Abstract
Insulin resistance is associated with central obesity and an increased risk of cardiovascular disease. Our objective is to examine the association between abdominal subcutaneous (SAT) and visceral adipose tissue (VAT) and insulin resistance, to determine which fat depot is a stronger correlate of insulin resistance, and to assess whether there was an interaction between SAT, VAT, and age, sex, or BMI. Participants without diabetes from the Framingham Heart Study (FHS), who underwent multidetector computed tomography to assess SAT and VAT (n = 3,093; 48% women; mean age 50.4 years; mean BMI 27.6 kg/m2), were evaluated. Insulin resistance was measured using the homeostasis model and defined as HOMAIR ≥75th percentile. Logistic regression models, adjusted for age, sex, smoking, alcohol, menopausal status, and hormone replacement therapy use, were used to assess the association between fat measures and insulin resistance. The odds ratio (OR) for insulin resistance per standard deviation increase in SAT was 2.5 (95% confidence interval (CI): 2.2–2.7; P < 0.0001), whereas the OR for insulin resistance per standard deviation increase in VAT was 3.5 (95% CI: 3.1–3.9; P < 0.0001). Overall, VAT was a stronger correlate of insulin resistance than SAT (P < 0.0001 for SAT vs. VAT comparison). After adjustment for BMI, the OR of insulin resistance for VAT was 2.2 (95% CI: 1.9–2.5; P < 0.0001). We observed an interaction between VAT and BMI for insulin (P interaction = 0.0004), proinsulin (P interaction = 0.003), and HOMAIR (P interaction = 0.003), where VAT had a stronger association in obese individuals. In conclusion, SAT and VAT are both correlates of insulin resistance; however, VAT is a stronger correlate of insulin resistance than SAT.
INTRODUCTION
Insulin resistance is associated with elevated levels of trigly cerides and systolic and diastolic blood pressure, C-reactive protein, and type 2 diabetes and with decreased levels of high-density lipoprotein-cholesterol (1,2). Additionally, insulin resistance has been shown to predict cardiovascular disease events above and beyond traditional risk factors (1,3). Insulin resistance is associated with increasing BMI. However, normal-weight individuals may also be insulin resistant, suggesting that overall adiposity is not the sole determinant of insulin resistance (4).
Although insulin resistance is correlated with BMI, it is more strongly associated with abdominal obesity, a key component of the metabolic syndrome (5). Traditionally, simple measurements, such as BMI and waist circumference, are used to assess abdominal obesity. However, regional adiposity may confer differential metabolic risk. Radiographic imaging allows for a more precise evaluation of abdominal obesity by quantifying the volume of visceral (VAT) and subcutaneous adipose tissue (SAT). Adipose tissue, especially VAT, has been shown to be strongly associated with cardiovascular disease risk factors (5,6).
Several studies have examined the association between abdominal obesity, as measured by SAT and VAT, and insulin resistance (7–18). However, the results of these studies have been inconsistent, with some showing that both SAT and VAT are correlated with insulin measures and others showing that only VAT is correlated with insulin measures. These prior studies are limited by their small sample sizes and highly selected patient populations, which may account for the discrepant results. Previous analyses in the Framingham Heart Study (FHS) have shown that both VAT and SAT are correlated with metabolic risk factors, including triglycerides, high-density lipoprotein-cholesterol, and systolic and diastolic blood pressure (6). We now propose to extend these findings by examining the association between abdominal SAT, VAT and various measures of insulin sensitivity in order to further elucidate the relationship between adipose tissue measures and insulin resistance in a large population-based study sample, including subgroup analyses by sex, age, and BMI.
METHODS AND PROCEDURES
study sample
The study design of the FHS cohorts has been previously described (6). Briefly, the FHS Original cohort began in 1948 with the enrollment of 5,209 men and women aged 28–62 years who subsequently underwent biennial examinations. In 1971, 5,124 offspring of the original participants and their spouses were enrolled into the Offspring cohort and underwent examinations approximately every 4 years. In 2002, 4,095 children of the offspring participants and their spouses were enrolled into the Third Generation cohort. Participants from the Offspring and Third Generation cohorts were invited to participate in the multidetector computed tomography substudy. Inclusion in this substudy was weighted toward individuals from larger FHS families who were residing in the New England area. To be eligible for the substudy, participants had to be ≥35 years old if male, ≥40 years old if female, nonpregnant, and have a body weight <160 kg. A total of 3,529 participants (1,422 from Offspring, 2,093 from Third Generation) underwent multidetector computed tomography scanning from 2002 to 2005. We excluded individuals with diabetes (type 1 or 2), who had missing data on VAT or SAT measurements, or who were missing measurements of both insulin and proinsulin, resulting in a final sample size of 3,093. The present analysis uses data from the seventh Offspring examination (1998–2001) and the first Third Generation examination (2002–2005). All subjects provided written informed consent, and the study has been approved by the Institutional Review Board at Boston University Medical Center.
