Summary
The Reelin ligand regulates a Dab1-dependent signaling pathway required for brain lamination and normal dendritogenesis, but the specific mechanisms underlying these actions remain unclear. We find that Stk25, a modifier of Reelin-Dab1 signaling, regulates Golgi morphology and neuronal polarization as part of an LKB1-Stk25-Golgi matrix protein 130 (GM130) signaling pathway. Overexpression of Stk25 induces Golgi condensation and multiple axons, both of which are rescued by Reelin treatment. Reelin stimulation of cultured neurons induces the extension of the Golgi into dendrites, which is suppressed by Stk25 overexpression. In vivo, Reelin and Dab1 are required for the normal extension of the Golgi apparatus into the apical dendrites of hippocampal and neocortical pyramidal neurons. This demonstrates that the balance between Reelin-Dab1 signaling and LKB1-Stk25-GM130 regulates Golgi dispersion, axon specification and dendrite growth, and provides insights into the importance of the Golgi apparatus for cell polarization.
Introduction
The development of the exquisite morphology of neurons is a carefully orchestrated process that optimizes the ability of individual neurons to receive signals, integrate them and transmit the output to target cells. Neuronal polarization, first observed as the rapid growth of a process that will ultimately become an axon, followed by the asymmetrical development of dendrites, are key steps in morphological and functional maturation (Arimura and Kaibuchi, 2005). Interestingly, the Golgi apparatus has been implicated in these different aspects of neuronal polarity. In the nascent neuron, the position of the Golgi and the adjoined centrosome correlates with the site of axon emergence, which becomes the future basal side of a mature pyramidal neuron (de Anda et al., 2010; de Anda et al., 2005; Zmuda and Rivas, 1998). Later, the Golgi apparatus is positioned on the apical side of pyramidal neurons, proximal to the major apical dendritic tree and opposite to the axon and minor basal dendrites (Horton et al., 2005). Dispersion of the Golgi apparatus away from the apical pole leads to a loss of dendrite asymmetry in these cells, with equal-sized apical and basal dendrites (Horton et al., 2005). Furthermore, specialized Golgi outposts, which populate dendrites, promote the elaboration of dendritic branches (Ye et al., 2007). However, it remains to be determined how Golgi positioning within neurons is regulated.
Mutations in the genes encoding the Reelin-Dab1 signaling pathway lead to profound defects in neuronal positioning and dendritogenesis during brain development (Niu et al., 2004; Rice et al., 2001). The lamination of the cerebral cortex, hippocampus and cerebellum is disorganized and appears approximately inverted compared to normal. Reelin is a secreted ligand that is produced in discreet layers in the developing brain (D'Arcangelo et al., 1995; Ogawa et al., 1995). Genetic and biochemical studies have shown that it regulates a signal transduction pathway requiring the ApoE receptors ApoER2 and VLDLR (D'Arcangelo et al., 1999; Hiesberger et al., 1999; Trommsdorff et al., 1999), the cytoplasmic adaptor protein Dab1 (Howell et al., 2000), and Src family kinases (Arnaud et al., 2003; Bock and Herz, 2003). Disparate functions have been proposed for Reelin-Dab1 signaling, though a clear biological response to clarify its role in brain development is lacking (Chai et al., 2009; Cooper, 2008; Forster et al., 2010; Sanada et al., 2004).
The severity of Dab1-dependent phenotypes depends on the genetic background (Brich et al., 2003). We have recently identified stk25 as a modifier of dab1-mutant phenotypes (manuscript in preparation). Here we characterize the role of Stk25 (also YSK1, Sok1) in nervous system development. Previous work has implicated Stk25 in regulating Golgi morphology through the Golgi matrix protein GM130 (Preisinger et al., 2004), which we confirm here. GM130 regulates the fusion of ER-to-Golgi vesicles with the Golgi cisternae and the fusion of Golgi cisternae into elongated ribbons (Barr and Short, 2003; Puthenveedu et al., 2006). Depletion or mitotic phosphorylation of GM130 leads to Golgi fragmentation and reduced efficiency of biosynthetic processing (Lowe et al., 1998; Marra et al., 2007; Puthenveedu et al., 2006).
The protein kinase LKB1 and its associated factors STRAD and MO25 are known to be important for neuronal polarization, axon specification and dendrite growth (Asada et al., 2007; Barnes et al., 2007; Shelly et al., 2007). In this study, we find that Stk25 is part of an LKB1 cell polarization pathway. Stk25, LKB1 and GM130 are shown to regulate Golgi morphology and axon initiation. In addition, we show that Stk25 and Reelin-Dab1 signaling have antagonistic effects on neuronal polarization and the morphology and subcellular distribution of the Golgi. Since the position of the Golgi plays roles in cell polarization, process extension and cell migration (Fidalgo et al., 2010; Horton et al., 2005; Yadav et al., 2009; Ye et al., 2007), this evidence is fundamental for understanding the molecular control of neuronal morphogenesis and provides new insights into the biological role of Reelin-Dab1 signaling.
Results
Stk25 regulates neuronal polarity
Stk25 has previously been shown to regulate the polarized migration of epithelial cells. Since other Ste20-like kinases have roles in neuronal polarization (Jacobs et al., 2007; Preisinger et al., 2004), we sought to assess a role for Stk25 in neuronal polarization using hippocampal neuronal cultures (Dotti and Banker, 1987). These neurons have a stereotypic morphology and program of differentiation, and respond to Reelin-Dab1 signaling (Matsuki et al., 2008). Soon after plating, they extend short uniform processes that have the potential to develop into either axons or dendrites (Arimura and Kaibuchi, 2007). By stage III, 48 to 72 h later, one of the processes can be identified as an axon while the other processes differentiate into dendrites.
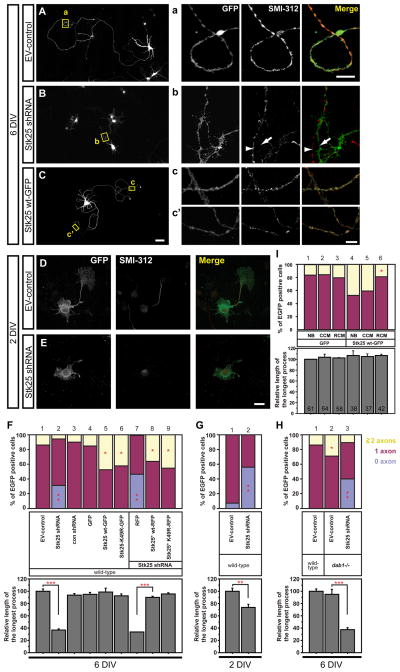
We reduced Stk25 levels by infection with a lentivirus carrying GFP and Stk25 shRNA, and identified axons 6 days later using SMI-312, a pan-axonal neurofilament marker. Depletion of Stk25 inhibited axon specification. At least 30% of the Stk25 shRNA lentivirus-infected, GFP-positive neurons lacked an axon (Fig. 1B, 1F lane 2), while axons were detected in all neurons infected with either empty vector (EV) or control shRNA vectors (Fig 1A, 1F lane 1, 3 and insets). The longest process in Stk25 shRNA-expressing cells was also much shorter than the long axons of control cells (Fig. 1A, 1B, 1F, lane 2), consistent with a failure to induce an axon.
Figure 1. Stk25 expression regulates axon differentiation in culture.
