome measures.29
This study has several limitations. First, the clinical information included in the database is limited to broad diagnostic categories based on the coding of administrative data. A study of more precise clinical variables, either independently or as a part of validated clinical algorithms, would allow for a more detailed analysis of patient deaths. Second, there exists the possibility of information bias due to the misclassification of clinical information and errors in data entry. Moreover, the study sample may be affected by a self-selection bias since the participation of hospitals in the PHIS collaborative is voluntary. Thus, hospitals with a focus on performance improvement activities are more likely to participate. Finally, while we have used standardized well-validated methods to determine the SMRs in this study, systematic biases in the calculation of the “expected” mortality variable are certainly possible. This variable was applied uniformly across all diagnoses and institutions leading to the same level of “scrutiny” for all institutions included in the analysis.
While there are limitations, we believe that this study provides a multi-institutional perspective from academic, children’s hospitals that can begin to inform the discussion on why children die. Furthermore, we believe that it can also help to inform specific improvement efforts at institutions that care for patients with these diagnoses. Finally, this approach can help to inform analyses at individual institutions to measure their SMRs and work on efforts to improve excess mortality.
Acknowledgments
Funding/Support: Dr. Slonim and Ms. Turenne were supported in part by AHRQ-KO-8 HS-14009. Dr. Shah received support from the National Institute of Allergy and Infectious Diseases (K01 AI73729) and the Robert Wood Johnson Foundation under its Physician Faculty Scholar Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions: Dr. Slonim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Slonim, Turenne, Khandelwal.
Acquisition of data: Slonim, He, Hall.
Analysis and interpretation of data: Slonim, Stockwell, Khandelwal, Turenne, Shah, Hall.
Drafting of the manuscript: Slonim, Stockwell, Khandelwal, Turenne, He, Shah, Hall.
Statistical analysis: Slonim, Turenne, He.
Obtained funding: Slonim.
Administrative, technical, or material support: Slonim, He, Khandelwal.
Study supervision: Slonim.
Financial Disclosures: Nothing to Disclose.
Additional Contributions: None of the individuals acknowledged received compensation for their contributions.
Contributor Information
Anthony D. Slonim, Email: aslonim@carilion.com, Childrens National Medical Center, Division of Critical Care Medicine, Washington, DC 20010. Associate Professor, Internal Medicine, Pediatrics and Public Health, The George Washington University School of Medicine, Washington DC.
Sachin Khandelwal, Email: skhandel@cnmc.org, Children’s National Medical Center, Washington, DC 20010.
Jianping He, Email: jhe@cnmc.org, Children’s National Medical Center, Washington, DC 20010.
Matthew Hall, Email: Matt.hall@chca.com, Child Health Corporation of America, Shawnee Mission, KS.
David C. Stockwell, Email: dstockwe@cnmc.org, Children’s National Medical Center, Washington, DC 20010.
Wendy M. Turenne, Email: wendyturenne@aol.com, Children’s National Medical Center, Washington, DC 20010.
Samir S. Shah, Email: shahs@email.chop.edu, Divisions of General Pediatrics and Inf. Diseases, The Childrens Hospital of Philadelphia, Departments of Pediatrics and Epidemiology, And the Center for Clinical Epid. and Biostatistics, University of Pennsylvania School of Medicine, Philadelphia, Pa.
References
- 1.Deaths: Final data 1999. National Center for Health Statistics; 2001a. [Accessed on August 18, 2009.]. Available at: http://www.nber.org/mortality/1999/docs/nvsr49_08.pdf. [PubMed] [Google Scholar]
- 2.Deaths by place of death, age, race, and sex: United states, 2002. National Center for Health Statistics; 2006. Available from: http://www.cdc.gov/nchs/data/dvs/mortfinal2002_work309.pdf. [Google Scholar]
- 3.Nasi T, Vince JD, Mokela D. Mortality in children admitted to port moresby general hospital: How can we improve our hospital outcomes? P N G Med J. 2003;46:113–124. [PubMed] [Google Scholar]
- 4.Carter BS, Howenstein M, Gilmer MJ, Throop P, France D, Whitlock JA. Circumstances surrounding the deaths of hospitalized children: Opportunities for pediatric palliative care. Pediatrics. 2004;114:e361–6. doi: 10.1542/peds.2003-0654-F. [DOI] [PubMed] [Google Scholar]
- 5.McCallum DE, Byrne P, Bruera E. How children die in hospital. J Pain Symptom Manage. 2000;20:417–423. doi: 10.1016/s0885-3924(00)00212-8. [DOI] [PubMed] [Google Scholar]