adiposity measurements
SAT and VAT were measured as previously described (19). Briefly, participants underwent multidetector computed tomography scanning using an eight-slice scanner (LightSpeed Ultra; General Electric, Milwaukee, WI). Twenty-five contiguous 5 mm thick slices (120 kV(p), 400 mA, gantry rotation time 500 ms, table feed 3:1) were acquired covering 125 mm above the S1 level. SAT and VAT were assessed by experienced technicians using a dedicated offline workstation (Aquarius 3D Workstation; TeraRecon, San Mateo, CA). The volume (cm3) of SAT and VAT was determined by manually tracing the abdominal wall separating the SAT and VAT compartments. In addition to SAT and VAT volume, slice-specific measures (L1/2, L2/3, L3/4, umbilicus, L4/5, iliac crest, and L5/S1) were available for a subset of participants (n = 161) (20). The correlations between the slice-specific 1 measures and insulin measures were similar to the correlation for the total volume measures. The correlations between the slice-specific measures and the insulin measures are presented in Supplementary Table S1 online.
BMI was calculated by dividing weight in kilograms by the square of height in meters. Waist circumference was measured at the level of the umbilicus and was recorded to the nearest quarter inch.
Insulin resistance and covariate measurements
Participants underwent measurement of fasting blood glucose, insulin, and proinsulin. Insulin and proinsulin were measured using radioimmunoassay in the Offspring cohort and enzyme-linked immunosorbent assay in the Third Generation cohort. For the Offspring cohort, the intra-assay coefficient of variation was 3.9% for insulin and 3.7% for proinsulin, and the interassay coefficient of variation had a range of 4.7–6.1% for insulin and 7.8–12.5% for proinsulin. For the Third Generation cohort, the intra-assay coefficient of variation was 2.7% for insulin and 2.4% for proinsulin, and the interassay coefficient of variation was 8.1% for insulin and 8.7% for proinsulin. Due to the differing methods used to measure insulin and proinsulin in the two cohorts, all values for the Third Generation cohort were standardized to those for the Offspring cohort. Diabetes was defined as a fasting blood glucose of ≥126 mg/dl or treatment with insulin or a hypoglycemic agent. The homeostasis model assessment of insulin resistance (HOMAIR) measurement was calculated for all participants. A participant was considered to have insulin resistance if they had a HOMAIR value in the top quartile (≥75th percentile) of the distribution in the study sample.
A participant was defined as a current cigarette smoker if they reported smoking ≥1 cigarette per day over the previous year. Regular alcohol consumption was defined as >14 drinks/week for men or >7 drinks/week for women. A woman was considered postmenopausal if they reported that their periods had stopped for ≥1 years.
statistical analysis
SAT and VAT were standardized (sex-specific) to a mean of zero and a standard deviation of one in order to facilitate comparisons between the two fat depots. Insulin, proinsulin, proinsulin\insulin ratio, and HOMAIR were log- (natural log, ln) transformed to improve normality. Age- and sex-adjusted Pearson correlation coefficients were calculated between adiposity measures and insulin measures. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression to examine the association between standard deviation increment in adipose tissue and insulin resistance (HOMAIR ≥75th percentile). β-coefficients and standard errors were calculated IR using linear regression models to assess the association between standard deviation change in SAT and VAT and continuous insulin measures. Linear regression models were also constructed to examine the association between tertiles of adipose tissue measurements and continuous insulin measures. All models were adjusted for age (years), sex, current cigarette smoking (yes vs. no), regular alcohol consumption (yes vs. no), menopausal status (yes vs. no), and hormone replacement therapy use (yes vs. no). The models for VAT were further adjusted for BMI and for waist circumference. Standardized values of SAT and VAT were included in a model together in order to calculate a Wald P value comparing the strength of the β-coefficients for SAT and VAT. Because the prior literature has shown that ectopic fat in the liver is more strongly correlated with insulin resistance than visceral fat, we conducted a secondary analysis adjusting our main VAT models for the liver enzymes alanine aminotransferase and aspartate aminotransferase (21,22).
Proc GLM was used to calculate multivariable-adjusted mean levels of insulin variables by tertiles of SAT and VAT. The interaction between tertiles of SAT and VAT on insulin measures was assessed by examining the significance of the cross-product term of ordinal variables for SAT and VAT. We additionally examined the interaction between standard deviation increments of SAT and VAT and the following factors: sex and age (≥50 years vs. <50 years). We additionally examined the interaction between VAT and BMI (<25 kg/m2, 25–29.9 kg/m2, ≥30 kg/ m2). The interaction was assessed by examining the significance of the cross-product term of standard deviation increments of SAT, VAT, sex, age-group, and BMI category (coded as an ordinal variable). Due to the multicollinearity between SAT and BMI, we were unable to examine the interaction between these two factors. All analyses were performed using SAS v.9.1 (SAS Institute, Cary, NC). A P value of <0.05 was considered statistically significant.