A Primary hippocampal neurons (E17.5) infected with the GFP-expressing EV-control virus had typical pyramidal neuron morphologies, including a long SMI-positive axon (inset a) and shorter dendrites. B Neurons infected with the Stk25 shRNA virus had shorter processes and frequently lacked long (>250 μm) SMI-positive processes that meet the criteria for axons (inset b). An SMI-positive process (arrowhead) from a non-infected neuron runs parallel to the GFP-positive process (arrow). C Cells overexpressing Stk25 wild-type (wt)-GFP had multiple SMI-positive axons (insets c, c’). D At stage III (2DIV), EV-control infected neurons have one dominant SMI-positive axon. E In contrast, Stk25 shRNA-expressing neurons often lacked SMI-positive, axon-like processes. F The number of neurons with 0, 1, 2 or more axons and the length of the longest processes were determined for neurons infected with the indicated viruses. For rescue experiments, neurons were coinfected with the Stk25 shRNA (GFP positive) and either RFP, Stk25* wt-RFP, or Stk25* K49R-RFP expressing viruses (lanes 7-9, Fig. S2). G At stage III (2DIV) many Stk25 shRNA-expressing neurons lacked axons as compared to a small percentage of EV-control infected neurons H The number of neurons with multiple axons was increased in dab1 −/− (lane 2) compared to wild-type neurons (lane 1, duplicated from F) and this was reduced by Stk25 shRNA expression (lanes 3). I Primary hippocampal neurons that were infected with either GFP or Stk25 wt-GFP expressing viruses were split into three groups and grown in either neurobasal (NB), control-conditioned (CCM) or Reelin-conditioned (RCM) media for six days. Statistical significance (*,**,***, p <0.0001, Student’s t-test, compared between the sample pairs: for F 1:2; 4:5,6,7; 7:8,9, for G 1:2, for H 1:2, 2:3; n >60; I 5:6 n indicated in bars). Bars C 50 μm; a, c’ 5 μm; E 20 μm. See also Fig. S1.
To assess whether axon absence was specifically caused by reduced Stk25 expression, we tested for rescue by Stk25 overexpression. Both kinase-active and kinase-inactive versions of an shRNA-resistant Stk25 (Stk25*) were expressed as red fluorescent protein (RFP) fusion proteins in cultures that were also infected with the GFP-expressing, Stk25 shRNA virus (Fig. S1A-S1D). Both kinase-active and kinase-inactive Stk25*-RFP rescued the axon-less phenotype caused by Stk25 knockdown (Fig. 1F, lanes 7–9). This suggests that the axon-less phenotype in Stk25 shRNA-expressing cells was the specific result of reducing Stk25 expression and that Stk25 kinase activity is not required for axon production.
To investigate whether Stk25 affected axon initiation or maintenance, we examined stage III hippocampal neurons (Fig. 1D, 1E). We found that 56±5% of Stk25 knockdown neurons lacked an axon compared to only 7±8% of control samples (Fig. 1G). The longest neurite in Stk25 knockdown neurons was also significantly shorter than the incipient axon in control cultures. Moreover, overexpression of Stk25 induced multiple axons. Expression of either the wild-type or kinase-inactive Stk25*-RFP fusion proteins, or an Stk25-green fluorescent protein (GFP) fusion that has previously been shown to be biologically active (Preisinger et al., 2004; Fig. S2B-S2E), induced multiple SMI-positive axons in approximately 45% – 50% of neurons as compared to 15±3% in GFP-alone expressing controls (Fig. 1C, 1F lanes 5, 6, 8, 9). Stk25 overexpression did not increase axon length (Fig. 1F). Taken together, the results show that Stk25 regulates axon initiation but not axon growth in cultured neurons.
Reelin-Dab1 signaling suppresses multiple axon production
Stk25 is expressed at relatively high levels in Reelin-Dab1 responsive cells in the developing cortical plate (Fig. S1E) and in the adult hippocampus and cerebellar Purkinje cells (Fig. S1F). Since we identified stk25 in a screen for modifiers of dab1 mutant phenotypes (manuscript in preparation), we examined whether Reelin-Dab1 signaling might have an undiscovered role in axon initiation. Hippocampal neurons were cultured from dab1−/− mutant embryos and infected with GFP-expressing lentiviruses to survey their morphology. Surprisingly, approximately 30% of the dab1−/− mutant neurons produced multiple axons as compared to approximately 15% of the wild-type neurons (Fig. 1H). To determine if the multiple axon phenotype in dab1−/− mutant neurons was sensitive to Stk25 expression level, we examined the effect of knocking down Stk25. Significantly fewer dab1−/− mutant neurons infected with the Stk25 shRNA-expressing lentivirus produced multiple axons than the GFP-expressing control sample (Fig. 1H). In addition, a significant number of the Stk25 shRNA-expressing neurons completely lacked axons. This shows that Reelin-Dab1 signaling regulates axon initiation and that the multiple axon phenotype in dab1−/− mutant mice is dependent upon Stk25 expression.
Congruent with this result, growth of neurons in the presence of Reelin suppressed the multiple axon phenotype caused by Stk25 overexpression (Fig. 1I). This treatment did not, however, lead to the loss of axon production, which would be expected if Stk25 function was abolished. None of these treatments affected axon length. Therefore, Reelin-Dab1 signaling appears to counteract the effects of high Stk25 expression without completely blocking its function in axon induction.
Stk25 regulates axon formation and dendrite asymmetry in vivo
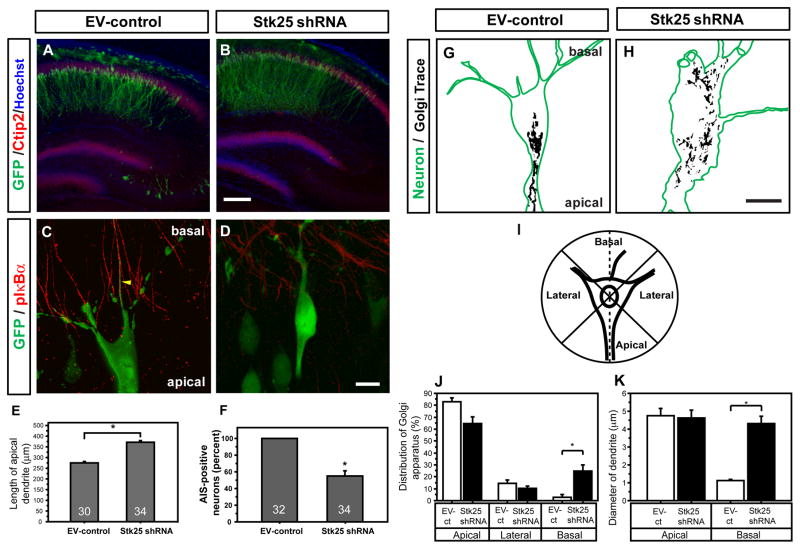
To investigate whether Stk25 regulates neuronal differentiation in vivo, we electroporated the Stk25 shRNA-expressing vector into the hippocampi of fetal mice. The brains of these mice were analyzed for GFP expression and neuronal polarization of Ctip2-positive, pyramidal neurons in the CA1 region of the hippocampus at postnatal day 7 (P7). Stk25 shRNA did not interfere with the positioning of neurons but their apical dendrites were significantly longer (Fig 2A, 2B, 2E). In addition, approximately 40% of the strongly GFP-positive, Stk25 shRNA-expressing neurons lacked identifiable axon initial segments, detected using anti-phospho-IκBα antibodies, suggesting that axons were either absent or failed to mature normally (Fig. 2D, 2F, movie S1). By comparison, all of the GFP-positive, EV-control electroporated neurons examined had axon initial segments (Fig. 2C, 2F, movie S1). This suggests that Stk25 regulates axon specification and dendrite growth in hippocampal pyramidal neurons in vivo.