- 6.Ramnarayan P, Craig F, Petros A, Pierce C. Characteristics of deaths occurring in hospitalised children: Changing trends. J Med Ethics. 2007;33:255–260. doi: 10.1136/jme.2005.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solloway M, LaFrance S, Bakitas M, Gerken M. A chart review of seven hundred eighty-two deaths in hospitals, nursing homes, and hospice/home care. J Palliat Med. 2005;8:789–796. doi: 10.1089/jpm.2005.8.789. [DOI] [PubMed] [Google Scholar]
- 8.Cantagrel S, Ducrocq S, Chedeville G, Marchand S. Mortality in a pediatric hospital. six-year retrospective study. Arch Pediatr. 2000;7:725–731. doi: 10.1016/s0929-693x(00)80152-9. [DOI] [PubMed] [Google Scholar]
- 9.Serwint JR, Nellis ME. Deaths of pediatric patients: Relevance to their medical home, an urban primary care clinic. Pediatrics. 2005;115:57–63. doi: 10.1542/peds.2004-0445. [DOI] [PubMed] [Google Scholar]
- 10.Surkan PJ, Kreicbergs U, Valdimarsdottir U, et al. Perceptions of inadequate health care and feelings of guilt in parents after the death of a child to a malignancy: A population-based long-term follow-up. J Palliat Med. 2006;9:317–331. doi: 10.1089/jpm.2006.9.317. [DOI] [PubMed] [Google Scholar]
- 11.Vernon DD, Dean JM, Timmons OD, Banner W, Jr, Allen-Webb EM. Modes of death in the pediatric intensive care unit: Withdrawal and limitation of supportive care. Crit Care Med. 1993;21:1798–1802. doi: 10.1097/00003246-199311000-00035. [DOI] [PubMed] [Google Scholar]
- 12.Move your dot™: Measuring, evaluating, and reducing hospital mortality rates (part 1) Boston: Institute for Healthcare Improvement; 2003. IHI Innovation Series white paper. [Google Scholar]
- 13.Whittington J, Simmonds T, Jacobsen D. Reducing hospital mortality rates (part 2) Cambridge, MA: Institute for Healthcare Improvement; 2005. IHI Innovation Series white paper. [Google Scholar]
- 14.Nathanson BH, Higgins TL, Teres D, Copes WS, Kramer A, Stark M. A revised method to assess intensive care unit clinical performance and resource utilization. Crit Care Med. 2007;35:1853–1862. doi: 10.1097/01.CCM.0000275272.57237.53. [DOI] [PubMed] [Google Scholar]
- 15.Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: An updated mortality probability admission model (MPM0-III) Crit Care Med. 2007;35:827–835. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 16.Teres D. The value and limits of severity adjusted mortality for ICU patients. J Crit Care. 2004;19:257–263. doi: 10.1016/j.jcrc.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed on October 20, 2009]; Available at: http://www.ahrq.gov/qual/mortality/Hughes.htm.
- 18.Iezzoni LI, editor. Risk adjustment for measuring healthcare outcomes. 2. Chicago, IL: Health Administration Press; [Accessed October 20, 2009]. 1997. Available at: http://www.ahrq.gov/qual/mortality/Hughessumm.htm. [Google Scholar]
- 19. [Accessed October 20, 2009]; Available at: http://www.ahrq.gov/qual/mortality/Foster.pdf.
- 20.Slonim AD. Medical errors in children hospitalized at academic children’s hospitals: An analysis using hierarchical modeling. Washington, DC: The School of Public Health and Health Services of The George Washington University; 2005. [Google Scholar]
- 21.Ruttimann UE, Patel KM, Pollack MM. Length of stay and efficiency in pediatric intensive care units. J Pediatr. 1998;133:79–85. doi: 10.1016/s0022-3476(98)70182-9. [DOI] [PubMed] [Google Scholar]
- 22.Ruttimann UE, Pollack MM. Variability in duration of stay in pediatric intensive care units: A multiinstitutional study. J Pediatr. 1996;128:35–44. doi: 10.1016/s0022-3476(96)70425-0. [DOI] [PubMed] [Google Scholar]
- 23.Pollack MM, Getson PR, Ruttimann UE, et al. Efficiency of intensive care. A comparative analysis of eight pediatric intensive care units. JAMA. 1987;258:1481–1486. [PubMed] [Google Scholar]
- 24.Pollack MM, Katz RW, Ruttimann UE, Getson PR. Improving the outcome and efficiency of intensive care: The impact of an intensivist. Crit Care Med. 1988;16:11–17. doi: 10.1097/00003246-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed June 14, 2007]; Available at: http://www.sas.com.
- 26.Feudtner C, Christakis DA, Zimmerman FJ, Muldoon JH, Neff JM, Koepsell TD. Characteristics of deaths occurring in children’s hospitals: Implications for supportive care services. Pediatrics. 2002;109:887–893. doi: 10.1542/peds.109.5.887. [DOI] [PubMed] [Google Scholar]
- 27. [Accessed August 7, 2007.]; Available at: http://www.ihi.org/NR/rdonlyres/EB78B6DB-0955-4C9C-A9B8-599E1E53DF6D/0/5MillionLivesCampaignBrochure0207.pdf.
- 28.Slonim AD, Marcin JP, Turenne WM, Hall M, Joseph JG. Pediatric Patient Safety Events during Hospitalization: Approaches to Accounting for Institution-Level Effects. Health Services Research. 2007;42:2275–93. doi: 10.1111/j.1475-6773.2007.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright J, Dugdale B, Hammond I, et al. Learning from death: A hospital mortality reduction programme. J R Soc Med. 2006;99:303–308. doi: 10.1258/jrsm.99.6.303. [DOI] [PMC free article] [PubMed] [Google Scholar]