RESULTS
Characteristics of the study sample are presented in Table 1. The mean volume of SAT was 2,607 cm3 among men and 3,135 cm3 among women. The mean volume of VAT was 2,189 cm3 among men and 1,326 cm3 among women.
Table 1.
Study sample characteristics
| Men (N = 1,602) |
Women (N = 1,491) |
|
|---|---|---|
| Continuous characteristics, mean (s.d.) | ||
| Age (years) | 49.3 (10.5) | 51.7 (9.6) |
| BMI (kg/m2) | 28.2 (4.3) | 26.9 (5.6) |
| Waist circumference (cm) | 100.2 (11.3) | 92.6 (15.0) |
| Abdominal subcutaneous adipose tissue (cm3) |
2,607 (1,182) | 3,135 (1,499) |
| Visceral adipose tissue (cm3) | 2,189 (988) | 1,326 (789) |
| Log insulin (pmol/l) | 4.44 (0.38) | 4.34 (0.37) |
| Log proinsulin (pmol/l) | 2.52 (0.50) | 2.31 (0.46) |
| Log proinsulin/insulin ratio | 0.57 (0.09) | 0.54 (0.09) |
| Log HOMAIR | 1.06 (0.41) | 0.91 (0.41) |
| Categorical characteristics, n (%) | ||
| Current smoker | 209 (13.1) | 188 (12.6) |
| Regular alcohol consumptiona | 218 (13.9) | 192 (13.1) |
| Postmenopausal | — | 740 (49.6) |
| Hormone replacement therapy use |
— | 301 (20.3) |
| Subcutaneous adipose tissue ≥90th percentile |
483 (30.2) | 447 (30.0) |
| Visceral adipose tissue ≥90th percentile |
635 (39.6) | 629 (42.2) |
| HOMAIR ≥75th percentile | 462 (30.6) | 265 (19.0) |
HOMAIR, homeostasis model assessment of insulin resistance.
Defined as >14 drinks/week for men, >7 drinks/week for women.
Insulin was positively correlated with SAT (age- and sex-adjusted Pearson correlation coefficient (r) = 0.41), VAT (r = 0.49), BMI (r = 0.48), and waist circumference (r = 0.48). Results were similar for the correlation of proinsulin with SAT (r = 0.39), VAT (r = 0.47), BMI (r = 0.47), and waist circumference (r = 0.46) and for the correlation of HOMAIR with SAT (r = 0.43), VAT (r = 0.52), BMI (r = 0.51), and waist circumference (r = 0.51). The magnitude of the correlation coefficients was lower for proinsulin/insulin ratio: SAT (r = 0.26), VAT (r = 0.30), BMI (r = 0.31), and waist circumference (r = 0.30). All correlation coefficients were highly statistically significant (P < 0.0001). Overall, the correlations were stronger for VAT as compared to SAT. The magnitude of the correlation coefficients was similar for VAT, BMI, and waist circumference.
The multivariable-adjusted OR of having insulin resistance for each standard deviation increase in SAT was 2.48 (95% CI: 2.24–2.74; P < 0.0001). The multivariable-adjusted OR of insulin resistance per standard deviation increase in VAT was 3.46 (95% CI: 3.08–3.90; P < 0.0001). When the model for VAT was further adjusted for BMI, the OR was 2.18 (95% CI: 1.88–2.52; P < 0.0001). When the model for VAT was adjusted for WC instead of BMI, the OR was 2.21 (95% CI: 1.90–2.56; P < 0.0001). When SAT and VAT were added to the multivariable model together, the OR for SAT was 1.53 (95% CI: 1.36–1.71; P < 0.0001) and the OR for VAT was 2.70 (95% CI: 2.36–3.08; P < 0.0001). VAT was a significantly stronger predictor of insulin resistance than SAT (P < 0.0001 for SAT vs. VAT comparison). Because VAT and BMI are both highly correlated with insulin measures, we also calculated the multivariable-adjusted OR for a one standard deviation increment (1 s.d. = 5 kg/m2) of BMI (OR = 3.20 (95% CI: 2.86–3.59; P < 0.0001)).
Table 2 shows the results from the linear regression of insulin resistance variables on the continuous fat measures (SAT and VAT). The β-coefficients in this model have the interpretation of the effect size of insulin resistance measure per one standardized standard deviation increase in SAT or VAT. Both SAT and VAT were statistically significant correlates (P < 0.0001) of all insulin variables (insulin, proinsulin, proinsulin/insulin ratio, and HOMAIR). However, the magnitudes of the effect estimates were greater for VAT as compared to SAT for all insulin resistance variables. When models for VAT were additionally adjusted for SAT, the β-coefficient for VAT was attenuated but remained statistically significant for all insulin measures. In a secondary analysis, when we adjusted the models for VAT for the liver enzymes alanine aminotransferase and aspartate aminotransferase, the β-coefficient for VAT did not change appreciably (data not shown).