Figure 2. Stk25 regulates neuronal polarity during brain development.
A EV-control vector (GFP positive, green) electroporated at E16.5 in utero was expressed in Ctip2-positive (red), hippocampal-pyramidal neurons at P7. B Stk25 shRNA-expressing neurons (GFP positive) were appropriately positioned in the CA1 layer, and their apical dendrites extended further than EV-control. C GFP-expressing, EV-control transfected CA1 neurons had the typical pyramidal shape and phospho-IκBα- (red), GFP-positive (green) axon initial segments (Sanchez-Ponce et al., 2008; movie S1). D In contrast, a high percentage of strongly GFP-positive, Stk25 shRNA-expressing neurons were often misshapen and lacked axon initial segments (movie S1). E Quantification of apical dendrite length in EV-control and Stk25 shRNA hippocampi. F Quantification of the number of GFP-, Ctip2-positive pyramidal neurons that had axon initial segments (n indicated in bar.) G In EV-control neurons, the Golgi apparatus (trace of GRASP65 signal) is concentrated on the apical side of the neuron (movie S2). H In Stk25 shRNA-expressing neurons, the Golgi apparatus was broadly distributed throughout the neuron (movie S2). I Scheme used to determine Golgi distribution in J. J The Golgi distribution in apical, lateral (combined) or basal quadrants was quantified. K The diameter of the largest apical and basal processes was determined (*p <0.0005, Student’s t-test, n≥12, neurons from 3 animals). Bars B 200 μm; D, H 10 μm. Error bars indicate SEM in all figures.
In addition to having longer apical dendrites, the basal dendrites of Stk25 shRNA-expressing neurons were also atypical. Normal pyramidal neurons have long, thick apical dendrites and much thinner and shorter basal dendrites (Horton et al., 2005; Fig. 2G, 2K, movie S2). The apical dendrites of Stk25 shRNA-expressing neurons had normal thickness, but the basal dendrites were thicker than normal (Fig. 2H, 2K, movie S2). We were not able to measure the length of the basal dendrites. Therefore, there is evidence for growth of both apical and basal dendrites, and this reduced the distinction between apical and basal dendrites in terms thickness. Therefore, Stk25 is needed for normal axon production and dendrite asymmetry in vivo.
Stk25 interacts with STRADα and acts on the LKB1 signaling pathway
The functions of Stk25 resemble those reported for LKB1-STRAD signaling (Barnes et al., 2007; Kishi et al., 2005; Shelly et al., 2007). This pathway has a prominent role in cell polarity control across numerous cell types from Caenorhabditis elegans to man. LKB1 is partially regulated by binding STRAD, which both shuttles it from the nucleus to the cytoplasm and stabilizes it. We therefore investigated whether Stk25 associates with the LKB1-STRAD signaling complex. By immunoprecipitating tagged fusion proteins coexpressed in HEK293T cells, we found that both wild-type and kinase-inactive HA-Stk25 coimmunoprecipitated with myc-STRADα (Fig. S2A). Identifying Stk25 as a direct or indirect STRAD-binding protein suggests a potential role for Stk25 on the LKB1 pathway.
To investigate whether Stk25 is important for LKB1 function, we took two approaches. We examined whether 1) Stk25 is required for LKB1-STRAD regulated epithelial cell polarization and 2) Stk25 overexpression rescues the LKB1 knockdown phenotype in neurons.
We first tested whether reduced Stk25 expression would inhibit the LKB1-STRAD- dependent polarization of W4 intestinal epithelial cells. These cells have been engineered to constitutively express LKB1 and express STRAD in response to doxycyline, which leads to their polarization (Baas et al., 2004). Most W4 cells infected with EV and control shRNA lentiviruses became polarized within 24 h of doxycycline treatment (Fig. S2C, S2E). In contrast, only 20% of cells infected by the humanized (h) Stk25 shRNA lentivirus were polarized by doxycycline treatment (Fig. S2C, S2E). Furthermore, expression of either wild-type or kinase-inactive Stk25*-RFP rescued STRAD-induced polarization in Stk25 shRNA-expressing W4 epithelial cells (Fig. S2F). Collectively, these experiments show that the Stk25 protein, not its kinase activity, is required for LKB1-STRAD regulated epithelial cell polarization.
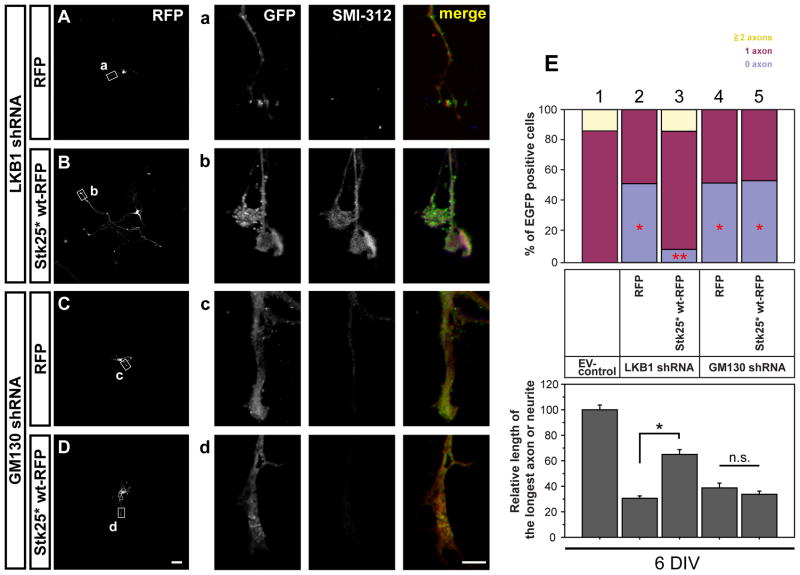
We then confirmed that LKB1 knockdown leads to a loss of axon initiation in cultured hippocampal neurons (Fig. 3A, Barnes et al., 2007; Shelly et al., 2007). We tested whether Stk25 can rescue or bypass the LKB1 requirement by overexpressing Stk25* wt-RFP in LKB1 shRNA-expressing neurons (Fig. 3B). Ninety-two percent of LKB1 knockdown neurons that expressed Stk25* wt-RFP produced at least one axon compared to only 48% of RFP-, LKB1 shRNA-coexpressing neurons (Fig. 3E). These results are consistent with a role of Stk25 on the LKB1 pathway to regulate axon induction.
Figure 3. Stk25-RFP overexpression rescues the neuronal polarization defect caused by LKB1 but not by GM130 knockdown.