Table 2.
Multivariable-adjusted β-coefficients for the increment in insulin measures per standard deviation increase in adipose tissue
| Abdominal subcutaneous adipose tissue |
Visceral adipose tissue |
P value comparing SAT vs. VAT |
|||||
|---|---|---|---|---|---|---|---|
| Outcome variable | β (s.e.) | P value | Partial r2 for SATb |
β (s.e.) | P value | Partial r2 for VATb |
|
| Log insulin | |||||||
| Multivariablea | 0.16 (0.01) | <0.0001 | 0.168 | 0.20 (0.01) | <0.0001 | 0.249 | <0.0001 |
| Multivariablea + BMI | — | — | — | 0.13 (0.01) | <0.0001 | 0.064 | — |
| Multivariablea + WC | — | — | — | 0.13 (0.01) | <0.0001 | 0.058 | — |
| Log proinsulin | |||||||
| Multivariablea | 0.19 (0.01) | <0.0001 | 0.157 | 0.25 (0.01) | <0.0001 | 0.238 | <0.0001 |
| Multivariablea + BMI | — | — | — | 0.15 (0.01) | <0.0001 | 0.055 | — |
| Multivariablea + WC | — | — | — | 0.16 (0.01) | <0.0001 | 0.056 | — |
| Log proinsulin/insulin ratio | |||||||
| Multivariablea | 0.02 (0.002) | <0.0001 | 0.062 | 0.03 (0.002) | <0.0001 | 0.096 | <0.0001 |
| Multivariablea + BMI | — | — | — | 0.02 (0.002) | <0.0001 | 0.018 | — |
| Multivariablea + WC | — | — | — | 0.02 (0.002) | <0.001 | 0.020 | — |
| Log HOMAIR | |||||||
| Multivariablea | 0.18 (0.01) | <0.0001 | 0.186 | 0.23 (0.01) | <0.0001 | 0.277 | <0.0001 |
| Multivariablea + BMI | — | — | — | 0.13 (0.01) | <0.0001 | 0.064 | — |
| Multivariablea + WC | — | — | — | 0.12 (0.01) | <0.001 | 0.058 | — |
SAT and VAT are standardized (sex-specific) to a mean of zero and a standard deviation of one.
HOMAIR, homeostasis model assessment of insulin resistance; SAT, abdominal subcutaneous adipose tissue; VAT, visceral adipose tissue; WC, waist circumference.
Adjusted for age, sex, current smoking, regular alcohol consumption, menopausal status, and hormone replacement therapy use.
Adjusted model r2 for model containing only covariates is 0.03 (log insulin), 0.06 (log proinsulin), 0.06 (log proinsulin/insulin ratio), and 0.04 (log HOMAIR).
We additionally constructed linear regression models to examine the association between standard deviation increment of BMI and WC and each of the four insulin measures. The β-coefficient (s.e.) for a one standard deviation increment of BMI was 0.19 (s.e.: 0.01; P < 0.0001) for insulin, 0.23 (s.e.: 0.01; P < 0.0001) for proinsulin, 0.03 (s.e.: 0.002; P < 0.0001) for proinsulin/insulin ratio, and 0.21 (s.e.: 0.01; P < 0.0001) for HOMAIR. The β-coefficient (s.e.) for a one standard deviation increment of WC was 0.19 (s.e.: 0.01; P < 0.0001) for insulin, 0.23 (s.e.: 0.01; P < 0.0001) for proinsulin, 0.03 (s.e.: 0.002; P < 0.0001) for proinsulin/insulin ratio, and 0.22 (s.e.: 0.01; P < 0.0001) for HOMAIR.
Table 3 shows the linear regression of insulin resistance variables on tertiles of SAT and VAT. Overall, the results were consistent with those shown in Table 2. For the SAT models, the β-coefficients were attenuated after further adjustment for VAT, but still remained statistically significant. For the VAT models, the β-coefficients were attenuated after further adjustment for BMI or waist circumference, but still remained statistically significant.
Table 3.