A Expression of LKB1 shRNA (GFP positive, green) in hippocampal neurons led to an increase in the number of neurons that lack an axon at 6DIV in cells also expressing RFP (red). a Longest process lacks SMI immunoreactivity. B In contrast, overexpressing Stk25* wt-RFP in LKB1 knockdown neurons rescued axon production. b Long, axon-like process is SMI-positive. C GM130 knockdown (GFP positive) also caused a reduction in axon production in RFP-positive cells. c No SMI-imunoreactivity was detected in processes of the GFP-, RFP- positive neuron. D Stk25* wt-RFP expression did not rescue axonogenesis in GM130 knockdown neurons. d Longest process is SMI negative. E Axon number and the length of the longest processes were quantified for the indicated treatment groups. (Lane 1 was duplicated from Fig. 1F lane 1). (* p<0.005 compared to lane 1, ** p=0.01 compared to lane 2, Student’s t-test) Bars: D, 50 μm; d, 5 μm. See also Fig. S2.
GM130 interacts with Stk25 and regulates axon induction
The Golgi matrix protein GM130, which has critical roles in regulating Golgi dynamics, was identified as an Stk25 binding partner in a yeast two-hybrid screen (Preisinger et al., 2004). We confirmed this interaction by coimmunoprecipitating tagged fusions of GM130 and Stk25 (Fig. S2B). Interestingly, kinase-inactive Stk25 consistently immunoprecipitated with GM130 more efficiently than wild-type, suggesting that Stk25-dependent phosphorylation may destabilize the complex.
Stk25 colocalizes with GM130 at the Golgi apparatus of HeLa cells (Preisinger et al., 2004). To determine if Stk25 localizes to the Golgi complex in neurons, we raised an antibody to a region of Stk25 that is divergent from the close relatives Mst3 and Mst4 (extended experimental procedures). Endogenous Stk25 expression overlapped with the GM130-positive cis-Golgi in neurons at stage III, coincident with axon specification (Fig. S2D).
To asses if GM130 plays a role in neuronal differentiation, we examined GM130 shRNA-expressing neurons for defects in polarity. Similar to Stk25 and LKB1 knockdown neurons, knockdown of GM130 reduced axon number at 6DIV (Fig. 3C). GM130 knockdown also caused a significant reduction in axon initiation in stage III (2DIV) neurons (data not shown). Stk25*-RFP overexpression in GM130-deficient cells did not rescue axon number at 6DIV (Fig. 3D), which suggests that GM130 is required for neuronal polarization downstream of Stk25.
Stk25, GM130 and LKB1 regulate Golgi distribution
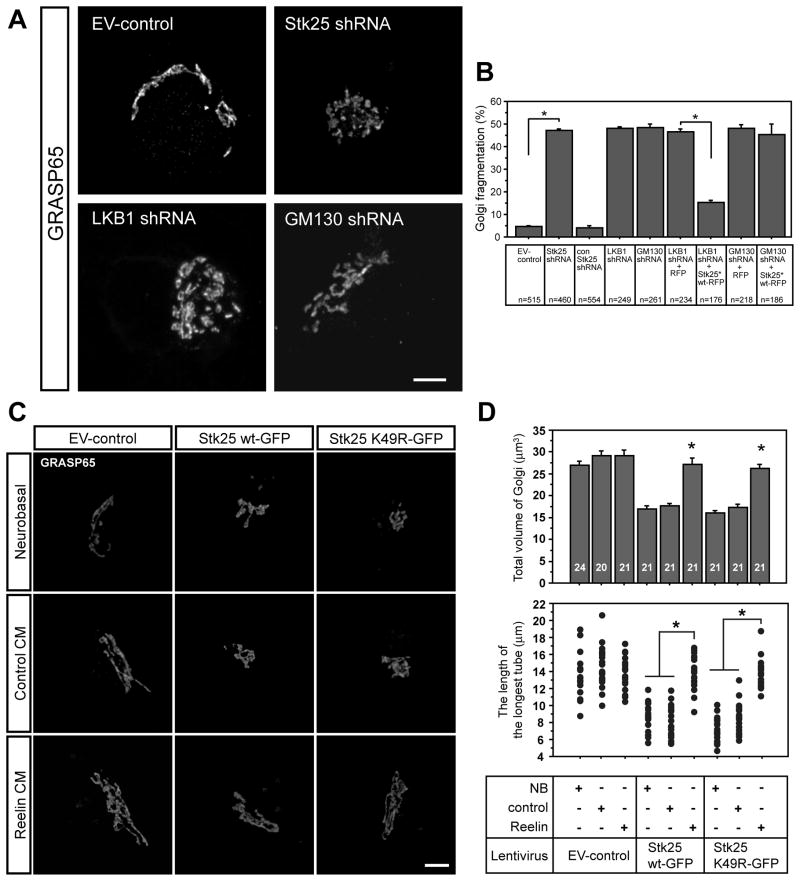
Previously it was shown that GM130 regulates Golgi morphology in HeLa cells (Puthenveedu et al., 2006). Since Stk25, LKB1 and GM130 regulate axon initiation, and the position of the Golgi apparatus early in differentiation normally coincides with axonal localization (de Anda et al., 2010; de Anda et al., 2005), we examined whether Stk25, LKB1 and GM130 regulate Golgi morphology (Fig. 4). Individually knocking down Stk25, LKB1 and GM130 in stage III primary hippocampal neurons resulted in dispersion of Golgi elements in a high percentage of cells, in contrast to the typical elongated morphology observed in the EV-control neurons (Fig. 4A, 4B, movie S3).
Figure 4. Golgi apparatus morphology is regulated by Stk25, LKB1 and GM130 expression and Reelin signaling.
A Stage III neurons that were infected with the EV-control virus had typical cis-Golgi ribbons (GRASP65, movie S3). In contrast, the cis-Golgi in Stk25 shRNA-, LKB1 shRNA- or GM130 shRNA-expressing neurons was fragmented (movie S5). GFP signal was omitted for clarity. B Significantly more Stk25 knockdown neurons had fragmented Golgi complexes compared to the EV-control and the control shRNA (n, as indicated). LKB1 and GM130 knockdown also caused significant Golgi fragmentation as compared to EV-control infected neurons. Stk25*-RFP expression rescued Golgi fragmentation in LKB1 shRNA but not GM130 shRNA-expressing neurons. C Neurons overexpressing either Stk25 wt-GFP or Stk25 K49R-GFP had condensed cis-Golgi (GRASP65 signal) compared to EV-controls when grown in either neurobasal or control-CM. Growth in Reelin-CM partially rescued the Golgi appearance in Stk25-overexpressing cells. GM130 and GRASP65 colocalized under all conditions (not shown). D Golgi volume (upper panel) and the length of the longest Golgi ribbon (lower panel) were determined (* p< 0.0001, Student’s t-test, n indicated in bars). Bars 5 μm. See also Fig. S3.
Interestingly, the Golgi fragmentation caused by LKB1 knockdown was rescued by Stk25*-RFP overexpression (Fig. 4B), suggesting that Stk25 overexpression can compensate for reductions in LKB1 signaling. In contrast, Golgi fragmentation in GM130 shRNA-expressing cells was not rescued by Stk25 overexpression (Fig. 4B). Overexpression of either Stk25 wt-GFP or Stk25 K49R-GFP led to the condensation of the Golgi into a smaller volume (Fig. 4C, neurobasal). Therefore, increasing or decreasing Stk25 expression from endogenous levels has different consequences for Golgi morphology, in addition to having the opposite effects on axon production. These results suggest an LKB1-Stk25-GM130 pathway for Golgi regulation in cultured neurons.