Multivariable-adjusted β-coefficients for the increment in insulin measures per one tertile increase in adipose tissue
| Adipose tissue tertile |
||||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 |
Tertile 2 |
Tertile 3 |
||||||
| Fat depot | Outcome variable | β (s.e.) | P value | β (s.e.) | P value | β (s.e.) | P value | P trend |
| SATa | Log insulin | |||||||
| Multivariableb | Ref. | — | 0.16 (0.02) | <0.0001 | 0.35 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + VAT | Ref. | — | 0.04 (0.02) | 0.005 | 0.12 (0.02) | <0.0001 | <0.0001 | |
| Log proinsulin | ||||||||
| Multivariableb | Ref. | — | 0.18 (0.02) | <0.0001 | 0.42 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + VAT | Ref. | — | 0.04 (0.02) | 0.07 | 0.12 (0.02) | <0.0001 | <0.0001 | |
| Log proinsulin/insulin ratio | ||||||||
| Multivariableb | Ref. | — | 0.02 (0.004) | <0.0001 | 0.05 (0.004) | <0.0001 | <0.0001 | |
| Multivariableb + VAT | Ref. | — | 0.005 (0.004) | 0.22 | 0.01 (0.005) | 0.009 | 0.008 | |
| Log HOMAIR | ||||||||
| Multivariableb | Ref. | — | 0.19 (0.02) | <0.0001 | 0.41 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + VAT | Ref. | — | 0.06 (0.02) | 0.0003 | 0.15 (0.02) | <0.0001 | <0.0001 | |
| VATc | Log insulin | |||||||
| Multivariableb | Ref. | — | 0.19 (0.02) | <0.0001 | 0.43 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + BMI | Ref. | — | 0.09 (0.02) | <0.0001 | 0.21 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + WC | Ref. | — | 0.08 (0.02) | <0.0001 | 0.20 (0.02) | <0.0001 | <0.0001 | |
| Log proinsulin | ||||||||
| Multivariableb | Ref. | — | 0.25 (0.02) | <0.0001 | 0.54 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + BMI | Ref. | — | 0.12 (0.02) | <0.0001 | 0.26 (0.03) | <0.0001 | <0.0001 | |
| Multivariableb + WC | Ref. | — | 0.12 (0.02) | <0.0001 | 0.26 (0.03) | <0.0001 | <0.0001 | |
| Log proinsulin/insulin ratio | ||||||||
| Multivariableb | Ref. | — | 0.03 (0.004) | <0.0001 | 0.06 (0.004) | <0.0001 | <0.0001 | |
| Multivariableb + BMI | Ref. | — | 0.02 (0.004) | 0.0001 | 0.03 (0.005) | <0.0001 | <0.0001 | |
| Multivariableb + WC | Ref. | — | 0.02 (0.004) | 0.0001 | 0.03 (0.005) | <0.0001 | <0.0001 | |
| Log HOMAIR | ||||||||
| Multivariableb | Ref. | — | 0.22 (0.02) | <0.0001 | 0.49 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + BMI | Ref. | — | 0.11 (0.02) | <0.0001 | 0.25 (0.02) | <0.0001 | <0.0001 | |
| Multivariableb + WC | Ref. | — | 0.10 (0.02) | <0.0001 | 0.24 (0.02) | <0.0001 | <0.0001 | |
Ref., reference; SAT, abdominal subcutaneous adipose tissue; VAT, visceral adipose tissue; WC, waist circumference.
Median values of SAT: tertile 1 (men, 1,597 cm3; women, 1,722 cm3); tertile 2 (men, 2,401 cm3; women, 2,861 cm3); tertile 3 (men, 3,600 cm3; women, 4,656 cm3).
Adjusted for age, sex, current smoking, regular alcohol consumption, menopausal status, and hormone replacement therapy use.
Median values of VAT: tertile 1 (men, 1,234 cm3; women, 543 cm3); tertile 2 (men, 2,092 cm3; women, 1,180 cm3); tertile 3 (men, 3,127 cm3; women, 2,104 cm3).
Table 4 shows the results of the interaction between SAT, VAT, and sex, age, and BMI on the insulin measures. There was no interaction between either SAT or VAT, and sex for any outcome. There was a statistically significant interaction between SAT and age for insulin (P = 0.002) and HOMAIR (P = 0.008), where there was a stronger association among participants ≥50 years of age. There was a statistically significant interaction between VAT and age for proinsulin (P = 0.01) and proinsulin/insulin ratio (P = 0.003), where there was a stronger association among the younger participants (<50 years of age). We observed an interaction between VAT and BMI for insulin (P = 0.0004), proinsulin (P = 0.003), and HOMAIR (P = 0.003). The strongest effect sizes were seen among obese participants.
Table 4.