Importantly, Stk25 knockdown in hippocampal pyramidal neurons also caused Golgi fragmentation in vivo, as determined by use of in utero electroporation. Normally, the Golgi is strictly localized to the apical side of the soma and forms outposts in the apical dendrite (Horton et al., 2005; Fig. 2G, 2J, movie S2). However, in Stk25 shRNA-expressing Ctip2-positive neurons, the Golgi apparatus was often broadly distributed throughout the soma (Fig. 2H, 2J, movie S2).
In summary, these results indicate that, Stk25, LKB1 and GM130 are required for normal Golgi morphology in neurons at a time when axons are first appearing. Furthermore, the fragmented Golgi phenotype correlated with the loss of axon production in neurons and both phenotypes were rescued by Stk25 overexpression in LKB1 knockdown cells.
Reelin signaling regulates Golgi morphology
Since Stk25 and Reelin have opposing effects on axon initiation (Fig. 1H) and Stk25 affects Golgi morphology (Fig. 4A, 4B), we investigated the role of Reelin in regulating Golgi morphology.
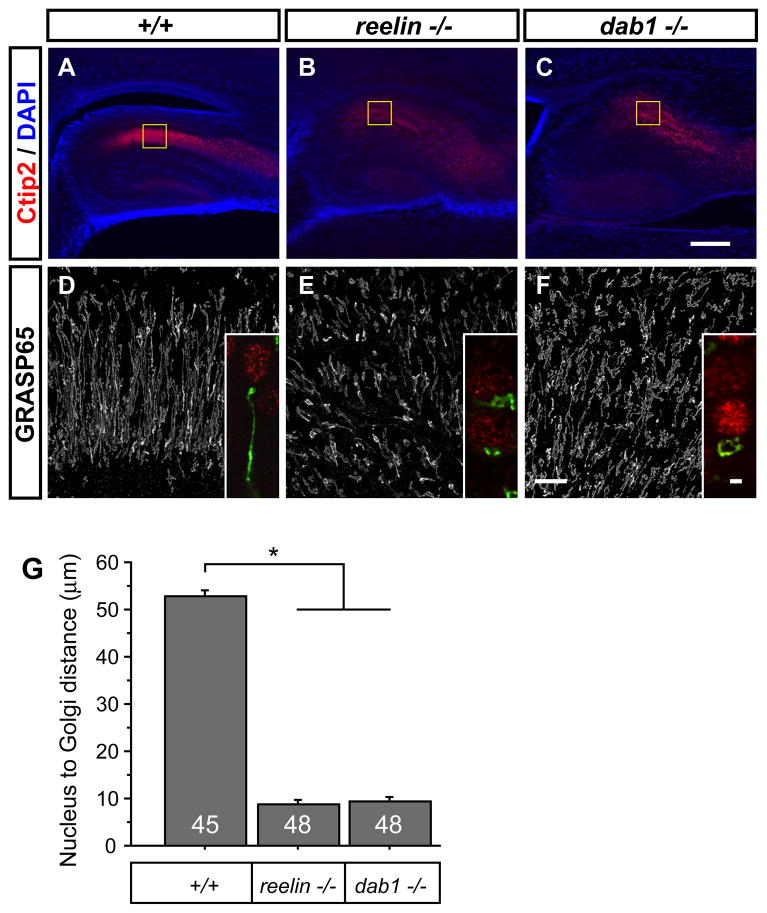
First we examined the appearance of the Golgi apparatus in hippocampal and neocortical pyramidal neurons of reelin−/− and dab1−/− mutant mice. In the pyramidal layer of the wild-type CA1 zone and in developing neocortical layers, the Golgi apparati were linearly organized and extended tens of microns into the apical processes (Fig. 5D, S4D, S4G, insets). The Golgi of the reelin-/− and dab1−/− mutants often appear convoluted near the nucleus rather than extended into a dendrite (Fig. 5E, 5F, S4E, S4F, insets). The distance from the Ctip2-positive nucleus to the tip of the Golgi ribbon was significantly decreased in reelin−/− and dab1−/− mutants as compared to wild-type (Fig. 5G, S4G), indicating that the reelin and dab1 genes either directly or indirectly regulate Golgi extension into the apical process of pyramidal neurons.
Figure 5. The Golgi apparatus extends into an apical process in neonatal hippocampus in a reelin- and dab1- dependent manner.
A Ctip2-positive CA1 neurons are organized into a tight lamella in wild-type brain. B Homozygous disruption of reelin or C dab1 causes dispersion of these neurons. D Confocal imaging through the CA1 region of the wild-type hippocampus revealed that the Golgi apparatus (white, or green, inset) extends radially into the presumptive apical dendrite of Ctip2-positive neurons (red, inset). E In equivalent reelin−/− or F dab1−/− mutant sections, the Golgi is more often convoluted proximal to the nucleus (inset). Insets were selected from regions where isolated cells could be distinquished. G The Golgi phenotype was quantified by measuring the distance from the nucleus to the furthest tip of the Golgi ribbon. (* p<0.0001, Student’s t-test, n indicated in bar from 3 animals per group). Bar 200 μm in C, 20 μm in F and 2 μm in inset. See also Fig. S4.
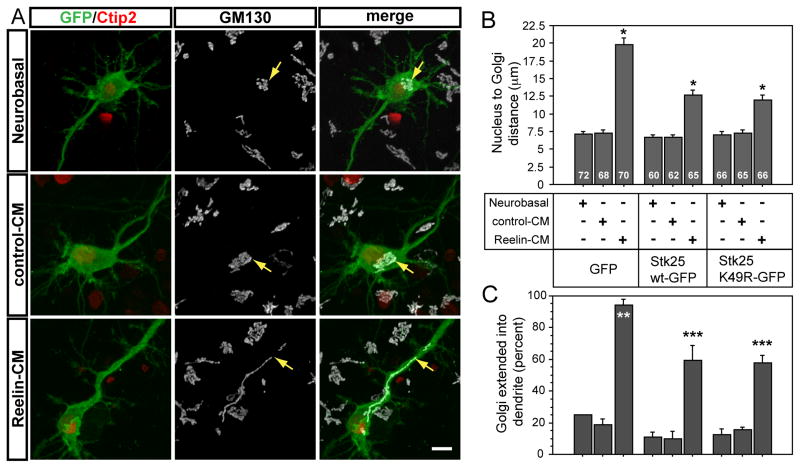
Since Reelin and Dab1 also regulate the proper layering of hippocampal pyramidal neurons (Caviness and Sidman, 1973; Goffinet, 1984; Rice et al., 2001; Fig. 5B, 5C), the effects of Reelin and Dab1 on Golgi deployment may be indirect. Therefore, we tested whether Reelin-Dab1 signaling acutely induces changes in Golgi morphology or localization by treating hippocampal neuron cultures with Reelin for 30 min. Hippocampal pyramidal neurons were infected with a low titer GFP-expressing lentivirus to help visualize individual neurons. The Golgi was largely localized close to the nucleus in control-CM and Neurobasal-treated Ctip2-positive pyramidal neurons (Fig. 6A, 6C). However, in approximately 80±5% of Reelin-CM treated neurons, the Golgi apparati extended into the largest dendritic process (Fig. 6A, 6C). The distance between the nucleus and the most distal portion of the Golgi ribbon from randomly selected Ctip2-positive neurons was significantly larger in the Reelin-CM treated samples compared to the control-CM and neurobasal treated samples (Fig. 6B). The Golgi apparatus is therefore rapidly deployed into dendrites in response to Reelin stimulation.
Figure 6. Reelin stimulation leads to rapid Golgi extension into dendrites.