Multivariable-adjusted β-coefficients for the increment in insulin measures per standard deviation increment of adipose tissue, stratified by sex, age, and BMI
| SAT |
VAT |
|||||
|---|---|---|---|---|---|---|
| Outcome variable | β (s.e.) | P value |
P value for interactiona |
β (s.e.) | P value |
P value for interactiona |
| Log insulin | ||||||
| Men | 0.16 (0.01) | <0.0001 | 0.21 (0.01) | <0.0001 | ||
| Women | 0.15 (0.01) | <0.0001 | 0.98 | 0.19 (0.01) | <0.0001 | 0.14 |
| Age <50 years | 0.14 (0.01) | <0.0001 | 0.19 (0.01) | <0.0001 | ||
| Age ≥50 years | 0.18 (0.01) | <0.0001 | 0.002 | 0.21 (0.01) | <0.0001 | 0.58 |
| BMI <25 kg/m2 | — | — | 0.13 (0.02) | <0.0001 | ||
| BMI 25–29.9 kg/m2 | — | — | 0.15 (0.01) | <0.0001 | ||
| BMI ≥30 kg/m2 | — | — | — | 0.15 (0.02) | <0.0001 | 0.0004 |
| Log proinsulin | ||||||
| Men | 0.18 (0.01) | <0.0001 | 0.25 (0.02) | <0.0001 | ||
| Women | 0.20 (0.01) | <0.0001 | 0.46 | 0.25 (0.01) | <0.0001 | 0.52 |
| Age <50 years | 0.18 (0.01) | <0.0001 | 0.27 (0.01) | <0.0001 | ||
| Age ≥50 years | 0.20 (0.01) | <0.0001 | 0.51 | 0.23 (0.01) | <0.0001 | 0.01 |
| BMI <25 kg/m2 | — | — | 0.15 (0.02) | <0.0001 | ||
| BMI 25–29.9 kg/m2 | — | — | 0.16 (0.02) | <0.0001 | ||
| BMI ≥30 kg/m2 | — | — | — | 0.20 (0.02) | <0.0001 | 0.003 |
| Log proinsulin/insulin ratio | ||||||
| Men | 0.019 (0.002) | <0.0001 | 0.027 (0.002) | <0.0001 | ||
| Women | 0.025 (0.002) | <0.0001 | 0.10 | 0.031 (0.002) | <0.0001 | 0.31 |
| Age <50 years | 0.023 (0.002) | <0.0001 | 0.035 (0.002) | <0.0001 | ||
| Age ≥50 years | 0.020 (0.003) | <0.0001 | 0.28 | 0.024 (0.003) | <0.0001 | 0.003 |
| BMI <25 kg/m2 | — | — | 0.019 (0.005) | 0.0005 | ||
| BMI 25–29.9 kg/m2 | — | — | 0.019 (0.003) | <0.0001 | ||
| BMI ≥30 kg/m2 | — | — | — | 0.022 (0.004) | <0.0001 | 0.44 |
| Log HOMAIR | ||||||
| Men | 0.17 (0.01) | <0.0001 | 0.23 (0.01) | <0.0001 | ||
| Women | 0.18 (0.01) | <0.0001 | 0.35 | 0.23 (0.01) | <0.0001 | 0.78 |
| Age <50 years | 0.16 (0.01) | <0.0001 | 0.23 (0.01) | <0.0001 | ||
| Age ≥50 years | 0.20 (0.01) | <0.0001 | 0.008 | 0.23 (0.01) | <0.0001 | 0.96 |
| BMI <25 kg/m2 | — | — | 0.15 (0.02) | <0.0001 | ||
| BMI 25–29.9 kg/m2 | — | — | 0.18 (0.01) | <0.0001 | ||
| BMI ≥30 kg/m2 | — | — | — | 0.17 (0.02) | <0.0001 | 0.003 |
Multivariable is adjusted for age, sex, current smoking, regular alcohol consumption, menopausal status, and hormone replacement therapy use. SAT and VAT are standardized (sex-specific) to a mean of zero and a standard deviation of one. We were unable to examine the interaction between SAT and BMI due to the high collinearity between these two variables.
HOMAIR, homeostasis model assessment of insulin resistance; SAT, abdominal subcutaneous adipose tissue; VAT, visceral adipose tissue.
Interaction between SAT or VAT and sex, age, or BMI category.
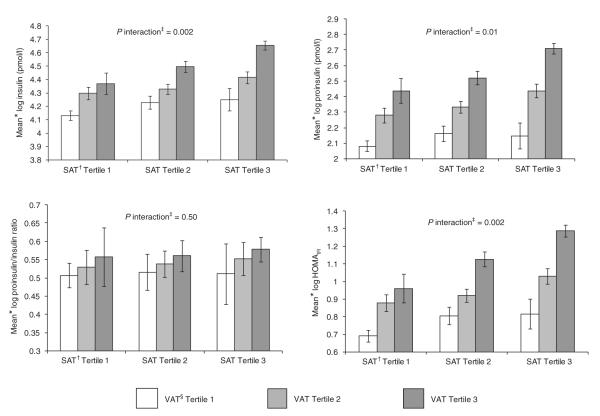
Figure 1 shows the interaction between SAT and VAT tertiles on the insulin measures. There was a significant interaction for insulin (P interaction = 0.002), proinsulin (P interaction = 0.01), and HOMAIR (P interaction = 0.002). Overall, individuals in the top tertile of both SAT and VAT had the highest levels of insulin, proinsulin, and HOMAIR. VAT was most strongly associated with insulin, proinsulin, and HOMAIR among those in the top tertile of SAT.
Figure 1.