Primary hippocampal neurons were infected with GFP-expressing viruses after 3DIV and stimulated 3 days later. A The Golgi apparati in Reelin-CM treated neurons extended tens of microns into dendrites, compared to little or no extension into dendrites of control-CM or neurobasal treated neurons. B The distance between the nucleus and the tip of the Golgi was measured for GFP-, Ctip2-positive neurons. Expression of Stk25 wt-GFP and Stk25 K49R-GFP caused a significant reduction in Reelin-induced Golgi extension. C The Golgi of most GFP-, Ctip2-positive Reelin-CM treated neurons extended at least 10 μm from the nucleus into or towards a dendrite. Significantly fewer Golgi were observed in the processes of control treated samples or Reelin-CM treated samples that also overexpressed Stk25. Yellow arrows indicate furthest tip of Golgi ribbon from nucleus. (* p<0.0001, ** p=0.0002, *** p<0.05 Student’s t-test, between Reelin-CM and control-treated samples and between GFP- and Stk25-expressing samples treated with Reelin-CM) Bar 10 μm.
We next evaluated whether the Golgi response to Reelin was sensitive to elevated Stk25 expression levels. Hippocampal neurons were infected with Stk25 wt-GFP or Stk25 K49R-GFP expressing viruses after 72 h in culture and treated analogously to experiments described above. Expression of either Stk25 wt-GFP and Stk25 K49R-GFP reduced but did not eliminate the Golgi extension in response to Reelin (Fig. 6B, 6C). Under these conditions, linear Golgi ribbons were observed extending into the dendrites, but on average this was approximately 50% the distance observed in the Reelin-treated, GFP-expressing cells (Fig. 6B). Furthermore, Reelin signaling suppressed Golgi compaction induced by Stk25 overexpression (Fig. 4C, D). In cultures that were grown in Reelin CM for two days (Fig. 4), we did not observe Golgi deployment into dendrites. This is not surprising since components of the Reelin-Dab1 pathway begin to be degraded with in a few hours. In 60 day-old animals Golgi extension into dendrites was also reduced (data not shown). Therefore Golgi deployment appears to be a transient, developmental phenomemon. Thus, similar to the manifestation of the multiple axon phenotype caused by Stk25 overexpression or loss of dab1 gene function, the degree of Golgi extension seems to be determined by a competition between Reelin-Dab1 signaling and Stk25 levels.
Discussion
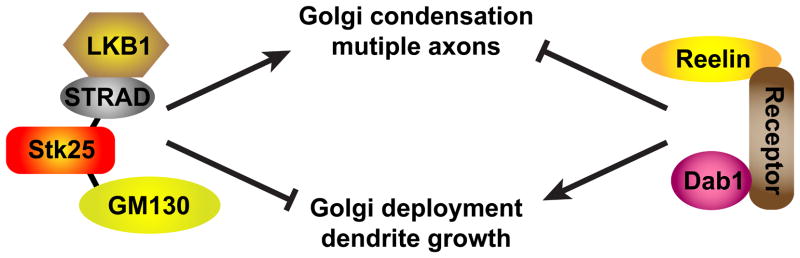
In this study, we find that Reelin-Dab1 signaling acts in an opposing manner to LKB1, GM130, and Stk25 to regulate the polarization of axons, dendrites, and Golgi apparati of hippocampal neurons, as shown in Fig. 7. Knocking down these three proteins led to Golgi fragmentation and inhibited axon initiation (Fig. 1, 3, 4). In contrast, Stk25 overexpression caused Golgi condensation and the formation of multiple axons (Fig. 1, 4). It also rescued axon production and Golgi fragmentation caused by LKB1 knockdown but did not rescue either phenotype caused by reduced GM130 expression (Fig. 3), suggesting that Stk25 functions as an intermediary between LKB1 and GM130. Stk25 directly or indirectly binds to the LKB1-STRAD complex and GM130, and may play a scaffolding role to link LKB1 signaling to GM130 and Golgi regulation (Fig. S2). Reelin-Dab1 signaling antagonizes the effects of Stk25 overexpression on Golgi morphology and neuronal polarization as well as inducing polarized deployment of the Golgi into the apical dendrite (Fig. 1, 4, 6, S1). Together this implicates the LKB1 pathway, GM130, Stk25, and Reelin-Dab1 signaling in Golgi regulation during neuronal polarization.
Figure 7. Model of Stk25 as a scaffolding protein acting competitively with Reelin-Dab1 signaling.
LKB1 is known to act in complex with STRAD to regulate cellular polarity (Alessi et al., 2006). Reelin, the receptors ApoER2 and VLDLR, and Dab1 also form a signaling complex (Hiesberger et al., 1999; Trommsdorff et al., 1998). STK25 coimmunoprecipitates with STRAD and GM130 (Fig. 2S). Overexpression of LKB1, and STRAD are known to induce the formation of multiple axons (Barnes et al., 2007; Shelly et al., 2007). Independent of its kinase activity, STK25 does so also and induces Golgi condensation (Fig. 1F, 4A). Knocking down LKB1, Stk25 or GM130 causes Golgi fragmentation/dispersion and lost axon production; the opposite to Golgi condensation and multiple axon formation (Fig. 1, 3, 4, Barnes et al., 2007; Shelly et al., 2007). The overexpression phenotypes are suppressed by Reelin stimulation. Dab1−/− neurons (Reelin signaling deficient) have multiple axons and shorter dendrites (Fig. 1F, Niu et al., 2004). Reelin stimulation induces Golgi deployment and dendrite growth, phenotypes suppressed by Stk25 expression/overexpression (Fig. 2, 6).
Involvement of the Golgi apparatus in neuronal polarization
The Golgi apparatus and centrosomes reorient as neurons migrate into the cortical plate (de Anda et al., 2010; Nichols and Olson, 2010). At the time of axon initiation, the centrosome is near the basal pole (rear) of the cell. It then moves to the opposite pole (front) and is important for extending an apical process that is used for radial migration (de Anda et al., 2010). The apical process subsequently transforms into the apical dendritic tree, with the Golgi and centrosomes at its base (Barnes et al., 2008; Horton et al., 2005). The same events presumably occur during migration of hippocampal pyramidal neurons in vivo. When hippocampal neurons are cultured, the centrosome position determines which neurite becomes an axon (de Anda et al., 2005). Later, the apical localization of the Golgi apparatus promotes the asymmetric growth of the apical compared to the basal dendrites (Horton et al., 2005). Consistent with this, Stk25 knockdown led to Golgi disorganization, inhibited axon induction, and lessened the asymmetry between the long, thick apical dendrite and short, slender basal dendrites (Fig. 2E, H, J, K).
The Golgi may influence axon initiation through nucleating microtubules, regulating secretory trafficking or interacting with the centrosome (Efimov et al., 2007; Pfenninger, 2009; Rosso et al., 2004; Sutterlin and Colanzi, 2010). It seems less likely that the Golgi is required to supply materials to sustain axon growth, since none of our manipulations affected axon length, only axon number. Therefore, the Golgi probably has a signaling or microtubule nucleation role in axon specification. Indeed, microtubule stabilization has been shown to enhance axon formation (Witte et al., 2008), and inhibiting post-Golgi trafficking disrupts axo-dendritic polarization (Bisbal et al., 2008; Yin et al., 2008). In dendrites, however, the Golgi may have a role in supplying materials for dendrite growth, since we detected effects on dendrite thickness and length (Fig. 2E, 2K). Deployment of the Golgi into the apical dendrite may initiate the formation of dendritic Golgi outposts, which have been shown to promote dendrite growth and branching (Horton et al., 2005; Ye et al., 2007).
We found that Stk25 functions in Golgi morphology and axon specification as part of an LKB1 pathway (Fig. 3, 4). LKB1, the mammalian Par-4 homolog, is an evolutionarily conserved cell polarity protein that is known to regulate axo-dendritic polarity in neurons (Barnes et al., 2008). LKB1 is activated upon binding STRAD and MO25 (Alessi et al., 2006). STRAD stabilized LKB1 in processes prior to axon production and in the nascent axon, suggesting a role in axon specification (Shelly et al., 2007). As a master kinase, LKB1 activates several downstream kinases that regulate various aspects of cell polarity. These include the Sad A and Sad B kinases, which are required for neuronal polarization (Barnes et al., 2007; Kishi et al., 2005). Mst4, another downstream kinase, is closely related to Stk25. Like Stk25, it binds to GM130 and is enriched in the Golgi apparatus (Preisinger et al., 2004). Both Mst4 and Stk25 are required downstream of LKB1-STRAD induction for polarized brush border formation in epithelial cells (ten Klooster et al., 2009; Fig. S2). However, while Mst4 kinase activity is required during this process, the kinase activity of Stk25 is not required to induce polarized brush border formation, regulate Golgi morphogenesis or polarization of hippocampal neurons (Fig. 1F, 4C, 4D). This suggests a kinase-independent scaffolding function for Stk25 (Fig. 7), which is reminiscent of the pseudokinase STRAD (Lizcano et al., 2004). GM130 appears to be necessary for Stk25 effects on Golgi and neuronal polarization; however, it may not be sufficient. By linking LKB1 signaling to GM130, Stk25 may directly regulate GM130 or indirectly modulate the activity of other Golgi proteins.
Reelin-Dab1 signaling regulates neuronal polarization and Golgi deployment
Our work also identifies an effect of Reelin-Dab1 signaling on Golgi morphology and axon formation, acting in opposition to LKB1-Stk25-GM130. The absence of Reelin or Dab1 inhibited Golgi deployment into the apical dendrite in vivo (Fig. 5, S4), and long-term growth in Reelin opposed Golgi condensation induced by Stk25 overexpression in vitro (Fig. 4). Similarly, Dab1 absence induced supernumerary axons in vitro (Fig. 1H), the opposite effect to depleting Stk25. However, Reelin-Dab1 and LKB1-Stk25-GM130 do not fit into a simple epistatic relationship. For example, Stk25 depletion reduces axon number even when Dab1 is absent, suggesting that Stk25 does not require Dab1 to regulate axon number (Fig. 1). This indicates that LKB1-Stk25-GM130 and Reelin-Dab1 act on the Golgi and axon initiation through different pathways, and the balance between the two pathways determines the outcome. In this respect, Golgi distribution is a quantitative trait, not all or none, and may be influenced by other factors. Indeed, extended Golgi were observed in a subset of neurons in reelin−/− and dab1−/− mutant brains (Fig. 5, S4). One possibility is that Reelin-Dab1 and LKB1-Stk25-GM130 regulate different aspects of Golgi morphology through different mechanisms. For example, Reelin-Dab1 may regulate ER-Golgi vesicle movement, and LKB1-Stk25-GM130 may affect vesicle fusion.
In sum, we have characterized Stk25, a modifier of the Reelin-Dab1 pathway, and shown it acts on the LKB1-STRAD pathway to regulate Golgi morphology and neuronal polarization. Stk25 may play a scaffolding role to link LKB1-STRAD to Golgi regulation through binding GM130, since the kinase activity was shown to be dispensable for neuronal polarization and Golgi morphogenesis. We find that Reelin-Dab1 signaling regulates Golgi morphology and deployment into dendrites in a competitive manner with Stk25. Golgi position has been shown to enhance local secretory trafficking (Horton et al., 2005; Ye et al., 2007); thus, this competition may regulate membrane and protein cargo flow into proximal dendrites. Our findings provide new insights into the regulation of morphogenic changes in neurons that drive neuronal polarization and brain lamination.
Experimental Procedures
Expression vectors
The lentiviral vectors used in this study were based on pLentiLox 3.7 (pLL3.7) vectors (Rubinson et al., 2003) with the following substitutions: 1) For shRNA experiments, instead of the CMV promoter, the CMV enhancer/Chicken β-actin promoter (Niwa et al., 1991) directs GFP expression, 2) For fusion protein experiments, instead of the U6 promoter the CMV enhancer/chicken β-actin promoter directs expression. The shRNA constructs include Stk25 shRNA AGGAGCTCCTGAAGCACAAAT and control shRNA AGTAGCTCCTAAAGCACACAT. The lentivirus production was as previously described (Matsuki et al., 2008). The knockdown viruses were confirmed to reduce expression of either Stk25, LKB1 or GM130 (Fig. S1, S3). The Stk25 K49R mutant has previously been reported to be kinase inactive, which we confirmed (Preisinger et al., 2004; and data not shown).
Animals
All animals were used in accordance with protocols approved by the Animal Care and Use Committees of SUNY Upstate Medical University, National Institutes of Neurological Disorders and Stroke, and the Fred Hutchinson Cancer Research Center, following NIH guidelines. Time pregnant mice (C57BL/6 for in vitro experiments and Swiss Webster for in utero electroporations) and rats (Sprague Dawley) were purchased from Charles River Laboratories and Taconic. The dab1 −/− (Howell et al., 1997) and reelin −/− (Jackson Labs) mice were on the C57BL/6 strain.
Immunocytochemistry
Immunocytochemistry was done according to published methods (Matsuki et al., 2008) and is detailed in the extended experimental procedures along with a list of the antibodies used. To measure Golgi volumes and length of the longest Golgi ribbon we immunostained the neurons with anti-GRASP65, anti-GFP, and anti-Ctip2, which recognizes a CA1 and layer V pyramidal neuron-specific transcription factor. The area of the Golgi apparatus was calculated for each Z-plain (Image Examiner, Zeiss), multiplied by the thickness of the section and summed to determine the volume.
Cell culture
Hippocampal neuronal cultures were isolated from E17.5 mice or E18.5 rats and grown in neurobasal supplemented with 2% B27 (Invitrogen, Matsuki et al., 2008). For polarity studies, neurons (1X104 cells per cm2) were infected with the respective viruses on the day of culturing, and replated 2 days later on poly-L-lysine coated coverslips placed over a monolayer of astrocytes. Axons were quantified at 2DIV or 6DIV as indicated, following standard criteria (Shelly et al., 2007). For Golgi deployment assays, rat cultured neurons (3X105 cells per cm2) were infected with low titer virus on day 3 and treated and fixed on day 6 in culture. Similar results were obtained with mouse neurons (data not shown). The control- and Reelin-conditioned media were collected and concentrated as previously described (Matsuki et al., 2008).