Multivariable-adjusted mean insulin variables by tertiles of abdominal subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). HOMAIR, homeostasis model assessment of insulin resistance. Note: Bars represent 95% confidence intervals. *Adjusted for age, sex, current smoking, regular alcohol consumption, menopausal status, and hormone replacement therapy use. †Median values of SAT: tertile 1 (men, 1,597 cm3; women, 1,722 cm3); tertile 2 (men, 2,401 cm3; women, 2,861 cm3); tertile 3 (men, 3,600 cm3; women, 4,656 cm3). ‡P interaction refers to the interaction between SAT and VAT. §Median values of VAT: tertile 1 (men, 1,234 cm3; women, 543 cm3); tertile 2 (men, 2,092 cm3; women, 1,180 cm3); tertile 3 (men, 3,127 cm3; women, 2,104 cm3).
DISCUSSION
Principal findings
In this study, we examined the association between abdominal SAT and VAT and insulin measures. Our findings are fivefold. First, both SAT and VAT were statistically significant correlates of insulin, proinsulin, proinsulin/insulin ratio, and HOMAIR. Second, VAT was a stronger correlate of insulin variables than SAT. Third, we observed an interaction between adipose tissue depots and age-group. Among older individuals (≥50 years), there was a stronger association between SAT and insulin and HOMAIR and a weaker association between VAT and proinsulin and proinsulin/insulin ratio. Fourth, we observed an interaction between VAT and BMI, where VAT had a greater effect on insulin, proinsulin, and HOMAIR among obese participants as compared to normal-weight participants. Fifth, we observed an interaction between SAT and VAT for insulin and proinsulin, where individuals with high SAT and high VAT had greater insulin and proinsulin levels than those with elevated levels of either depot alone.
In the context of the current literature
The association between abdominal obesity, as measured by SAT and VAT, and insulin measures has been examined in several small cross-sectional studies (7–17). The majority of these studies were performed in highly selective patient populations, such as obese individuals, postmenopausal women, and specific ethnic/racial groups. Most have shown that both SAT and VAT are correlates of insulin measures (7–13). However, some studies have found that only VAT is a correlate of insulin resistance measures (14–17). Few studies have formally tested the difference between SAT and VAT. Of those that did, most found that VAT was a stronger correlate of insulin resistance than SAT (14,16,17). Interestingly, the studies that demonstrated that VAT was a stronger correlate of insulin resistance than SAT were all done in study samples of obese participants (14,16,17). This is consistent with our observation that there was a stronger association between VAT and insulin, proinsulin, and HOMAIR among obese participants as compared to normal-weight participants. However, some studies did show that SAT was a stronger correlate or remained as a significant predictor in multivariable models after adjusting for VAT (8,12), which we did not observe in the present study. The major limitation of these studies is their small sample size, which may account for the disparate results. Only one, the Insulin Resistance Atherosclerosis Study Family Study (13), had a sample size >100, whereas our study had a sample size of nearly 3,000 individuals. Prior work has demonstrated that volumetric measurements of SAT and VAT are superior to single-slice measurements (23). However, it has been suggested that a single-slice measurement is sufficient for SAT, but that the location of the measurement for VAT affects the degree of association (24). However, we did not observe any notable differences in the correlation of insulin measures and volumetric fat measures as compared to single-slice measures. It is also possible the varying types of study samples may account for the disparate results.
Overall, our results were consistent with the majority of prior studies in that it showed that both SAT and VAT were correlates of insulin resistance measures and that the strength of the association for VAT was greater than that of SAT. We also did observe an interaction between SAT and VAT that was demonstrated in prior studies (16,17). Our study extends the current literature by examining the association between adipose tissue depots and insulin measures in a large population-based study. We included four different insulin measures in the analysis, which characterize both aspects of hyperglycemia: impairments in insulin sensitivity and defects in pancreatic β-cell secretory function. High levels of insulin and HOMAIR are measures of insulin resistance, whereas high proinsulin and proinsulin/insulin ratio are measures of β-cell secretory function. Because it has been suggested that proinsulin and proinsulin/insulin ratio are markers of insulin resistance, we included them in our study to provide a more comprehensive analysis (25).
Biological mechanisms
Several mechanisms have been proposed to explain the link between excess adipose tissue and insulin resistance. It is known that both SAT and VAT release free fatty acids into the circulation, and elevated levels of free fatty acids have been associated with insulin resistance (26,27). Elevated free fatty acid levels have several adverse effects including inhibition of insulin-stimulated glucose uptake, glycogen synthesis, and glucose oxidation (28). Additionally, visceral fat, in particular, has been correlated with endothelial dysfunction and with C-reactive protein levels, which may indicate an inflammatory component to the adverse effects of VAT (29,30).
It has been established that adipose tissue acts as an endocrine organ (31). SAT and VAT have different endocrine functions, which may account for their varying associations with risk of adverse outcomes. VAT is more inversely associated with adiponectin levels than SAT (31,32). Additionally, VAT releases higher levels of IL-6 and PAI-1, relative to SAT, which also may account for VAT’s stronger association with insulin resistance (31). It has been suggested that there may be a causal link between both IL-6 and PAI-1 and obesity and insulin resistance because levels of these inflammatory markers decrease after weight loss (31). However, it is important to note that IL-6 and PAI-1 are secreted by SAT, albeit in lower levels than VAT, which may explain the interaction we observed between SAT and VAT on insulin resistance.
strengths and limitations
Our study extends the current literature by comparing the relative strength of SAT vs. VAT on insulin resistance measures in a large population-based study. One of the limitations of this study is that it is cross-sectional in design, so we cannot infer causality from the results.
Additionally, the study sample is predominantly white, thus these results may not be generalizable to other racial or ethnic groups. Furthermore, we used HOMAIR instead of the gold standard insulin clamp measure to assess insulin sensitivity. Finally, we were unable to distinguish between superficial and deep SAT, which have been shown to have differing associations with insulin resistance (9).
conclusions
Both SAT and VAT are correlates of insulin resistance. However, VAT is more strongly associated with insulin resistance than SAT. VAT is associated with approximately a twofold increased risk of insulin resistance after accounting for traditional adiposity measures.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (N01-HC-25195). R.S.V. was supported in part by R01DK080739. J.B.M. is supported by NIDDK K24 DK080140.
Footnotes
SUPPLEMENTARY MATERIAL Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
DISCLOSURE J.B.M. currently has research grants from GlaxoSmithKline and Sanofi-Aventis, and has consulting agreements with Eli Lilly, Interleukin Genetics, Kalypsys, and Outcomes Science. The other authors declared no conflict of interest.
REFERENCES
- 1.Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–324. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108(Suppl 6a):9S–14S. doi: 10.1016/s0002-9343(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 3.Jeppesen J, Hansen TW, Rasmussen S, et al. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49:2112–2119. doi: 10.1016/j.jacc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metab Clin Exp. 2004;53:495–499. doi: 10.1016/j.metabol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 7.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 9.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 10.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 11.Sites CK, Calles-Escandón J, Brochu M, et al. Relation of regional fat distribution to insulin sensitivity in postmenopausal women. Fertil Steril. 2000;73:61–65. doi: 10.1016/s0015-0282(99)00453-7. [DOI] [PubMed] [Google Scholar]
- 12.Tulloch-Reid MK, Hanson RL, Sebring NG, et al. Both subcutaneous and visceral adipose tissue correlate highly with insulin resistance in african americans. Obes Res. 2004;12:1352–1359. doi: 10.1038/oby.2004.170. [DOI] [PubMed] [Google Scholar]
- 13.Wagenknecht LE, Langefeld CD, Scherzinger AL, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–2496. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 14.Brochu M, Starling RD, Tchernof A, et al. Visceral adipose tissue is an independent correlate of glucose disposal in older obese postmenopausal women. J Clin Endocrinol Metab. 2000;85:2378–2384. doi: 10.1210/jcem.85.7.6685. [DOI] [PubMed] [Google Scholar]
- 15.Rendell M, Hulthén UL, Törnquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab. 2001;86:744–749. doi: 10.1210/jcem.86.2.7260. [DOI] [PubMed] [Google Scholar]
- 16.Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87:5044–5051. doi: 10.1210/jc.2002-020570. [DOI] [PubMed] [Google Scholar]
- 17.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282:E657–E663. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Boyko EJ, McNeely MJ, et al. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 19.Maurovich-Horvat P, Massaro J, Fox CS, et al. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 20.Irlbeck T, Massaro JM, Bamberg F, et al. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2009.279. e-pub ahead of print 12 January 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 22.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 23.Ellis KJ, Grund B, Visnegarwala F, et al. Strategies for Management of Anti-Retroviral Therapy (SMART) Study Group. Visceral and subcutaneous adiposity measurements in adults: influence of measurement site. Obesity (Silver Spring) 2007;15:1441–1447. doi: 10.1038/oby.2007.172. [DOI] [PubMed] [Google Scholar]
- 24.Kuk JL, Church TS, Blair SN, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with the metabolic syndrome? Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- 25.Kim NH, Kim DL, Choi KM, Baik SH, Choi DS. Serum insulin, proinsulin and proinsulin/insulin ratio in type 2 diabetic patients: as an index of beta-cell function or insulin resistance. Korean J Intern Med. 2000;15:195–201. doi: 10.3904/kjim.2000.15.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48:1586–1592. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 27.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boden G. Free fatty acids-the link between obesity and insulin resistance. Endocr Pract. 2001;7:44–51. doi: 10.4158/EP.7.1.44. [DOI] [PubMed] [Google Scholar]
- 29.Lemieux I, Pascot A, Prud’homme D, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto M, Akishita M, Eto M, et al. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Int J Obes Relat Metab Disord. 1998;22:477–484. doi: 10.1038/sj.ijo.0800620. [DOI] [PubMed] [Google Scholar]
- 31.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 32.Kwon K, Jung SH, Choi C, Park SH. Reciprocal association between visceral obesity and adiponectin: in healthy premenopausal women. Int J Cardiol. 2005;101:385–390. doi: 10.1016/j.ijcard.2004.03.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.