Analysis of in utero electroporated brains
To knock down Stk25 expression, DNA was injected into the lateral ventricle of E17.5 embryos of Swiss Webster mice in utero and electroporated (70 mV) as previously described (Olson et al., 2006) with the electrode paddles oriented to direct the DNA into the hippocampus. Perfused brains were processed for analysis on P7. Floating sections (70–100 μm) were immunostained with antibodies described in figure legends. Confocal images were collected with overlapping optical sections through 30 μm, which were flattened for display. We assessed whether axon initial segments or Golgi elements belonged to a particular GFP-positive neuron (Fig. 2), by examining movies of either 3D-rendered images or Z-sections (movies S1, S2). Golgi areas (Fig. 2G, 2H) were produced by thresholding (Adobe Photoshop) flattened, 2D-negative images to match the GRASP65 signal channel in the original and discarding the signal extraneous to the GFP-positive cells (movie S2). Process diameters were measured 12 μm from the nucleus (Fig. 2K). These measurements were done using Image Examiner (Zeiss). Measurement of dendrite lengths was done using the softWoRx (AppliedPrecision).
Supplementary Material
Acknowledgments
We would like to thank Zainab Mansaray and Kristin Giamanco for experimental assistance, Michael Zuber for comments on the manuscript, Hans Clevers for cell lines, Louis Cantley and Jun-ichi Miyazaki for DNA vectors, Arvydas Matiukas and Melissa Pepling for assistance with confocal microscopy and Bonnie Lee Howell for editing. This work was supported by funds from the NINDS intramural program and SUNY Upstate Medical University to B.W.H. and NIH grants NS066071 to E.C.O., NS069660 to R.T.M., CA41072 to J.A.C. and NIA intramural funds for M.R.C. None of the authors have financial conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Förster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- Asada N, Sanada K, Fukada Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J Neurosci. 2007;27:11769–11775. doi: 10.1523/JNEUROSCI.1938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, Sanes JR, Polleux F. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Solecki D, Polleux F. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr Opin Neurobiol. 2008;18:44–52. doi: 10.1016/j.conb.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA, Short B. Golgins in the structure and dynamics of the Golgi apparatus. Curr Opin Cell Biol. 2003;15:405–413. doi: 10.1016/s0955-0674(03)00054-1. [DOI] [PubMed] [Google Scholar]
- Bisbal M, Conde C, Donoso M, Bollati F, Sesma J, Quiroga S, Diaz Anel A, Malhotra V, Marzolo MP, Caceres A. Protein kinase D regulates trafficking of dendritic membrane proteins in developing neurons. J Neurosci. 2008;28:9297–9308. doi: 10.1523/JNEUROSCI.1879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock HH, Herz J. Reelin activates Src family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- Brich J, Shie FS, Howell BW, Li R, Tus K, Wakeland EK, Jin LW, Mumby M, Churchill G, Herz J, et al. Genetic modulation of tau phosphorylation in the mouse. J Neurosci. 2003;23:187–192. doi: 10.1523/JNEUROSCI.23-01-00187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VSJ, Sidman RL. Retrohippocampal, hippocampal and related structures of the forebrain in the reeler mutant mouse. J Comp Neurol. 1973;147:235–254. doi: 10.1002/cne.901470206. [DOI] [PubMed] [Google Scholar]
- Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci. 2009;29:288–299. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–119. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- de Anda FC, Meletis K, Ge X, Rei D, Tsai LH. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci. 2010;30:10391–10406. doi: 10.1523/JNEUROSCI.0381-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG. Centrosome localization determines neuronal polarity. Nature. 2005;436:704–708. doi: 10.1038/nature03811. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Banker GA. Experimentally induced alteration in the polarity of developing neurons. Nature. 1987;330:254–256. doi: 10.1038/330254a0. [DOI] [PubMed] [Google Scholar]
- Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12:917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo M, Fraile M, Pires A, Force T, Pombo C, Zalvide J. CCM3/PDCD10 stabilizes GCKIII proteins to promote Golgi assembly and cell orientation. J Cell Sci. 2010;123:1274–1284. doi: 10.1242/jcs.061341. [DOI] [PubMed] [Google Scholar]
- Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S. Emerging topics in Reelin function. Eur J Neurosci. 2010;31:1511–1518. doi: 10.1111/j.1460-9568.2010.07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet AM. Events governing organization of postmigratory neurons: studies on brain development in normal and reeler mice. Brain Res. 1984;319:261–296. doi: 10.1016/0165-0173(84)90013-4. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- Jacobs T, Causeret F, Nishimura YV, Terao M, Norman A, Hoshino M, Nikolic M. Localized activation of p21-activated kinase controls neuronal polarity and morphology. J Neurosci. 2007;27:8604–8615. doi: 10.1523/JNEUROSCI.0765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M, Rabouille C, Nakamura N, Watson R, Jackman M, Jamsa E, Rahman D, Pappin DJ, Warren G. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell. 1998;94:783–793. doi: 10.1016/s0092-8674(00)81737-7. [DOI] [PubMed] [Google Scholar]
- Marra P, Salvatore L, Mironov A, Jr, Di Campli A, Di Tullio G, Trucco A, Beznoussenko G, Mironov A, De Matteis MA. The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol Biol Cell. 2007;18:1595–1608. doi: 10.1091/mbc.E06-10-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki T, Pramatarova A, Howell BW. Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis. J Cell Sci. 2008;121:1869–1875. doi: 10.1242/jcs.027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AJ, Olson EC. Reelin Promotes Neuronal Orientation and Dendritogenesis during Preplate Splitting. Cereb Cortex. 2010;20:2213–2223. doi: 10.1093/cercor/bhp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D'Arcangelo G. Reelin Promotes Hippocampal Dendrite Development through the VLDLR/ApoER2-Dab1 Pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Olson EC, Kim S, Walsh CA. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci. 2006;26:1767–1775. doi: 10.1523/JNEUROSCI.3000-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nat Rev Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- Preisinger C, Short B, De Corte V, Bruyneel E, Haas A, Kopajtich R, Gettemans J, Barr FA. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol. 2004;164:1009–1020. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8:238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- Rice DS, Nusinowitz S, Azimi AM, Martinez A, Soriano E, Curran T. The reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron. 2001;31:929–941. doi: 10.1016/s0896-6273(01)00436-6. [DOI] [PubMed] [Google Scholar]
- Rosso S, Bollati F, Bisbal M, Peretti D, Sumi T, Nakamura T, Quiroga S, Ferreira A, Caceres A. LIMK1 regulates Golgi dynamics, traffic of Golgi-derived vesicles, and process extension in primary cultured neurons. Mol Biol Cell. 2004;15:3433–3449. doi: 10.1091/mbc.E03-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- Sanada K, Gupta A, Tsai LH. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron. 2004;42:197–211. doi: 10.1016/s0896-6273(04)00222-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Tapia M, Munoz A, Garrido JJ. New role of IKK alpha/beta phosphorylated I kappa B alpha in axon outgrowth and axon initial segment development. Mol Cell Neurosci. 2008;37:832–844. doi: 10.1016/j.mcn.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Sutterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol. 2010;188:621–628. doi: 10.1083/jcb.200910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, Colland F, de Koning J, Maurice MM, Hornbeck P, et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell. 2009;16:551–562. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor-2. Cell. 1999:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728–1736. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DM, Huang YH, Zhu YB, Wang Y. Both the establishment and maintenance of neuronal polarity require the activity of protein kinase D in the Golgi apparatus. J Neurosci. 2008;28:8832–8843. doi: 10.1523/JNEUROSCI.1291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda JF, Rivas RJ. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskeleton. 1998;41:18–38. doi: 10.1002/(SICI)1097-0169(1998)41:1<18::AID-CM2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.